Abstract
Cumulative evidence suggests a link between specific autoimmune diseases (AD), including idiopathic inflammatory myopathies (IIM), and SARS-CoV-2 infection or COVID-19 vaccination. Anti-synthetase syndrome (ASS), a subset of IIM, is defined by the presence of autoantibodies against aminoacyl-tRNA synthetase (anti-ARS) and is strongly associated with interstitial lung disease (ILD), a major contributor to severe complications and reduced survival. We present four clinical cases of patients who developed autoantibodies against threonyl (PL-7) and alanyl (PL-12) synthetases associated with ASS-ILD shortly after SARS-CoV-2 infection or COVID-19 vaccination. Anti-ARS autoantibodies were identified using three complementary methods: immunoblotting, western blotting (WB) and the method considered the gold standard, immunoprecipitation (IP), which ensures accurate interpretation of results. The study highlights the clinical and pathogenic overlap between ASS-ILD and SARS-CoV-2-related lung involvement.Both conditions share similar high-resolution computed tomography (HRCT) patterns, including inflammation and pulmonary fibrosis (PF), driven by IFN-γ signaling, which complicates accurate diagnosis. Our results provide novel insights into the temporal association of SARS-CoV-2 and vaccine exposure with ASS-ILD, focusing on possible molecular mimicry between viral proteins and ARS molecules as a potential mechanism. Understanding the involvement of specific anti-ARS autoantibodies (PL-7 and PL-12) and the identification of genetic predispositions (HLA-B∗08:01 and HLA-DRB1∗03:01) in these patients may be key to underpinning these autoimmune manifestations. The study underscores the importance of a multidisciplinary approach and vigilant follow-up to optimize diagnosis and management. Further research is essential to elucidate the causal relationships and molecular mechanisms behind these observations.
Keywords: Idiopathic inflammatory myopathies, Anti-synthetase syndrome, Interstitial lung disease, COVID-19, SARS-CoV-2 vaccine
Highlights
-
•
Evidence suggests links between autoimmune diseases, such as IIM, and COVID-19 infection or vaccination.
-
•
Autoantibodies in ASS may develop via molecular mimicry between SARS-CoV-2 proteins and ARS molecules on specific HLA alleles.
-
•
The severity of lung disease may be associated with the titer and specific type of anti-ARS autoantibodies (PL-7 or PL-12).
-
•
Early treatment and multidisciplinary approach are key for ILD-ASS patients, especially those with an autoimmune background.
Abbreviations
- •AD
autoimmune diseases
- •ANA
antinuclear antibodies
- •ARS
aminoacyl-tRNA synthetase
- •ASS
antisynthetase syndrome
- •COVID-19
coronavirus disease 2019
- •CK
creatine kinase
- •CRP
C-reactive protein
- •DM
dermatomyositis
- •HLA
human leukocyte antigen
- •HRCT
high-resolution computed tomography
- •IIF
indirect immunofluorescence
- •ILD
interstitial lung disease
- •IIM
idiopathic inflammatory myopathies
- •IP
immunoprecipitation
- •Jo-1
anti-histidyl-RNAt-synthetase
- •LDH
lactate dehydrogenase
- •MCP
mycophenolate mofetil
- •NSIP
nonspecific interstitial pneumonia
- •OP
organizing pneumonia
- •PF
pulmonary fibrosis
- •PFT
pulmonary function testing
- •PL-7
anti-threonyl synthetase
- •PL-12
anti-alanyl synthetase
- •RT-PCR
reverse transcription polymerase chain reaction
- •UIP
usual interstitial pneumonia
- •WB
western blotting
1. Introduction
Anti-synthetase syndrome (ASS) comprises a subgroup of idiopathic inflammatory myopathies (IIM) featured by the presence of autoantibodies targeting aminoacyl-tRNA synthetase (anti-ARS) [1]. These autoantibodies are central to the pathogenesis of ASS, influencing both its immunological mechanisms and clinical manifestations. The hallmark clinical presentation of ASS is characterized by the triad of arthritis, myositis, and interstitial lung disease (ILD). Other supporting features, such as Raynaud's phenomenon, “mechanic's hands”, and fever, have been also associated [2].
Among the defining criteria for ASS, ILD is particularly significant due to its impact on morbidity and mortality. However, diagnosing ILD in ASS remains challenging, as radiological findings on high-resolution computed tomography (HRCT) often lack specificity and may mimic other conditions, including coronavirus disease 2019 (COVID-19) pneumonia [[3], [4], [5]]. Several studies have demonstrated that the most commonly observed patterns of ILD in ASS include non-specific interstitial pneumonia (NSIP) and organizing pneumonia (OP), with evolution to progressive pulmonary fibrosis (PF) also reported [[6], [7], [8]]. These imaging patterns may closely resemble those observed in COVID-19 pneumonia, where the predominant findings include persistent ground-glass opacities with or without interstitial thickening, often accompanied by features consistent with OP or NSIP. In severe cases, progression to fibrosis may occur, complicating the differential diagnosis and delaying early treatment, thereby underscoring the critical role of a comprehensive physical examination [9,10].
To date, eight anti-ARS autoantibodies have been identified in patients with ASS, with the most common being anti-histidyl-tRNA-synthetase (Jo-1), found in approximately 70 % of diagnosed ASS cases. Other anti-ARS autoantibodies, such as anti-threonyl (PL-7) and anti-alanyl (PL-12) synthetases, are less common, comprising about 4 % of cases. However, they are frequently associated with isolated ILD, severe complications and poorer survival rate [[11], [12], [13], [14]].
Cumulative investigations have linked specific autoimmune diseases (AD), including IIM, with COVID-19 [[15], [16], [17], [18]]. Our group and others have observed an increased in the incidence of MSA with a diagnosis of IIM since the onset of the COVID-19 pandemic [19,20]. Molecular mimicry between SARS-CoV-2 proteins and human self-antigens has been proposed as a potential mechanism for triggering ASS following SARS-CoV-2 infection or vaccination [5,[21], [22], [23]]. In this context, immunogenic peptides from the SARS-CoV-2 spike glycoprotein presented by specific human leukocyte antigen (HLA) molecules to T lymphocytes, may provoke the development of anti-ARS autoantibodies.
