Abstract
Background
Femoral head fractures result from high-energy trauma and may be associated with posterior dislocation of the hip joint. An appropriate surgical approach is essential for improving clinical outcomes and preventing complications. This study aims to compare the clinical efficacy of the modified Smith-Peterson (mS-P) approach and Ganz surgical dislocation (GSD) approach in the treatment of Pipkin I fractures.
Methods
We conducted a retrospective analysis of patients diagnosed with Pipkin I fractures at Pidu District People’s Hospital from June 2010 to May 2020. Of these, 11 cases were treated with the mS-P approach and 12 cases were treated with the GSD approach. All patients were followed for 12–55 months, with a mean duration of 37.7 months. Basic demographic information, perioperative-related records, postoperative complications, and outcomes at the final follow-up were compared. Functional evaluations included the Thompson-Epstein Score, the Modified Harris Hip Score (MHHS), and the Vail Hip Score (VHS).
Results
Overall, 21 patients were included in the study. We found that the mS-P approach was associated with a smaller incision, shorter operative time, and reduced intraoperative blood loss compared to the GSD approach (P < 0.05). The mean MHHS was 83.5 ± 9.14 and 75 ± 9.22 in the mS-P group and GSD group, respectively, P = 0.048. The mean VHS was 83.9 ± 7.4 and 74.4 ± 11.28 in the mS-P group and GSD group, respectively, P = 0.034. Thompson-Epstein scores in the mS-P group were excellent (6 patients), good (3 patients), and fair (1 patient). In the GSD group, the scores were excellent (3 patients), good (5 patients), and fair (2 patients). Among the postoperative complications, avascular necrosis (AVN) occurred in 1 patient in each of the mS-P and GSD groups. Heterotopic ossification (HO) occurred in 5 patients in each of the mS-P and GSD groups. 2 patients in the mS-P group developed post-traumatic osteoarthritis (PTOA), compared to 5 patients in the GSD group. One patient in the GSD group developed a sciatic nerve injury (SNI).
Conclusions
This study suggests that the mS-P approach offers advantages over the GSD approach in terms of surgical efficiency and surgical trauma. However, longer-term follow-up is required to fully assess complications and functional outcomes.
Keywords: Pipkin fracture, Modified Smith-Peterson approach, Ganz surgical dislocation, Surgical treatment
Introduction
Femoral head fractures are intra-articular fractures, with 5-15% of patients have associated with hip dislocation [1–3]. In most instances, fractures of the femoral head are result from high-energy trauma with a posterior dislocation of the femoral head [3–6]. Hip dislocation accompanied by femoral head fracture is frequently associated with a high rate of complications, including avascular necrosis (AVN), heterotopic ossification (HO), and post-traumatic osteoarthritis (PTOA) [3, 5, 7]. Therefore, prompt and accurate treatment of the injury and continuous follow-up are required.
Conventionally, open reduction and internal fixation (ORIF) including the lateral (Ludloff), anterior (Smith-Petersen), posterior (Kocher-Langenbeck) and anterolateral (Watson-Jones) approaches, has been applied for femoral head fractures [1, 3, 8]. In 1957, Pipkin published a new classification system for femoral head fractures [9]. Pipkin III and IV fractures often follow a fracture of the neck of the femur or a fracture of the acetabulum, which requires total hip replacement or internal fixation, making an anterior approach difficult to achieve [10]. For Pipkin I fractures, there is still no consensus on the treatment, especially regarding which surgical approach is best.
Current evidence suggests that complications such as HO, AVN, and PTOA can limit patients’ hip function and postoperative outcomes [11–13]. We therefore used the modified Smith-Peterson (mS-P) approach or the Ganz surgical dislocation (GSD) approach, as described by Ganz et al. [14], to investigate the differences between anterior and posterior approaches in terms of their effects on surgical procedures, postoperative function and complications.
