Abstract
Background and aim
Whereas Yttrium-90 selective internal radiation therapy (Y-90 SIRT) was shown to improve local tumor control in non-Asian population, the efficacy of this therapy for Asian population in real-world setting remains poorly detailed. We aimed to determine outcomes and identify predictors of response in hepatocellular carcinoma (HCC) patients treated by Y-90 SIRT.
Methods
We retrospectively enrolled 52 HCC patients receiving Y-90 SIRT at our tertiary center between 2014 and 2019. Overall survival (OS), progression free survival (PFS), and predictive factors were determined by Kaplan–Meier method and Cox-proportional hazard analysis.
Results
Of the 52 patients (81% male, mean age 64.9 years), 71% and 29% were classified as Barcelona Clinic Liver Cancer stage C and B HCC, respectively; 63% had portal vein thrombosis, and 35% had objective tumor response defined by the modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria. OS and PFS were 11.0 and 2.4 months, respectively. Two patients were successfully down-staged and further underwent surgical resection. Multifocal lesion, alpha-fetoprotein (AFP) ≥200 ng/mL, and Eastern Cooperative Oncology Group (ECOG) score ≥1 were significantly associated with poor survival, with adjusted hazard ratio (95% confidence interval) of 7.7 (2.0–29.8), 5.4 (2.0–14.7), and 3.1 (1.0–9.6), respectively (all in P < 0.05).
Conclusions
Y-90 SIRT is an effective treatment for the local tumor control of HCC without serious adverse events. Single lesion, AFP level and ECOG status were predictors of response.
Keywords: Liver cancer, Survival outcome, Transarterial radioembolization (TARE), Unresectable hepatocellular carcinoma, Yttrium-90 selective internal radiation therapy (Y-90 SIRT)
1. Introduction
Liver cancer represents the seventh most common cancer and the fourth most common cause of cancer-related mortality worldwide.1,2 Ninety percent of primary liver malignancy is hepatocellular carcinoma (HCC).3 Approximately 54% of HCC patients presents at an advanced stage, meaning that curative treatment options are not feasible and resulting in poor outcomes.4
Yttrium-90 (Y-90) radioembolization, also known as selective internal radiation therapy (SIRT), is a minimally invasive treatment for HCC. Microspheres loaded with radioisotope Y-90 are delivered to the tumor by trans-catheter intra-arterial injection. Βeta particles emitted from Y-90 cause direct cytotoxic effect to the tumor with minimal damage to the non-tumoral liver parenchyma.5 The potential benefits of Y-90 SIRT for cancer treatment were reported in a small cohort of patients with unresectable pancreatic cancer and HCC as early as in 1960s.6 Over the past decades, Y-90 SIRT has been increasingly used as a treatment option for local tumor control in patients developing unresectable HCC. Notably, efficacy and good safety profile of Y-90 SIRT were consistently evidenced in patients with early and intermediate stage HCC.7, 8, 9, 10 Y-90 SIRT can be considered as a bridge to liver transplantation or an alternative therapy to radiofrequency ablation (RFA) for early stage HCC (Barcelona Clinic Liver Cancer (BCLC) stage A).10 For intermediate stage (BCLC stage B) HCC, although the guidelines recommend conventional transarterial chemoembolization (cTACE),3,11,12 a recent randomized trial showed that Y-90 SIRT was superior to cTACE in terms of increasing duration of time-to-progression (TTP) and improving local tumor control.10 In comparison with cTACE, Y-90 SIRT is more efficient for downstaging the United Network for Organ Sharing (UNOS) stage T3 HCC to T2 HCC, and Y-90 SIRT reduced the drop-out rate from a transplant waiting list, albeit the lack of survival benefits.10,13 Additionally, patients receiving Y-90 SIRT treatment offered superior quality of life and exhibited low toxicity.9
Y-90 SIRT can be safely used in advanced stage HCC patients with portal vein thrombosis (PVT) or BCLC stage C, according to the minimal embolic effect of the Y-90 microspheres to hepatic arteries.14 Although, sorafenib is currently the standard treatment modality for BCLC stage C HCC, a previous trial highlighted that overall survival (OS) was similar for patients treated with Y-90 SIRT or this inhibitor drug. However, Y-90 SIRT treatment triggered had a higher tumor response rate, lesser adverse effects and better quality of life.15
Accordingly, Y-90 SIRT can be recommended for the treatment of all HCC stages. The outcomes of Y-90 SIRT in real-world practice remains few described. In this retrospective observational cohort study, we aimed to determine survival outcome and identify predictors of response in non-transplanted patients with unresectable HCC treated by Y-90 SIRT in a tertiary care hospital.
2. Patients and methods
2.1. Patients and study design
This single-center observational study was approved by the Institutional Research Committee, Faculty of Medicine, Chulalongkorn University (IRB No. 658/61). We retrospectively reviewed medical records of unresectable HCC patients admitted to the King Chulalongkorn Memorial Hospital for Y-90 SIRT during the period of January 2014 to April 2019. Written informed consent was waived due to the retrospective nature of the present study. HCC was diagnosed by pathology or imaging criteria according to the American Association for the Study of Liver Diseases (AASLD) guidelines.11 The HCC treatment option was decided by a multidisciplinary tumor board. Prior to the Y-90 SIRT procedure, patients underwent Technetium-99m-labeled macro-aggregated albumin scintigraphy (99mTc-MAA) and angiography of the hepatic artery to measure hepato-pulmonary shunt fraction. The imaging was used as well to calculate the dose of Y-90 administrated to the tumor and the adjacent liver parenchyma together with the tracer distribution. Prophylactic embolization of hepaticoenteric arteries was performed to prevent the dispersion of Y-90 resin microspheres to other organs in gastrointestinal tracts or pancreas. Patients showing severe pulmonary shunting (pulmonary shunt fraction >20%) and extrahepatic uptake of 99mTc-MAA were excluded from the Y-90 SIRT procedure.16 Within 1 month after evaluation, patients were admitted for treatment with Y-90 resin microsphere (SIR-spheres®; Sirtex Medical Pty. Ltd., St Leonards, NSW, Australia). Unilobar, sequential bilobar, or whole liver was exposed to Y-90 injected through intra- or extrahepatic collateral arteries, depending on tumor size and location. These protocols can be defined as follows: (i) unilobar approach: selective injection of Y-90 resin microspheres into either right hepatic artery (RHA) or left hepatic artery (LHA); (ii) sequential bilobar approach: selective injection of Y-90 resin microspheres into RHA (or LHA) followed by a second Y-90 administration into LHA (or RHA) 4–6 weeks later; (iii) whole liver approach: injection of Y-90 microsphere into both RHA and LHA in a single session; and (iv) extrahepatic approach: injection of Y-90 microsphere into collateral arteries other than hepatic arteries that vascularize tumor.
