Abstract
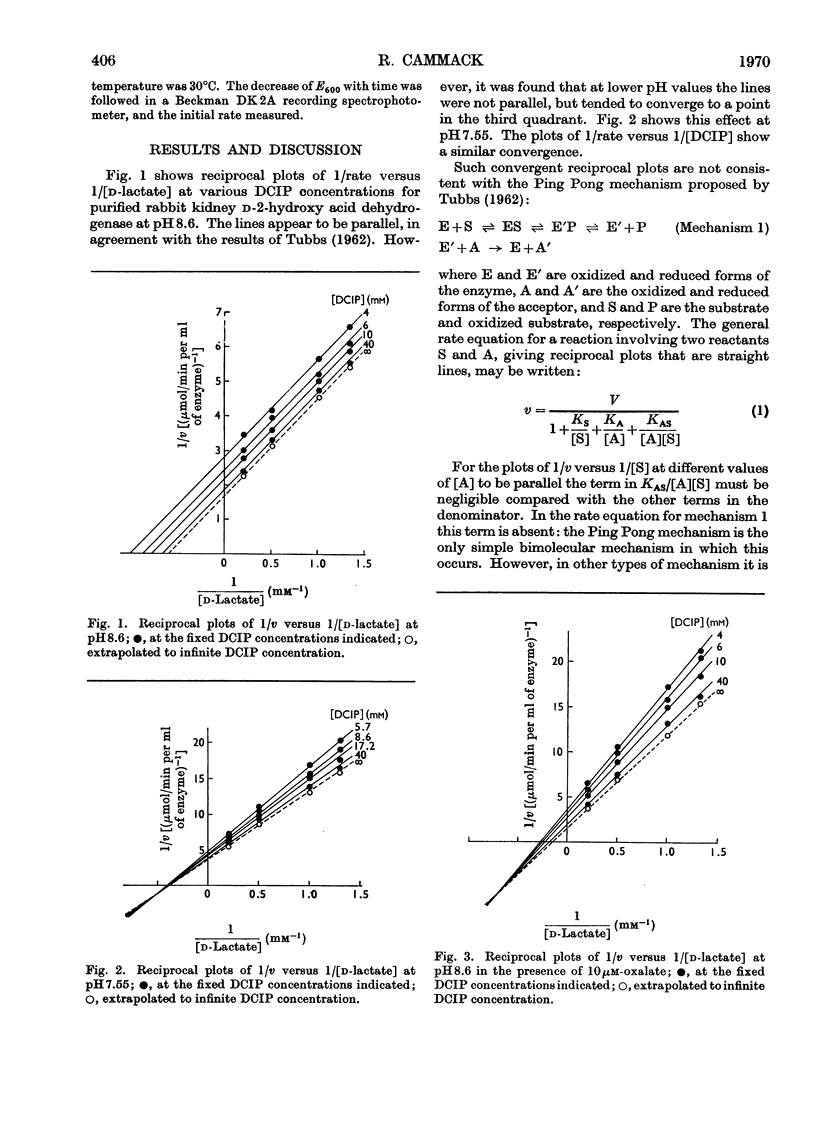
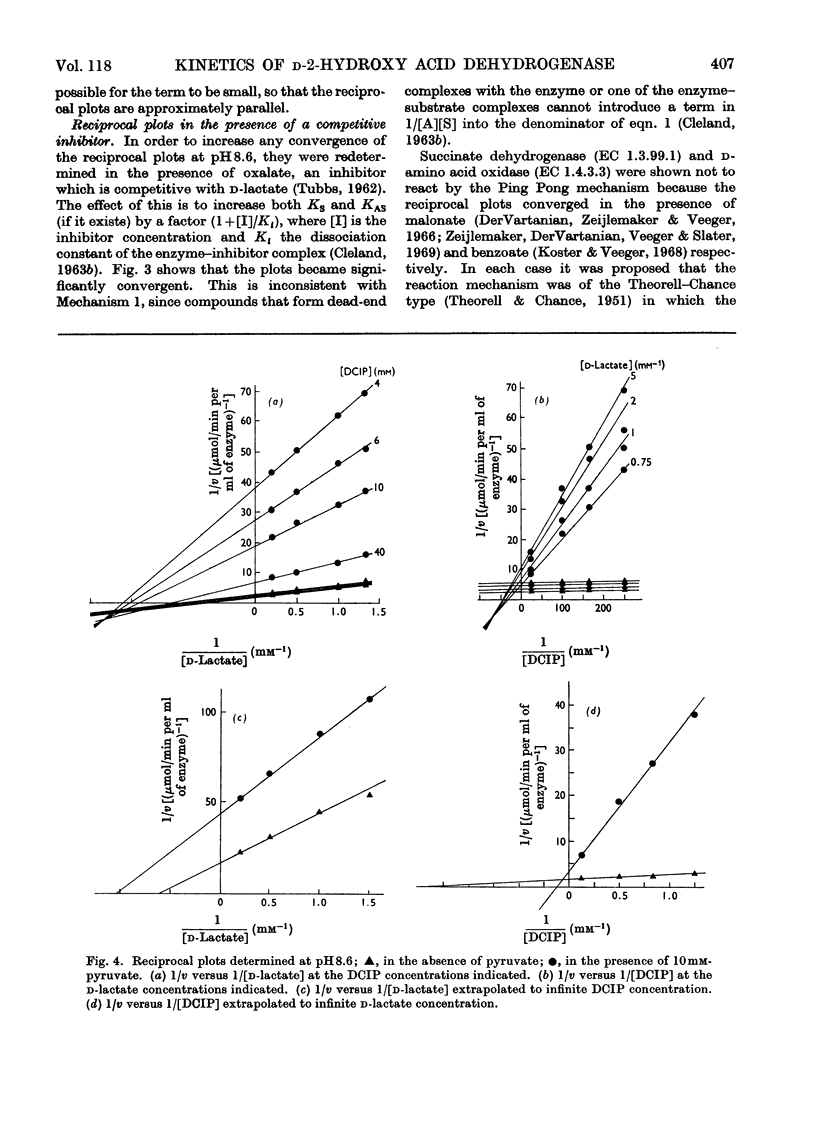
1. The reaction of d-2-hydroxy acid dehydrogenase with d-lactate and 2,6-dichlorophenol-indophenol (DCIP) at pH8.6 yields reciprocal plots of 1/rate versus 1/[d-lactate], at different DCIP concentrations, which appear to be parallel. However, at pH7.55, or in the presence of the competitive inhibitor oxalate at pH8.6, the plots are convergent. This is inconsistent with the mechanism previously proposed for this enzyme. 2. The pattern of inhibition by the product, pyruvate, is consistent with either an Ordered mechanism or an Iso Theorell–Chance mechanism. 3. The observation that the enzyme forms a complex with d-lactate favours the Ordered reaction. In this, first d-lactate and then DCIP bind to the enzyme to form a ternary complex, from which pyruvate and reduced DCIP dissociate in that order.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. II. Inhibition: nomenclature and theory. Biochim Biophys Acta. 1963 Feb 12;67:173–187. doi: 10.1016/0006-3002(63)91815-8. [DOI] [PubMed] [Google Scholar]
- Cammack R. Assay, purification and properties of mammalian D-2-hydroxy acid dehydrogenase. Biochem J. 1969 Oct;115(1):55–64. doi: 10.1042/bj1150055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HINKSON J. W., MAHLER H. R. Studies on the mechanism of enzyme-catalyzed oxidation-reduction reactions. VI. Kinetic studies with yeast L-lactate dehydrogenase. Biochemistry. 