In this study, we present four cases of ILD or similar pulmonary involvement diagnosed as ASS associated with anti-PL-12 or anti-PL-7 autoantibodies, occurring shortly after SARS-CoV-2 infection or COVID-19 vaccination. The clinical and demographic data of these patients are described in Table 1.
Table 1.
Clinical and demographic data.
|
Age at symptom onset |
CASE Nº 1 |
CASE Nº 2 |
CASE Nº 3 |
CASE Nº 4 |
|---|---|---|---|---|
| 60 | 55 | 69 | 63 | |
| Gender | Female | Female | Female | Female |
| Personal history | Ex-smoker for 20 years Hypertension Autoimmune hypothyroidism |
Psoriatic dermatitis Episode of pericarditis 30 years ago Sarcoidosis |
Hypertension Autoimmune hypothyroidism | Ex-smoker for 20 years Helicobacter Pylori infection Periorbital facial eczema First episode of right middle lobe pneumonia (2019) |
| Family history | No family history of thrombotic, oncological, or autoimmune diseases | Sister and aunt with cutaneous psoriasis. Father with giant cell arteritis. Daughter with juvenile rheumatoid arthritis | No family history of thrombotic, oncological, or autoimmune diseases | Mother with liver cancer |
| SARS-CoV-2 infection (time) | Post symptom onset (June 01, 2022) | Post symptom onset (January 10, 2022) | Prior to symptom onset (September 01, 2020) | Prior to symptom onset (March 01, 2020) |
| COVID-19 vaccine Type (time) | 1st dose AstraZeneca (April 20, 2021) 2nd dose AstraZeneca (June 30, 2021) | 1st dose Janssen (May 27, 2021) 2nd dose Pfizer (November 26, 2021) | 1st dose Pfizer (April 29, 2021) 2nd dose Pfizer (May 20, 2021) 3rd dose Pfizer (January 03, 2022) | 1st dose Pfizer (January 19, 2021) 2nd dose Pfizer (February 09, 2021) 3rd dose Pfizer (February 07, 2022) |
| Time from SARS-CoV-2 infection or vaccination to symptom onset | 14 days after 2nd dose | 10 days after 2nd dose | 1 day after SARS-CoV-2 infection | 1 day after SARS-CoV-2 infection |
| Clinical features | Dyspnea Asthenia Myalgia Raynaud's phenomenon Suspected mechanic's hands |
Dyspnea Asthenia Fever Polyarthritis | Dyspnea Fever Arthralgias Xerostomia | Dyspnea Arthralgias Myalgia Fever Raynaud's phenomenon |
| Chest CT scan diagnosis | ILD-NSIP and progression to pulmonary fibrosis | OP | ILD-NSIP and progression to pulmonary fibrosis | Interstitial pneumonia |
| Laboratory data (Normal values) | ||||
| Leukocytes (4-10 × 103/μL) | 12.4 × 103 | 11.9 × 103 | 11.2 × 103 | 12.35 × 103 |
| Neutrophils (2-7 x103/μL) | 10.5 × 103 | normal | 9.3 × 103 | 9.2 × 103 |
| Lymphocytes (1-3 x103/μL) | normal | normal | normal | normal |
| Hemoglobin (11.7–16 g/dL) | 11.3 | 11.4 | normal | normal |
| D-dimer (215–500 ng/mL) | 2099 | 2053 | 1297 | normal |
| CRP (1–3 mg/L) | 25.8 | 7.7 | 26 | normal |
| Ferritin (10–150 ng/ml) | normal | normal | 396 | 163 |
| ALT (3–35 U/L) | 56 | 42 | 142 | normal |
| AST (3–35 U/L) | 55 | 47 | 106 | normal |
| GGT (1–39 U/L) | normal | 325 | normal | normal |
| LDH (208–378 U/L) | 484 | 425 | 701 | 558 |
| CK (10–155 U/L) | normal | normal | normal | 572 |
| Autoimmune profile | ANA 1:640 (AC-19) Anti-PL-12 (+++) Anti-TPO Anti-TG |
ANA negative (AC-0) Anti-PL-7 (+) Anti-Ro-52 (++) |
ANA 1:640 (AC-19) Anti-PL-12 (+++) Anti-Ro-52 (+++) | ANA negative (AC-0) Anti-PL-7 (++) Anti-PM-Scl75 (+) |
| HLA genetic typing | HLA-A∗01:01; 30:02 | HLA-A∗01:01; 24:03 | HLA-A∗26:01; 26:05 | HLA-A∗11:01; 26:01 |
| HLA-B∗08:01; 18:01 | HLA-B∗38:01; 57:01 | HLA-B∗27:03; 44:02 | HLA-B∗35:01; 44:03 | |
| HLA-DRB1∗03:01; 03:01 | HLA-DRB1∗11:01; 13:01 | HLA-DRB1∗01:01; 12:01 | HLA-DRB1∗07:01; 07:01 | |
| HLA-DQA∗05:01; 05:01 | HLA-DQA∗05:05; 01:03 | HLA-DQA∗01:01; 05:05 | HLA-DQA∗02:01; 02:01 | |
| HLA-DQB∗02:01; 02:01 | HLA-DQB∗03:01; 06:03 | HLA-DQB∗05:01; 03:01 | HLADQB∗02:02; 02:02 | |
| Treatment at diagnosis | Cyclophosphamide MCP Prednisone Nintedanib |
Antibiotherapy: levofloxacin and amoxicillin clavulanic) Hydroxychloroquine Beclomethasone dipropionate anhydrous and formoterol fumarate dihydrate (inhaled) | Antibiotherapy: cefditoren and levofloxacin MCP Prednisone N-Acetylcysteine Anticholinergic Ipratropium bromide |
Antibiotherapy (azithromycin, cefditoren and levofloxacin) |
| Outcomes | Pulmonary fibrosis | Resolution of pulmonary infiltrates | Improvement pulmonary fibrosis | Resolution of ILD |
ILD: Interstitial lung disease; NSIP: nonspecific interstitial pneumonia; COP: cryptogenic organizing pneumonia; MCP: mycophenolate mofetil. Laboratory data values in red indicate that they are elevated above the normal range and in blue below the normal range.