The administered radioactivity dose was calculated using the following formula: activity (Gigabecquerel (GBq)) = (body surface area (m2)-0.2) + (tumor volume/total liver volume). This formula was used for any Y-90 SIRT protocol performed between January 2014 and July 2018.17 Since July 2018, partition model version 1.1 (Sirtex Medical Pty. Ltd., St Leonards, NSW, Australia) replaced the above formula for dosing the radioactivity. Patients were routinely followed up at 1 and 2 weeks after the procedure for blood testing, adverse events monitoring and toxicity assessment. Computed tomography (CT) and/or magnetic resonance imaging (MRI) were performed to assess the treatment response at 1 month post-treatment and every 3 months afterwards.
Clinical information was abstracted from electronic medical records, including baseline characteristics, underlying etiology of liver disease, Child-Pugh class, Eastern Cooperative Oncology Group (ECOG) performance status, liver tumor characteristics (size, number, location and distribution, vascular involvement), BCLC stage, liver chemistries, alpha-fetoprotein (AFP) level, radiologic reports of dynamic contrast upper abdominal CT and/or MRI, Y-90 SIRT technique and dose (GBq), hepato-pulmonary shunt fraction, length of hospital stay, and any other treatment before or after Y-90 SIRT. Response to Y-90 SIRT using radiologic data was assessed using the modified Response Evaluation Criteria in Solid Tumors (mRECIST).3 According to the mRECIST criteria, patients who had complete or partial radiologic response were counted as responders while those who had stable or progressive disease were defined as non-responders.
2.2. Statistical analyses
Mean (±standard deviation (SD)) and median (range or 25th and 75th percentiles) were reported as appropriate. The primary outcome of the study was the OS, calculated from the date of first Y-90 SIRT until death due to any causes or last visit date. The secondary outcome was the progression free survival (PFS), calculated from the date of the first Y-90 SIRT to the date of disease progression at any organs by radiologic evidence or death due to any causes. OS and PFS were estimated using the Kaplan–Meier method, and compared using the log-rank test. Factors associated with survival were determined using the Cox proportional hazards analysis. Laboratory toxicities were evaluated using pre-treatment and post-treatment parameters and compared by the paired t-test. All analyses were performed using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY, USA). P-value <0.05 was considered as significant.
3. Results
3.1. Patients’ characteristics
For this observational study, 61 HCC patients were eligible for Y-90 SIRT. At the pretreatment evaluation, 4 (6.6%) patients were excluded from the protocol (2 had high pulmonary shunt fraction, 1 had poor tumoral radiotracer uptake, and 1 had large arterioportal shunt). Another (1.6%) patient was excluded due to rapid extensive progression of PVT. Before the initiation of Y-90 SIRT, three other patients died of upper gastrointestinal bleeding, ruptured HCC and rapid liver function deterioration and 1 patient was lost to follow-up. The remaining 52 patients scheduled for Y-90 SIRT were included in the analysis. In total, 66 Y-90 SIRT sessions were performed with an average session number of 1.3 per patient (range 1–3).
Baseline patient and tumor characteristics are shown in Table 1. Forty-two (81%) were male with a mean age of 64.9 years. Hepatitis B virus infection was the most common etiology of cirrhosis (n = 23, 44%), followed by non-alcoholic fatty liver disease (n = 9, 17%), and hepatitis C virus infection (n = 7, 13%). All patients except one had ECOG score of 0–1.
Table 1.
Baseline characteristics of the patients treated with Y-90 SIRT (N = 52).
| Characteristics | Value |
|---|---|
| Age (years) | 64.9 ± 11.1 |
| Male, n (%) | 42 (81) |
| Etiology of cirrhosis, n (%) | |
| Hepatitis B virus infection | 23 (44) |
| Non-alcoholic fatty liver disease | 9 (17) |
| Hepatitis C virus infection | 7 (13) |
| Alcoholic cirrhosis | 6 (12) |
| Cryptogenic | 6 (12) |
| Non-cirrhosis | 1 (2) |
| Child-Pugh class, n (%) | |
| A | 45 (86) |
| B7 | 5 (10) |
| B8 | 1 (2) |
| B9 | 1 (2) |
| ECOG, n (%) | |
| 0 | 26 (50) |
| 1 | 25 (48) |
| 2 | 1 (2) |
| Liver function | |
| Total bilirubin (mg/dL) | 0.83 (0.23–2.18) |
| Albumin (g/dL) | 3.7 (2.6–4.4) |
| AFP, n (%) | |
| <200 ng/mL | 23 (46) |
| ≥200 ng/mL | 27 (54) |
| Distribution of HCC, n (%) | |
| Bilobar | 25 (48) |
| Unilobar | 27 (52) |
| Number of lesions, n (%) | |
| Single | 7 (13) |
| 2–3 | 8 (15) |
| Multifocal | 37 (71) |
| Maximum size, n (%) | |
| <5 cm | 8 (15) |
| 5–10 cm | 22 (42) |
| >10 cm | 9 (17) |
| Infiltrative lesion | 13 (25) |
| Portal vein thrombosis, n (%) | |
| Absent | 19 (37) |
| Main branch | 13 (25) |
| Segmental branch | 20 (38) |
| BCLC stage, n (%) | |
| B | 15 (29) |
| C | 37 (71) |
| Prior treatment, n (%)a | |
| None | 22 (42) |
| TACE alone | 10 (19) |
| TACE with othersb | 16 (31) |
| Others without TACEc | 4 (8) |
Data were expressed as the mean ± standard deviation or median (range).
Abbreviations: AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; EBRT, external beam radiation therapy; ECOG, Eastern Cooperative Oncology Group; HCC, hepatocellular carcinoma; RFA, radiofrequency ablation; TACE, transarterial chemoembolization.
Most patients received more than one treatment as follows: cTACE (n = 26), resection (n = 9), RFA (n = 7), EBRT (n = 6), systemic therapy with sorafenib or nivolumab (n = 5), drug-eluting beads TACE (n = 2), and liver transplantation (n = 1).
TACE with others: TACE with RFA (n = 5), resection (n = 2), systemic therapy (n = 1), drug eluting beads (n = 1), EBRT and systemic therapy (n = 3), resection and EBRT (n = 2), resection and systemic therapy (n = 1), resection and RFA (n = 1).
