Abstract
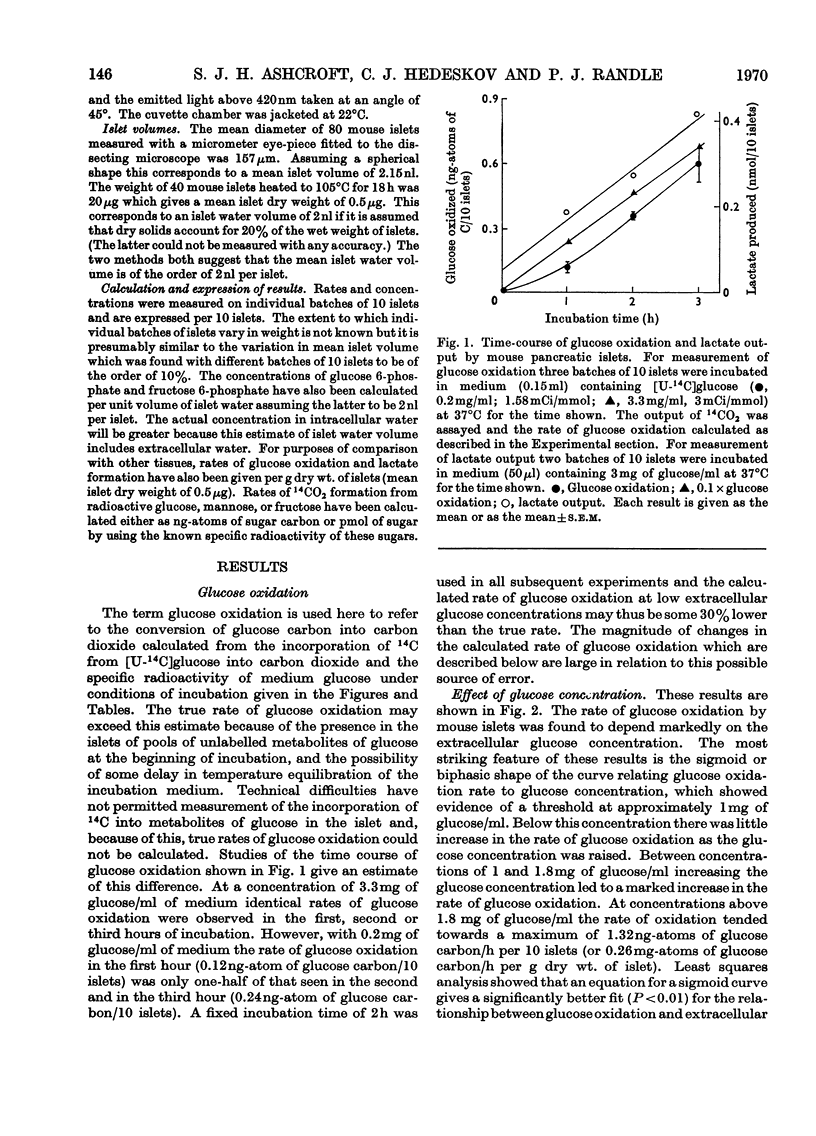
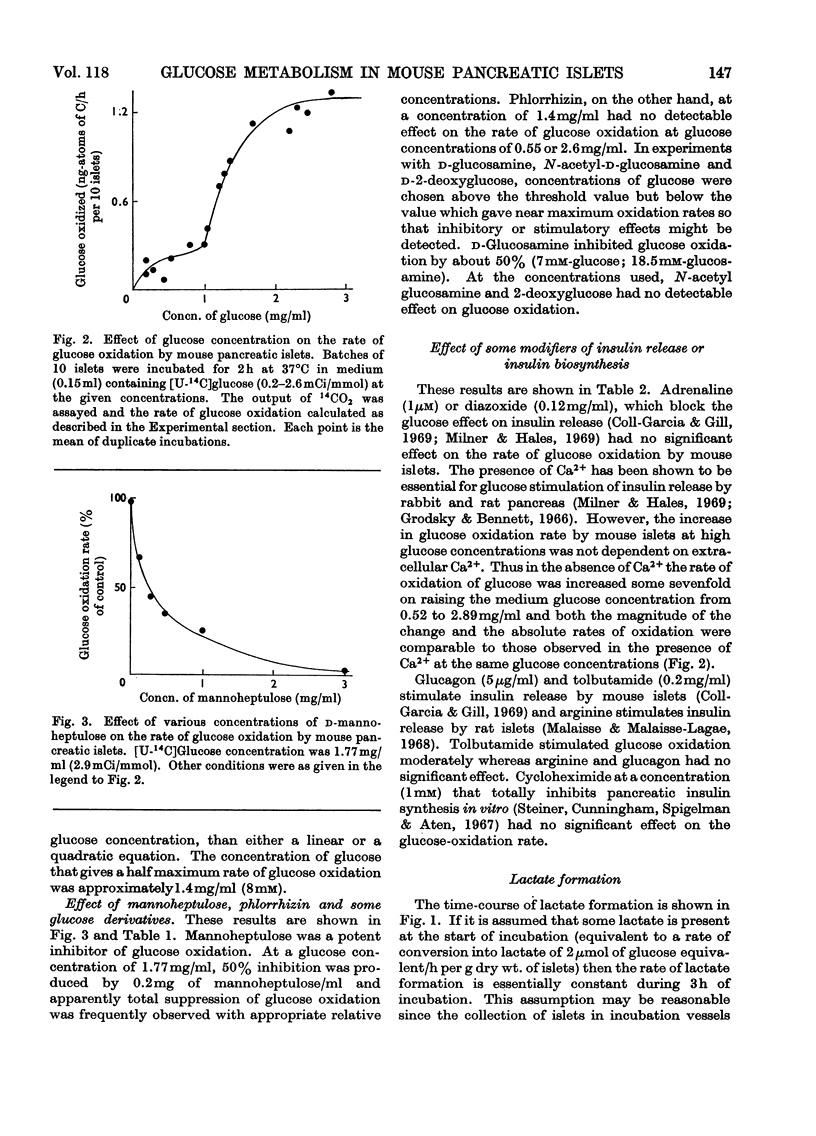
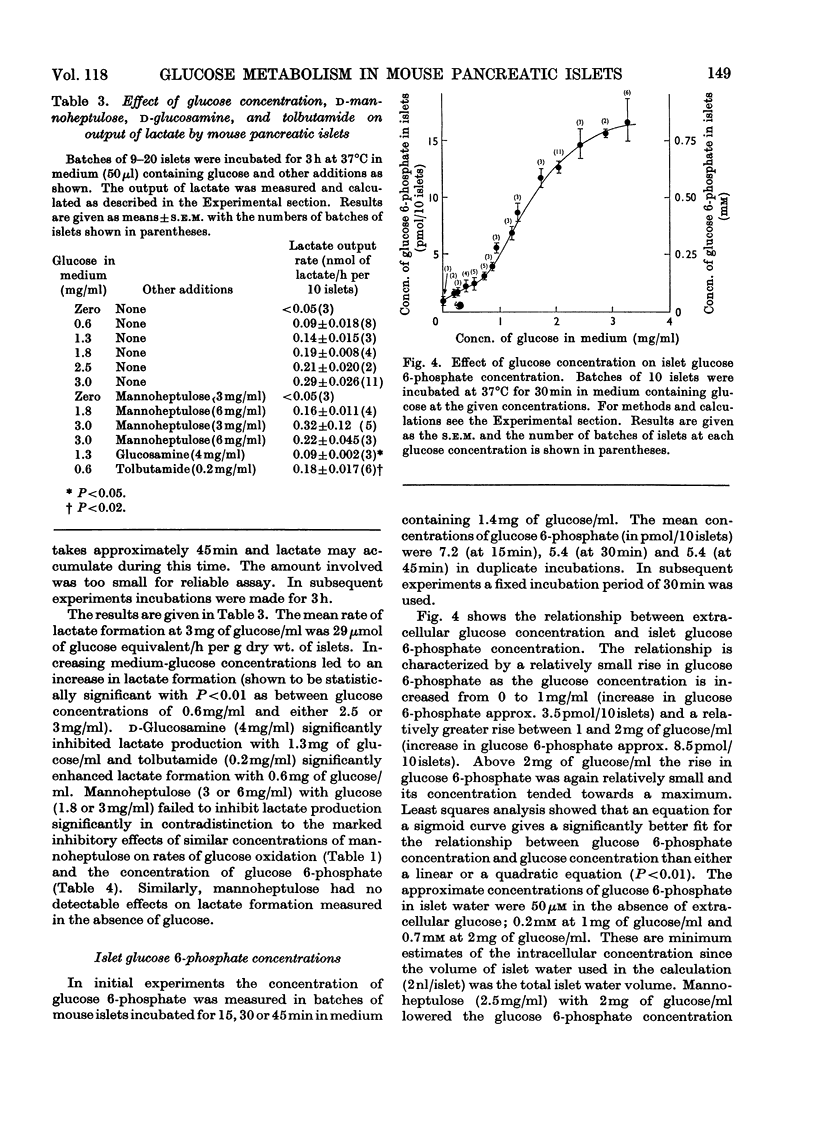
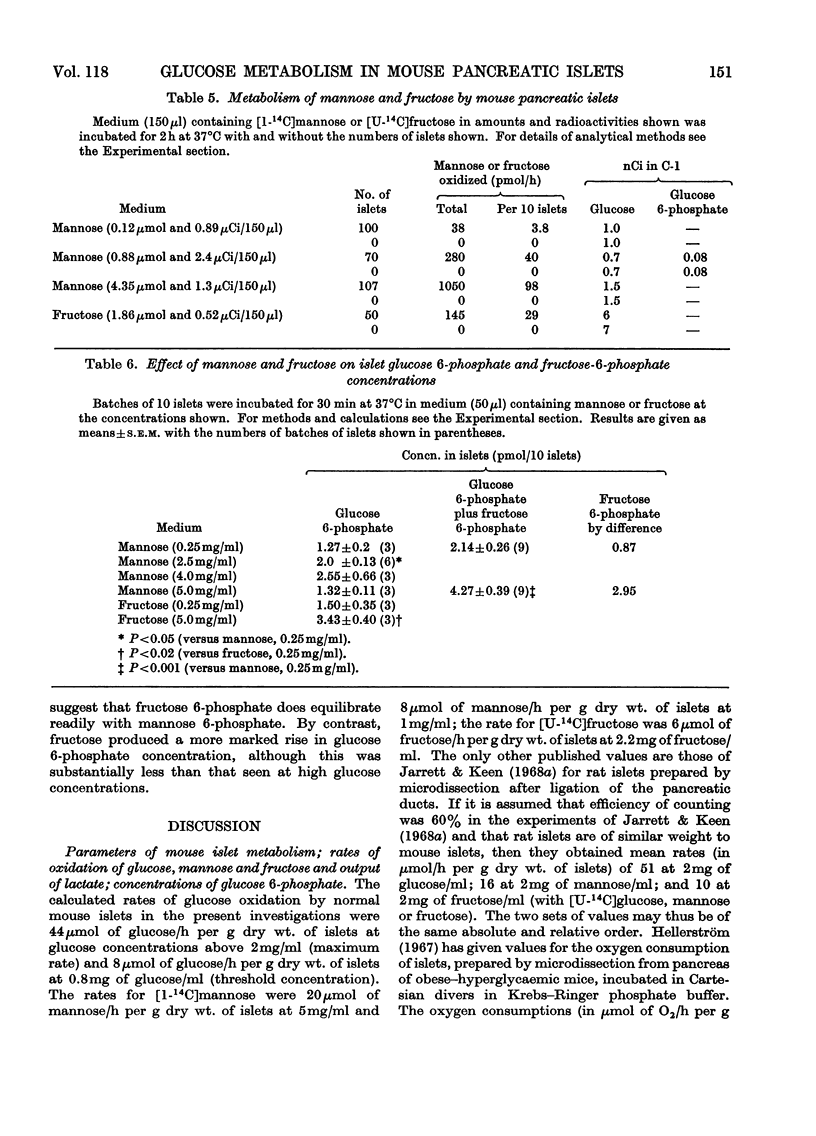
1. Rates of glucose oxidation, lactate output and the intracellular concentration of glucose 6-phosphate were measured in mouse pancreatic islets incubated in vitro. 2. Glucose oxidation rate, measured as the formation of 14CO2 from [U-14C]glucose, was markedly dependent on extracellular glucose concentration. It was especially sensitive to glucose concentrations between 1 and 2mg/ml. Glucose oxidation was inhibited by mannoheptulose and glucosamine but not by phlorrhizin, 2-deoxyglucose or N-acetylglucosamine. Glucose oxidation was slightly stimulated by tolbutamide but was not significantly affected by adrenaline, diazoxide or absence of Ca2+ (all of which may inhibit glucose-stimulated insulin release), by arginine or glucagon (which may stimulate insulin release) or by cycloheximide (which may inhibit insulin synthesis). 3. Rates of lactate formation were dependent on the extracellular glucose concentration and were decreased by glucosamine though not by mannoheptulose; tolbutamide increased the rate of lactate output. 