Methods
Patients
We retrospectively reviewed the records of 21 patients with Pipkin I fractures treated surgically via ORIF (10 cases of mS-P approach and 11 cases of GSD approach) at Pidu District People’s Hospital from June 2010 to May 2020. All surgeries were performed by the same two experienced hip surgeons to reduce variability. Inclusion criteria included patients with Pipkin I femoral head fractures with displacement greater than 2 mm, treated by ORIF using either the mS-P or GSD approach, and who had at least 12 months of clinical follow-up. Patients with arthritis, AVN, or those with a follow-up time of less than one year were excluded.
Radiographic evaluation
All patients were diagnosed with posterior dislocation of the hip joint and femoral head fractures using complete X-ray or CT 3D reconstruction in the emergency department (Figs. 1 and 2). After admission, manual reduction was performed for 5 patients in the emergency department and for 16 patients under general anesthesia after fasting. Following reduction, all patients underwent skeletal traction through the supracondylar femoral bone.
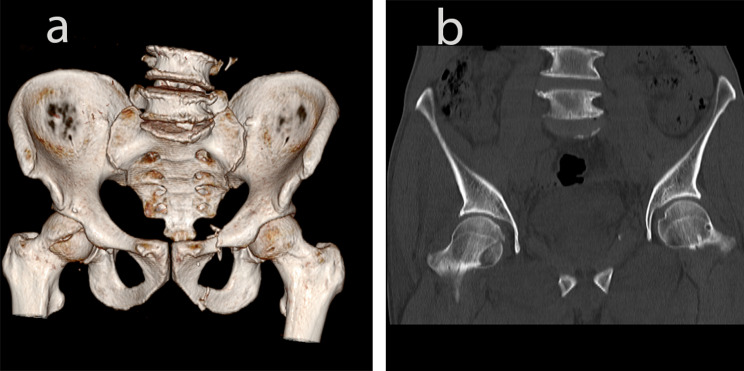
Fig. 1.
A 29-year-old young woman with left femoral head fracture (Pipkin I) injured in a car accident. a-b: Preoperative CT three-dimensional reconstruction
Fig. 2.
A 57-year-old male with right femoral head fracture (Pipkin I) injured in a car accident. a-b: Preoperative CT three-dimensional reconstruction
Surgical intervention
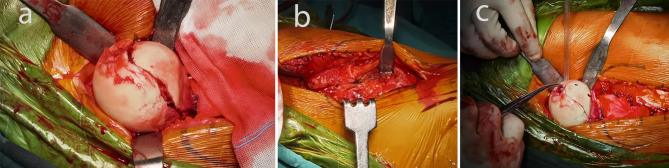
mS-P approach: Patients were positioned supine. The incision is located in the line from the anterior superior iliac spine to the outer edge of the patella. It begins at the middle of the iliac crest and extended distally for approximately 10 cm [15, 16]. Care was taken to protect the lateral femoral cutaneous nerve and while exposing the tensor fascia lata. Sharp and blunt dissection was performed along the space between the tensor fascia lata and sartorius, and between the gluteus medius and rectus femoris. The anterior space of the hip capsule was exposed and the vessels around the joint capsule were protected. The capsule was incised in a “Z” shape, and the femoral head was exposed by externally rotating the affected limb with hip flexion. After the fracture was reduced, the Kirschner wire was temporarily fixed, and C-arm fluoroscopy confirmed a satisfactory reduction. Subchondral headless Herbert screws or 4 mm cannulated screws were then inserted for fixation (Fig. 3). C-arm fluoroscopy was used to confirm satisfactory fracture reduction, as well as optimal screw positioning and depth. The fracture haematoma and bone fragments within the hip capsule were carefully removed. After repeated irrigation of the joint cavity, the wound was sutured layer by layer.
Fig. 3.
a: Complete exposure of the femoral head through mS-P approach; b, c: Fixation femoral head
GSD approach: Patients were positioned laterally. The incision extended from the posterior superior iliac spine through the apex of the greater trochanter. The gluteus maximus fascia was incised longitudinally, and the gluteus maximus was retracted to expose the proximal femur. An osteotomy, approximately 1.5 cm thick, was performed parallel to the femoral axis. After removing the residual muscle and soft tissue attachment, the greater trochanter was lifted proximally to expose the hip capsule anteriorly. A “Z” incision was made in the capsule to expose the femoral head fracture site. Following ligament resection, the femoral head was reduced and stabilized using 2–3 subchondral headless Herbert screws or 4 mm cannulated screws as needed. C-arm fluoroscopy confirmed the reduction quality, screw position, and depth. The greater trochanter was reattached using 4.5-mm screws (Fig. 4).
