Abstract
Background
Systemic inflammation is closely correlated with the progression of cancer. Inflammation-related indicators has been recognized as outcome predictors in triple-negative breast cancer (TNBC). Our study aimed to investigate the predictive value of several inflammation-related indicators in TNBC patients underwent chemotherapy (NAC).
Methods
100 TNBC patients were enrolled in the study. Levels of interleukin-6 (IL-6) were detected by enzyme-linked immunosorbent assays (ELISAs). Platelet-to-lymphocyte ratio (PLR) and immune inflammatory index (SII) were obtained from blood routine. Levels of Ki-67 expression were detected by immunohistochemistry (IHC). Mentioned indicators were divided into two groups based on their median values. The correlation between these indicators and NAC efficacy was analyzed using t tests. Prognostic risk scores were calculated by univariate Cox regression analysis and Lasso-penalized Cox regression. The patients were divided into low- and high-risk groups based on the median risk score. Survival curves were obtained by Kaplan–Meier method. Models for univariate and multivariate Cox regression analyses were performed to determine the independent risk factors. A nomogram was used for the prediction of 1-, 2-, and 3-year disease-free survival (DFS). Accuracy of the prognostic model was validated by receiver operating characteristic curve (ROC).
Results
IL-6, PLR, SII, and Ki-67 levels were associated with neoadjuvant efficacy. IL-6, PLR, SII, Ki-67, and lymphocyte count were associated with DFS. The risk score for each TNBC patient was obtained using LASSO regression analysis to construct a prognostic model. In the prognostic model, patients in the high-risk score group showed worse DFS than those in the low-risk group. Risk score and tumor size were independently correlated with outcomes in multivariate Cox regression analysis. A nomogram was constructed using IL-6, PLR, SII, Ki-67, and Miller-Payne (MP) scores. Calibration curves demonstrated good consistency between the actual and predictive values of the nomogram.
Conclusion
A prognostic model was established by combining four prognosis-related indicators in TNBC patients who underwent NAC.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40001-025-02328-6.
Keywords: Triple-negative breast cancer, Inflammation-related indicators, Prognostic model, Nomogram
Introduction
Breast cancer (BC) is a heavy burden on women worldwide. The latest data reported that the incidence rate of BC ranks second among women, and the mortality rate ranks seventh, with a gradually increasing trend [1]. Triple-negative breast cancer (TNBC) is widely acknowledged for its high invasiveness, recurrence, and metastasis rates after surgery and drug resistance. The survival time of most patients is less than 18 months [2]. In most cases, TNBC used for immunohistochemistry lacks the expression of estrogen receptors (ER) and progesterone receptors (RP), and human epidermal growth factor receptor-2 (HER-2) [3, 4] has low (1+ or 2+FISH−) or negative expression.
Due to the lack of targeted and endocrine therapy, chemotherapy remains the standard adjuvant treatment for both operable patients and recurrent or metastatic patients with TNBC [5]. Neoadjuvant chemotherapy (NAC) has been widely used to improve the prognosis of patients with operable early-stage TNBC. The current pathological complete response (pCR) rate of NAC is 40–50% [6]. It is gratifying that immune checkpoint inhibitors (ICIs) have made encouraging improvement in TNBC treatment in the past decades [7–9]. Recently, based on the phase 3 KEYNOTE-522 trial, pembrolizumab in combination with NAC has been announced as a new standard regimen for high-risk early-stage TNBC [8]. NAC combined with ICIs can improve the pCR rate and event-free survival (EFS) in TNBC patients with early-stage disease, but due to the high price, not all patients can afford it [8, 10].
Systemic inflammation, particularly chronic inflammation, is prominently linked to the occurrence, progression, metastasis, and recurrence of malignant tumors [11]. Chronic inflammation causes continuous tissue damage and repair, and cell metaplasia often occurs during this long-term process. If cell metaplasia cannot be reversed, it will further develop into atypical hyperplasia [12]. In this state, the tissues and organs may become cancerous. Inflammatory mediators, particularly interleukin-6 (IL-6), play vital roles in cancer progression. Previous studies have demonstrated that inflammatory factors and chemokines in tumor cells or tumor-associated leukocytes can lead to tumor progression [11]. Many inflammation-related indicators, including C-reactive protein (CPR) level, platelet-lymphocyte ratio (PLR), and neutrophil–lymphocyte ratio (NLR), have been recognized as prognostic indicators for the outcomes of BC patients [13, 14]. Compared with traditional single indicators, the systemic immune-inflammation index (SII), a composite immune-inflammatory indicator, comprehensively reflects inflammation and immune status [15]. Previous studies have shown that SII is advantageous in outcome prediction in patients with TNBC [16, 17].