4. Islet glucose 6-phosphate concentration was also markedly dependent on extracellular glucose concentration and was diminished by mannoheptulose or glucosamine; tolbutamide and glucagon were without significant effect. Mannose increased islet fructose 6-phosphate concentration but had little effect on islet glucose 6-phosphate concentration. Fructose increased islet glucose 6-phosphate concentration but to a much smaller extent than did glucose. 5. [1-14C]Mannose and [U-14C]fructose were also oxidized by islets but less rapidly than glucose. Conversion of [1-14C]mannose into [1-14C]glucose 6-phosphate or [1-14C]glucose could not be detected. It is concluded that metabolism of mannose is associated with poor equilibration between fructose 6-phosphate and glucose 6-phosphate. 6. These results are consistent with the idea that glucose utilization in mouse islets may be limited by the rate of glucose phosphorylation, that mannoheptulose and glucosamine may inhibit glucose phosphorylation and that effects of glucose on insulin release may be mediated through metabolism of the sugar.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft S. J., Randle P. J. Glucose-6-phosphatase activity of mouse pancreatic islets. Nature. 1968 Aug 24;219(5156):857–858. doi: 10.1038/219857a0. [DOI] [PubMed] [Google Scholar]
- COORE H. G., RANDLE P. J., SIMON E., KRAICER P. F., SHELESNYAK M. C. Block of insulin secretion from the pancreas by D-mannoheptulose. Nature. 1963 Mar 30;197:1264–1266. doi: 10.1038/1971264a0. [DOI] [PubMed] [Google Scholar]