1963 Mar-Apr;2:209–216. doi: 10.1021/bi00902a001. [DOI] [PubMed] [Google Scholar]
- Koster J. F., Veeger C. On the catalytic mechanism of D-amino-acid oxidase. Biochim Biophys Acta. 1968 Jan 8;151(1):11–19. doi: 10.1016/0005-2744(68)90156-3. [DOI] [PubMed] [Google Scholar]
- SAVAGE N. Preparation and properties of highly purified diaphorase. Biochem J. 1957 Sep;67(1):146–155. doi: 10.1042/bj0670146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TUBBS P. K. Effects of inhibitors on mitochondrial D-alpha-hydroxy acid dehydrogenase. Biochem J. 1962 Jan;82:36–42. doi: 10.1042/bj0820036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TUBBS P. K., GREVILLE G. D. The oxidation of D-alpha-hydroxy acids in animal tissues. Biochem J. 1961 Oct;81:104–114. doi: 10.1042/bj0810104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeijlemaker W. P., Dervartanian D. V., Veeger C., Slater E. C. Studies on succinate dehydrogenase. IV. Kinetics of the overall reaction catalysed by preparations of the purified enzyme. Biochim Biophys Acta. 1969 Apr 22;178(2):213–224. doi: 10.1016/0005-2744(69)90391-x. [DOI] [PubMed] [Google Scholar]