These cases underscore the importance of multidisciplinary evaluation and early diagnosis, particularly in patients with underlying autoimmune predispositions or genetic susceptibility identified through HLA typing. This highlights the need for heightened clinical vigilance and comprehensive diagnostic approaches to effectively manage such complex and overlapping conditions.
1.1. Case report Nº 1
A 60-year-old Caucasian female, presented at the Pneumology Department 14 days after receiving the 2nd dose of the ChAdOx1 nCoV-19 (Oxford/AstraZeneca) vaccine. She reported symptoms including an acute onset of shortness of breath, non-productive cough, asthenia, muscle pain and weakness, painful erythematous papular lesions on her fingertips, and generalized pruritus. She had received the 1st vaccine dose 40 days prior without complications, and no other environmental exposures were reported.
Physical examination revealed dry crackles in the lower lung fields. Elevated levels of C-reactive protein (CRP) and lactate dehydrogenase (LDH) were noted, along with HRCT chest findings compatible with multiple bilateral pulmonary infiltrates of bilateral basal predominance with ground-glass opacities. Reverse transcription polymerase chain reaction (RT-PCR) for SARS-CoV- 2 was negative. Treatment with bronchodilators was initiated, and she was referred to the Interstitial Pulmonary Pathology Consultation for further evaluation.
One month later, she returned to the Emergency Department with worsening dyspnea and dysphagia. On physical examination, she presented with tachycardia (110 bpm), high blood pressure (140/90 mmHg), and low peripheral oxygen saturation (94 %). CT-angiography revealed pulmonary thromboembolism in both segmental arteries of the right upper lobes along with persistent HRCT chest findings. Pulmonary function testing (PFT) showed a restrictive pattern (FVC 60 %, FEV1/FVC 84 %, DLCO 28 %). Bronchoscopy with bronchoalveolar lavage was negative for infections. Flow cytometry analysis demonstrated elevated CD8+ T cells (55 %) and an inverted CD4/CD8 ratio (0.71). Laboratory tests revealed mild anemia, leukocytosis with neutrophilia, and elevation of D-dimer, and a higher increase in CRP compared to previous results. Alanine transaminase (ALT), aspartate transaminase (AST), and LDH were also elevated. RT-PCR test was negative for SARS-CoV-2. Empirical treatment included low-molecular-weight heparin (tinzaparin 10,000 IU) and antibiotic prophylaxis with levofloxacin 500 mg for 14 days. Following discharge, the patient continued to experience dyspnea and asthenia. Physical examination revealed mid-basal bilateral crackles, swollen fingers with lesions at the nail base, and mild Raynaud's syndrome. Furthermore, she presented dry and cracked hands. Chest X-ray and HRCT chest confirmed ILD diagnosis compatible with nonspecific interstitial pneumonia (NSIP).
Due to the high suspicion of ASS, a complete immunological study was requested. Screening analysis showed elevated serum IgG levels and positive antinuclear antibodies (ANA) by indirect immunofluorescence (IIF) with a fine dense cytoplasmic mottled pattern (AC-19) at a titer of 1:640 [Fig. 1A]. Further autoantibody characterization revealed positive anti-PL-12 antibodies [197 (+++)] by commercial immunoblotting (EUROIMMUN AG, Lübeck, Germany) [Fig. 2A] confirmed by western blotting (WB) using purified recombinant protein of human alanyl-tRNA synthetase kindly provided by EUROIMMUN AG, Lübeck, Germany [Fig. 2B] and by RNA immunoprecipitation (IP) [Fig. 2C]. HLA typing was also performed [Table 1].
Fig. 1.
Indirect immunofluorescence (IIF) on Hep-2 cells. Fine dense cytoplasmic mottled pattern (AC-19) at 1:640 titer in serum from reported cases Nº1 (A) and Nº3 (B).
Fig. 2.
Results for anti-PL-12 autoantibodies detection in patients Nº1 and Nº3. Anti-PL-12 autoantibodies were detected by commercial immunoblot and evaluated automatically with the EUROLineScan software showing results of very strong intensity (+++) (A). Anti-PL-12 autoantibodies of both patients were confirmed by western blotting (WB) using recombinant protein of human alanyl-tRNA synthetase (B). Gold standard RNA immunoprecipitation (IP) technique for detection of tRNA synthetases in native form with migration in the lower zone of the agarose gel. Samples from patients Nº1 (lane A) and Nº3 (lane B) show bands compatible with PL-12 tRNA synthetase. Serum from patient without known autoantibodies is used as a negative control (lane C) and serum from patient with known anti-PL12 autoantibodies is used as a positive control (lane D) (C).
The final diagnosis was compatible with ASS, and immunosuppressive treatment was initiated with intravenous cyclophosphamide at a dose of 500 mg/m2 6 cycles, followed by mycophenolate mofetil (MCP) and oral prednisone starting at 50 mg with a tapering schedule down to 5 mg. However, due to clinical and radiological progression of NSIP to pulmonary fibrosis (PF) confirmed by HRCT [Fig. 3], she was also initiated with nintedanib at a dose of 150 mg, 1 tablet every 12 hours, with close monitoring.
Fig. 3.
High-resolution computed tomography (HRCT) chest findings from patient Nº 1. Fibrotic NSIP pattern: The results show bilateral and symmetric interstitial lung involvement, predominantly in both lung bases. The involvement consists of bronchiectasis and cylindrical bronchiectasis, as well as septal thickening and faint ground-glass attenuation opacities.
1.2. Case report Nº 2
A 56-year-old Caucasian female experienced a dermatitis flare during the COVID-19 pandemic that resolved after receiving the first dose of the Ad26.CoV2.S COVID-19 vaccine (Janssen), without a previous history of SARS-CoV-2 infection.
