Abstract
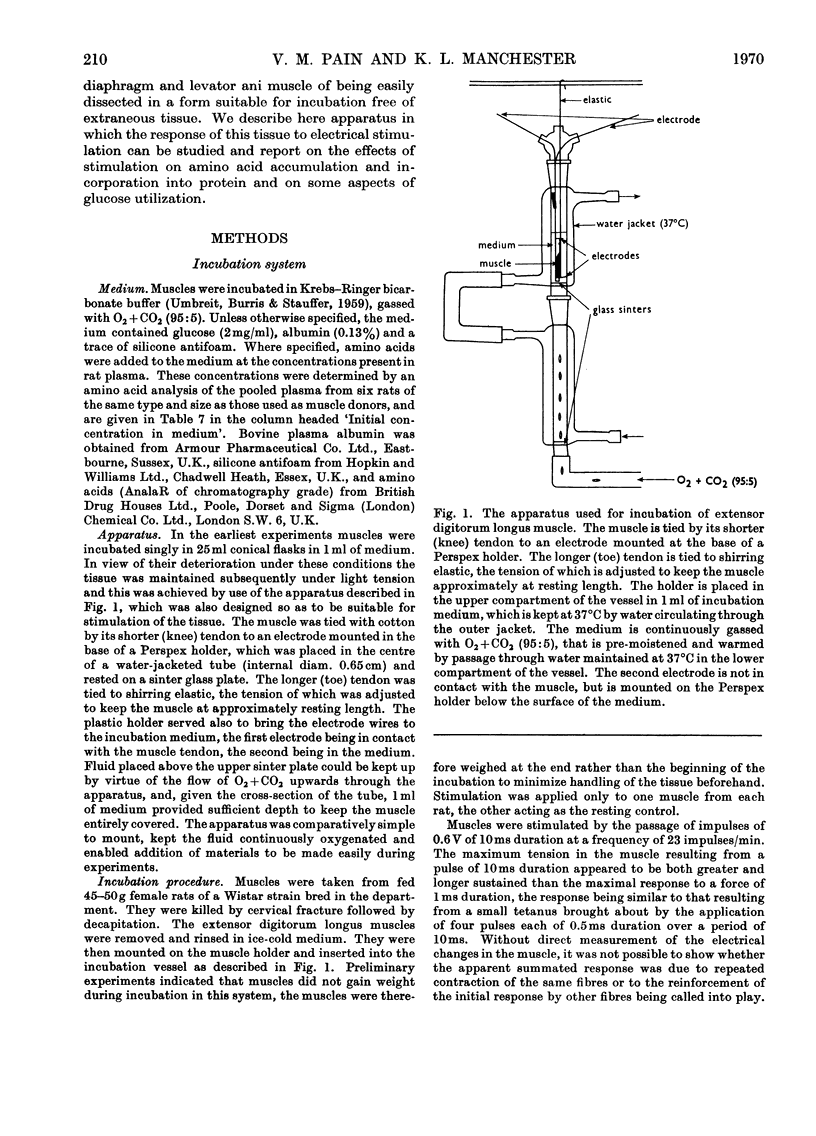
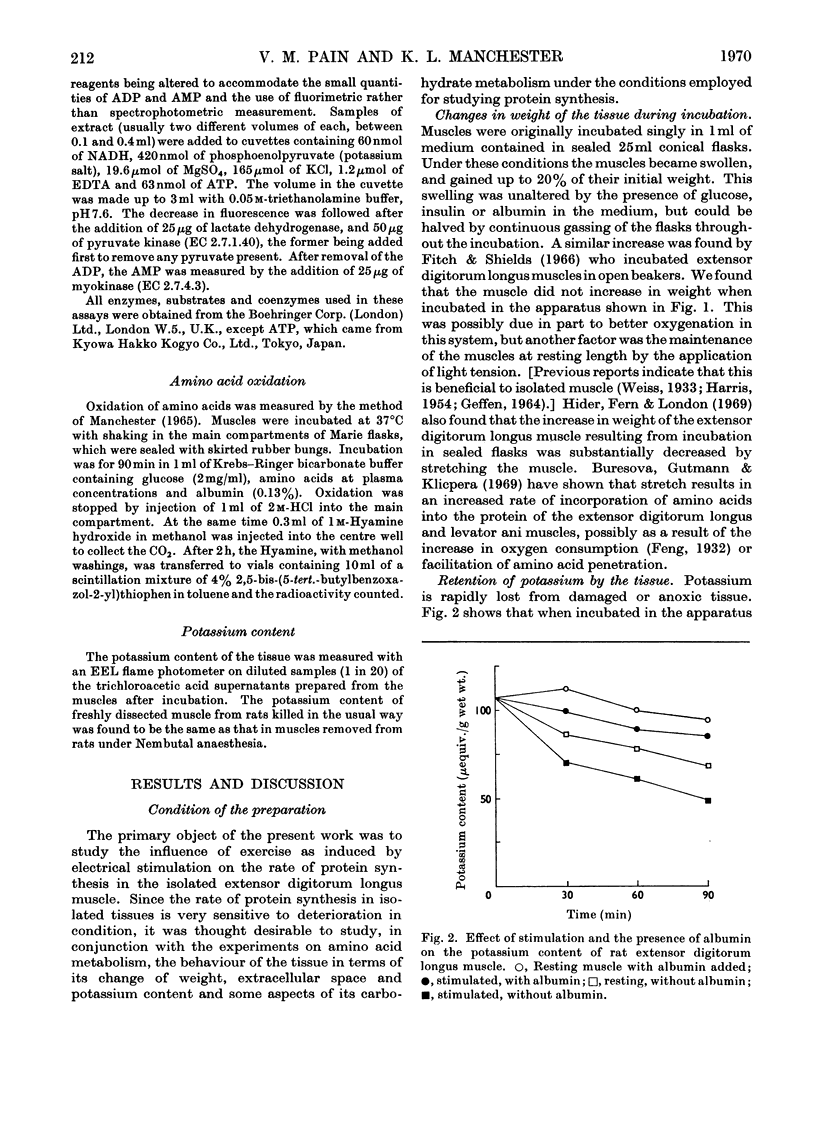
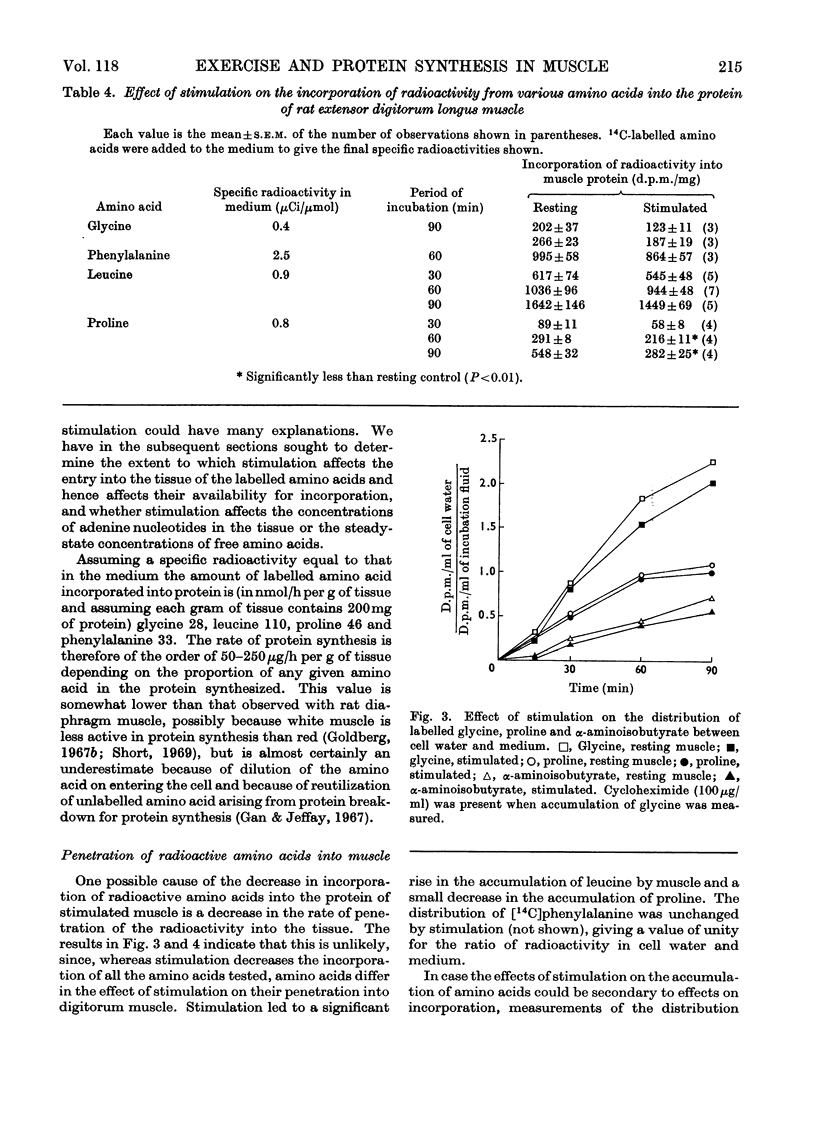
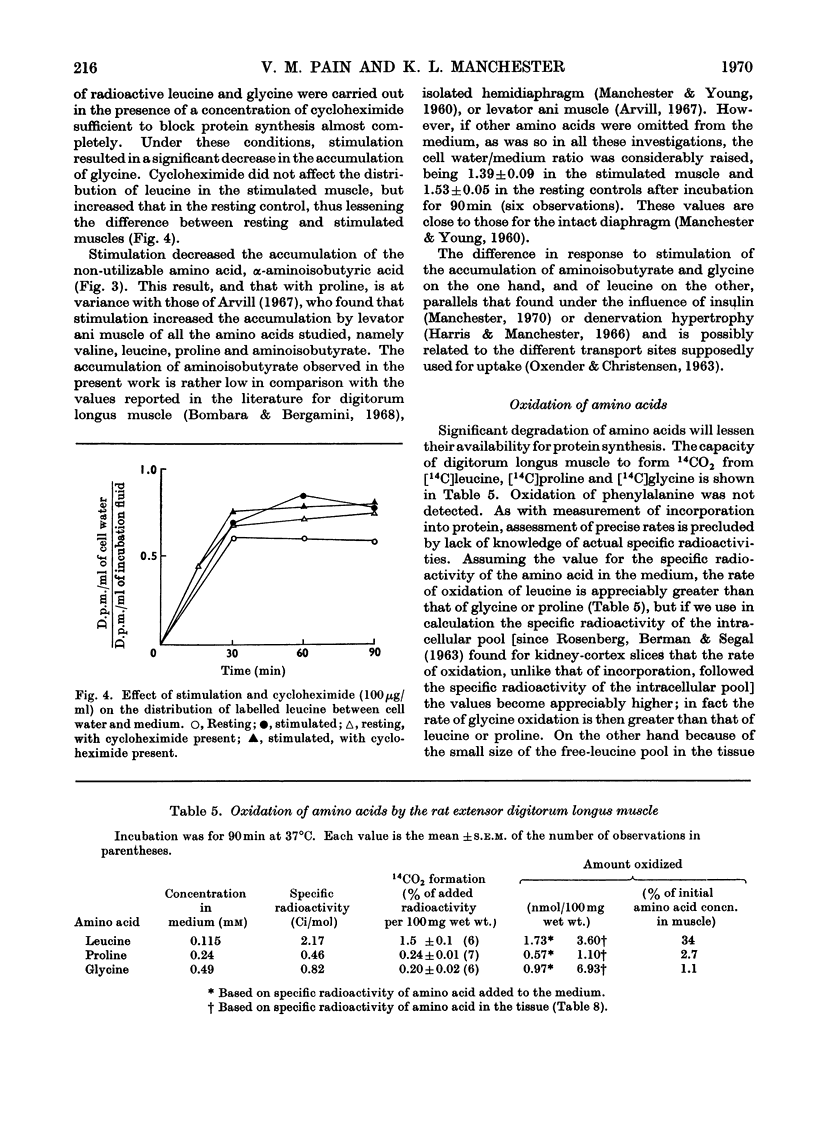
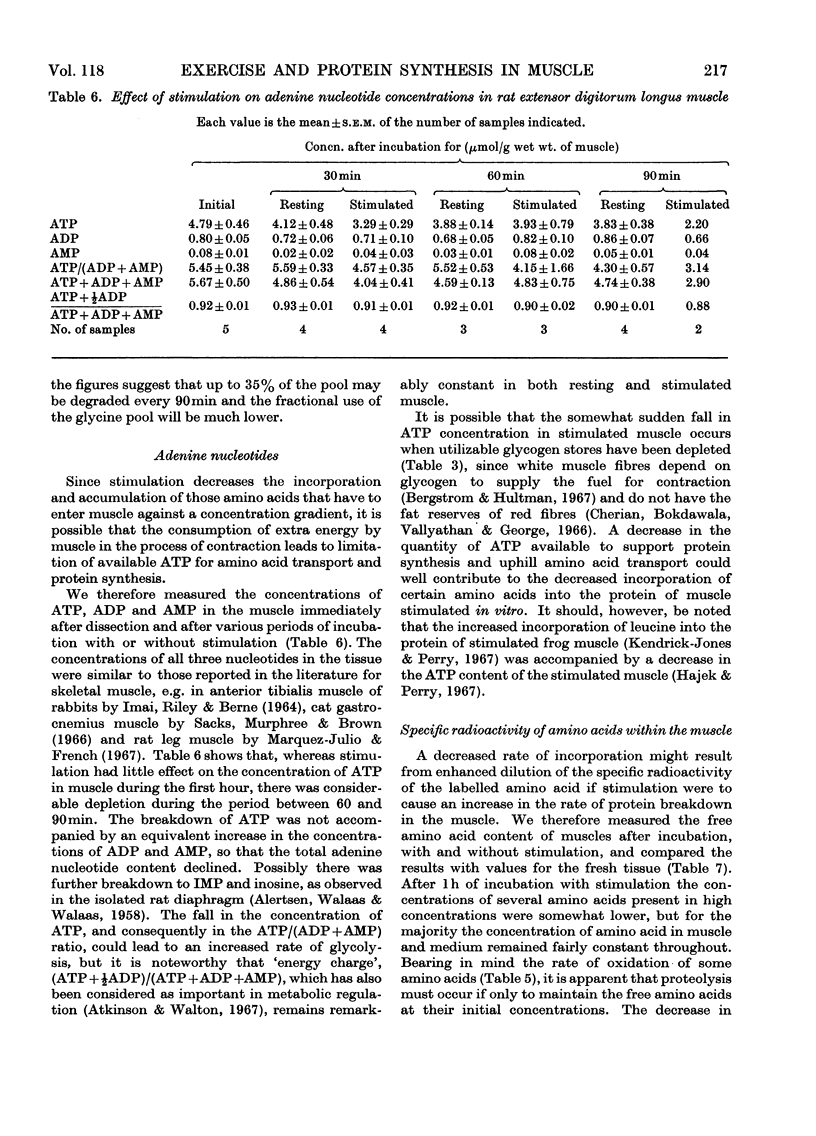
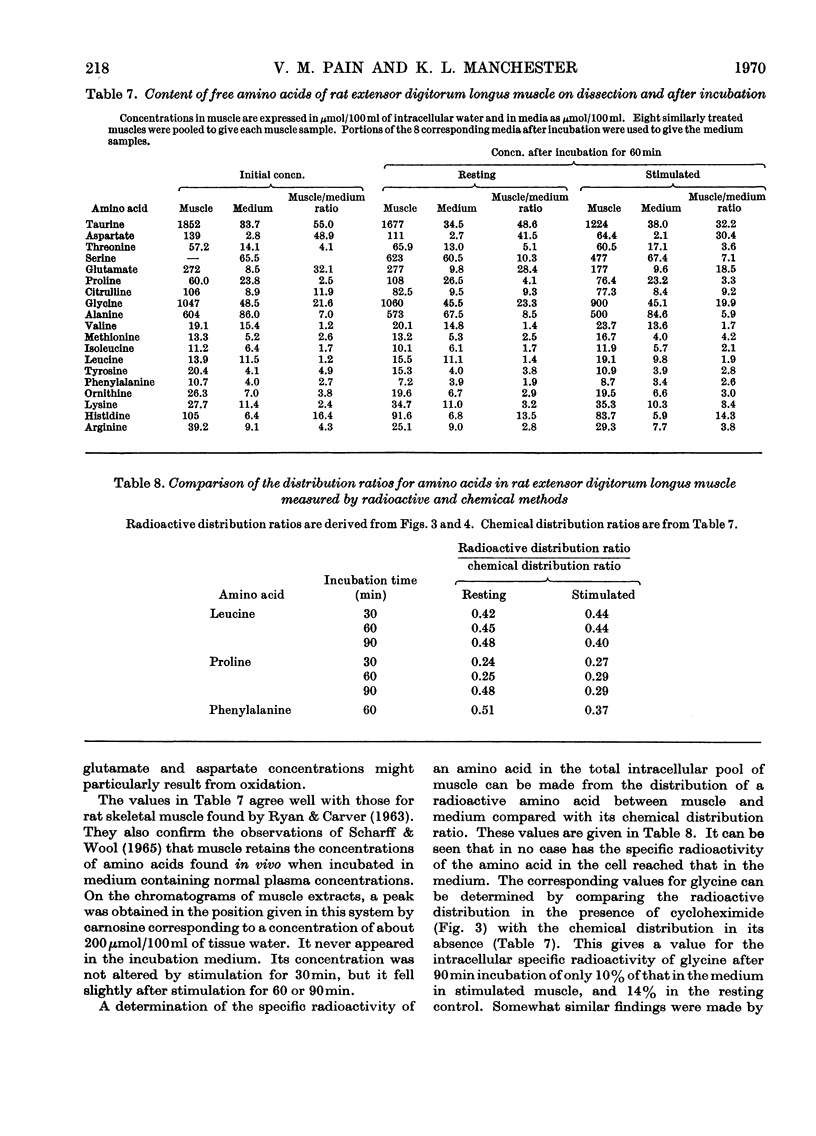
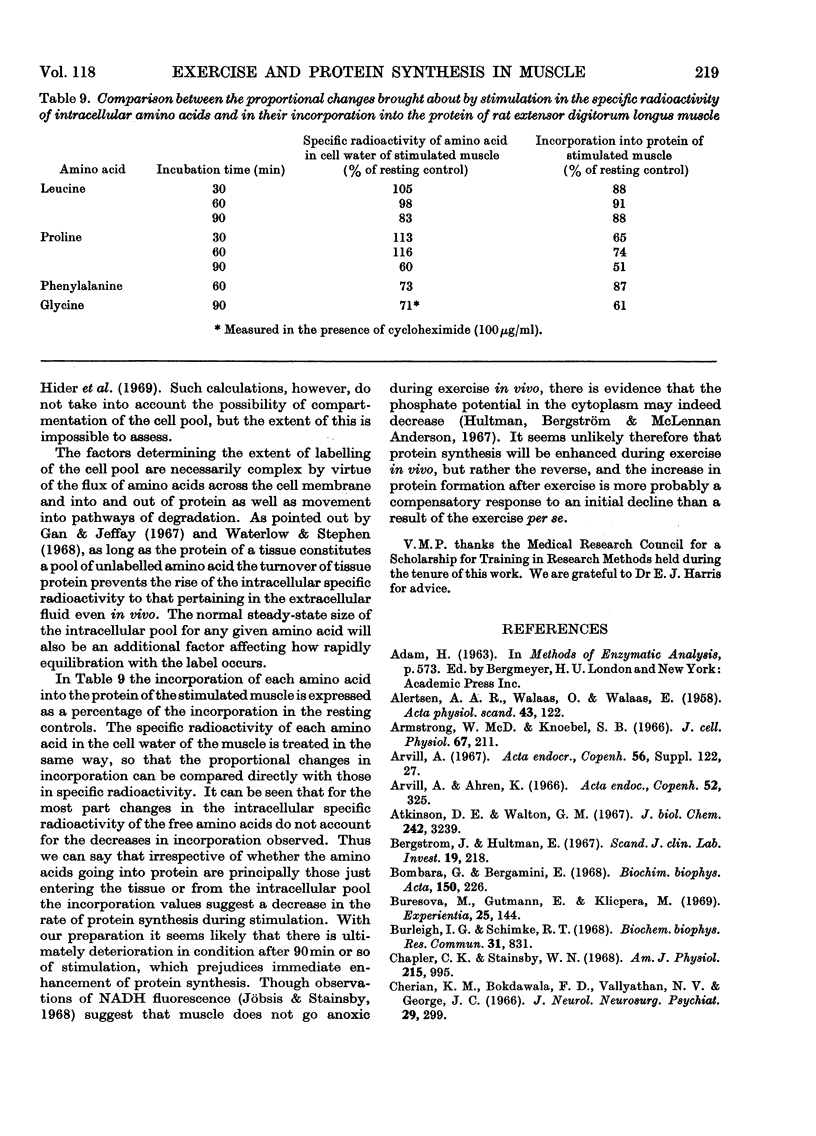
1. Apparatus is described in which rat extensor digitorum longus muscle can be incubated in buffer under conditions of light tension and be subject to contractures induced by electrical stimulation in vitro. Under these conditions the tissue retains its weight, its content of potassium and size of the extracellular space at values similar to those in vivo. 2. Though uptake of glucose was enhanced on addition of insulin, there was little increase in glucose consumption on stimulation. Breakdown of glycogen and enhancement of lactate output were found on stimulation. 3. Incorporation into protein of several labelled amino acids was diminished during stimulation. Accumulation of [14C]leucine was enhanced whereas that of glycine was decreased. 4. There were no very consistent changes in the content of free unlabelled amino acids during incubation with or without stimulation. Comparison of actual amino acid concentrations in tissue and incubation mixture with accumulation of 14C-labelled amino acid indicated that full equilibration of the cell pool of amino amino acids with the medium is slow. 5. Substantial oxidation of several 14C-labelled acids was observed. 