Previous researches have focused on single inflammation-related indicator, which lacked a full disclosure of impacts on the outcome of TNBC patients underwent NAC. Our study aimed to investigate the prognostic predictive value of several inflammation-related indicators and establish a more individualized and comprehensive evaluation model of NAC efficacy in TNBC patients. Moreover, a nomogram was performed to quantify the impact of each indicator to the outcome.
Materials & methods
Sample size calculation
The sample size was calculated by PASS (2021, v21.0.3). The original sample size was determined to be 81, which would provide 80% power, with a 2-sided significance level of α = 0.05, both Ctrl loss and Trt loss of 0.05, while the Hazard ratio (HR) of high-risk group was twice of the low-risk group. Our research included 100 TNBC cases in practice.
Subjects
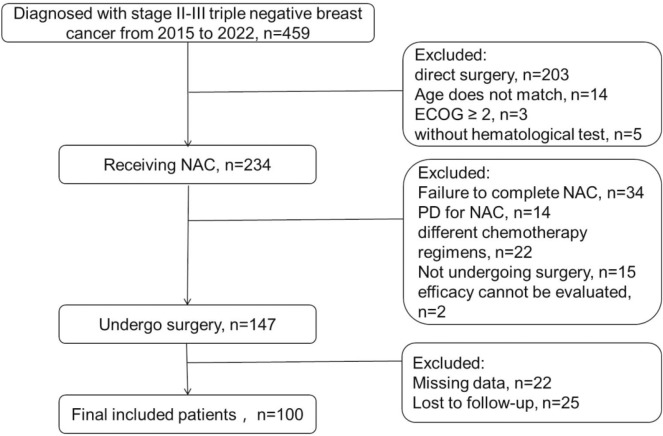
In practice, 100 patients diagnosed with TNBC in Suzhou Municipal Hospital, the Affiliated Suzhou Hospital of Nanjing Medical University were enrolled in this retrospective study. The study was approved by the Ethics Committee of Suzhou Municipal Hospital, and was performed in accordance with the Declaration of Helsinki, with all participants providing their written informed consent. The case-screening flowchart was displayed in Fig. 1.
Fig. 1.
The case-screening flowchart
Inclusion & exclusion criteria
Inclusion criteria: 1. Patients aged 18–75 (18 and 75 years); 2, the biopsy pathology before treatment was ER- and PR-negative; HER-2 expression according to immunohistochemistry was 0, 1+, or 2+ and FISH−; 3, the patient underwent eight cycles of standard NAC; 4, standard radical mastectomy for BC after NAC; 5, Eastern Cooperative Oncology Group (ECOG) score of 0–1. Exclusion criteria: 1. Patients who did not undergo NAC or direct surgery; 2, patients who failed to complete NAC or were unable to tolerate NAC; 3, no surgery after completion of NAC; 4, before treatment, the patient’s hematological parameters could not be assessed; 5, the efficacy cannot be evaluated after NAC.
Blood samples
Peripheral blood samples of all patients were collected within 1 week before the biopsy. Blood samples were collected between 6:00 am and 7:30 a.m. to eliminate the influence of circadian rhythm on inflammatory indicators. A hematology analyzer (Sysmex XE-9100; Sysmex, Kobe, Japan) was used to analyze routine blood samples to detect platelet (PLT), neutrophils, lymphocytes, monocytes, and eosinophils levels. A fully automatic biochemical analyzer (Hitachi 7600; Hitachi, Tokyo, Japan) was used to detect CRP levels. Levels of IL-6 were detected by using enzyme-linked immunosorbent assays (ELISAs) (R&D Systems, Inc., Shanghai, China). The definitions of NLR, PLR and SII were shown as follows: NLR = neutrophil/lymphocyte counts; PLR = platelet/lymphocyte counts; SII = platelet × neutrophil/lymphocyte counts.
Immunohistochemistry
TNBC tissue samples were obtained from the patients who had surgery at Suzhou Municipal Hospital and all patients provided written informed consent prior to being operation on. Levels of Ki-67 expression were measured by immunohistochemistry (IHC). Primary antibodies (Ki-67, 1:1000, GB151499, servicebio, China) were used to specifically bind to antigens under 4 °C lasting for 12 h. And we applied immunopotentiator and secondary antibody modified by horseradish peroxidase to combine the primary antibody. Samples stained by 3,3′-diaminobenzidine (DAB) and dehydrated under alcohol were continued to be counterstained by hematoxylin. Lastly, these slices were faxed by neutral gum. Positive or negative staining of a certain protein in one FFPE slide was independently assessed by two experienced pathologists and supervised by a clinician.
