Abstract
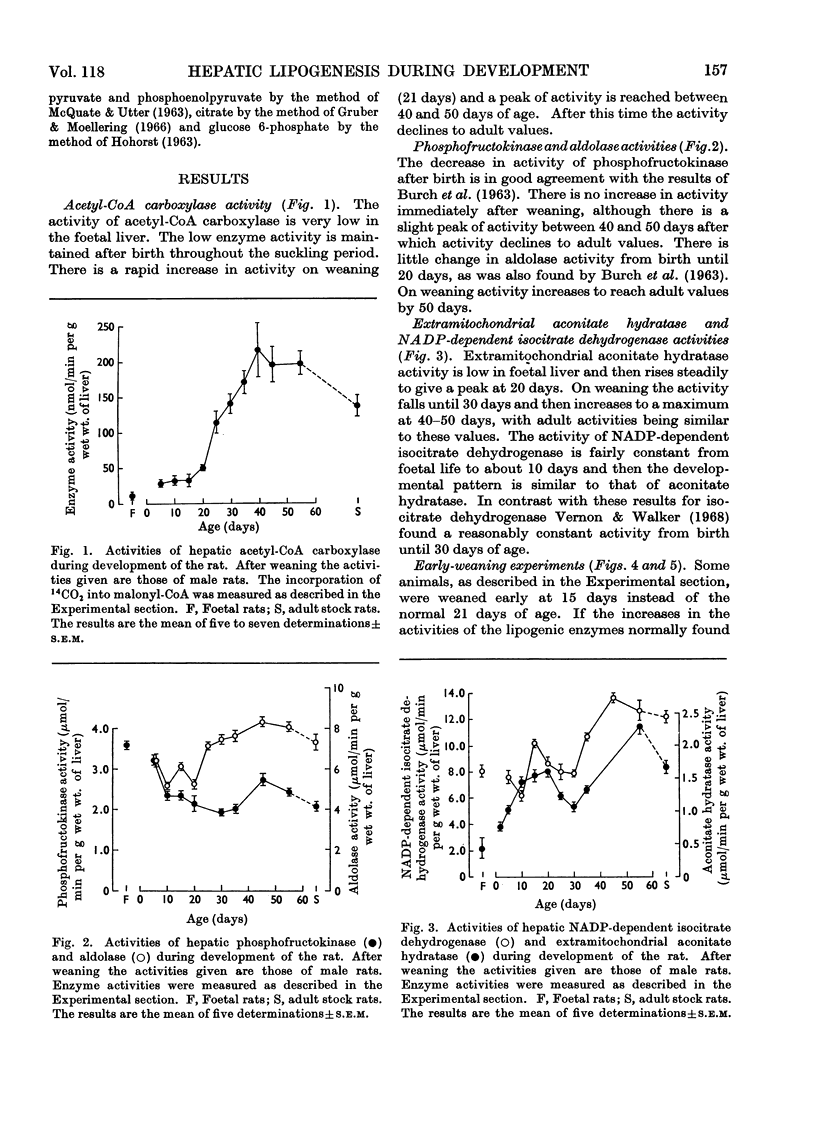
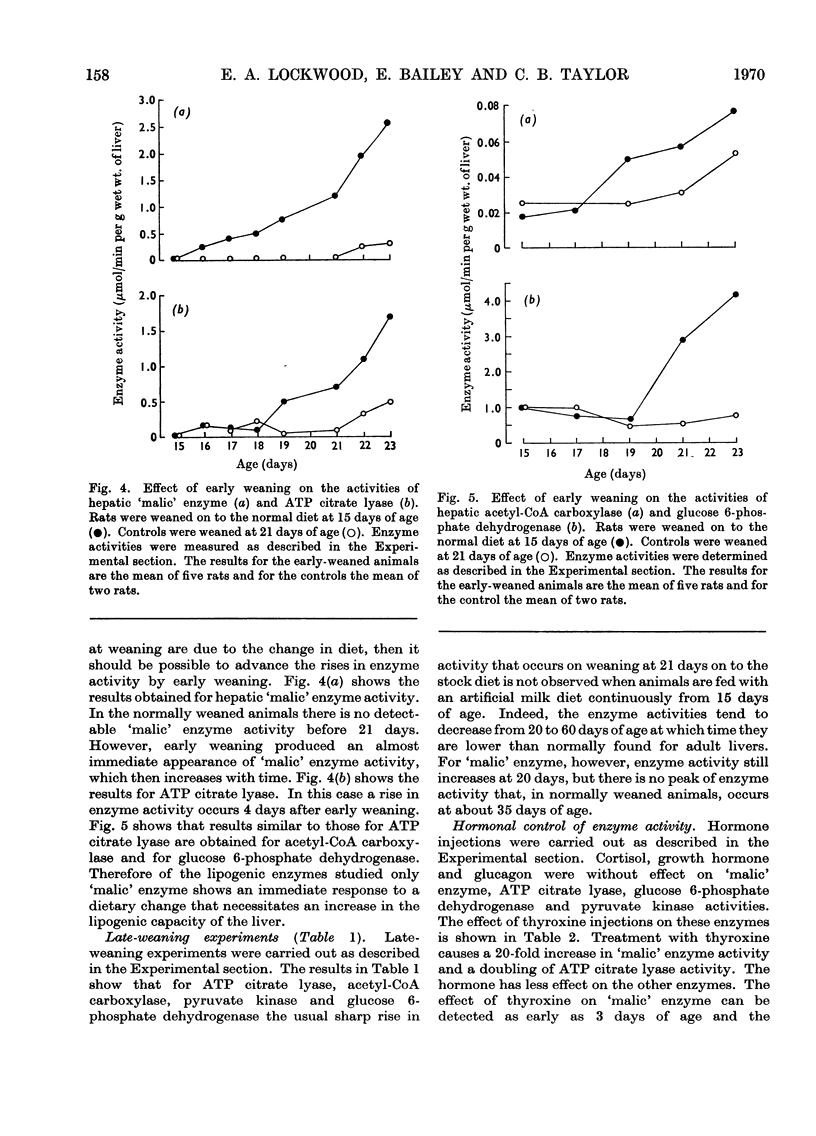
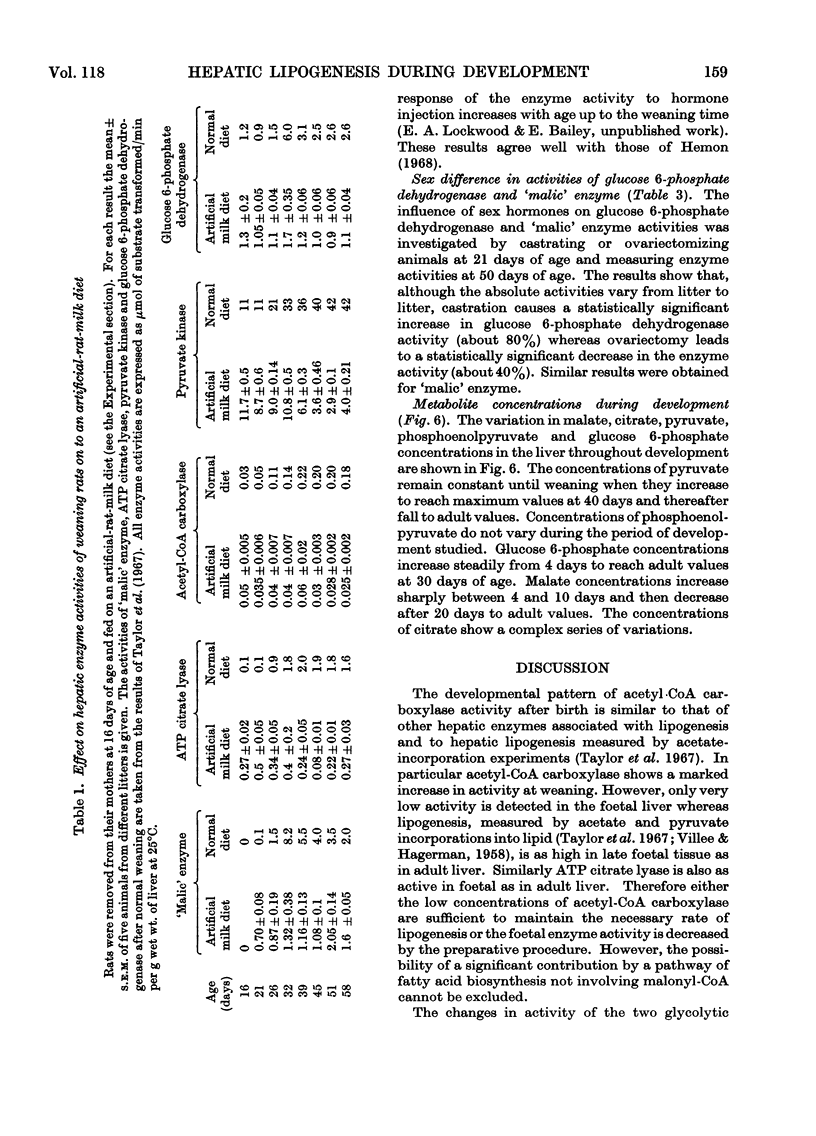
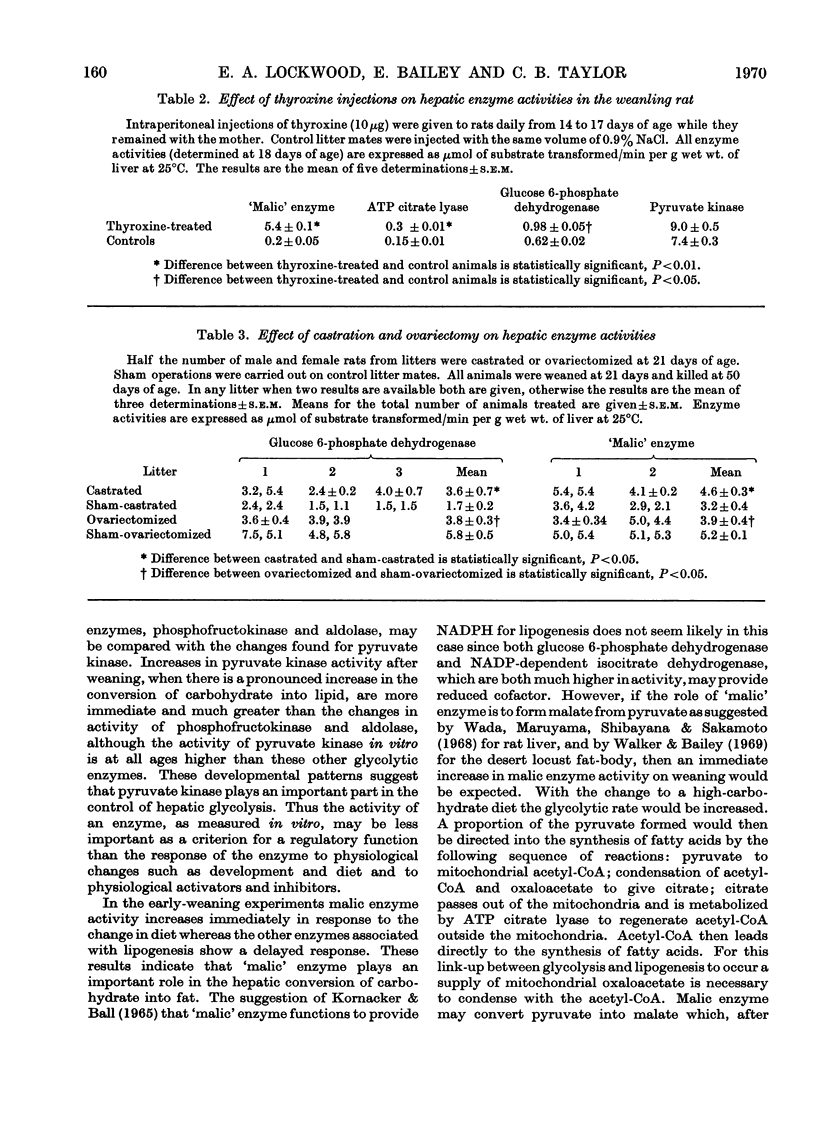
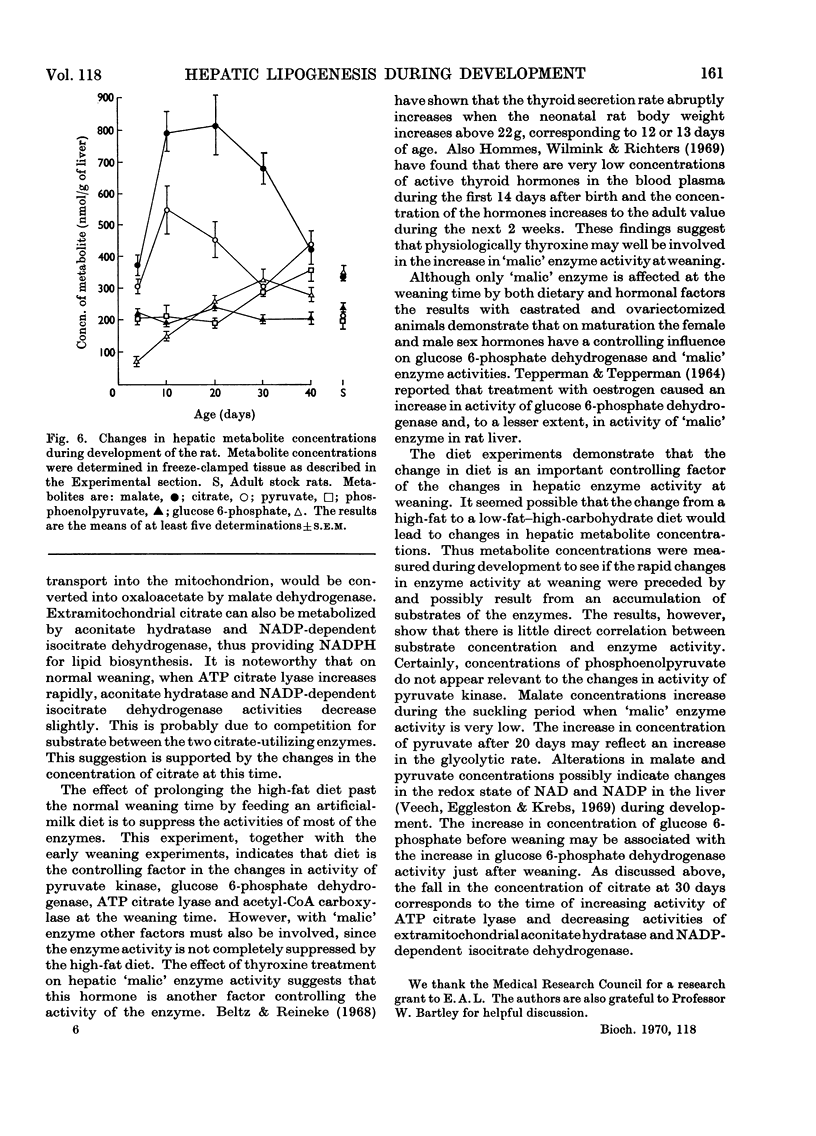
1. Changes in the activities of acetyl-CoA carboxylase (EC 6.4.1.2), phosphofructokinase (EC 2.7.1.11), aldolase (EC 4.1.2.13), extramitochondrial aconitate hydratase (EC 4.2.1.3) and NADP-dependent isocitrate dehydrogenase (EC 1.1.1.42) have been measured in the livers of developing rats from late foetal life to maturity. 2. The effect of altering the weaning time on some enzymes associated with lipogenesis has been studied. Weaning rats at 15 days of age instead of 21 days results in an immediate increase in the activity of `malic' enzyme (EC 1.1.1.40) whereas the activities of glucose 6-phosphate dehydrogenase (EC 1.1.1.49) and ATP citrate lyase (EC 4.1.3.8) did not increase until 4–5 days and acetyl-CoA carboxylase 2–3 days after early weaning. Weaning rats on to an artificial-milk diet led to complete repression of the rise in activity of hepatic enzymes associated with lipogenesis normally found on weaning, except for `malic' enzyme, which increased in activity after 20 days of age. 3. The effect