Fig. 4.
a: Complete exposure of the femoral head through Ganz osteotomy; b, c: Fixation femoral head
Postoperative protocol
Low-molecular-weight heparin was administered 6 h after surgery to prevent deep vein thrombosis, and indomethacin was given orally on the second post-operative day to prevent heterotopic ossification. Prophylactic antibiotics were routinely administered for 24 h post-surgery. If the procedure extends beyond 4 h or significant blood loss occurs, the medication should be re-administered every 4 h [17, 18]. Skin traction was routinely applied, with weights of 3–5 kg. Patients were advised to perform quadriceps isometric exercises within 24 h postoperatively, and hip flexion and extension exercises commenced at 4–6 weeks. All patients remained non-weight-bearing for 6–8 weeks, followed by gradual progression to full weight-bearing activities over the next 4–6 months as indicated by X-ray results. Radiographs were performed to evaluate fracture healing, osteotomy site integrity, implant stability, and the development of osteonecrosis.
Clinical evaluation
Clinical outcomes were assessed using the Thompson-Epstein function evaluation system [19], Modified Harris Hip function score (MHHS) [20], and Vail Hip Score (VHS) [21] to monitor hip function recovery.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation. Categorical variables were expressed as numbers or percentages. Normality was assessed using the Shapiro-Wilk test. Continuous variables were analyzed using independent samples t-tests or Mann-Whitney U-tests. Categorical variables were evaluated using chi-square tests. All tests were considered statistically significant with a p-value < 0.05. All analyses were performed using SPSS version 29.0.
Results
General results
The demographic characteristics of the 21 patients are summarized in Table 1. The mS-P group included 10 patients (6 males and 4 females), while the GSD group included 11 patients (8 males and 3 females). The mean age was 44 ± 11.82 (range, 26–65 years) and 44.2 ± 11.72 (range, 29–67 years) in the mS-P group and GSD group, respectively, P = 0.972.
Table 1.
General information of patients
| mS-P (n = 10) | Ganz (n = 11) | P value | |
|---|---|---|---|
| Age, years, Mean ± SD | 44 ± 11.82 | 44.2 ± 11.72 | 0.972 |
| Sex, n (%) | 0.877 | ||
| Male | 6 (60) | 8 (72.7) | |
| Female | 4 (40) | 3 (27.3) | |
| Side of injury, n (%) | 0.730 | ||
| Left | 2 (20) | 4 (36.4) | |
| Right | 8 (80) | 7 (63.6) | |
| Mode of trauma, n (%) | 0.903 | ||
| Traffic accident | 7 (70) | 9 (81.8) | |
| High fall injury | 3 (30) | 2 (18.2) | |
|
Follow-up period, months Mean ± SD |
35.9 ± 13.73 | 39.4 ± 11.63 | 0.543 |
| Associated injuries | |||
| Epidural hemorrhage | 2 | - | |
| Contralateral femoral shaft fracture | 1 | - | |
| Distal radius fracture | 1 | 2 | |
| Rib fracture | 4 | 3 | |
| Cerebral contusion with cerebral hemorrhage | - | 1 | |
| Ankle fracture | - | 2 | |
Perioperative outcomes
The mean BMI was 22.6 ± 2.48 (range, 18.49–26.07 kg/m2) and 24.2 ± 2.68 (range, 20.43–28.1 kg/m2) in the mS-P group and GSD group, respectively, P = 0.181 (Table 2). The mean incision length was 10.65 ± 1.68 (range, 7–11 cm) and 14.45 ± 0.907 (range, 13–16 cm) in the mS-P group and GSD group, respectively, P < 0.001. The mean operative time was 91 ± 9.66 (range, 75–105 min) and 108.6 ± 8.97 (range, 95–125 min) in the mS-P group and GSD group, respectively, P < 0.001. The mean intraoperative blood loss was 162.5 ± 19.33 (range, 125–190 mL) and 285.45 ± 46.93 (range, 210–365 mL) in the mS-P group and GSD group, respectively, P < 0.001 (Table 3).
