Abstract
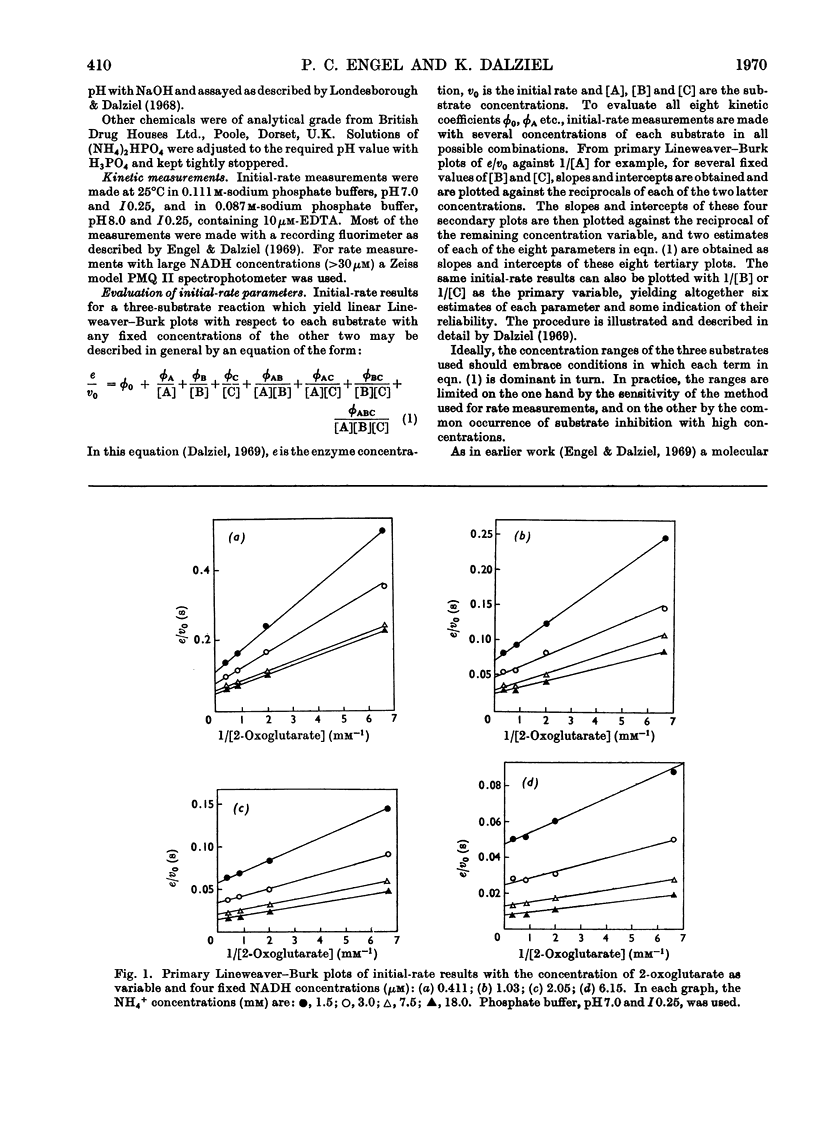
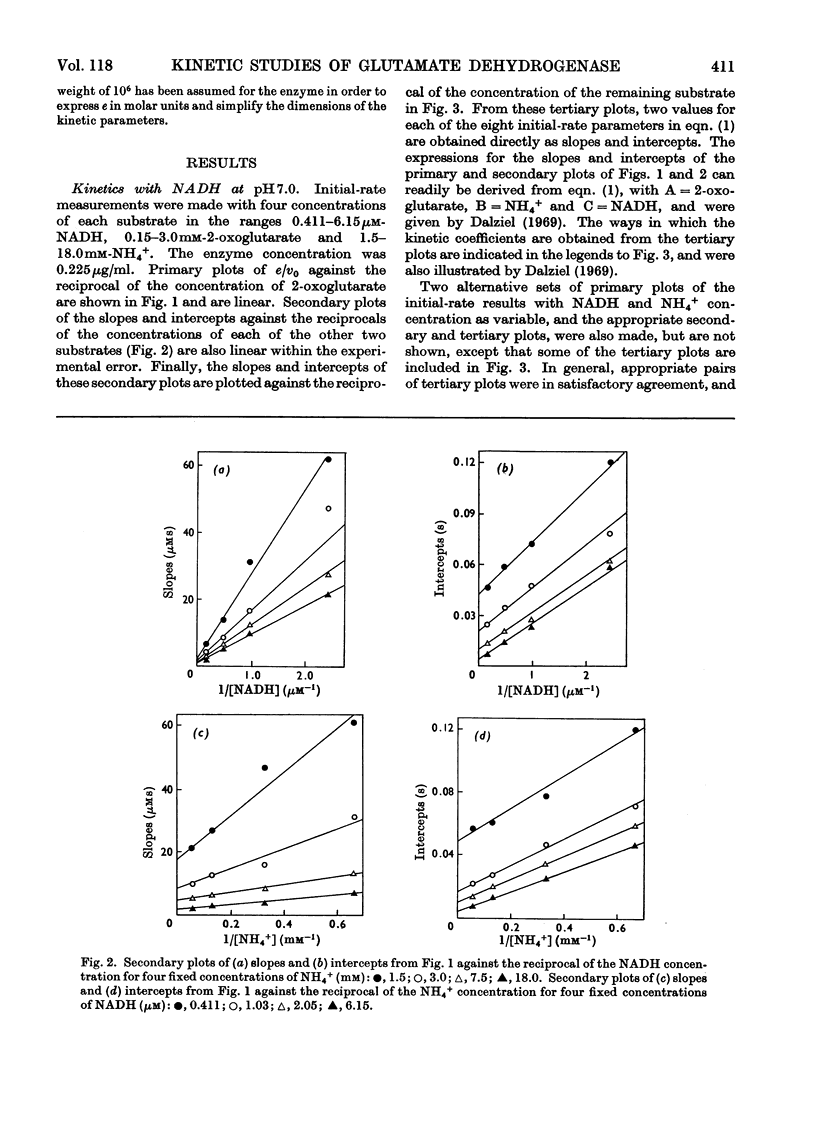
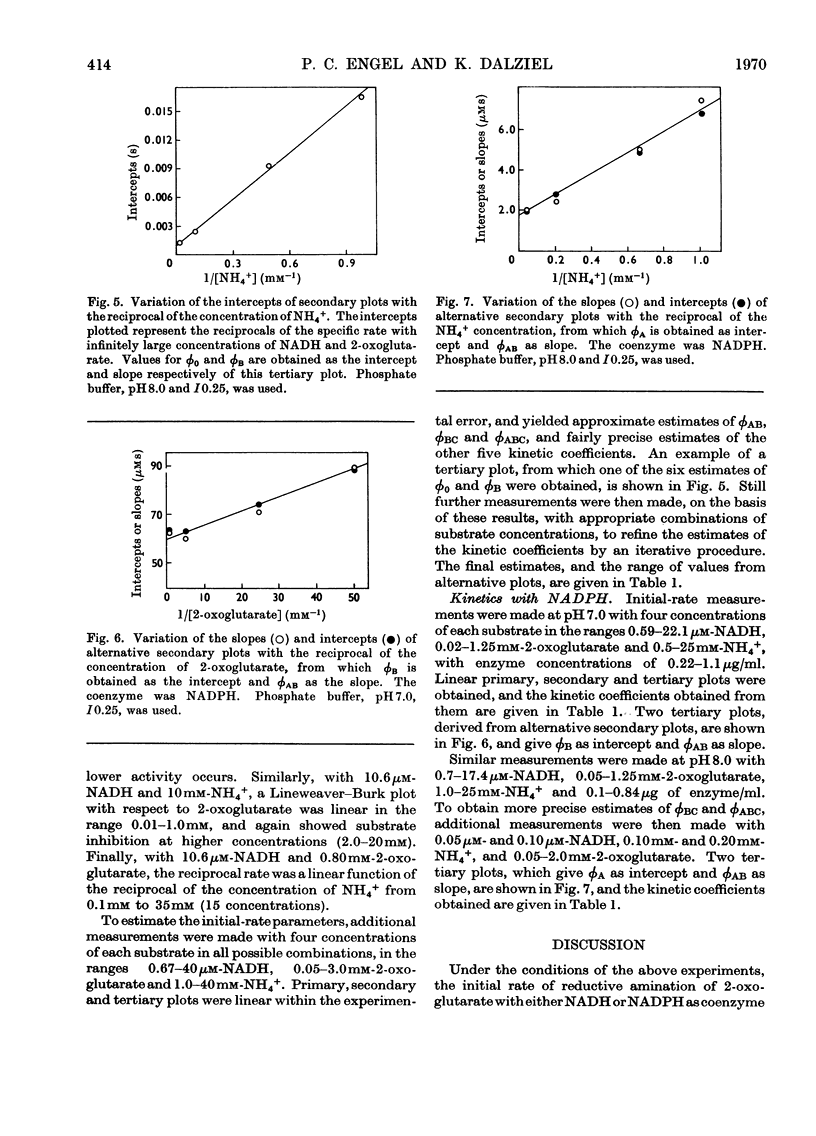
1. Kinetic studies of the reductive amination of 2-oxoglutarate catalysed by glutamate dehydrogenase with NADH and NADPH as coenzyme were made at pH7.0 and pH 8.0. The concentrations of both substrates and coenzymes were simultaneously varied over wide ranges. Lineweaver–Burk plots with respect to each substrate and coenzyme were linear, except that with high concentrations of 2-oxoglutarate or coenzyme inhibition occurred. There was no evidence of the negative homotropic interactions between the enzyme subunits that were revealed in previous kinetic studies of the reverse reaction. 2. The initial-rate results are shown to be inconsistent with any of the six possible compulsory-order mechanisms for this three-substrate reaction, and it is concluded that a random-order mechanism is the most likely one. On the basis of this mechanism, the dissociation constants of all the binary, ternary and quaternary complexes of the enzyme and substrates are calculated from initial-rate parameters. 3. The results are discussed in relation to those of earlier workers who concluded that the mechanism is of the compulsory-order type.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHURCHICH J. E., WOLD F. THE EFFECT OF DIOXANE ON THE DISSOCIATION AND ACTIVITY OF GLUTAMIC DEHYDROGENASE. Biochemistry. 