- Coll-Garcia E., Gill J. R. Insulin release by isolated pancreatic islets of the mouse incubated in vitro. Diabetologia. 1969 Apr;5(2):61–66. doi: 10.1007/BF01211999. [DOI] [PubMed] [Google Scholar]
- Coore H. G., Randle P. J. Inhibition of glucose phosphorylation by mannoheptulose. Biochem J. 1964 Apr;91(1):56–59. doi: 10.1042/bj0910056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coore H. G., Randle P. J. Regulation of insulin secretion studied with pieces of rabbit pancreas incubated in vitro. Biochem J. 1964 Oct;93(1):66–78. doi: 10.1042/bj0930066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodsky G. M., Bennett L. L. Cation requirements for insulin secretion in the isolated perfused pancreas. Diabetes. 1966 Dec;15(12):910–913. doi: 10.2337/diab.15.12.910. [DOI] [PubMed] [Google Scholar]
- Hellerström C. Effects of carbohydrates on the oxygen consumption of isolated pancreatic islets of mice. Endocrinology. 1967 Jul;81(1):105–112. doi: 10.1210/endo-81-1-105. [DOI] [PubMed] [Google Scholar]
- Hernández A., Sols A. Transport and phosphorylation of sugars in adipose tissue. Biochem J. 1963 Jan;86(1):166–172. doi: 10.1042/bj0860166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell S. L., Taylor K. W. Effects of diazoxide on insulin secretion in vitro. Lancet. 1966 Jan 15;1(7429):128–129. doi: 10.1016/s0140-6736(66)91263-3. [DOI] [PubMed] [Google Scholar]