Table 2.
Patients’ admission assessment and perioperative-related data
| Parameters | Total (n) = 21 | Type | P | |
|---|---|---|---|---|
| GSD (n) = 11 | mS-P (n) = 10 | |||
| Height (cm) | 169 ± 9.01 | 171 ± 8.57 | 168 ± 9.56 | 0.379 |
| Weight (kg) | 67.1 ± 8.11 | 70.6 ± 7.84 | 63.3 ± 6.82 | 0.033 |
| BMI (kg/m2) | 23.4 ± 2.65 | 24.2 ± 2.68 | 22.6 ± 2.48 | 0.181 |
| TIA (hour) | 4.95 ± 2.56 | 4.00 ± 2.28 | 6.00 ± 2.54 | 0.074 |
| TISR (hour) | 7.69 ± 2.38 | 7.09 ± 2.18 | 8.35 ± 2.54 | 0.241 |
| PH (g/L) | 108 ± 11.5 | 107 ± 12.9 | 108 ± 10.5 | 0.841 |
| TAO (day) | 5.57 ± 0.98 | 5.45 ± 1.13 | 5.70 ± 0.82 | 0.574 |
BMI, Body mass index; TIA, Time from Injury to Admission; TISR, Time from Injury to Successful Reduction; PH, Preoperative Hemoglobin; TAO, Time from admission to Operation
Table 3.
Comparison of surgical efficiency and surgical trauma between GSD and m S-P approaches
| Parameters | Total (n) = 21 | Type | P | |
|---|---|---|---|---|
| GSD (n) = 11 | mS-P (n) = 10 | |||
| Incision length (cm) | 12.02 ± 2.80 | 14.45 ± 0.907 | 10.65 ± 1.68 | < 0.001 |
| Operative time (min) | 100.2 ± 12.79 | 108.6 ± 8.97 | 91 ± 9.66 | < 0.001 |
| Intraoperative blood loss (mL) | 226.9 ± 72.3 | 285.45 ± 46.93 | 162.5 ± 19.33 | < 0.001 |
Follow-up results
The mean follow-up was 35.9 ± 13.73 (range, 12–55 months) and 39.4 ± 11.63 (range, 18–55 months) in the mS-P group and GSD group, respectively, P = 0.543. Table 4 shows the follow-up function and complications. The mean MHHS was 83.5 ± 9.14 (range, 70–95) and 75 ± 9.22 (range, 60–95) in the mS-P group and GSD group, respectively, P = 0.048. The mean VHS was 83.9 ± 7.4 (range, 73–93) and 74.4 ± 11.28 (range, 55–93) in the mS-P group and GSD group, respectively, P = 0.034. According to the Thompson-Epstein Score, the mS-P group included patients classified as Excellent (6 patients), Good (3 patients), and Fair (1 patient). In the GSD group, the classifications were Excellent (3 patients), Good (5 patients), Fair (2 patients). Typical cases of mS-P follow-up are shown in Figs. 5 and 6. Typical cases of GSD follow-up are shown in Figs. 7 and 8.
Table 4.