6. The ATP content of the tissue declined a little during incubation and somewhat faster after a period of stimulation. 7. The results are discussed in relation to the way in which exercise can induce muscle hypertrophy.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALERTSEN A. R., WALAAS O., WALAAS E. Enzymic conversion of mononucleotides by rat diaphragm in vitro. Acta Physiol Scand. 1958 Aug 25;43(2):122–134. doi: 10.1111/j.1748-1716.1958.tb01582.x. [DOI] [PubMed] [Google Scholar]
- Armstrong W. M., Knoebel S. B. The effect of serum albumin on the efflux of K-42 from frog sartorius muscle. J Cell Physiol. 1966 Apr;67(2):211–216. doi: 10.1002/jcp.1040670202. [DOI] [PubMed] [Google Scholar]
- Arvill A., Hjalmarson A. Effects of growth hormone on the metabolism of the isolated levator ani muscle of the rat. Acta Endocrinol (Copenh) 1967;56(Suppl):15+–15+. [PubMed] [Google Scholar]
- Atkinson D. E., Walton G. M. Adenosine triphosphate conservation in metabolic regulation. Rat liver citrate cleavage enzyme. J Biol Chem. 1967 Jul 10;242(13):3239–3241. [PubMed] [Google Scholar]
- Bergström J., Hultman E. A study of the glycogen metabolism during exercise in man. Scand J Clin Lab Invest. 1967;19(3):218–228. doi: 10.3109/00365516709090629. [DOI] [PubMed] [Google Scholar]