Construction of the prognostic model and validation model
Prognostic risk scores were calculated using univariate Cox regression analysis and Lasso-penalized Cox regression [18]. The formula is as follows:
In the formula, k represents the number of prognostic indicators involved in the model, Ci represents the coefficient of the prognostic indicators in multivariate Cox regression analysis, and Vi represents the specific value of the prognostic indicator.
Follow-up
All the patients were followed up by physical examination and imaging examination every 3 months for the first 2 years, and every 6 months after 2 years; afterwards, patients were followed up once per year till death or May 2023. The median follow-up time was 17.4 months, ranging from 7.8 to 48.0 months. Disease-free survival (DFS) was estimated from the date of diagnosis to the date of postoperative recurrence, metastasis, last clinical assessment, or death.
Statistical analysis
Statistic analysis was performed using GraphPad Prism (version 8.0, San Diego, USA) and SPSS software (version 19.0, Illinois, USA). Receiver operating characteristic (ROC) curves was performed to get the optimal cut-of values for sensitivity and 1-specificity with highest Youden’s index. An area of 0.5–0.7 indicated a low predicting value, 0.7–0.9 indicated a medium predicting value, and over 0.9 indicated a high predicting value. Survival data analysis was performed using Kaplan–Meier curves, while statistical analysis was performed using the log-rank test. The correlation between indicators and NAC efficacy was analyzed using the t test. Models for univariate and multivariate Cox regression analyses determined independent risk factors. A nomogram was established using the rms package in R software (version 4.0.3; https://www.r-project.org/) for predicting 1-, 2-, and 3-year DFS. The nomogram was quantified in terms of discrimination and calibration. Statistical significance was set at p < 0.05.
Results
Clinical characteristics
This study included 100 patients diagnosed with TNBC who underwent NAC combined with surgery. The case-screening flowchart was shown in Fig. 1. The median age of participants was 52. There were 8, 37, and 55 patients with tumor stages T1, T2, and T3, respectively. A total of 82 patients had lymph node metastasis at diagnosis, whereas the other 18 did not. Among the tumor immunohistochemistry results, 45 patients were HER-2 negative, 35 were 1+, and 20 were 2+FISH−. A total of 76 patients had a high Ki-67 level (>30), and 24 had a low level (≤30). The clinical and pathological characteristics of the patients with TNBC were presented in Table 1.
Table 1.
Clinical features
| Age (years) | Median | 52 |
| Range | 23–75 | |
| Tumor size | T1 | 8 |
| T2 | 37 | |
| T3 | 55 | |
| HER-2 | 0 | 45 |
| 1 | 35 | |
| 2 (FISH−) | 20 | |
| N | Have | 82 |
| None | 18 | |
| Ki-67 | ≤30 | 24 |
| >30 | 76 |
The relationship between inflammation indicators and the efficacy of NAC
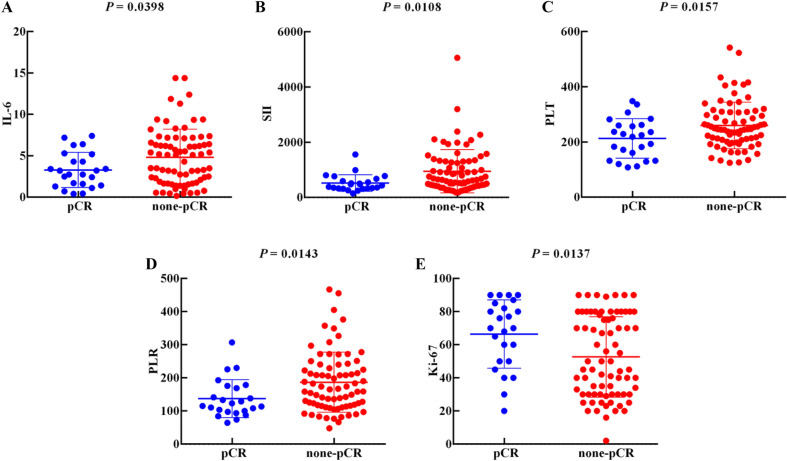
IL-6 (p = 0.0398), SII (p = 0.0108), PLT (p = 0.0157), and PLR (p = 0.0143) were significantly lower in the pCR group than in the non-pCR group, whereas Ki-67 (p = 0.0137) in the pCR group was distinctly higher than that in the non-pCR group, as displayed in Fig. 2. However, levels of CRP, NLR, neutrophils, lymphocytes, monocytes, and esinophils showed no difference between the pCR group and non-pCR group (Figure S1).
Fig. 2.
