Abstract
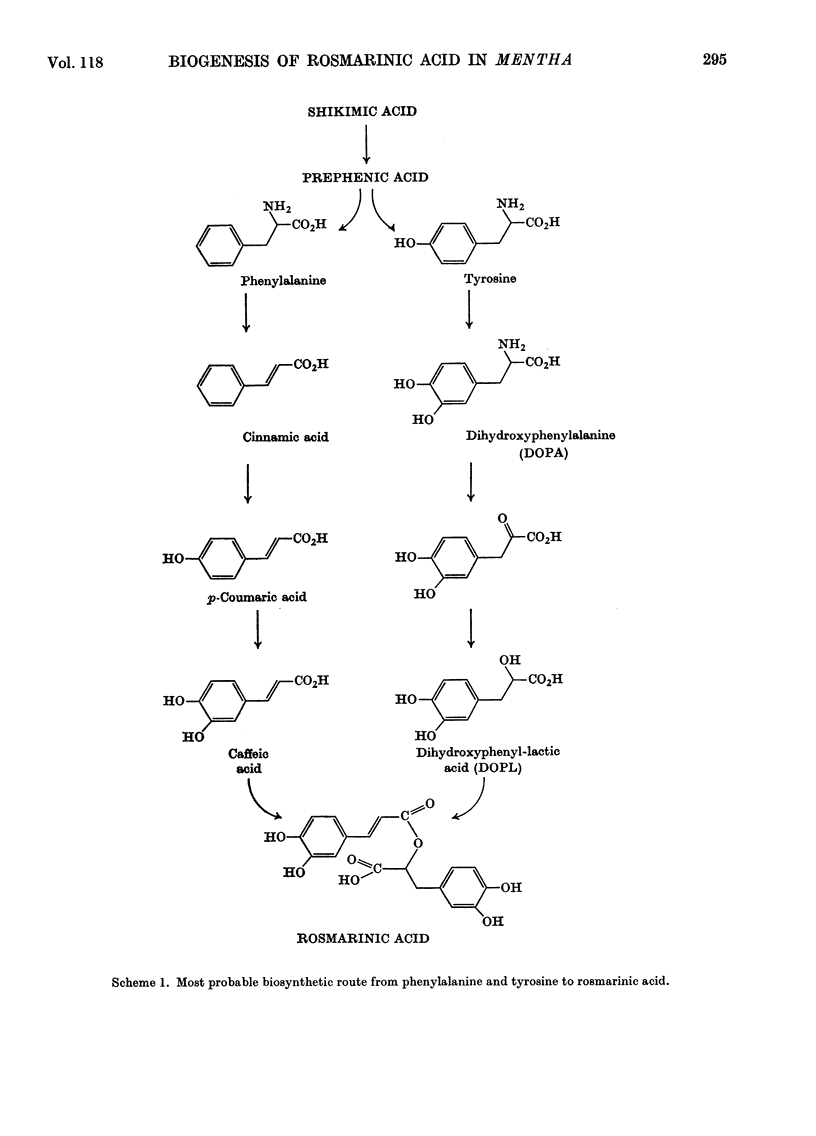
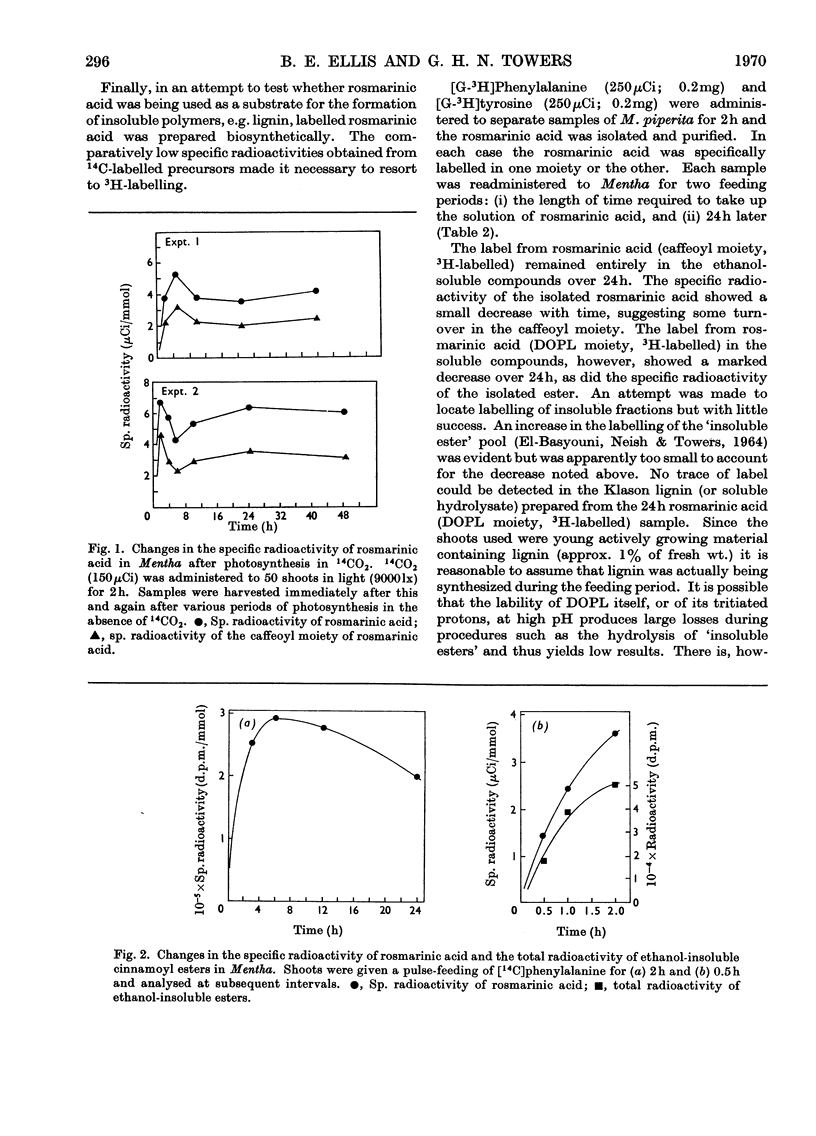
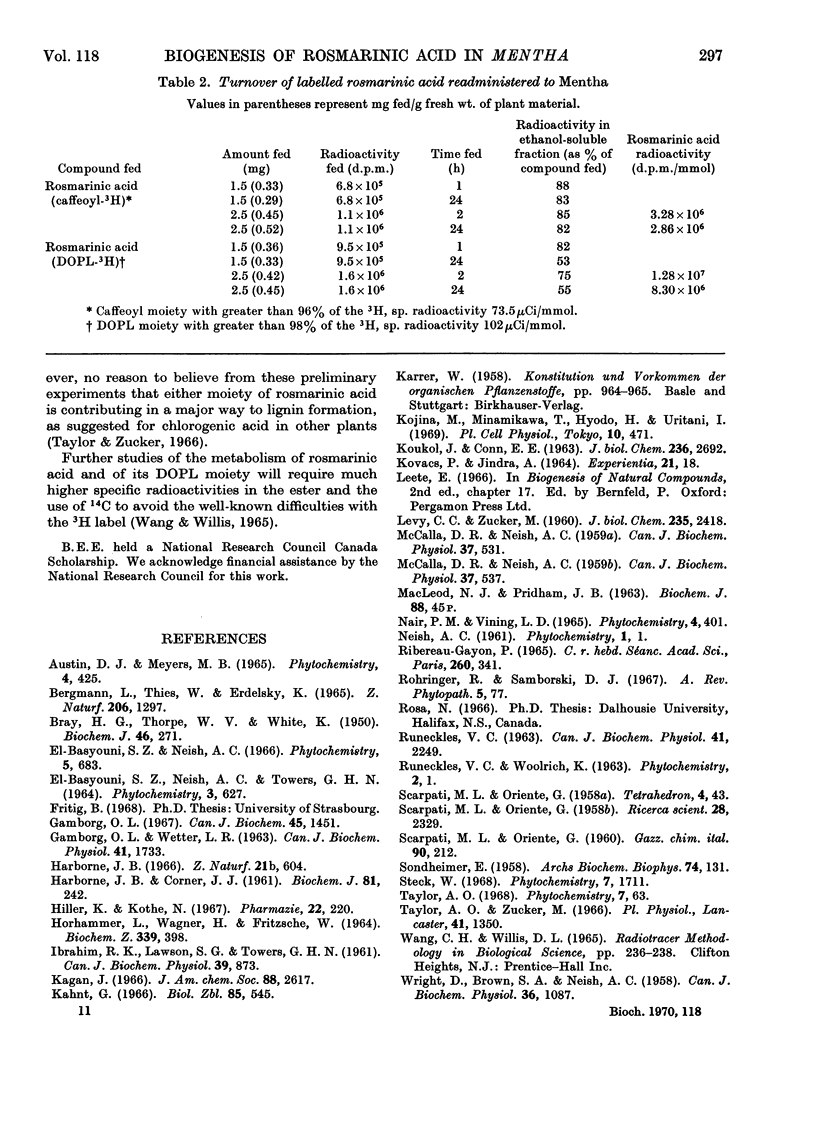
The biogenesis of rosmarinic acid (α-O-caffeoyl-3,4-dihydroxyphenyl-lactic acid), the second most common ester of caffeic acid in the plant kingdom, was studied in Mentha arvense and Mentha piperita. Administration of 14C-labelled compounds showed that, whereas the caffeoyl moiety was formed from phenylalanine via cinnamic acid and p-coumaric acid, the 3,4-dihydroxyphenyl-lactic acid moiety was formed from tyrosine and 3,4-dihydroxyphenylalanine. Time-course studies and the use of labelled rosmarinic acid showed that endogenous rosmarinic acid had a low turnover rate. The caffeoyl moiety did not appear to contribute to the formation of insoluble polymers, as has been suggested for chlorogenic acid in other plants.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bray H. G., Thorpe W. V., White K. The fate of certain organic acids and amides in the rabbit. 10. The application of paper chromatography to metabolic studies of hydroxybenzoic acids and amides. Biochem J. 1950 Mar;46(3):271–275. doi: 10.1042/bj0460271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamborg O. L. Aromatic metabolism in plants. V. The biosynthesis of chlorogenic acid and lignin in potato cell cultures. Can J Biochem. 1967 Sep;45(9):1451–1457. doi: 10.1139/o67-171. [DOI] [PubMed] [Google Scholar]