- Bombara G., Bergamini E. Alpha-aminoisobutyric acid uptake in vitro by the rat extensor digitorum longus muscle after denervation and tenotomy. Biochim Biophys Acta. 1968 Mar 1;150(2):226–236. doi: 10.1016/0005-2736(68)90166-1. [DOI] [PubMed] [Google Scholar]
- Buresová M., Gutmann E., Klicpera M. Effect of tension upon rate of incorporation of amino acids into proteins of cross-striated muscle. Experientia. 1969 Feb 15;25(2):144–145. doi: 10.1007/BF01899088. [DOI] [PubMed] [Google Scholar]
- Burleigh G., Schimke R. T. On the activities of some enzymes concerned with glycolysis and glycogenolysis in extracts of rabbit skeletal muscles. Biochem Biophys Res Commun. 1968 Jun 10;31(5):831–836. doi: 10.1016/0006-291x(68)90638-4. [DOI] [PubMed] [Google Scholar]
- Chapler C. K., Stainsby W. N. Carbohydrate metabolism in contracting dog skeletal muscle in situ. Am J Physiol. 1968 Nov;215(5):995–1004. doi: 10.1152/ajplegacy.1968.215.5.995. [DOI] [PubMed] [Google Scholar]
- Cherian K. M., Bokdawala F. D., Vallyathan N. V., George J. C. Effect of denervation on the red and white fibres of the pectoralis muscle of the pigeon. J Neurol Neurosurg Psychiatry. 1966 Aug;29(4):299–309. doi: 10.1136/jnnp.29.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese R. Sodium fluxes in diaphragm muscle and the effects of insulin and serum proteins. J Physiol. 1968 Jul;197(2):255–278. doi: 10.1113/jphysiol.1968.sp008558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng T. P. The effect of length on the resting metabolism of muscle. J Physiol. 