1963 Jul-Aug;2:781–786. doi: 10.1021/bi00904a027. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- Corman L., Prescott L. M., Kaplan N. O. Purification and kinetic characteristics of dogfish liver glutamate dehydrogenase. J Biol Chem. 1967 Apr 10;242(7):1383–1390. [PubMed] [Google Scholar]
- DALZIEL K. Some observations on the preparation and properties of dihydronicotinamide-adenine dinucleotide. Biochem J. 1962 Aug;84:240–244. doi: 10.1042/bj0840240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalziel K. The interpretation of kinetic data for enzyme-catalysed reactions involving three substrates. Biochem J. 1969 Sep;114(3):547–556. doi: 10.1042/bj1140547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalziel Keith, Engel Paul C. Antagonistic homotropic interactions as a possible explanation of coenzyme activation of glutamate dehydrogenase. FEBS Lett. 1968 Oct;1(5):349–352. doi: 10.1016/0014-5793(68)80153-x. [DOI] [PubMed] [Google Scholar]
- Engel P. C., Dalziel K. Kinetic studies of glutamate dehydrogenase with glutamate and norvaline as substrates. Coenzyme activation and negative homotropic interactions in allosteric enzymes. Biochem J. 1969 Dec;115(4):621–631. doi: 10.1042/bj1150621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDEN C. Glutamic dehydrogenase. III. The order of substrate addition in the enzymatic reaction. J Biol Chem. 1959 Nov;234:2891–2896. [PubMed] [Google Scholar]