Ten days after receiving her second dose of the BNT162b2 COVID-19 vaccine (Pfizer), she developed flu-like symptoms and respiratory distress, including a non-productive cough, asthenia, and fever. Physical examination revealed bilateral basal dry crackles and a peripheral oxygen saturation of 92 %. Bloodwork showed mild anemia and leukocytosis. Chest X-ray displayed patchy infiltrates in the middle and base of the left lung [Fig. 4A]. A subsequent HRCT scan revealed patchy bilateral alveolar consolidations, accompanied by peripheral ground-glass opacities in the middle and lower lung fields, suggestive of SARS-CoV-2 pneumonia or organized pneumonia (OP) [Fig. 5]. Despite negative RT-PCR for SARS-CoV-2, positive IgM serology for Chlamydia (1.2) prompted antibiotic treatment with amoxicillin 500 mg every 8 hours for 10 days and clarithromycin 500 mg every 12 hours for 7 days. Blood tests revealed elevated CRP and D-Dimer levels.
Fig. 4.
Chest X-ray findings from patient Nº2. Results showed patchy bilateral alveolar consolidations (A). Subsequent results demonstrated resolution of previous pulmonary infiltrates (B).
Fig. 5.
High-resolution computed tomography (HRCT) chest findings from patient Nº 2. Organizing pneumonia pattern: The results show subtle areas of ground glass attenuation in the lower lobes and a small consolidation area of nodular morphology with air bronchogram in the right basal pyramid.
Two months later, respiratory distress persisted. No evidence of pulmonary thromboembolism was detected on computed tomography angiography. A study of atypical sputum cultures, as well as Mantoux testing and urinary antigen tests for pneumococcus and Legionella, all yielded negative results. The patient exhibited slightly increased levels of ALT, AST, GGT and LDH. Despite a negative ANA test (AC-0), the myositis autoantibody panel showed positivity for anti- PL-7 [27 (+)] and anti-Ro52 [60 (++)] [Fig. 6A]. However, the determination of anti-PL7 antibodies by WB [Fig. 6B] and RNA IP [Fig. 6C] was negative. HLA typing results were also obtained [Table 1]. Due to suspected ASS and concomitant infectious disease, treatment included hydroxychloroquine 200 mg, levofloxacin 500 mg and amoxicillin clavulanic 850 mg for 6 weeks.
Fig. 6.
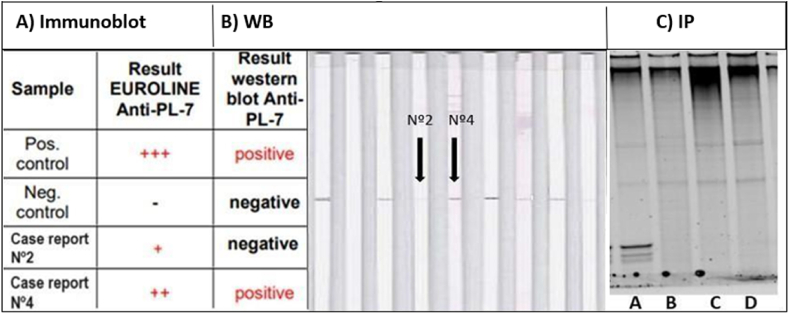
Results for anti-PL-7 autoantibodies detection in patients Nº2 and Nº4. The analytical methods used were the same as for the detection of anti-PL12 antibodies. Anti-PL-7 autoantibodies were detected by commercial immunoblot showing results of medium and low intensity (++/+) (A). Anti-PL-7 autoantibodies of patient Nº2 were not confirmed by WB, however the result was positive in patient Nº4 (B). Patient samples Nº2 (lane C) and Nº4 (lane D) show no bands compatible with anti-PL-7 antibodies by IP. Lane A shows a serum from a patient with known anti-PL-7 autoantibodies used as a positive control and lane B shows a serum from a patient without known autoantibodies as a negative control (C).
One month after concluding the treatment, she presented symptoms of non-specific polyarthritis, knee inflammation, and slight pain of the metatarsophalangeal joints, so treatment with hydroxychloroquine was restarted. Azathioprine 50mg daily was also initiated, but it had to be stopped due to gastrointestinal intolerance. Additionally, she was prescribed the Foster Nexthaler inhaler 100/6 μg for 1 month.
By December 2022, chest X-ray and pulmonary function tests demonstrated resolution of prior infiltrates and normalized lung volumes and capacity, indicating clinical improvement in the patient's respiratory status [Fig. 4B].
1.3. Case report Nº 3
A 69-year-old Caucasian female presented with acute respiratory symptoms (dyspnea, non-productive cough) during the early COVID-19 pandemic, suggestive of SARS-CoV-2 infection. Despite the absence of initial RT-PCR confirmation, she received antibiotic and mucolytic therapy with cefuroxime 500 mg for 6 days and ambroxol 15mg/5 ml for 10 days due to worsening dyspnea associated with a productive cough of 15 days, leading to temporary improvement. Initially, she was diagnosed with community-acquired pneumonia.
Two months later, she developed worsening respiratory symptoms associated with febrile syndrome and joint pain in both wrists and left hand. At the Emergency Department, blood tests showed elevated D-dimer, ferritin, and LDH levels. The RT-PCR for SARS-CoV-2 was negative. Positive serology for IgG and negative for IgM against SARS-CoV-2 confirmed past COVID-19 infection. PFT revealed a restrictive pattern with FEV1 at 99 %, FVC at 106 %, FEV1/FVC ratio at.
77 % and DLCO at 58 %. Further investigations showed a bilateral and symmetric reticular interstitial pattern of subpleural and bilateral basal distribution on lung HRCT, prompting video-assisted thoracoscopic biopsy. The biopsy revealed microcyst formation with epithelium-lined lining, metaplastic ciliate, and PF that disrupted normal pulmonary architecture. Chronic inflammatory infiltrates of probable lymphoplasmacytic origin were identified in areas of cystic dilation. Although COVID-19 pneumonia can lead to secondary PF, this pattern was atypical, and other possibilities, including usual interstitial pneumonia (UIP), had to be excluded. Treatment included oxygen supplementation up to 2L/min, levofloxacin 500 mg plus ceftidorene 400 mg every 24 hours during 10 days, prednisone 30 mg every 24 hours with a tapering schedule until discontinuation, N-Acetylcysteine 600 mg every 8 hours and Ipratropium bromide 200 mg.