- HARBORNE J. B., CORNER J. J. Plant polyphenols. 4. Hydroxycinnamic acid-sugar derivatives. Biochem J. 1961 Nov;81:242–250. doi: 10.1042/bj0810242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOERHAMMER L., WAGNER H., FRITZSCHE W. ZUR BIOSYNTHESE DER BETACYANE. I. Biochem Z. 1964 Mar 12;339:398–400. [PubMed] [Google Scholar]
- IBRAHIM R. K., LAWSON S. G., TOWERS G. H. Formation of labeled sugars from L-tyrosine-C14 in some higher plants. Can J Biochem Physiol. 1961 May;39:873–880. doi: 10.1139/o61-087. [DOI] [PubMed] [Google Scholar]
- KOUKOL J., CONN E. E. The metabolism of aromatic compounds in higher plants. IV. Purification and properties of the phenylalanine deaminase of Hordeum vulgare. J Biol Chem. 1961 Oct;236:2692–2698. [PubMed] [Google Scholar]
- KOVACS P., JINDRA A. BIOSYNTHESIS OF ALKALOIDS. ON THE TRANSFORMATION OF TYROSINE TO 3,4-DIHYDROXYPHENYLALANINE IN PAPAVER SOMNIFERUM L. PLANTS. Experientia. 1965 Jan 15;21:18–19. doi: 10.1007/BF02136358. [DOI] [PubMed] [Google Scholar]
- LEVY C. C., ZUCKER M. Cinnamyl and p-coumaryl esters as intermediates in the biosynthesis of chlorogenic acid. J Biol Chem. 1960 Aug;235:2418–2425. [PubMed] [Google Scholar]