1932 Apr 26;74(4):441–454. doi: 10.1113/jphysiol.1932.sp002860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch C. D., Shields R. P. Creatine metabolism in skeletal muscle. I. Creatine movement across muscle membranes. J Biol Chem. 1966 Aug 10;241(15):3611–3614. [PubMed] [Google Scholar]
- GOLDSPINK G. THE COMBINED EFFECTS OF EXERCISE AND REDUCED FOOD INTAKE ON SKELETAL MUSCLE FIBERS. J Cell Physiol. 1964 Apr;63:209–216. doi: 10.1002/jcp.1030630211. [DOI] [PubMed] [Google Scholar]
- Gan J. C., Jeffay H. Origins and metabolism of the intracellular amino acid pools in rat liver and muscle. Biochim Biophys Acta. 1967 Nov 28;148(2):448–459. doi: 10.1016/0304-4165(67)90141-9. [DOI] [PubMed] [Google Scholar]
- Geffen L. B. Optimum length for contraction of rat circulated limb muscles. Arch Int Physiol Biochim. 1964 Nov;72(5):825–834. doi: 10.3109/13813456409066459. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. Protein synthesis in tonic and phasic skeletal muscles. Nature. 1967 Dec 23;216(5121):1219–1220. doi: 10.1038/2161219a0. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. Role of insulin in work-induced growth of skeletal muscle. Endocrinology. 1968 Nov;83(5):1071–1073. doi: 10.1210/endo-83-5-1071. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. Work-induced growth of skeletal muscle in normal and hypophysectomized rats. Am J Physiol. 1967 Nov;213(5):1193–1198. doi: 10.1152/ajplegacy.1967.213.5.1193. [DOI] [PubMed] [Google Scholar]