Months later, the patient experienced myalgia in the lower extremities, and a chest HRCT indicated ILD with features suggestive of NSIP, with progression to pulmonary fibrosis. [Fig. 7]. Upon physical examination, the patient reported xerostomia. Blood tests showed neutrophilia and AST and LDH enzymes were slightly elevated. ANA was positive with a fine dense cytoplasmic mottled pattern (AC-19) at a titer of 1:640 [Fig. 1B]. Additionally, the myositis autoantibodies panel detected positive anti-PL-12 antibodies [185 (+++)] and anti-Ro52 antibodies [160 (+++)] [Fig. 2A]. The presence of anti-PL-12 autoantibody was further confirmed by WB [Fig. 2B] and RNA IP [Fig. 2C]. Due to the positivity of anti-Ro52 antibodies (anti-Ro-60 and anti-La negative) and the presence of xerostomia, a biopsy of the minor salivary gland was performed, with results compatible with Sjögren's disease. HLA typing was also performed [Table 1].
Fig. 7.
High-resolution computed tomography (HRCT) chest findings from patient Nº 3. Fibrotic NSIP pattern: The results display a bilateral and symmetrical reticular interstitial pattern with a subpleural distribution and bibasal predominance. The condition is characterized by thickening of the inter- and intra-lobular septa, bronchiectasis and traction bronchiolectasis, with a bibasal predominance.
Regarding treatment, due to the progression of PF, the dose of MCP was increased to 750 mg every 12 hours, in addition to prednisone 5 mg.
1.4. Case report Nº 4
A 63-year-old Caucasian female presented to the Emergency Department with a 15-days onset of arthralgias, myalgias, abdominal pain, anosmia, dysgeusia, and fever. Physical examination revealed a blood pressure of 133/71 mmHg, heart rate of 89 beats per minute, temperature of 37 °C and normal peripheral oxygen saturation (98 %). Cardiac and pulmonary auscultation showed no abnormalities. CK levels were elevated, and RT-PCR confirmed SARS-CoV-2 infection. Chest X-ray and HRCT revealed bilateral basal opacities with a diagnosis of interstitial pneumonia. The patient was treated with hydroxychloroquine 200 mg, azithromycin 500 mg and ceftidorene 400 mg, with led to improvement in symptoms.
Months later, she returned to the Emergency Department with catarrhal symptoms, persistent fever, generalized muscle weakness, and left-sided chest pain. She had elevated ferritin levels and serology screening (IgA + IgM) and IgG were positive for SARS-CoV-2, but RT-PCR for SARS- CoV- 2 was negative. Chest X-ray showed resolution of previous opacities. Due to the persistence of muscular and respiratory symptoms, she visited her primary care physician. She also reported an erythematous rash on the upper trunk, mainly on the facial level, which improved after topic corticosteroid application, Raynaud's phenomenon and a significant increase in the levels of CK and LDH enzymes compared to previous determinations. ANA were negative (AC-0). The myositis autoantibodies panel revealed anti-PL-7 [30 (++)] and anti-PM-Scl75 [17 (+)] auto-antibodies [Fig. 6A]. Anti-PL-7 autoantibodies were confirmed by WB [Fig. 6B] but not by RNA IP [Fig. 6C]. HLA typing was also performed [Table 1].
In a subsequent visit to the emergency department, she presented with another catarrhal episode, fever, cough with greenish expectoration and poor general condition. Blood tests showed neutrophilia. Her PFT was normal, and her lung X-ray and HRCT imaging ruled out ILD but showed bronchiectasis in the left middle lobe and lingula, some with endobronchial secretions and associated distal subsegmental atelectasis. Levofloxacin 500 mg and paracetamol 1g were prescribed.
2. Literature review and discussion
In this manuscript, we present four case reports illustrating the development of ILD or related pulmonary involvement associated with ASS and anti-ARS autoantibodies, specifically anti-PL-12 and anti-PL-7. These complications occurred shortly after receiving a COVID-19 vaccine (cases Nº1 and Nº2) or contracting SARS-CoV-2 infection (cases Nº3 and Nº4). Those cases highlight the clinical and immunological complexity of ASS, particularly its overlap with SARS-CoV-2-related pulmonary involvement, and raise important questions about the interplay between viral infections, vaccines, and autoimmune mechanisms. Furthermore, because establishing a direct causal link between the development of ASS and the COVID-19 pandemic is challenging, we will discuss some molecular mechanisms involved in the development of anti-ARS autoantibodies.
The diagnosis of ASS requires a comprehensive, multidisciplinary approach involving rheumatology, pulmonary, immunology, pathology and radiographic evaluation, occasionally relying on muscle and/or lung biopsy results. While the 2017 ACR/EULAR criteria [24] include only anti-Jo1 autoantibodies for classifying IMM, other anti-ARS autoantibodies, such as anti-PL-12 and anti-PL-7, are widely recognized in clinical practice as hallmark indicators of ASS disease [25,26]. In this study, three different methods were utilized for the analysis and characterization of anti-ARS autoantibodies: immunoblotting, WB, and IP, with the latter recognized as the gold standard [27]. Notably, the detection of anti-PL-7 autoantibodies in patients Nº2 and Nº4 exhibited weak positivity by immunoblotting method. The lack of confirmation by WB and/or IP makes these findings less certain, leaving it inconclusive whether the result represents a false positive or reflects a higher sensitivity of the myositis autoantibodies panel. Moreover, although the clinical presentation of patients Nº2 and Nº4 was compatible with ASS, pulmonary involvement in these cases resolved without immunosuppressant treatment. These findings underscore the heterogeneity of ASS and emphasize the necessity of comprehensive diagnostic approaches to accurately differentiate it from other overlapping conditions, including COVID-19 pneumonia.