of intraperitoneal injections of glucagon, cortisol, growth hormone and thyroxine on the same hepatic enzymes has been investigated. Only thyroxine had any effect on enzyme activities and caused a 20-fold increase in `malic' enzyme activity and a twofold increase in ATP citrate lyase activity. 4. The activities of hepatic glucose 6-phosphate dehydrogenase and `malic' enzyme are higher in adult female than in adult male rats and it has been shown that this sex difference in enzyme activities is due to both male and female sex hormones. 5. Hepatic malate, citrate, pyruvate, glucose 6-phosphate and phosphoenolpyruvate concentrations have been measured throughout development. 6. The results are discussed in relation to the dietary and hormonal control of hepatic enzyme activities during development.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURCH H. B., LOWRY O. H., KUHLMAN A. M., SKERJANCE J., DIAMANT E. J., LOWRY S. R., VON DIPPE P. Changes in patterns of enzymes of carbohydrate metabolism in the developing rat liver. J Biol Chem. 1963 Jul;238:2267–2273. [PubMed] [Google Scholar]
- Ballard F. J., Hanson R. W. Changes in lipid synthesis in rat liver during development. Biochem J. 1967 Mar;102(3):952–958. doi: 10.1042/bj1020952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz A. D., Reineke E. P. Thyroid secretion rate in the neonatal rat. Gen Comp Endocrinol. 1968 Feb;10(1):103–108. doi: 10.1016/0016-6480(68)90015-4. [DOI] [PubMed] [Google Scholar]
- Gregolin C., Ryder E., Lane M. D. Liver acetyl coenzyme A carboxylase. I. Isolation and cat- alytic properties. J Biol Chem. 1968 Aug 25;243(16):4227–4235. [PubMed] [Google Scholar]
- Guarriera-Bobyleva V., Buffa P. The inhibition by fluorocitrate of rat liver mitochondrial and extramitochondrial aconitate hydratase. Biochem J. 1969 Aug;113(5):853–860. doi: 10.1042/bj1130853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemon P. Malate dehydrogenase (decarboxylating) (NADP) and alpha-glycerophosphate oxidase in the developing rat. Biochim Biophys Acta. 1968 Mar 25;151(3):681–683. doi: 10.1016/0005-2744(68)90016-8. [DOI] [PubMed] [Google Scholar]
- Hommes F. A., Wilmink C. W., Richters A. The development of thyroid function in the rat. Biol Neonat. 1969;14(1):69–73. doi: 10.1159/000240171. [DOI] [PubMed] [Google Scholar]