- Grossman S. H., Manchester K. L. Response to insulin by guinea-pig taenia coli. Nature. 1966 Sep 17;211(5055):1300–1301. doi: 10.1038/2111300a0. [DOI] [PubMed] [Google Scholar]
- HELANDER E. A. Influence of exercise and restricted activity on the protein composition of skeletal muscle. Biochem J. 1961 Mar;78:478–482. doi: 10.1042/bj0780478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamosch M., Lesch M., Baron J., Kaufman S. Enhanced protein synthesis in a cell-free system from hypertrophied skeletal muscle. Science. 1967 Aug 25;157(3791):935–937. doi: 10.1126/science.157.3791.935. [DOI] [PubMed] [Google Scholar]
- Harris E. J., Manchester K. L. The effects of potassium ions and denervation on protein synthesis and the transport of amino acids in muscle. Biochem J. 1966 Oct;101(1):135–145. doi: 10.1042/bj1010135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hider R. C., Fern E. B., London D. R. Relationship between intracellular amino acids and protein synthesis in the extensor digitorum longus muscle of rats. Biochem J. 1969 Sep;114(2):171–178. doi: 10.1042/bj1140171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IMAI S., RILEY A. L., BERNE R. M. EFFECT OF ISCHEMIA ON ADENINE NUCLEOTIDES IN CARDIAC AND SKELETAL MUSCLE. Circ Res. 1964 Nov;15:443–450. doi: 10.1161/01.res.15.5.443. [DOI] [PubMed] [Google Scholar]