The prevalence of autoantibodies against ARS in patients with ASS varies widely, demonstrating high specificity and a strong association with complicated ILD, which significantly affects patient survival, as highlighted by Hervier et al. [28]. The most commonly described patterns of ILD associated with ASS on HRCT include NSIP, observed in patients Nº1 and Nº3; OP, observed in patient Nº2; and progressive PF in severe cases, as seen in patients Nº1 and Nº3. These imaging patterns may closely resemble those observed in COVID-19 pneumonia [9]. Pulmonary fibrosis, a recognized complication of COVID-19 and a significant risk factor for adverse outcomes [29]. Moreover, SARS-CoV-2 infection has been associated with acute exacerbations of ILD, resulting in a poorer prognosis compared to non-COVID-19-related cases [30]. Our observations suggest that the severity of pulmonary involvement may be influenced by the specificity and levels of anti-ARS autoantibodies. For example, in cases Nº1 and Nº3, the strong positivity for anti-PL-12 was associated with progression to pulmonary fibrosis in ASS-ILD.
Based on the findings of Chen et al. [31], our results further support the hypothesis that SARS-CoV-2 may act as a catalyst for autoimmune responses through mechanisms such as molecular mimicry and immune dysregulation. This reinforces their observation of a temporal association between SARS-CoV-2 infection and the onset of ASS and highlights the critical importance of increased clinical vigilance in patients with ILD and autoimmune features following COVID-19.
There is a growing body of evidence suggesting that ARS could function as RNA sensors and elicit immune responses against pathogen infections [32]. However, the pathogenic role of anti-ARS autoantibodies remains to be fully elucidated. Megremis et al. [33] proposed that the enrichment of SARS-CoV-2 virus-derived-immunogenic peptides observed in patients with dermatomyositis (DM) could contribute to the development of musculoskeletal AD.
Viral infections are widely recognized as triggers for AD, including IIM [34]. Additionally, both ASS- ILD and COVID-19 appear to share several pathogenic mechanisms, particularly through the activation of the IFN-γ signaling pathway [35]. In ASS, IFN-γ-induced expression of HLA-II molecules in necrotic perifascicular myofibers has been observed [36], which subsequently activates the JAK/STAT pathway associated with AD [37]. The JAK2/STAT3 axis plays a critical role in ILD, with STAT3 overexpression contributing to PF [38]. The IFN-γ pathway emerges as a central driver of inflammation and PF in ILD-ASS and SARS-CoV-2 pneumonia [39]. This shared pathogenic mechanism underscores the potential link between viral infections, autoimmune responses, and the development of ILD.
We hypothesize that one plausible mechanism for explaining autoimmunity involves molecular mimicry between SARS-CoV-2 proteins, such as the SARS-CoV-2 spike glycoprotein found in both the virus and COVID-19 vaccines, and ARS polypeptides. This potential mimicry might result in the presentation of these peptides by antigen-presenting cells to T lymphocytes, facilitated by specific HLA molecules. Interestingly, three of the patients had an autoimmune background and certain HLA genetic susceptibility associated with different AD, including ASS, and a higher prevalence of severe SARS-CoV-2 infection (HLA-B∗08:01 and HLA-DRB1∗03:01 in patient Nº1; HLA-B∗27 in patient Nº3 and HLA- B∗44 in patients Nº3 and Nº4).
The link between HLA and AD susceptibility is well-established. Previous studies have demonstrated that the H1N1 influenza vaccination served as triggering factor for narcolepsy, particularly in individuals carrying the HLA- DQB1∗06:02 allele [40]. Similarly, in patients with IIM, certain HLA alleles have been associated with the development of myositis autoantibodies. Several studies [[41], [42], [43]] have documented an increased prevalence of HLA-DRB1∗03:01, HLA-DQA1∗05:01, and HLA-B∗08:01 alleles among individuals diagnosed with ASS who tested positive for anti-ARS autoantibodies and/or exhibited chronic interstitial lung disease (ILD) compared to healthy individuals. Among our reported cases, patient Nº1 presented both HLA-DRB1∗03:01 and HLA-B∗08:01 alleles, which may suggest a genetic predisposition to the development of ASS-ILD. Furthermore, Remuzgo-Martinez et al. also found a lower prevalence of HLA-DRB1∗07:01 in patients with ASS. Patient Nº4, who presented HLA- DRB1∗07:01, along with the uncertain positivity of PL-7 and resolution of pulmonary involvement without the need for immunosuppressants, raises doubts about the diagnosis of ASS in this case.
Consequently, HLA typing could serve as a complementary tool for diagnosing ASS-associated ILD from other conditions, such as SARS-CoV-2 infection. However, the lack of shared alleles among our patients underscores the complexity of this relationship and highlights the need for further research with larger cohorts to clarify the genetic associations and their implications for disease susceptibility and progression.
While our findings provide valuable insights, several limitations must be acknowledged. The retrospective nature of the study, coupled with incomplete clinical histories for some patients referred from other institutions due to the pandemic, limited our ability to obtain comprehensive data. Notably, in patient 4, the absence of imaging studies further restricted our capacity to fully assess the extent and progression of pulmonary involvement. Despite these challenges, our study reinforces the need for heightened clinical vigilance and systematic evaluation of autoantibodies in patients presenting with post-SARS-CoV-2 pulmonary symptoms.
As stated by other authors, a temporal association between events does not necessarily imply causality, and extensive epidemiological studies would be essential to establish any causal relationship definitively [44]. A comprehensive clinical and pathological examination of additional patients is required to elucidate the potential association between SARS-CoV-2 infection or vaccination and IIM. However, in cases where vaccinated individuals present with severe and persistent muscle pain and weakness, along with exacerbation of pulmonary symptoms, clinicians should consider the possibility of vaccine-induced IIM. Utilizing an autoantibodies work-up can serve as a straightforward and rapid method to guide diagnosis in such instances.
It is important to emphasize that our findings do not diminish the importance of vaccines, rather they contribute to medical knowledge by underscoring the significance of a multidisciplinary approach and early intervention, particularly in patients with autoimmune backgrounds and genetic susceptibility indicated by HLA typing.
Given the severe prognosis and diverse clinical manifestations of ASS, this study underscores the critical need for a standardized protocol to manage suspected ILD-ASS cases effectively. The occurrence of these four cases, closely linked in time to vaccination or SARS-CoV-2 infection, highlights the importance of vigilant and active surveillance to identify potential ASS development. Larger-scale studies are essential to establish causality and clarify the mechanisms involved, ultimately guiding the development of personalized diagnostic and therapeutic strategies for ASS-ILD.