Others without TACE: resection (n = 1), resection with EBRT (n = 1), RFA (n = 1), EBRT (n = 1).
HCC was diagnosed by histopathology in 16 (31%) patients. Tumors were classified as BCLC stages B and C in 15 (29%) and 37 (71%) patients, respectively. Patients with bilobar involvement or multifocal lesions (>3 lesions) represented 48% (n = 25) and 71% (n = 37), respectively. Median maximum size of tumor was 6.4 cm (range: 1.5–22.0 cm). PVT was found in 33 (63%) patients. Median AFP level was 280 ng/mL (25th and 75th percentiles: 9–1889 ng/mL).
Out of the 52 patients, 22 (42%) underwent Y-90 SIRT as the first-line therapy for HCC, while 30 (58%) received multiple treatments prior to Y-90 SIRT (Table 1). cTACE was the most common previous treatment (n = 26, average 2 sessions per patient, range 1–5 sessions), followed by surgical resection (n = 9), RFA (n = 7), external beam radiation (n = 6), systemic therapy with sorafenib or nivolumab (n = 5), drug-eluting beads TACE (n = 2), and liver transplantation (n = 1).
3.2. Y-90 SIRT treatment
Fifty-two patients underwent 63 Y-90 SIRT sessions (mean 1.2, median 1, range 1–3). The radiotherapy was delivered in 2 or 3 sessions for 10 and 2 patients, respectively. Median time from HCC diagnosis to Y-90 SIRT was 3.6 months (25th and 75th percentiles: 1.5–13.2, range: 0.8–53.5). Median time from pre-treatment evaluation to Y-90 SIRT was 0.5 months (25th and 75th percentiles: 0.5–0.7, range: 0.3–1.1). Median pulmonary shunt fraction was 5.20% (range: 1.32–17.70). Median radioactivity dose per treatment session was 1.76 GBq (range: 0.36–4.90). Whole liver radioembolization was performed in 48% of patients while 46% underwent unilobar treatment. Y-90 SIRT of whole liver with extrahepatic approach was performed in 10 (19%) cases (Table 2). Median length of hospital stay was 2 days (range: 1–8).
Table 2.
Clinical outcomes of the patients treated with Y-90 SIRT.
| Results | Value |
|---|---|
| Pre-treatment evaluation | |
| Lung shunting (%) | 5.20 (1.32–17.70) |
| Treatment procedure | |
| Length of stay (days) | 2 (1–8) |
| Radioactivity dose (GBq) | 1.76 (0.36–4.90) |
| Technique, n (%) | |
| Unilobar | 17 (32.7) |
| Sequential bilobar | 3 (5.8) |
| Whole liver | 22 (42.3) |
| Unilobar with extrahepatic | 7 (13.5) |
| Whole liver with extrahepatic | 3 (5.8) |
| Duration of protocol | |
| Diagnosis to treatment (months) | 3.6 (0.8–53.5) |
| Pretreatment evaluation to treatment (months) | 0.5 (0.3–1.1) |
| Objective response by mRECIST, n (%) | |
| Complete response | 1 (2) |
| Partial response | 17 (33) |
| Stable disease | 10 (19) |
| Progressive disease | 24 (46) |
| Further treatment after first Y-90 SIRT, n (%)a | |
| None | 24 (46.2) |
| Repeated Y-90 SIRT | 9 (17.4) |
| TACE ± others | 10 (19.2) |
| Targeted therapy only | 5 (9.6) |
| Resection | 2 (3.8) |
| Other modalities | 2 (3.8) |
| Outcomes at the end of study, n (%) | |
| Tumor related deaths | 19 (36) |
| Liver decompensation related deaths | 16 (31) |
| Non-tumor related deaths | 5 (10) |
| Lost to follow up | 4 (8) |
| Alive | 8 (15) |
Data were expressed as median (range) or n (%).
Abbreviations: GBq, gigabecquerel; TACE, transarterial chemoembolization; Y-90 SIRT, yttrium-90 selective internal radiation therapy.
Some patients received more than one treatment modalities.
3.3. Objective response rate
Treatment response rate, assessed by objective radiologic response, was 35% (n = 18). One (2%) patient achieved a complete response and a partial response was observed in 17 (33%) patients; while 10 (19%) and 24 (46%) patients had stable or progressive disease, respectively. Among those who had progressive or recurrent disease, 28 (54%) patients received additional treatments including cTACE (n = 10), sorafenib or lenvatinib (n = 6), RFA (n = 3), surgical resection (n = 2), nivolumab (n = 2), external radiation therapy (n = 1) and percutaneous ethanol injection (n = 1).