GSD and m S-P approaches of postoperative function and complication occurrence
| mS-P (n = 10) | Ganz (n = 11) | P value | |
|---|---|---|---|
| MHHS, Mean ± SD | 83.5 ± 9.14 | 75 ± 9.22 | 0.048 |
| Thompson-Epstein Score, n (%) | 0.425 | ||
| Excellent |
6 (60) 3 (30) 1 (10) - |
3 (27.2) 5 (45.5) 2 (18.2) 1 (9.1) |
|
| Good | |||
| Fair | |||
| poor | |||
| VHS, Mean ± SD | 83.9 ± 7.4 | 74.4 ± 11.28 | 0.034 |
| Complications, n (%) | 1.000 | ||
| AVN |
1 (10) 5 (50) 3 2 - 2 (20) - |
1 (9.1) 5 (45.5) 2 2 1 5 (45.5) 1 (9.1) |
|
| HO | |||
| Brooker I | |||
| Brooker II | |||
| Brooker IV | |||
| PTOA | |||
| SNI | |||
MHHS, Modified Harris Hip Score; VHS, Vail Hip Score; AVN, Avascular Necrosis; HO, Heterotopic Ossification; PTOA, Post-Traumatic Osteoarthritis; SNI, Sciatic Nerve Injury
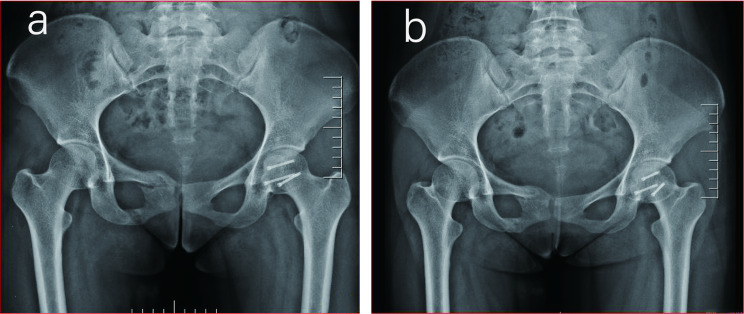
Fig. 5.
mS-P approach radiograph: a: 6 months after surgery; b: 18 months after surgery
Fig. 6.
GSD osteotomy radiograph: a: 10 months after surgery; b: 54 months after surgery
Fig. 7.
mS-P approach: Postoperative functional images at 18 months
Fig. 8.
GSD osteotomy: Postoperative functional images at 54 months
Complications
Table 4 shows the complications in patients with different surgical approaches. In the mS-P group, 1 patient (10%) developed AVN, diagnosed by pelvic X-ray one year post-surgery, and underwent total hip replacement the following year. HO was observed in 5 patients (50%), including 3 cases of Brooker I and 2 of Brooker II. Additionally, 2 patients (20%) presented with PTOA and were managed with oral glucosamine hydrochloride and celecoxib for pain relief.
Complications
In the GSD group, 5 patients (45.5%) developed HO (2 cases of Brooker I, 2 of Brooker II, and 1 of Brooker IV). PTOA was identified in 5 patients (45.5%), and AVN was detected in 1 patient (9.1%) at 34 months, leading to total hip replacement 21 months later. One patient (9.1%) experienced SNI, with limited response to mecobalamin for neurotrophic support. No cases of internal fixation failure or greater trochanteric osteotomy nonunion were observed.
Discussion
In early follow-up, we found that the mS-P approach was superior to the GSD method in terms of operative time, intraoperative blood loss, and incision length. It also showed a trend toward better early hip functional recovery. Therefore, in the treatment of Pipkin I fractures, a surgical method that is relatively less invasive and can fully expose the fracture site may be chosen based on the patient’s specific situation.
The Pipkin classification system is widely used clinically, based on the fracture line location in the femoral head’s weight-bearing area and associated fractures of the femoral neck or acetabular rim [9]. However, controversy remains regarding surgical indications, timing, approach, and whether the fracture fragment should be removed [5, 22, 23]. Some studies reported poor hip functional recovery post-Pipkin fracture, as assessed by functional scores [5]. Clinical reduction and internal fixation should be performed as early as possible to preserve the femoral head blood supply and minimize complications for patients without surgical contraindications [10]. Current indications for open reduction include poor reduction after hip dislocation, femoral head comminution, hip instability, sciatic nerve injury, and femoral neck involvement [4, 9, 24, 25]. Although treatments range from fragment removal to ORIF and hip replacement, most experts recommend ORIF for Pipkin I fractures [4, 10, 23, 25]. Closed reduction within six hours of injury has been advocated by some to reduce AVN and arthritis risk, although repeated attempts are discouraged due to the risk of displacement and cartilage damage [26–28]. Our average reduction time was 8 h. Given that Pipkin fractures are often high-energy injuries with associated severe injuries, immediate surgical treatment is generally not recommended [24, 28]. Marchetti et al. [23] found no significant outcome difference between reduction within 24 h and later. Early surgical intervention, ideally within three days post-admission, has been linked to better healing and reduced necrosis risk [29]. It has also been suggested that the femoral head heals slowly and is prone to AVN, so surgery should be scheduled as soon as the condition allows [30]. In this study, patients were treated with ORIF an average of five days post-injury.