- Fahien L. A., Strmecki M. Studies of gluconeogenic mitochondrial enzymes. 3. The conversion of alpha-ketoglutarate to glutamate by bovine liver mitochondrial glutamate dehydrogenase and glutamate-oxaloacetate transaminase. Arch Biochem Biophys. 1969 Mar;130(1):468–477. doi: 10.1016/0003-9861(69)90059-9. [DOI] [PubMed] [Google Scholar]
- Hochreiter M. C., Schellenberg K. A. Alpha-iminoglutarate formation by beef liver L-glutamate dehydrogenase. Detection by borohydride or dithionite reduction to glutamate. J Am Chem Soc. 1969 Nov 5;91(23):6530–6531. doi: 10.1021/ja01051a084. [DOI] [PubMed] [Google Scholar]
- Londesborough J. C., Dalziel K. The equilibrium constant of the isocitrate dehydrogenase reaction. Biochem J. 1968 Nov;110(2):217–222. doi: 10.1042/bj1100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison J. F., Cleland W. W. Isotope exchange studies of the mechanism of the reaction catalyzed by adenosine triphosphate: creatine phosphotransferase. J Biol Chem. 1966 Feb 10;241(3):673–683. [PubMed] [Google Scholar]
- OLSON J. A., ANFINSEN C. B. Kinetic and equilibrium studies on crystalline 1-glutamic acid dehydrogenase. J Biol Chem. 1953 Jun;202(2):841–856. [PubMed] [Google Scholar]
- Williamson D. H., Lund P., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967 May;103(2):514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]