3.4. OS and PFS
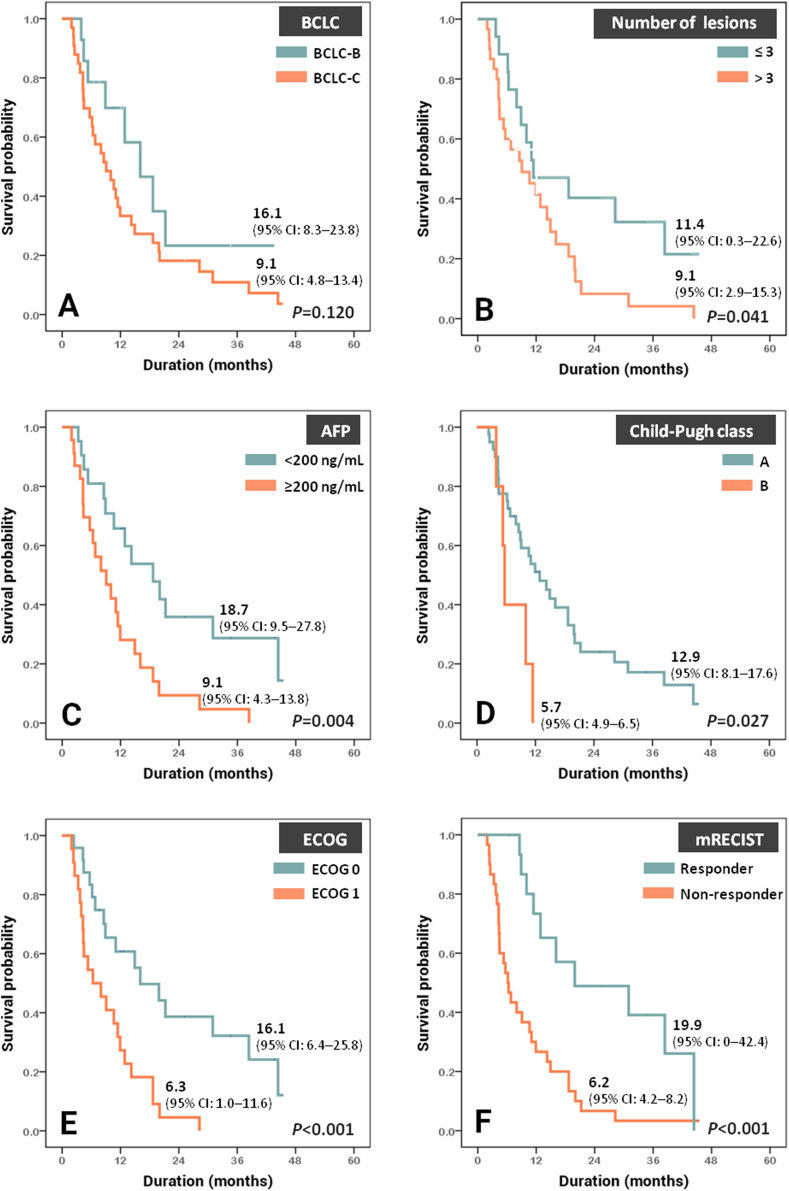
Median OS was 11.0 (95% confidence interval (95% CI), 7.8–14.3) months whereas median PFS was 2.4 (95% CI, 1.0–3.9) months. Y-90 SIRT had a tendency to provide longer life expectancy in patients with BCLC stage B (n = 15) than in patient with BCLC stage C (n = 37), i.e. 16.1 (95% CI, 8.3–23.8) vs. 9.1 (95% CI, 4.8–13.4) months, but the difference was not statistically significant (P = 0.12, Fig. 1A). When categorized on the basis of tumor parameters, patients with multifocal lesions and those with AFP ≥200 ng/mL had significantly worse survival (P = 0.041 and 0.004, Fig. 1B and C). Patients with Child-Pugh A cirrhosis (n = 45) had significantly better survival than those with Child-Pugh B cirrhosis (n = 7), i.e. 12.9 (95% CI, 8.1–17.6) vs. 5.7 (95% CI, 4.9–6.5) months, P = 0.027 (Fig. 1D). Likewise, ECOG 0 patients or those who had objective tumor response had significantly longer survival than ECOG 1 patients or those who did not have objective response (Fig. 1E and F).
Fig. 1.
Kaplan–Meier curves demonstrating overall survival classified by (A) BCLC staging, (B) number of lesions, (C) AFP, (D) Child-Pugh class, (E) ECOG performance status, and (F) tumor response by mRECIST criteria. Abbreviations: AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; ECOG, Eastern Cooperative Oncology Group; mRECIST, modified Response Evaluation Criteria in Solid Tumors.
Mean follow-up time in this study was 12.5 months. Forty patients had died during the follow up period. The most common cause of death was tumor progression (n = 19, 36%), followed by liver decompensation (n = 16, 31%), and non-tumor non-liver related events (n = 5, 10%). Four patients were lost to follow up. At the end of study, eight patients remained alive.
3.5. Factors associated with poor survival
Table 3 shows the predictive factors of survival for Y-90 SIRT. Univariate analysis showed that Child-Pugh class, ECOG performance status, size and number of lesions, AFP level, and objective response were significantly associated with survival. Multivariate analysis following adjustment for age and gender evidenced that multifocal lesions, AFP ≥200 ng/mL, and ECOG status were significantly associated with poor survival, with adjusted hazard ratio (aHR) (95% CI) of 7.7 (2.0–29.8), 5.4 (2.0–14.7), and 3.1 (1.0–9.6), P = 0.003, <0.001, 0.047, respectively. Objective tumor response was the only factor significantly associated with PFS, with aHR (95% CI) of 10.0 (3.1–32.5), P < 0.001.
Table 3.
Factors associated with overall survival and progression-free survival.
| Predictor | Category | Overall survival |
Progression free survival |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | aHR (95% CI)∗ | P | HR (95% CI) | P | aHR (95% CI)∗ | P | ||
| Age (years) | 1.0 (1.0–1.0) | 0.800 | 1.0 (0.9–1.0) | 0.910 | |||||
| Gender | Female | 1.1 (0.5–2.7) | 0.800 | 1.2 (0.5–2.8) | 0.750 | ||||
| Male | 1 (reference) | 1 (reference) | |||||||
| Etiology of cirrhosis | |||||||||
| Alcohol vs. non-alcohol | 0.6 (0.2–1.5) | 0.270 | 1.1 (0.4–2.9) | 0.800 | |||||
| HBV vs. non-HBV | 1.2 (0.6–2.3) | 0.550 | 0.9 (0.5–1.7) | 0.760 | |||||
| HCV vs. non-HCV | 0.8 (0.3–2.0) | 0.570 | 1.1 (0.4–2.8) | 0.870 | |||||
| NASH vs. non-NASH | 0.8 (0.3–2.7) | 0.740 | 0.7 (0.2–2.4) | 0.570 | |||||
| Cryptogenic vs. non-cryptogenic | 1.6 (0.7–3.6) | 0.290 | 1.1 (0.5–2.5) | 0.840 | |||||
| ECOG | 0 | 1 (reference) | 1 (reference) | 1 (reference) | |||||
| 1 | 3.1 (1.6–6.3) | 0.001 | 3.1 (1.0–9.6) | 0.047 | 2.2 (1.1–4.4) | 0.022 | 0.4 (0.2–1.0) | 0.050 | |
| Albumin (g/dL) | <3.0 | 2.9 (0.9–10.1) | 0.088 | 1.2 (0.4–4.0) | 0.750 | ||||
| ≥3.0 | 1 (reference) | 1 (reference) | |||||||
| Child-Pugh | A | 1 (reference) | 1 (reference) | 1 (reference) | |||||
| B | 2.9 (1.1–7.9) | 0.036 | 3.1 (0.9–11.1) | 0.080 | 1.2 (0.6–4.6) | 0.280 | |||
| Distribution | Unilobar | 1 (reference) | 1 (reference) | ||||||
| Bilobar | 1.3 (0.7–2.4) | 0.440 | 1.1 (0.6–2.1) | 0.750 | |||||
| Number of lesions | Single | 1 (reference) | 1 (reference) | 1 (reference) | |||||
| 2–3 | 2.5 (0.7–9.4) | 0.160 | 3.7 (0.7–20.7) | 0.130 | 2.9 (0.7–13.0) | 0.160 | |||
| Multifocal | 3.1 (1.1–8.9) | 0.039 | 7.7 (2.0–29.8) | 0.003 | 3.1 (0.9–10.8) | 0.090 | |||
| Maximum size (cm) | <5 | 1 (reference) | 1 (reference) | 1 (reference) | |||||
| 5–10 | 0.5 (0.2–1.3) | 0.180 | 0.5 (0.1–3.0) | 0.420 | 0.5 (0.2–1.2) | 0.100 | |||
| >10 | 0.4 (0.1–1.2) | 0.100 | 0.3 (0.1–1.5) | 0.290 | 0.3 (0.1–0.9) | 0.400 | |||
| Infiltration | 0.3 (0.1–1.0) | 0.033 | 0.2 (0–1.2) | 0.080 | 0.6 (0.2–1.6) | 0.290 | |||
| Metastases | Absent | 1 (reference) | 1 (reference) | ||||||
| Present | 1.0 (0.4–2.5) | 0.970 | 2.1 (0.9–5.2) | 0.110 | |||||
| PVT | Absent | 1 (reference) | 1 (reference) | 1 (reference) | |||||
| Present | 1.4 (0.7–2.8) | 0.320 | 2.0 (1.0–4.1) | 0.042 | 0.4 (0.1–1.5) | 0.190 | |||
| AFP (ng/mL) | <200 | 1 (reference) | 1 (reference) | 1 (reference) | |||||
| ≥200 | 2.7 (1.3–5.4) | 0.006 | 5.4 (2.0–14.7) | <0.001 | 2.0 (1.0–3.9) | 0.050 | |||
| BCLC stage | B | 1 (reference) | 1 (reference) | 1 (reference) | |||||
| C | 1.8 (0.8–4.0) | 0.127 | 3.1 (1.4–6.8) | 0.005 | 4.0 (1.0–16.7) | 0.060 | |||
| Technique approach | |||||||||
| Unilobar | 1 (reference) | 1 (reference) | |||||||
| Sequential bilobar | 1.2 (0.3–4.4) | 0.780 | 1.3 (0.4–4.7) | 0.690 | |||||
| Whole liver | 1.2 (0.6–2.5) | 0.680 | 1.5 (0.7–3.1) | 0.300 | |||||
| Unilobar with extrahepatic | 0.9 (0.3–2.6) | 0.880 | 0.8 (0.3–2.2) | 0.620 | |||||