The relationship between inflammation-related indicators and the efficacy of NAC: A IL-6 (p = 0.0398); B S II (p = 0.0108); C PLT (p = 0.0157); D PLR (p = 0.0143); E Ki-67 (p = 0.0137)
Prognostic relevance of inflammation indicators and clinical features
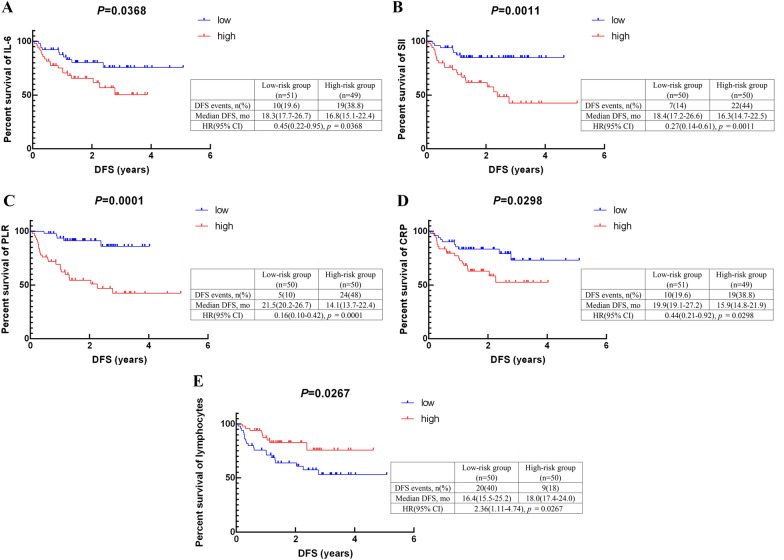
Indicators were divided into two groups based on their median values. 100 patients were divided into the low IL-6 (≤3.5 pg/mL) group (=51) and the high IL-6 (>3.5 pg/mL) group (=49); the low SII (≤587.0) group (=50) and the high SII (>587.0) group (=50); the low PLR (≤152.1) group (=50) and the high PLR (>152.1) group (=50); the low PLT (≤239.5 × 109/L) group (=50) and the high PLT (>239.5 × 109/L) group (=50); the low NLR (≤2.494) group (=50) and the high NLR (>2.494) group (=50); the low CRP (≤4.6 mg/L) group (=51) and the high CRP (>4.6 mg/L) group (=49); the low neutrophils (≤4.215 × 109/L) group (=50) and the high neutrophils (>4.215 × 109/L) group (=50); the low lymphocytes (≤1.545 × 109/L) group (=50) and the high lymphocytes (>1.545 × 109/L) group (=50); the low monocytes (≤0.412 × 109/L) group (=51) and the high monocytes (>0.412 × 109/L) group (=49); the low eosinophils (≤0.1 × 109/L) group (=52) and the high eosinophils (>0.1 × 109/L) group (=48). The high-level IL-6 (p = 0.0368), SII (p = 0.0011), PLR (p = 0.0001), and CRP (p = 0.0298) levels were significantly correlated with worse DFS compared to the low-level group. Additionally, the prognosis of the group with high lymphocytes (p = 0.0267) levels was better than that of the low-level group, as showed in Fig. 3. Meanwhile, levels of PLT (p = 0.0802), NLR (p = 0.0542), neutrophils (p = 0.0539), monocytes (p = 0.0623), and eosinophils (p = 0.2400) had no correlation with DFS (Figure S2).
Fig. 3.
The relationship between inflammation-related indicators values and DFS of TNBC patients: A the DFS according to IL-6 (p = 0.0368); B the DFS according to S II (p = 0.0011). C the DFS according to PLR (p = 0.0001); D the DFS according to CRP (p = 0.0298); E the DFS according to lymphocytes (p = 0.0267). DFS: disease-free survival; HR: hazard ratio; mo: month
Development of a prognostic model
The present study suggests that IL-6, SII, PLR, and Ki-67 can serve as predictive indicators of NAC efficacy and postoperative DFS. Univariate Cox regression and least absolute shrinkage and selection operator (LASSO) regression were used to construct an optimization model utilizing these four indicators. The risk model formula based on prognostic parameters is as follows:
Risk score = (IL-6 value * 0.05196) + (SII value * 0.00029) + (Ki-67 value * −0.01468) + (PLR value * 0.00109).
The patients were divided into low- and high-risk groups based on the median risk score. Survival curves were analyzed using the Kaplan–Meier method. Patients in the low-risk score group exhibited a significantly better DFS than those in the high-risk score group (Fig. 4A; p = 0.0007; HR, 3.630; 95% CI: 1.787–7.375). All participants were randomly assigned to either the test or training group using R package to validate the accuracy of the prognostic model. Prognostic analysis was conducted for both groups of patients. Regardless of whether or not the test was performed (Fig. 4B; p = 0.0262; HR, 2.936; 95% CI: 1.137–7.749) or training (Fig. 4C; p = 0.0111; HR, 3.228; 95% CI: 1.169–8.912) group, patients with a low-risk score consistently demonstrated better DFS.