- Karpatkin S., Samuels A. Effect of insulin and muscle contraction on protein synthesis in frog sartorius. Arch Biochem Biophys. 1967 Sep;121(3):695–702. doi: 10.1016/0003-9861(67)90055-0. [DOI] [PubMed] [Google Scholar]
- Kendrick-Jones J., Perry S. V. Protein synthesis and enzyme response to contractile activity in skeletal muscle. Nature. 1967 Jan 28;213(5074):406–408. doi: 10.1038/213406a0. [DOI] [PubMed] [Google Scholar]
- Kipnis D. M., Parrish J. E. Role of Na+ and K+ on sugar (2-deoxyglucose) and amino acid (alpha-aminoisobutyric acid) transport in striated muscle. Fed Proc. 1965 Sep-Oct;24(5):1051–1059. [PubMed] [Google Scholar]
- LAWRIE R. A. Effect of enforced exercise on myoglobin concentration in muscle. Nature. 1953 Jun 13;171(4363):1069–1070. doi: 10.1038/1711069a0. [DOI] [PubMed] [Google Scholar]
- MANCHESTER K. L., YOUNG F. G. The effect of insulin in vitro on the accumulation of amino acids by isolated rat diaphragm. Biochem J. 1960 Jun;75:487–495. doi: 10.1042/bj0750487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchester K. L. Some factors affecting the response of muscle to insulin. Biochem J. 1966 Mar;98(3):711–719. doi: 10.1042/bj0980711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchester K. L. The control by insulin of amino acid accumulation in muscle. Biochem J. 1970 Apr;117(3):457–465. doi: 10.1042/bj1170457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez-Julio A., French I. W. The effect of ether, pentobarbital, and decapitation on various metabolites of rat skeletal muscle. Can J Biochem. 