- Kornacker M. S., Ball E. G. Citrate cleavage in adipose tissue. Proc Natl Acad Sci U S A. 1965 Sep;54(3):899–904. doi: 10.1073/pnas.54.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANG C. A. RESPIRATORY ENZYMES IN THE HEART AND LIVER OF THE PRENATAL AND POSTNATAL RAT. Biochem J. 1965 May;95:365–371. doi: 10.1042/bj0950365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto R. E., Bartley W. The effect of age and sex on glutathione reductase and glutathione peroxidase activities and on aerobic glutathione oxidation in rat liver homogenates. Biochem J. 1969 Mar;112(1):109–115. doi: 10.1042/bj1120109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEPPERMAN H. M., TEPPERMAN J. PATTERNS OF DIETARY AND HORMONAL INDUCTION OF CERTAIN NADP-LINKED LIVER ENZYMES. Am J Physiol. 1964 Feb;206:357–361. doi: 10.1152/ajplegacy.1964.206.2.357. [DOI] [PubMed] [Google Scholar]
- Taylor C. B., Bailey E., Bartley W. Changes in hepatic lipigenesis during development of the rat. Biochem J. 1967 Nov;105(2):717–722. doi: 10.1042/bj1050717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNDERWOOD A. H., NEWSHOLME E. A. PROPERTIES OF PHOSPHOFRUCTOKINASE FROM RAT LIVER AND THEIR RELATION TO THE CONTROL OF GLYCOLYSIS AND GLUCONEOGENESIS. Biochem J. 1965 Jun;95:868–875. doi: 10.1042/bj0950868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAGELOS P. R., ALBERTS A. W., MARTIN D. B. Studies on the mechnism of activation of acetyl coenzyme A carboxylase by citrate. J Biol Chem. 1963 Feb;238:533–540. [PubMed] [Google Scholar]
- VILLEE C. A., HAGERMAN D. D. Effect of oxygen deprivation on the metabolism of fetal and adult tissues. Am J Physiol. 1958 Sep;194(3):457–464. doi: 10.1152/ajplegacy.1958.194.3.457. [DOI] [PubMed] [Google Scholar]
- Veech R. L., Eggleston L. V., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide phosphate in the cytoplasm of rat liver. Biochem J. 1969 Dec;115(4):609–619. doi: 10.1042/bj1150609a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon R. G., Walker D. G. Changes in activity of some enzymes involved in glucose utilization and formation in developing rat liver. Biochem J. 1968 Jan;106(2):321–329. doi: 10.1042/bj1060321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada F., Maruyama E., Shibayama K., Sakamoto Y. Physiological role of malic enzymes in the liver. J Biochem. 1968 Jun;63(6):805–807. doi: 10.1093/oxfordjournals.jbchem.a128849. [DOI] [PubMed] [Google Scholar]
- Walker P. R., Bailey E. Role of "malic enzyme" in lipogenesis. Biochim Biophys Acta. 1969 Dec 17;187(4):591–593. [PubMed] [Google Scholar]