CRediT authorship contribution statement
Laura García-Bravo: Writing – original draft, Project administration, Investigation, Conceptualization. Ángela Villegas: Validation, Resources, Investigation. Bárbara López Uceda: Methodology. Anaís Mariscal: Validation, Supervision, Investigation. Cristina Vadillo: Resources, Investigation. M Asunción Nieto Barbero: Resources, Investigation. Juan Luis Rodríguez-Hermosa: Resources, Investigation. Beatriz Mediero Valeros: Methodology. José Carlos Plaza-Hernández: Resources, Investigation. Miguel Fernández-Arquero: Supervision, Methodology, Investigation. María Guzmán-Fulgencio: Validation. Gloria Candelas-Rodríguez: Visualization, Validation, Investigation, Conceptualization. Silvia Sánchez-Ramón: Writing – review & editing, Validation, Supervision, Conceptualization. Juliana Ochoa-Grullón: Writing – review & editing, Supervision, Conceptualization.
Ethical approval
All procedures conducted in this study involving human participants adhered to the ethical standards set forth by the institutional and/or national research committee, as well as the 1964 Helsinki Declaration and its subsequent amendments or equivalent ethical guidelines.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Cavagna L., Nuño L., Sciré C.A., et al. AENEAS collaborative group Serum Jo-1 autoantibody and isolated arthritis in the antisynthetase syndrome: a review of the literature and report of the experience of AENEAS collaborative group. Clin. Rev. Allergy Immunol. 2017;52:71–80. doi: 10.1007/s12016-016-8544-0. [DOI] [PubMed] [Google Scholar]
- 2.Mirrakhimov A.E. Antisynthetase syndrome: a review of etiopathogenesis, diagnosis, and management. Curr. Med. Chem. 2015;22(16):1963–1975. doi: 10.2174/0929867322666150527102028. [DOI] [PubMed] [Google Scholar]
- 3.Goel N., Goyal N., Kumar R. Long COVID mimicking interstitial lung disease: a case series. Curr Health Sci J. 2021;47(3):469–473. doi: 10.12865/CHSJ.47.03.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duzgun S.A., Durhan G., Demirkazik F.B., Akpinar M.G., Ariyurek O.M. COVID-19 pneumonia: the great radiological mimicker. Insights Imaging. 2020;11(1):118. doi: 10.1186/s13244-020-00933-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elsayed M., Abdelgabar A., Karmani J., Majid M. A case of antisynthetase syndrome initially presented with interstitial lung disease mimicking COVID-19. J. Med. Cases. 2023;14(1):25–30. doi: 10.14740/jmc4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baratella E., Marrocchio C., Cifaldi R., et al. Interstitial lung disease in patients with antisynthetase syndrome: a retrospective case series study. Jpn. J. Radiol. 2021;39:40–46. doi: 10.1007/s11604-020-01030-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marie I., Hatron P.Y., Dominique S., et al. Interstitial lung disease in antisynthetase syndrome: initial and follow-up HRCT findings. Eur. Respir. J. 2020;56(5) doi: 10.1183/13993003.02062-2020. [DOI] [Google Scholar]
- 8.Kalluri M., Oddis C.V. Interstitial lung disease in antisynthetase syndrome. Front. Med. 2020;7:875. doi: 10.3389/fmed.2020.00875. [DOI] [Google Scholar]
- 9.Caruso D., Polidori T., Guido G., et al. Typical and atypical COVID-19 computed tomography findings. World J Clin Cases. 2020;8(15):3177–3187. doi: 10.12998/wjcc.v8.i15.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X.Y., Chen J., Zhi L.J., et al. Anti-synthase syndrome associated with SARS-Cov-2 infection. BMC Pulm. Med. 2024;24(1):179. doi: 10.1186/s12890-024-02966-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinal-Fernandez I., Casal-Dominguez M., Huapaya J.A., et al. A longitudinal cohort study of the anti-synthetase syndrome: increased severity of interstitial lung disease in black patients and patients with anti-PL7 and anti-PL12 autoantibodies. Rheumatology. 2017;56(6):999–1007. doi: 10.1093/rheumatology/kew430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hervier B., Devilliers H., Stanciu R., et al. Hierarchical cluster and survival analyses of antisynthetase syndrome: phenotype and outcome are correlated with anti-tRNA synthetase antibody specificity. Autoimmun. Rev. 2012;12(2):210–217. doi: 10.1016/j.autrev.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Marie I., Josse S., Decaux O., et al. Comparison of long-term outcomes between anti-Jo1 and anti-PL7/PL12-positive patients with antisynthetase syndrome. Autoimmun. Rev. 2012;11(10):739–745. doi: 10.1016/j.autrev.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Solomon J., Swigris J.J., Brown K.K. Myositis-related interstitial lung disease and antisynthetase syndrome. J. Bras. Pneumol. 2011;37(1):100–109. doi: 10.1590/S1806-37132011000100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gracia-Ramos A.E., Martin-Nares E., Hernández-Molina G. New onset of autoimmune diseases following COVID-19 diagnosis. Cells. 2021;10(12):3592. doi: 10.3390/cells10123592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Safary A., Esalatmanesh K., Eftekharsadat A.T., et al. Autoimmune inflammatory rheumatic diseases post-COVID-19 vaccination. Int Immunopharmacol. 2022;110 doi: 10.1016/j.intimp.2022.109061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galeotti C., Bayry J. Autoimmune and inflammatory diseases following COVID-19. Nat. Rev. Rheumatol. 2020;16(8):413–414. doi: 10.1038/s41584-020-0448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodríguez Y., Rojas M., Beltrán S., et al. Autoimmune and autoinflammatory conditions after COVID-19 vaccination: new case reports and updated literature review. J. Autoimmun. 2022;132 doi: 10.1016/j.jaut.2022.102898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.García-Bravo L., Calle-Rubio M., Fernández-Arquero M., et al. Association of anti-SARS-CoV-2 vaccine with increased incidence of myositis-related anti-RNA-synthetase autoantibodies. J Transl Autoimmun. 2022;5 doi: 10.1016/j.jtauto.2022.100160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kharouf F., Kenig A., Bohbot E., et al. Increased rates of idiopathic inflammatory myopathies during the COVID-19 pandemic: a single-centre experience. Clin. Exp. Rheumatol. 2023;41(2):316–321. doi: 10.55563/clinexprheumatol/970881. [DOI] [PubMed] [Google Scholar]