Fig. 4.
The prognosis of low- and high-risk groups in TNBC patients: A the low-risk score group exhibited a significantly better DFS than those in the high-risk score group (p = 0.0007); accuracy of the prognostic model validated by B testing group (p = 0.0262) and C training group (p = 0.0111). DFS: Disease-free survival; HR: hazard ratio; mo: month
Cox regression analysis of the prognostic model
Univariate regression analysis revealed that a high-risk score (HR, 3.932; 95% CI: 1.676–9.224; p = 0.002) and small tumor size (HR, 2.408; 95% CI: 1.061–5.463; p = 0.036) were correlated with worse DFS, as listed in Table 2. Multifactor analysis identified a high-risk score (HR, 4.597; 95% CI: 1.782–11.858; p = 0.002) and small tumor size (HR, 2.485; 95% CI: 1.084–5.696; p = 0.032) as independent risk factors (Table 2).
Table 2.
COX regression analysis of prognostic model
| p value | Hazard ratio | |
|---|---|---|
| Univariate analyses | ||
| Risk scores | 0.002 | 3.932 (1.676–9.224) |
| Tumor size | 0.036 | 2.408 (1.061–5.463) |
| Age (years) | 0.588 | 0.815 (0.389–1.710) |
| HER-2 | 0.633 | 1.198 (0.571–2.511) |
| Multivariate analyses | ||
| Risk scores | 0.002 | 4.597 (1.782–11.858) |
| Tumor size | 0.032 | 2.458 (1.084–5.696) |
| Age (years) | – | – |
| HER-2 | – | – |
Evaluation of prognostic model accuracy
The accuracy of the prognostic model was further validated using the ROC curve, as displayed in Fig. 5. The cut-off value of risk score was 0.103, with a specificity of 0.873, and a sensitivity of 0.724, and area under the curve was 0.790.
Fig. 5.

The ROC curve of the prognostic model
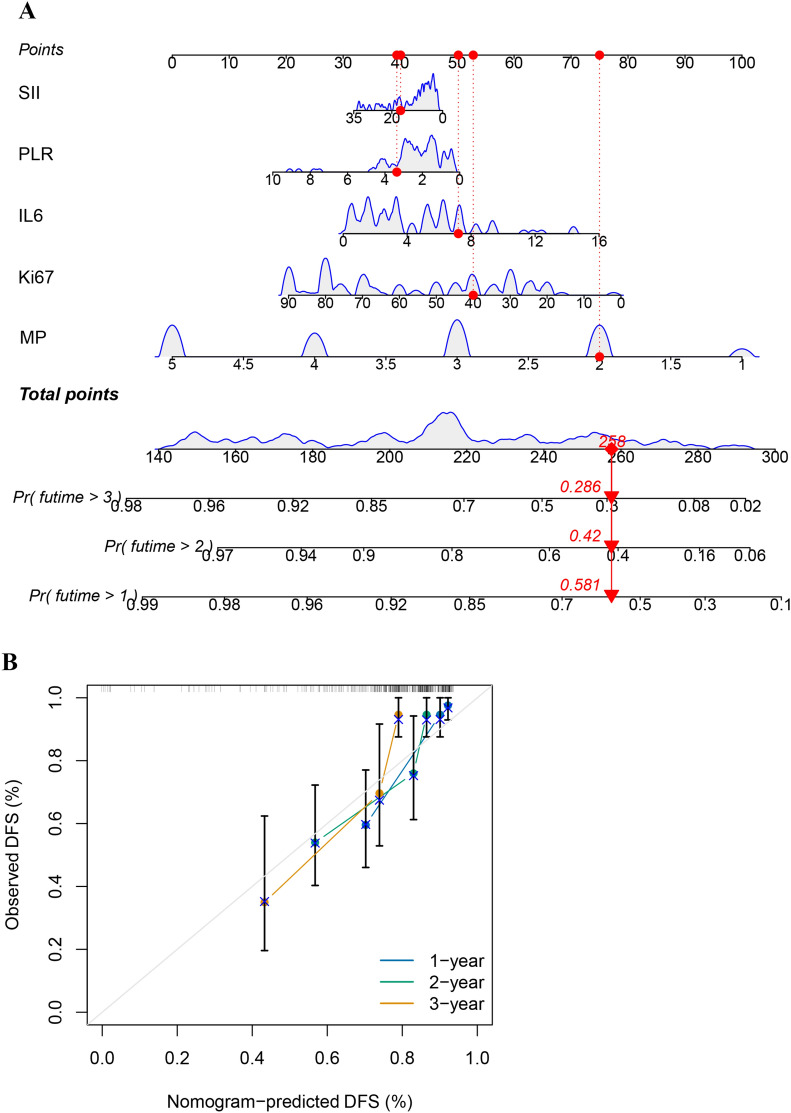
Establishment and validation of the nomogram
Four indicators were utilized along with the Miller–Payne (MP) scores [19] to develop a nomogram for predicting the 1-, 2-, and 3-year DFS. Calibration curves were generated to illustrate acceptable consistency between the actual and nomogram-predicted survival rates (Fig. 6).