- Jarrett R. J., Keen H. Glucose metabolism of isolated mammalian islets of Langerhans; effects of glucagon, 2-deoxy-D-glucose and D-mannoheptulose. Diabetologia. 1968 Nov;4(5):249–252. doi: 10.1007/BF01309895. [DOI] [PubMed] [Google Scholar]
- Jarrett R. J., Keen H. Oxidation of sugars, other than glucose, by isolated mammalian islets of Langerhans. Metabolism. 1968 Feb;17(2):155–157. doi: 10.1016/0026-0495(68)90142-x. [DOI] [PubMed] [Google Scholar]
- LACY P. E. Electron microscopy of the beta cell of the pancreas. Am J Med. 1961 Dec;31:851–859. doi: 10.1016/0002-9343(61)90024-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., SCHULZ D. W., ROCK M. K. The measurement of pyridine nucleotides by enzymatic cycling. J Biol Chem. 1961 Oct;236:2746–2755. [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F. Stimulation of insulin secretion by noncarbohydrate metabolites. J Lab Clin Med. 1968 Sep;72(3):438–448. [PubMed] [Google Scholar]
- Malaisse W., Malaisse-Lagae F., Wright P. H. A new method for the measurement in vitro of pancreatic insulin secretion. Endocrinology. 1967 Jan;80(1):99–108. doi: 10.1210/endo-80-1-99. [DOI] [PubMed] [Google Scholar]