| Whole liver with extrahepatic | 1.0 (0.1–7.9) | 0.990 | 0.4 (0.1–3.1) | 0.390 | |||||
| Objective responsea | Responder | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||||
| Non-responder | 3.3 (1.6–6.9) | <0.001 | 3.4 (1.0–11.8) | 0.052 | 5.4 (2.4–12.2) | <0.001 | 10.0 (3.1–32.5) | <0.001 | |
Abbreviations: AFP, alpha-fetoprotein; aHR, adjusted hazard ratio; BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HBV, Hepatitis B virus; HCV, Hepatitis C virus; HR, hazard ratio; NASH, non-alcoholic steatohepatitis; PVT, portal vein thrombosis.
∗Adjusted for age and gender.
Responders (complete response or partial response) and non-responders (stable disease or progressive disease).
3.6. Clinical and laboratory toxicities
Fatigue and abdominal pain after the procedure was reported in 3 (6%) and 3 (6%) patients, respectively. One (2%) patient developed herpes zoster infection. No episode of sepsis, diarrhea, perihepatic effusion, pleural effusion, radiation-induced pneumonitis, or cholecystitis was reported in this study. None of the patients experienced liver failure or hepatic encephalopathy after treatment.
Complete blood count, prothrombin time as well as liver and renal function test were performed at 1-week post-Y-90 SIRT. Compared to baseline values, white blood cell count, bilirubin and alkaline phosphatase (ALT) levels were significantly increased while albumin was statistically decreased; however, the changes were trivial and not considered to be clinically meaningful (Supplemental Table 1).
4. Discussion
HCC represents one of major public health problem in worldwide. Prognosis of HCC patients is generally poor, the 5-year survival rate being less than 20%.2,18 Surgical resection and liver transplantation are the most effective treatments for early stage HCC. However, these modalities are applicable to only a small proportion of the HCC patients because most of the patients are diagnosed in intermediate or advanced stages, or have co-existing cirrhosis with clinically significant portal hypertension which precludes surgical resection. Novel locoregional and systemic therapies have been increasingly used as treatments for unresectable HCC. Y-90 SIRT is currently offered in several countries worldwide as locoregional treatment for HCC. The present study reported that Y-90 SIRT had a good safety profile and induced positive outcomes in patients with intermediate and advanced stage HCC in a real-life setting. Factors associated with the positive effects of Y-90 SIRT were also identified and encompassed single lesion, AFP <200 ng/mL, and good ECOG performance status.
Previous studies reported that OS and PFS of patients treated with Y-90 SIRT ranged from 12.8 to 16.4 months, and 7.9–11.0 months, respectively.19, 20, 21, 22 Here, the estimated OS and PFS of the present cohort were relatively shorter, i.e. 11.0 and 2.4 months, respectively. The shorter survivals of our cohort could be explained by a higher proportion of patients with more aggressive disease, 71% of the patients in our cohort had advanced HCC (BCLC stage C), whereas BCLC stage C was present in 51–56% of the patients in previous studies.19,20,22 Additionally, 25% of patients in the present cohort had infiltrative HCC, a factor associated with poor prognosis.20,22 This hypothesis was supported by the finding that when stratified by HCC stage, our patients with BCLC stage B had longer median OS than those with BCLC stage C (16.1 months vs. 9.1 months) and these numbers were quite comparable to the survivals of BCLC stages B and C patients previously shown in other prospective cohorts, ranging from 16.4 to 17.2 months and 10 to 12 months, and in a real-world cohort of 18.5 months and 11.2 months, for BCLC stages B and C, respectively.19,20,22,23
cTACE and Y-90 SIRT are the main therapies for local tumor control in unresectable HCC. For patients with BCLC stage B HCC, cTACE is the first line treatment recommended by the American, European, and Asian guidelines.3,11,12 However, in real-life settings, the cTACE cannot be applied to all patients at this tumor stage, i.e. cTACE is incompatible due to technical considerations or concerns about treatment-related toxicities. In these circumstances, Y-90 SIRT may serve as an alternative therapeutic option as demonstrated by our findings that intermediate HCC patients who were offered Y-90 SIRT as the first-line therapy (cTACE naïve group) had a good outcome with median survival of 11.1 months. Additionally, Y-90 SIRT can be used as a rescue therapy for some patients with BCLC B who are initially treated with cTACE but are refractory to the treatment. In our cohort, 50% were treated by Y-90 SIRT after they did not response to TACE, i.e. having disease progression as shown in radiologic imaging. We found that patients with BCLC B stage who were refractory to cTACE had OS of 9.1 months after Y-90 SIRT. To determine whether the previous treatment with TACE affected the effect of Y-90 SIRT, the survival of patients who were previously treated with TACE and that of patients who were naïve to TACE were compared. We found that the median survivals were 9.1 and 11.0 months in patients who were refractory to TACE and patients who were naïve to TACE, respectively, P = 0.44 (data not shown). This finding suggested that prior TACE unlikely affected the effect of Y-90 SIRT. Taken together, these findings suggested that Y-90 SIRT may serve as an alternative for those who were not good candidates for TACE or rescue option for those who were refractory to cTACE.