1967 Sep;45(9):1323–1327. doi: 10.1139/o67-154. [DOI] [PubMed] [Google Scholar]
- OXENDER D. L., CHRISTENSEN H. N. DISTINCT MEDIATING SYSTEMS FOR THE TRANSPORT OF NEUTRAL AMINO ACIDS BY THE EHRLICH CELL. J Biol Chem. 1963 Nov;238:3686–3699. [PubMed] [Google Scholar]
- ROSENBERG L. E., BERMAN M., SEGAL S. Studies of the kinetics of amino acid transport, incorporation into portein and oxidation in kidney-cortex slices. Biochim Biophys Acta. 1963 Jun 4;71:664–675. doi: 10.1016/0006-3002(63)91140-5. [DOI] [PubMed] [Google Scholar]
- RYAN W. L., CARVER M. J. IMMEDIATED AND PROLONGED EFFECTS OF HYDROCORTISONE ON THE FREE AMINO ACIDS OF RAT SKELETAL MUSCLE. Proc Soc Exp Biol Med. 1963 Dec;114:816–819. doi: 10.3181/00379727-114-28808. [DOI] [PubMed] [Google Scholar]
- SRETER F. A., WOO G. CELL WATER, SODIUM, AND POTASSIUM IN RED AND WHITE MAMMALIAN MUSCLES. Am J Physiol. 1963 Dec;205:1290–1294. doi: 10.1152/ajplegacy.1963.205.6.1290. [DOI] [PubMed] [Google Scholar]
- STEWART D. M. Protein composition of denervated muscle. Am J Physiol. 1962 Feb;202:281–284. doi: 10.1152/ajplegacy.1962.202.2.281. [DOI] [PubMed] [Google Scholar]