Fig. 6.
Nomogram analysis of the prognostic model: A nomogram of the prognostic model; B nomogram predicted DFS of TNBC patients underwent NAC
Discussion
TNBC is one of the most lethal tumors in women. Neoadjuvant therapy presents an opportunity to improve the prognosis of patients with operable early-stage TNBC. However, there remains a cohort that is susceptible to metastasis post-surgery or even during chemotherapy. This study investigated common inflammation-related indicators, including IL-6, PLR, SII, and pre-chemotherapy platelet levels, as potential predictors of NAC efficacy. Furthermore, IL6, PLR, and SII were identified as the prognostic factors for postoperative DFS. Notably, among all clinical features detailed in Table 1, Ki-67 emerged as a significant predictor of both NAC efficacy and DFS. Consequently, four prognostic factors capable of reflecting the efficacy of NAC and predicting DFS were identified. Using univariate Cox and LASSO regression, a risk model was constructed using these four prognostic indicators. Patients classified in the low-risk group consistently demonstrated better DFS than their counterparts in the high-risk group. Additionally, the study successfully developed and validated a nomogram utilizing these prognostic factors, further enhancing prognostic accuracy in clinical settings.
Inflammation plays a pivotal role in cancer progression. The TME, which is characterized by inflammatory cells and mediators, significantly contributes to tumor initiation and progression. Chronic inflammation is acknowledged as a catalyst in the development of solid tumors such as BC [20]. Inflammation can also affect the immune system. Tumor-associated inflammation can facilitate the evasion of tumor cells via immune surveillance. It can also drive the alteration of the TME to a milieu, favoring tumor growth and directly transmitting pro-tumorigenic signals to epithelial and cancer cells [21].
Interleukin-6 (IL-6) is a key regulator of inflammation and immune responses. Within the tumor immune microenvironment (TIME), IL-6 plays a pivotal role as a major immunosuppressive factor, dampening the activity of dendritic cells (DCs) and CD8+ T cells [22, 23]. Dysregulation of the IL-6/JAK/STAT3 signaling pathway is crucial in driving tumor initiation, progression, metastasis, and recurrence. Despite numerous clinical investigations targeting IL-6/JAK/STAT3 in the treatment of TNBC [24, 25], no approved drugs that specifically target this pathway exist.
Studies such as those by Tawara et al. have underscored a significant association between elevated IL-6 levels and poor outcomes in TNBC [26]. CRP, an inflammatory biomarker synthesized in the liver, serves as an indicator of inflammatory response intensity. Previous research has consistently demonstrated a positive correlation between elevated CRP levels and the invasiveness, recurrence, and poor prognosis of BC. Furthermore, increased CRP expression has been observed in highly aggressive TNBC cell lines [13]. Studies have shown that knockout of the CRP gene markedly suppresses the proliferation, migration, and invasion of TNBC cell lines [27].
In the current study, not all inflammation-related indicators effectively reflected short-term or long-term efficacy in patients. Given the intricate nature of inflammation and the interplay of multiple inflammatory markers, our understanding of their roles in the onset and progression of TNBC remains incomplete. This complexity likely explains why not all indicators serve as reliable outcome predictors.
The SII has garnered attention for its ability to provide a comprehensive reflection of inflammatory status. It is a composite indicator that incorporates neutrophils, lymphocytes, and platelets, thus surpassing the predictive capacity of single or dual inflammatory markers. Hence, the SII was included in our study, revealing a significant correlation between elevated SII levels and poorer NAC efficacy and DFS in patients with TNBC. Consistent with findings from a meta-analysis involving 13 BC studies, including two focused on TNBC, this study underscores that patients with high SII scores experience worse DFS than those with low score [13]. Additionally, these patients exhibited poor OS outcomes.