cTACE is contraindicated in BCLC B patients who have main PVT as it can potentially cause hepatic deterioration from impaired hepatic perfusion. This main limitation of cTACE can be overcome by Y-90 SIRT, although the presence of PVT could negatively affect the clinical outcomes of Y-90 SIRT.20 In line with previous studies, we found that OS of patients with PVT was shorter than those without PVT, i.e. 9.1 vs. 14.2 months, respectively, but this survival difference did not reach statistical significance (P = 0.320), likely due to the small number of patients in the cohort.
Regarding BCLC stage C, there were 37 patients, of whom 28 patients were naïve to targeted therapy and received Y-90 SIRT as the first-line treatment. Although systemic therapy is the gold standard for this stage, these patients preferred Y-90 SIRT after discussion with our multidisciplinary team because the cost of targeted therapy was very much higher than that of Y-90 SIRT in our country, and it was previously shown that although Y-90 SIRT provided comparable survival outcome to sorafenib, it had less adverse effects and provided more favorable quality of life than sorafenib.15 We found that, the survival of the BCLC stage C patients treated with Y-90 SIRT were very similar to the previously reported survival of BCLC stage C patients treated with targeted therapy, i.e. 9.1 vs. 10.7 months, respectively.24 Y-90 SIRT can therefore be considered as a treatment option for some selected patients with BCLC stage C.
Interestingly, two patients in this cohort were successfully down-staged and further underwent surgical resection after Y-90 SIRT. The first patient was classified as BCLC stage B presenting a single tumor of 11.5 cm in size without PVT. He received Y-90 SIRT as the first line treatment, followed by surgical resection at 7 months post-Y-90 SIRT. At 18 months post-surgery, he developed recurrent small tumor which was successfully treated with microwave ablation. At 19 months after the last treatment, he remained in remission, without any recurrence. The second patient had infiltrative HCC with portal vein tumor thrombus and was initially treated with Y-90 SIRT. Three months later, he underwent surgical resection. The disease was in remission for 18 months until the patient developed a neck mass which was diagnosed as metastatic HCC by biopsy examination. He was treated with lenvatinib and remained on treatment at the time of writing this manuscript. These findings illustrated the potential role of Y-90 SIRT to facilitate curative surgical resection. As compared to previous studies, our downstaging rate seemed to be low (3.8% vs. 32–85%).13,25,26 This was likely because our study cohort had a higher proportion of patients with more advanced HCCs than previous studies.13,25, 26, 27 Our study cohort had a much larger proportion of patients with PVT than those reported in previous studies (63% vs. 0%). Given the more aggressive tumor burden, our downstaging rate was therefore lower than those reported in previous studies.
As Y-90 SIRT is relatively expensive, appropriate patient selection could potentially maximize effectiveness of the treatment. ECOG status, number of lesion and AFP levels were shown to be prognostic factors of Y-90 SIRT.20,22,28 In agreement with previously reported data, we found that factors associated with favorable outcomes were ECOG status 0, number of lesions ≤3 and baseline AFP <200 ng/mL (Fig. 1). Particularly, our results showed that AFP ≥200 ng/mL was associated with a 5.4-fold increased risk of death after Y-90 SIRT.
The main strength of our study is that we confirmed the clinical benefits of Y-90 SIRT for HCC treatment even under real-world medical settings (Table 4).19, 20, 21, 22,29, 30, 31, 32, 33, 34 Our cohort represented heterogeneous group of intermediate and advanced HCC patients. Some patients received various treatments before and after Y-90 SIRT, highlighting the fact that, to get the best outcome, personalized treatments are required for each individual HCC patient. The retrospective nature of the study introduced inevitably some limitations. Multiple factors including patient's preference, clinician's decision as well as the patient and disease status might introduce selection bias to recommend Y-90 SIRT for these HCC patients. Tumor volume, which may affect clinical outcomes, was not evaluated in the present study due to technical difficulties and high inter-observer variability in such determination.30,35 Instead, we selected the largest tumor size criteria to evaluate the impact of tumor burden on survival. In contrast to previous works, we found that tumor size did not affect OS.10,22 This unexpected result may be explained by the fact that some patients had more than 1 tumor and the longest diameter of tumor, contrary to the tumor volume, does not truly reflect the tumor burden. Finally, even though comprehensive data collection was done, some information was not available, i.e., patient's quality of life.
Table 4.
Demographic data and clinical outcomes of Y-90 SIRT for HCC treatment in previously published studies and the current study.