- Magee W. L., Berry J. F., Strickland K. P., Rossiter R. J. Labelling of phospholipids from inorganic [P]phosphate in brain preparations. Effect of acetylcholine, chlorpromazine and azacyclonol. Biochem J. 1963 Jul;88(1):45–52. doi: 10.1042/bj0880045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCALLA D. R., NEISH A. C. Metabolism of phenylpropanoid compounds in Salvia. I. Biosynthesis of phenylalanine and tyrosine. Can J Biochem Physiol. 1959 Apr;37(4):531–536. [PubMed] [Google Scholar]
- McCALLA D. R., NEISH A. C. Metabolism of phenylpropanoid compounds in Salvia. II. Biosynthesis of phenolic cinnamic acids. Can J Biochem Physiol. 1959 Apr;37(4):537–547. [PubMed] [Google Scholar]
- RIBEREAU GAYON P. IDENTIFICATION D'ESTERS DES ACIDES CINNAMIQUES ET DE L'ACIDE TARTIQUE DANS LES LIMBES ET LES BAIES E V. VINIFERA. C R Hebd Seances Acad Sci. 1965 Jan 4;260:341–343. [PubMed] [Google Scholar]
- SONDHEIMER E. On the distribution of caffeic acid and the chlorogenic acid isomers in plants. Arch Biochem Biophys. 1958 Mar;74(1):131–138. doi: 10.1016/0003-9861(58)90207-8. [DOI] [PubMed] [Google Scholar]
- Taylor A. O., Zucker M. Turnover and metabolism of chlorogenic Acid in xanthium leaves and potato tubers. Plant Physiol. 1966 Oct;41(8):1350–1359. doi: 10.1104/pp.41.8.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]