However, the present study has some limitations. First, the sample size of TNBC cases included in the retrospective analysis was relatively small, limiting its generalizability. This is because this study primarily reflects a single geographic region and lacks external validation data. Second, the study did not include patients who underwent treatment with immune checkpoint inhibitors in combination with chemotherapy. Future investigations should aim to address these limitations by collecting and analyzing data from patients receiving such combined therapies. Moreover, due to time constraints and limited number of TNBC cases, validation for our prognostic model will be performed in the further research. These efforts will contribute to a more comprehensive understanding of the efficacy and outcomes of diverse treatment modalities for patients with TNBC.
Conclusion
In summary, the analysis conducted on multiple inflammation-related indicators among patients with TNBC undergoing NAC revealed significant associations between IL-6, PLR, and SII levels and NAC efficacy. Additionally, this study found significant associations between IL-6, PLR, and SII levels and postoperative DFS among patients with TNBC undergoing NAC. These inflammation-related indicators offer an economic, safe, and simple means of assessment. Compared to purely predictive model in other researches, the nomogram showed the relationship of each variables, the contribution and impact of each predictor on outcome in a visualized way. With high accuracy, the easy-to-use nomogram can be a convenient tool for individualized evaluation of NAC effectiveness in TNBC patients. Thus, our nomogram demonstrated high clinical feasibility and potential for widespread clinical use. We hope our study will provide a foundation for future TNBC treatment strategies.
Supplementary Information
Additional file 1. Figure S1. The relationship between inflammation-related indicators and the efficacy of NAC: (A) CRP (p = 0.6709); (B) NLR (p = 0.2941); (C) neutrophils (p = 0.1308); (D) lymphocytes (p = 0.8230); (E) monocytes (p = 0.2739); (F) eosinophils (p = 0.4574).
Additional file 2. Figure S2. The relationship between inflammation-related indicators values and DFS of TNBC patients: (A) The DFS according to PLT (p = 0.0802); (B) The DFS according to NLR (p = 0.0542); (C) The DFS according to neutrophils (p = 0.0539); (D) The DFS according to monocytes (p = 0.0623); (E) The DFS according to eosinophils (p = 0.2400).
Acknowledgements
We gratefully acknowledged Dr. Haihua Shi and Dr. You Meng for their valuable contributions to this study.
Author contributions
(I) Conception and design: J. Z., H. S., Y. M.; (II) Administrative support: H.S., Y. M.; (III) Provision of study materials or patients: J. Z., J. C., Y.M.; (IV) Collection and assembly of data: W.W.; (V) Data analysis and interpretation: Y. W., Z.Z.; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.
Funding
This study was supported by the Science and Technology Project Foundation of Suzhou (grant No. SKJY2021126); Clinical Oncology Research Foundation of Beijing CSCO (grant No. Y-Young2021-0087).
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethical approval and consent to participate
The study was approved by the Ethics Committee of Suzhou Municipal Hospital, and was performed in accordance with the Declaration of Helsinki, with all participants providing their written informed consent.
Consent to publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jie Zhu, Jiale Cheng, and Yuyuan Ma have contributed equally to this work.
Contributor Information
Wenjie Wang, Email: suda_wangwenjie@163.com.
Haihua Shi, Email: doctorhaihua0309@163.com.
You Meng, Email: ymeng2025@njmu.edu.cn.
References
- 1.Han BF, Zheng RS, Zheng HM, Wang SM, Sun KX, Chen R, et al. Cancer incidence and mortality in China, 2022. Zhonghua Zhong Liu Za Zhi. 2024;46(3):221–31. [DOI] [PubMed] [Google Scholar]
- 2.Yin L, Duan J-J, Bian XW, Yu SC. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020;22(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36(20):2105–22. [DOI] [PubMed] [Google Scholar]
- 4.Allison KH, Hammond MEH, Dowsett M, McKernin SE, Carey LA, Fitzgibbons PL, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. 2020;38(12):1346–66. [DOI] [PubMed] [Google Scholar]
- 5.van den Ende NS, Nguyen A, Jager A, Kok M, Debets R, van Deurzen CHM, et al. Triple-negative breast cancer and predictive markers of response to neoadjuvant chemotherapy: a systematic review. Int J Mol Sci. 2023;24(3):2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abuhadra N, Stecklein S, Sharma P, Moulder S. Early-stage triple-negative breast cancer: time to optimize personalized strategies. Oncologist. 2022;27(1):30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emens LA. Breast cancer immunotherapy: facts and hopes. Clin Cancer Res. 2018;24(3):511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmid P, Cortes J, PusztaiL MH, Kümmel S, Bergh J, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810–21. [DOI] [PubMed] [Google Scholar]