In our study, the mean surgical incision length was 10.65 cm for the mS-P approach and 14.45 cm for the GSD approach. Khan et al. [31] performed periacetabular osteotomies in 168 patients using the mS-P approach. The mean length of the surgical incision was 9.34 cm. Hosny et al. [32] suggested that the incision for the GSD approach should be around 15 cm. This may be because the mS-P approach is relatively shallow compared with the posterior approach. It requires only the retraction of thin tissues such as the tensor fascia lata and rectus femoris [33]. The Ganz approach often requires stripping more muscles, such as gluteus maximus and gluteus medius, or exposing deeper structures [34].
The operative time and intraoperative blood loss were 91 min and 162.5 mL for the mS-P approach, and 108.6 min and 285.45 mL for the GSD approach. Jiang et al. [35] reported a mean operative time of 61.96 min and a mean intraoperative blood loss of 46.09 mL using the mS-P method. Mostafa et al. [29] found that the mean operative time and intraoperative blood loss for the Ganz method were 120 min and 283 mL, respectively. This may be because the mS-P approach exposes the femoral head by bluntly separating the muscle space. It is less invasive and allows for direct fracture fixation [33, 36]. The GSD approach requires more extensive muscle pulling and osteotomy to expose the surgical area [32, 37]. In contrast, the GSD approach is more cumbersome [38]. Overall, our data and previous studies suggest that the mS-P approach has advantages in operative time and intraoperative blood loss.
In our early follow-up, the mean MHHS for patients in the mS-P and GSD groups was 83.5 ± 9.14 and 75 ± 9.22, respectively. The mean VHS was 83.9 ± 7.4 for the mS-P group and 74.4 ± 11.28 for the GSD group. The Thompson-Epstein scores of “excellent and good” were 90% for the mS-P approach and 72.7% for the GSD approach. Mostafa et al. [29] treated patients with Pipkin I and II fractures using the GSD approach. They reported Thompson-Epstein scores of “excellent and good” in 83.3% (10 of 12). Abdelazeem et al. [39] reported that Pipkin I fractures treated with the medial approach achieved an 83.33% “excellent and good” Thompson-Epstein score. Another study reported that patients with Pipkin I and II fractures treated with the anteromedial approach had an 83.33% rate of “excellent and good” Thompson-Epstein scores. Their mean Harris score was 85.08 ± 5.73 [40]. Harris scores for Pipkin I fractures treated with hip arthroscopy averaged 94 [41].
The femoral head is primarily supplied by the medial femoral circumflex artery (MFCA). Isolating the external rotator muscle during dislocation may damage the MFCA in 7-24% of cases [42–45]. The mS-P approach minimizes muscle trauma, leading to faster recovery and reduced risk of MFCA damage, trochanteric osteotomy, and nonunion or malunion complications [46, 47]. However, Epstein et al. [1] argued that the posterior approach may injure blood vessels in patients with posterior dislocations. The anterior approach may damage the blood supply in front of the femoral head and increase the risk of AVN [1, 22–24, 46]. In the 153 patients studied by Giannoudis et al. [6], the AVN incidence rates were 5.3% for the mS-P approach and 8.3% for the GSD approach, respectively. The risk of AVN in the posterior approach was 3.2 times higher than in the anterior approaches [6, 14, 24, 48]. In our study, the AVN rates for the GSD and mS-P approaches were 9.1% and 10%, aligning with prior findings. The GSD approach, however, requires more surgical skills and anatomical expertise, with a potential risk of osteotomy nonunion [49, 50].
Although there was a statistically significant difference in weight between the two groups of mS-P and GSD patients, there was no difference in BMI. Regenbogen et al. [51] concluded that those with lower age and BMI were suitable for internal fixation after following 45 patients with femoral fractures for more than five years. Our findings indicate that in patients with higher BMI, the mS-P approach may be challenging due to limited surgical field exposure, as it does not transect the rectus femoris muscle.