- 21.Kouranloo K., Dey M., Elwell H., Nune A. A systematic review of the incidence, management, and prognosis of new-onset autoimmune connective tissue diseases after COVID-19. Rheumatol. Int. 2023;43(7):1221–1243. doi: 10.1007/s00296-023-05290-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peña C., Kalara N., Velagapudi P. A case of antisynthetase syndrome in the setting of SARS-CoV-2 infection. Cureus. 2023;15(6) doi: 10.7759/cureus.40588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Segal Y., Shoenfeld Y. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell. Mol. Immunol. 2018;15:586–594. doi: 10.1038/cmi.2017.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lundberg I.E., Tjärnlund A., Bottai M., et al. 2017 European League against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann. Rheum. Dis. 2017;76(12):1955–1964. doi: 10.1136/annrheumdis-2017-211468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahler M., Miller F.W., Fritzler M.J. Idiopathic inflammatory myopathies and the anti-synthetase syndrome: a comprehensive review. Autoimmun. Rev. 2014;13(4–5):367–371. doi: 10.1016/j.autrev.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casal-Dominguez M., Pinal-Fernandez I., Pak K., et al. Performance of the 2017 European Alliance of Associations for Rheumatology/American College of Rheumatology classification criteria for idiopathic inflammatory myopathies in patients with myositis-specific autoantibodies. Arthritis Rheumatol. 2022;74(3):508–517. doi: 10.1002/art.42010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cervantes-Arévalo C., Escobar-Valderrama J., Martínez-Figueroa A. Utility of antibody identification in inflammatory myopathies. Neurología Argentina. 2023;15(4):123–130. doi: 10.1016/j.neuarg.2023.07.005. [DOI] [Google Scholar]
- 28.Hervier B., Uzunhan Y. Inflammatory myopathy-related interstitial lung disease: from pathophysiology to treatment. Front. Med. 2020;6:326. doi: 10.3389/fmed.2019.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.George P.M., Wells A.U., Jenkins R.G. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir. Med. 2020;8(8):807–815. doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drake T.M., Docherty A.B., Harrison E.M., et al. Outcome of hospitalization for COVID-19 in patients with interstitial lung disease: an international multicenter study. Am. J. Respir. Crit. Care Med. 2020;202(12):1656–1665. doi: 10.1164/rccm.202007-2794OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen X.Y., Chen J., Zhi L.J., et al. Anti-synthase syndrome associated with SARS-CoV-2 infection. BMC Pulm. Med. 2024;24(1):179. doi: 10.1186/s12890-024-02966-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahn Y.H., Park S., Choi J.J., et al. Secreted tryptophanyl-tRNA synthetase: insights into its function and immunogenic potential. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.735275. [DOI] [Google Scholar]
- 33.Megremis S., Walker T.D.J., He X., et al. Antibodies against immunogenic epitopes with high sequence identity to SARS-CoV-2 in patients with autoimmune dermatomyositis. Ann. Rheum. Dis. 2020;79(10):1383–1386. doi: 10.1136/annrheumdis-2020-217522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saud A., Naveen R., Aggarwal R., Gupta L. COVID-19 and myositis: what we know so far. Curr. Rheumatol. Rep. 2021;23(8):63. doi: 10.1007/s11926-021-01000-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.La Rocca G., Ferro F., Baldini C., et al. Targeting intracellular pathways in idiopathic inflammatory myopathies: a narrative review. Front. Med. 2023;10 doi: 10.3389/fmed.2023.1158768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uruha A., Goebel H.H., Stenzel W. Updates on the immunopathology in idiopathic inflammatory myopathies. Curr. Rheumatol. Rep. 2021;23(56) doi: 10.1007/s11926-021-01020-8. [DOI] [PubMed] [Google Scholar]
- 37.Xin P., Xu X., Deng C., et al. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int Immunopharmacol. 2020;80 doi: 10.1016/j.intimp.2020.106210. [DOI] [PubMed] [Google Scholar]
- 38.Huo R., Guo Q., Hu J., et al. Therapeutic potential of janus kinase inhibitors for the management of interstitial lung disease. Drug Des. Dev. Ther. 2022;16:991–998. doi: 10.2147/DDDT.S356326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sposito B., Broggi A., Pandolfi L., et al. The interferon landscape along the respiratory tract impacts the severity of COVID-19. Cell. 2021;184(19):4953–4968.e16. doi: 10.1016/j.cell.2021.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szakács A., Darin N., Hallböök T. Increased childhood incidence of narcolepsy in western Sweden after H1N1 influenza vaccination. Neurology. 2013;80(14):1315–1321. doi: 10.1212/WNL.0b013e31828ab26f. [DOI] [PubMed] [Google Scholar]
- 41.Rothwell S., Chinoy H., Lamb J.A., et al. Focused HLA analysis in Caucasians with myositis identifies significant associations with autoantibody subgroups. Ann. Rheum. Dis. 2019;78:996–1002. doi: 10.1136/annrheumdis-2018-214433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Remuzgo-Martínez S., Atienza-Mateo B., Ocejo-Vinyals J.G., et al. HLA association with the susceptibility to antisynthetase syndrome. Joint Bone Spine. 2021;88(3) doi: 10.1016/j.jbspin.2020.105115. [DOI] [PubMed] [Google Scholar]
- 43.Yanagihara T., Inoue Y. Insights into pathogenesis and clinical implications in myositis-associated interstitial lung diseases. Curr. Opin. Pulm. Med. 2020;26(5):507–517. doi: 10.1097/MCP.0000000000000698. [DOI] [PubMed] [Google Scholar]
- 44.Ding Y., Ge Y. Inflammatory myopathy following coronavirus disease 2019 vaccination: a systematic review. Front. Public Health. 2022;10 doi: 10.3389/fpubh.2022.1007637. [DOI] [PMC free article] [PubMed] [Google Scholar]