- 9.Conforti F, Pala L, Sala I, Oriecuia C, De Pas T, Specchia C, et al. Evaluation of pathological complete response as surrogate endpoint in neoadjuvant randomised clinical trials of early stage breast cancer: systematic review and meta-analysis. BMJ. 2021;375: e066381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mittendorf EA, Zhang H, Barrios CH, Saji S, Jung KH, Hegg R, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet. 2020;396(10257):1090–100. [DOI] [PubMed] [Google Scholar]
- 11.Singh N, Baby D, Rajgur JP, Patil PB, Thakkannavar SS, Pujari VB, et al. Inflammation and cancer. Ann Afr Med. 2019;18(3):121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cordon-Cardo C, Prives C. At the crossroads of inflammation and tumorigenesis. J Exp Med. 1999;190(10):1367–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim E-S, Kim SY, Moon A. C-reactive protein signaling pathways in tumor progression. Biomol Ther. 2023;31(5):473–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lou CY, Jin F, Zhao Q, Qi HM. Correlation of serum NLR, PLR and HALP with efficacy of neoadjuvant chemotherapy and prognosis of triple-negative breast cancer. Am J Transl Res. 2022;14(5):3240–6. [PMC free article] [PubMed] [Google Scholar]
- 15.Yilmaz A, Mirili C, Bilici C, Tekin SB. A novel predictor in patients with gastrointestinal stromal tumors: systemic immune-inflammation index (SII). J BUON. 2019;24(5):2127–35. [PubMed] [Google Scholar]
- 16.Liu JX, Shi ZZ, Bai YS, Liu L, Cheng KL, et al. Prognostic significance of systemic immune-inflammation index in triple-negative breast cancer. Cancer Manag Res. 2019;11:4471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hua X, Long Z-Q, Zhang YL, Wen W, Guo L, Xia W, et al. Prognostic value of preoperative systemic immune-inflammation index in breast cancer: a propensity score-matching study. Front Oncol. 2020;10:580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tibshirani R. The lasso method for variable selection in the Cox model. Stat Med. 1997;16(4):385–95. [DOI] [PubMed] [Google Scholar]
- 19.Ogston KN, Miller ID, Payne S, Hutcheon AW, Sarkar TK, Smith I, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast. 2003;12(5):320–7. [DOI] [PubMed] [Google Scholar]
- 20.Zhang YG, Niu J-T, Wu HW, Si X, Zhang SJ, Li DH, et al. Actin-binding proteins as potential biomarkers for chronic inflammation-induced cancer diagnosis and therapy. Anal Cell Pathol. 2021;2021:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu ZY, Aiping S, Song D, Han B, Zhang ZR, Ma L, et al. Resistin confers resistance to doxorubicin-induced apoptosis in human breast cancer cells through autophagy induction. Am J Cancer Res. 2017;7(3):574–83. [PMC free article] [PubMed] [Google Scholar]
- 23.Chomarat P, Banchereau J, Davoust J, Palucka AK. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000;1(6):510–4. [DOI] [PubMed] [Google Scholar]
- 24.Stover DG, Gil Del Alcazar CR, Brock J, Guo H, Overmoyer B, Balko J, et al. Phase II study of ruxolitinib, a selective JAK1/2 inhibitor, in patients with metastatic triple-negative breast cancer. NPJ Breast Cancer. 2018;4(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Senolt L. JAK inhibition in the treatment of inflammatory rheumatic diseases. Vnitr Lek. 2023;69(3):181–8. [DOI] [PubMed] [Google Scholar]
- 26.Tawara K, Hannah S, Emathinger J, Wolf C, LaJoie D, Hedeen D, et al. HIGH expression of OSM and IL-6 are associated with decreased breast cancer survival: synergistic induction of IL-6 secretion by OSM and IL-1β. Oncotarget. 2019;10(21):2068–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim ES, Kim SY, Koh M, Lee HM, Kim K, Jung J, et al. C-reactive protein binds to integrin α2 and Fcγ receptor I, leading to breast cell adhesion and breast cancer progression. Oncogene. 2017;37(1):28–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Figure S1. The relationship between inflammation-related indicators and the efficacy of NAC: (A) CRP (p = 0.6709); (B) NLR (p = 0.2941); (C) neutrophils (p = 0.1308); (D) lymphocytes (p = 0.8230); (E) monocytes (p = 0.2739); (F) eosinophils (p = 0.4574).
Additional file 2. Figure S2. The relationship between inflammation-related indicators values and DFS of TNBC patients: (A) The DFS according to PLT (p = 0.0802); (B) The DFS according to NLR (p = 0.0542); (C) The DFS according to neutrophils (p = 0.0539); (D) The DFS according to monocytes (p = 0.0623); (E) The DFS according to eosinophils (p = 0.2400).
Data Availability Statement
No datasets were generated or analysed during the current study.