- STUBBS S. S., BLANCHAER M. C. GLYCOGEN PHOSPHORYLASE AND GLYCOGEN SYNTHETASE ACTIVITY IN RED AND WHITE SKELETAL MUSCLE OF THE GUINEA PIG. Can J Biochem. 1965 Apr;43:463–468. doi: 10.1139/o65-054. [DOI] [PubMed] [Google Scholar]
- Scharff R., Wool I. G. Accumulation of amino acids in muscle of perfused rat heart. Effect of insulin. Biochem J. 1965 Oct;97(1):257–271. doi: 10.1042/bj0970257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S. S., Oratz M., Rothschild M. A. Protein synthesis in the overloaded mammalian heart. Am J Physiol. 1966 Aug;211(2):314–318. doi: 10.1152/ajplegacy.1966.211.2.314. [DOI] [PubMed] [Google Scholar]
- Short F. A. Protein synthesis by red and white muscle in vitro: effect of insulin and animal age. Am J Physiol. 1969 Jul;217(1):307–309. doi: 10.1152/ajplegacy.1969.217.1.307. [DOI] [PubMed] [Google Scholar]
- Szabo A. J., Mahler R. J., Szabo O. Glucose uptake by the isolated perfused rat hind limb during rest and exercise. Horm Metab Res. 1969 Jul;1(4):156–161. doi: 10.1055/s-0028-1095147. [DOI] [PubMed] [Google Scholar]
- Waterlow J. C., Stephen J. M. The effect of low protein diets on the turn-over rates of serums, liver and muscle proteins in the rat, measured by continuous infusion of L-[14C]lysine. Clin Sci. 1968 Oct;35(2):287–305. [PubMed] [Google Scholar]
- ZIERLER K. L. Effect of insulin on membrane potential and potassium content of rat muscle. Am J Physiol. 1959 Sep;197:515–523. doi: 10.1152/ajplegacy.1959.197.3.515. [DOI] [PubMed] [Google Scholar]