- Matschinsky F. M., Ellerman J. E. Metabolism of glucose in the islets of Langerhans. J Biol Chem. 1968 May 25;243(10):2730–2736. [PubMed] [Google Scholar]
- Milner R. D., Hales C. N. The interaction of various inhibitors and stimuli of insulin release studied with rabbit pancreas in vitro. Biochem J. 1969 Jul;113(3):473–479. doi: 10.1042/bj1130473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague W., Taylor K. W. Islet-cell metabolism during insulin release. Effects of glucose, citrate, octanoate, tolbutamide, glucagon and theophylline. Biochem J. 1969 Nov;115(2):257–262. doi: 10.1042/bj1150257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague W., Taylor K. W. Pentitols and insulin release by isolated rat islets of Langerhans. Biochem J. 1968 Sep;109(3):333–339. doi: 10.1042/bj1090333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOLS A., CRANE R. K. Substrate specificity of brain hexokinase. J Biol Chem. 1954 Oct;210(2):581–595. [PubMed] [Google Scholar]
- Steiner D. F., Cunningham D., Spigelman L., Aten B. Insulin biosynthesis: evidence for a precursor. Science. 1967 Aug 11;157(3789):697–700. doi: 10.1126/science.157.3789.697. [DOI] [PubMed] [Google Scholar]
- TäljedalIB Presence, induction and possible role of glucose 6-phosphatase in mammalian pancreatic islets. Biochem J. 1969 Sep;114(2):387–394. doi: 10.1042/bj1140387. [DOI] [PMC free article] [PubMed] [Google Scholar]