| Sangro et al.20 | Salem et al.22 | Mantry et al.32 | Bhangoo et al.31 | Hilgard etal.19 | Mazzaferro et al.21 | Khor et al.34 | Lee et al.29 | Woo et al.33 | Floridi et al.30 | The current study | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Enrollment year | 2003–2009 | 2004–2008 | 2004–2013 | 2005–2014 | 2006–2009 | 2007–2009 | 2008–2012 | 2009–2013 | 2009–2011 | 2009–2015 | 2014–2019 |
| Study design | Retrospective real-world cohort | Prospective real-world cohort | Retrospective real-world cohort | Retrospective real-world cohort | Retrospective real-world cohort | Prospective phase 2 study | Retrospective real-world cohort | Retrospective real-world cohort | Retrospective real-world cohort | Retrospective real-world cohort | Retrospective real-world cohort |
| Country | Europe (multicenter) | USA | USA | USA | Germany | Italy | Singapore | China | Korea (Two centers) | Italy | Thailand |
| Number | 325 | 291 | 111 | 17 | 108 | 52 | 103 | 30 | 50 | 43 | 52 |
| Age (years)a | 65 ± 11 | 65 (26–90) | 66 ± 10 | 68 (50–83) | 65 ± 12 | 64 (27–82) | 62 ± 10 | 63 (36–84) | 66 (33–91) | 66 ± 10 | 65 ± 11 |
| Male (%) | 82 | 77 | 77 | 100 | 80 | 94 | 75 | 87 | 80 | 86 | 81 |
| ECOG 0, 1 (%) | 54, 33 | 56, 36 | 90, 8 | N/A | 51, 44 | 60, 40 | 40, 53 | N/A | N/A | N/A | 50, 48 |
| Multifocal (%) | 76 | 73 | N/A | 82 | N/A | 69 | 81 | 100 | 7 | 79 | 71 |
| Bilobar (%) | 53 | 48 | 50 | 47 | N/A | 6 | 58 | N/A | 8 | 63 | 48 |
| CP A, B (%) | 83, 18 | 45, 52 | 74, 23 | 53, 41 | 77, 22 | 83, 17 | 59, 38 | 80, 20 | 96, 4 | 86, 9 | 86, 14 |
| BCLC | |||||||||||
| A (%) | 16 | 17 | 34 | 6 | 2 | 0 | 1 | 0 | 40 | 12 | 0 |
| B (%) | 27 | 28 | 46 | 65 | 47 | 33 | 27.2 | 60 | 24 | 74 | 29 |
| C (%) | 56 | 52 | 20 | 24 | 51 | 7 | 69 | 40 | 36 | 14 | 71 |
| PVT (%) | 23 | 43 | 15 | 24 | 31 | 67 | 31 | 40 | 30 | 14 | 63 |
| Prior treatment (%) |
55 |
13 |
N/A |
53 |
37 |
29 |
36 |
40 |
6 |
47 |
58 |
| Response rate by mRECIST criteria | Not assessed | CR 23%, PR 34%b |
CR 11%, PR 14%, stable disease 20%, PD 7%c |
CR 0%, PR 24%, stable disease 24%, PD 35%, no data 18% |
CR 6%, PR 35%, stable disease 48%, PD 10%c |
CR 10%, PR 31%, stable disease 38%, PD 21%b |
CR 0%, PR 21%, stable disease 39%, PD 40%c |
CR 3%, PR 27%, stable disease 20%, PD 50% |
CR 20%, PR 38%, stable disease 27%, PD 8% |
CR 8%, PR 8%, stable disease 42%, PD 36% |
CR 2%, PR 33%, stable disease 19%, PD 46% |
| OS (median (95% CI), months) | 12.8 (10.9–15.7) | N/A | 13.1 (10.3–18.4) | 8.4 (1.3–21.1) | 16.4 (12.1–∞) | 15.0 (12.0–18.0) | 14.4 (11.0–22.2) | 13.2 | 40.9 (10.2–71.6) | 27.7 (15.8–27.7) | 11.0 (7.8–14.3) |
| PFS (median (95% CI), months) | N/A | 7.9 (6.0–10.3) | 9.8 (6.8–14.8) | N/A | 10.0 (6.1–16.4) | 11.0 (6–NC) | 5.3 (4.1–10.0) | 3.3 | 5.8 (0.9–46.1) | 16.8 (11.1–22.4) | 2.4 (1.0–3.9) |
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; CP, Child-Pugh class; CR, complete response; EASL, European Association for the Study of the Liver; ECOG, Eastern Cooperative Oncology Group; mRECIST, modified Response Evaluation Criteria in Solid Tumors; N/A, not available; NC, not calculable; OS, overall survival; PD, progressive disease; PFS, progression free survival; PR, partial response; PVT, portal vein thrombosis; USA, United States of America.
Age was expressed as the mean ± standard deviation or median (range).
EASL
RECIST.
5. Conclusion
Y-90 SIRT is an effective treatment for the local tumor control of HCC without serious adverse events. Our experience shows that Y-90 SIRT can be applied to several conditions of HCC. It can be used as an alternative treatment modality for patients with intermediate or advanced stage HCC who are not eligible for cTACE or targeted therapy or used as a rescue therapy for patients not responding to standard treatment options. Accordingly to the current research progression, combination therapy, including TACE, immunotherapy and systemic therapy, has been shown to provide a better prognosis for HCC. Whether combination of Y-90 SIRT with other therapies provides a better outcome than Y-90 SIRT alone needs to be further investigated.
Authors’ contributions
Conceptualization: J. Chaikajornwat and R. Chaiteerakij. Data acquistion: N. Pinjaroen. Data curation: J. Chaikajornwat and W. Tanasoontrarat. Writing and original draft preparation: J. Chaikajornwat. Writing, reviewing and editing: R. Chaiteerakij. Statistical consultation: C. Phathong. Manuscript writing and final approval of the manuscript: all authors.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements
This research was supported by Chulalongkorn University CU-GRS-62-02-30-01. Authors thank the research team of the Department of Medicine, Faculty of Medicine, Chulalongkorn University for language-editing the final manuscript.
Footnotes
Edited by Yuxia Jiang, Peiling Zhu and Genshu Wang.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.livres.2021.07.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380:1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Robinson A., Tavakoli H., Liu B., Bhuket T., Wong R.J. Advanced hepatocellular carcinoma tumor stage at diagnosis in the 1945-1965 birth cohort reflects poor use of hepatocellular carcinoma screening. Hepatol Commun. 2018;2:1147–1155. doi: 10.1002/hep4.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kallini J.R., Gabr A., Salem R., Lewandowski R.J. Transarterial radioembolization with Yttrium-90 for the treatment of hepatocellular carcinoma. Adv Ther. 2016;33:699–714. doi: 10.1007/s12325-016-0324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ariel I.M. Treatment of inoperable primary pancreatic and liver cancer by the intra-arterial administration of radioactive isotopes (Y90 radiating microspheres) Ann Surg. 1965;162:267–278. doi: 10.1097/00000658-196508000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salem R., Lewandowski R.J., Kulik L., et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140:497–507. doi: 10.1053/j.gastro.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El Fouly A., Ertle J., El Dorry A., et al. In intermediate stage hepatocellular carcinoma: radioembolization with yttrium 90 or chemoembolization? Liver Int. 2015;35:627–635. doi: 10.1111/liv.12637. [DOI] [PubMed] [Google Scholar]
- 9.Salem R., Gilbertsen M., Butt Z., et al. Increased quality of life among hepatocellular carcinoma patients treated with radioembolization, compared with chemoembolization. Clin Gastroenterol Hepatol. 2013;11:1358–1365. doi: 10.1016/j.cgh.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 10.Salem R., Gordon A.C., Mouli S., et al. Y90 radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2016;151:1155–1163. doi: 10.1053/j.gastro.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heimbach J.K., Kulik L.M., Finn R.S., et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 12.Omata M., Cheng A.L., Kokudo N., et al. Asia-pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewandowski R.J., Kulik L.M., Riaz A., et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009;9:1920–1928. doi: 10.1111/j.1600-6143.2009.02695.x. [DOI] [PubMed] [Google Scholar]
- 14.Kulik L.M., Carr B.I., Mulcahy M.F., et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47:71–81. doi: 10.1002/hep.21980. [DOI] [PubMed] [Google Scholar]
- 15.Vilgrain V., Pereira H., Assenat E., et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1624–1636. doi: 10.1016/S1470-2045(17)30683-6. [DOI] [PubMed] [Google Scholar]
- 16.Somma F., Stoia V., Serra N., D’Angelo R., Gatta G., Fiore F. Yttrium-90 trans-arterial radioembolization in advanced-stage HCC: the impact of portal vein thrombosis on survival. PLoS One. 2019;14 doi: 10.1371/journal.pone.0216935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho S., Lau W.Y., Leung T.W., et al. Partition model for estimating radiation doses from yttrium-90 microspheres in treating hepatic tumours. Eur J Nucl Med. 1996;23:947–952. doi: 10.1007/BF01084369. [DOI] [PubMed] [Google Scholar]
- 18.Wang C.Y., Li S. Clinical characteristics and prognosis of 2887 patients with hepatocellular carcinoma: a single center 14 years experience from China. Medicine (Baltimore) 2019;9 doi: 10.1097/MD.0000000000014070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hilgard P., Hamami M., Fouly A.E., et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology. 2010;52:1741–1749. doi: 10.1002/hep.23944. [DOI] [PubMed] [Google Scholar]
- 20.Sangro B., Carpanese L., Cianni R., et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54:868–878. doi: 10.1002/hep.24451. [DOI] [PubMed] [Google Scholar]
- 21.Mazzaferro V., Sposito C., Bhoori S., et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology. 2013;57:1826–1837. doi: 10.1002/hep.26014. [DOI] [PubMed] [Google Scholar]
- 22.Salem R., Lewandowski R.J., Mulcahy M.F., et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Rognoni C., Ciani O., Sommariva S., Tarricone R. Real-world data for the evaluation of transarterial radioembolization versus sorafenib in hepatocellular carcinoma: a cost-effectiveness analysis. Value Health. 2017;20:336–344. doi: 10.1016/j.jval.2016.09.2397. [DOI] [PubMed] [Google Scholar]
- 24.Llovet J.M., Ricci S., Mazzaferro V., et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 25.Vouche M., Habib A., Ward T.J., et al. Unresectable solitary hepatocellular carcinoma not amenable to radiofrequency ablation: multicenter radiology-pathology correlation and survival of radiation segmentectomy. Hepatology. 2014;60:192–201. doi: 10.1002/hep.27057. [DOI] [PubMed] [Google Scholar]
- 26.Kulik L., Vouche M., Koppe S., et al. Prospective randomized pilot study of Y90+/-sorafenib as bridge to transplantation in hepatocellular carcinoma. J Hepatol. 2014;61:309–317. doi: 10.1016/j.jhep.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 27.Abdelfattah M.R., Al-Sebayel M., Broering D., Alsuhaibani H. Radioembolization using yttrium-90 microspheres as bridging and downstaging treatment for unresectable hepatocellular carcinoma before liver transplantation: initial single-center experience. Transplant Proc. 2015;47:408–411. doi: 10.1016/j.transproceed.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Abouchaleh N., Gabr A., Ali R., et al. 90Y radioembolization for locally advanced hepatocellular carcinoma with portal vein thrombosis: long-term outcomes in a 185-patient cohort. J Nucl Med. 2018;59:1042–1048. doi: 10.2967/jnumed.117.199752. [DOI] [PubMed] [Google Scholar]
- 29.Lee V.H., Leung D.K., Luk M.Y., et al. Yttrium-90 radioembolization for advanced inoperable hepatocellular carcinoma. OncoTargets Ther. 2015;8:3457–3464. doi: 10.2147/OTT.S92473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Floridi C., Pesapane F., Angileri S.A., et al. Yttrium-90 radioembolization treatment for unresectable hepatocellular carcinoma: a single-centre prognostic factors analysis. Med Oncol. 2017;34:174. doi: 10.1007/s12032-017-1021-3. [DOI] [PubMed] [Google Scholar]
- 31.Bhangoo M.S., Karnani D.R., Hein P.N., et al. Radioembolization with Yttrium-90 microspheres for patients with unresectable hepatocellular carcinoma. J Gastrointest Oncol. 2015;6:469–478. doi: 10.3978/j.issn.2078-6891.2015.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mantry P.S., Mehta A., Madani B., Mejia A., Shahin I. Selective internal radiation therapy using yttrium-90 resin microspheres in patients with unresectable hepatocellular carcinoma: a retrospective study. J Gastrointest Oncol. 2017;8:799–807. doi: 10.21037/jgo.2017.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woo H.Y., Kim D.Y., Heo J., et al. Effect of yttrium-90 radioembolization on outcomes in Asian patients with early to advanced stage hepatocellular carcinoma. Hepatol Res. 2017;47:387–397. doi: 10.1111/hepr.12759. [DOI] [PubMed] [Google Scholar]
- 34.Khor A.Y., Toh Y., Allen J.C., et al. Survival and pattern of tumor progression with yttrium-90 microsphere radioembolization in predominantly hepatitis B Asian patients with hepatocellular carcinoma. Hepatol Int. 2014;8:395–404. doi: 10.1007/s12072-014-9533-9. [DOI] [PubMed] [Google Scholar]
- 35.Zhao B., Tan Y., Bell D.J., et al. Exploring intra- and inter-reader variability in uni-dimensional, bi-dimensional, and volumetric measurements of solid tumors on CT scans reconstructed at different slice intervals. Eur J Radiol. 2013;82:959–968. doi: 10.1016/j.ejrad.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.