From our results, the incidence of PTOA in the mS-P group was 20%, whereas it was 45.5% in the GSD group. Previous research found PTOA incidence in anterior surgery patients to be 20.3 times higher than in GSD patients, with higher rates in Pipkin IV and III fractures, and lower rates in Pipkin I fractures [6]. Moreover, Hanke et al. [52] suggested that the incidence of OA may also be related to the degree of cartilage damage to the femoral head at the time of injury. HO incidence following femoral head fractures ranges from 6 to 80%, with rates of 47.2% in GSD, 44.7% in S-P, and 32.3% in K-L approaches. Posterior approaches may reduce HO incidence relative to anterior approaches [6, 53, 54]. Guo et al. [55] found HO rates to be lower in GSD patients (33.3%) than in those treated with anterior or posterior approaches (42.1% and 36.9%, respectively), suggesting that while anterior approaches elevate HO risk, posterior approaches raise AVN risk. However, our results indicated a similar incidence of HO for the mS-P approach and the GSD approach, which were 50% and 45.5%, respectively. The risk of sciatic nerve injury remains for the posterior approach. Shakya et al. [56] showed a 5% (two of 42 cases) incidence of sciatic nerve injury and recommended the anterior approach for Pipkin I and II fractures. Our results showed a 9.1% incidence of sciatic nerve injury (one of 11 cases) with the GSD approach.
There are some limitations of this study: firstly, as a retrospective study, sample selection bias and judgement of causality cannot be avoided. Secondly, we only included patients with Pipkin I fractures; however, further studies are needed for other types of femoral fractures. Our sample size was small due to the low prevalence of the disease. Therefore, future studies should include larger sample sizes and long-term follow-up.
Conclusion
This study suggests that the mS-P approach offers advantages over the GSD approach in terms of surgical efficiency and surgical trauma. However, longer-term follow-up is required to fully assess complications and functional outcomes.
Acknowledgements
Not applicable.
Abbreviations
- mS-P
Modified Smith-Peterson
- GSD
Ganz Surgical Dislocation
- K-L
Kocher-Langenbeck
- VHS
Vail Hip Score
- MHHS
Modified Harris Hip Score
- ORIF
Open Reduction and Internal Fixation
- AVN
Avascular Necrosis
- HO
Heterotopic Ossification
- PTOA
Post-Traumatic Osteoarthritis
- SNI
Sciatic Nerve Injury
- MFCA
Medial Femoral Circumflex Artery
- TIA
Time from Injury to Admission
- TISR
Time from Injury to Successful Reduction
- PH
Preoperative Hemoglobin
- TAO
Time from admission to Operation
- BMI
Body mass index
Author contributions
Z.H.W. contributed to conceptualization, wrote the manuscript, statistics analysis, project administration, and final editing. D.W.contributed to data curation, supervision, performed the examination, review, and editing. W.S.T. contributed to correspondence supervision, prepared Figs. 1, 2, 3, 4, 5, 6, 7 and 8; Tables 1, 2, 3 and 4. Y.Y.contributed to data collection and performed the examination and review, final editing. All authors reviewed the manuscript.
Funding
This work was supported by Sichuan Science and Technology Program(2023NSFSC0659).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of the Clinical Research and Biomedical Ethical Committee of Pidu District People’s Hospital (No:202302). All the participants provided written informed consent to attend the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Epstein HC, Wiss DA, Cozen L. Posterior fracture dislocation of the hip with fractures of the femoral head. Clin Orthop Relat Res. 1985;201:9–17. [PubMed] [Google Scholar]
- 2.Hougaard K, Thomsen PB. Traumatic posterior fracture-dislocation of the hip with fracture of the femoral head or neck, or both. J bone Joint Surg Am Volume. 1988;70(2):233–9. [PubMed] [Google Scholar]
- 3.Sahin V, Karakaş ES, Aksu S, Atlihan D, Turk CY, Halici M. Traumatic dislocation and fracture-dislocation of the hip: a long-term follow-up study. J Trauma. 2003;54(3):520–9. [DOI] [PubMed] [Google Scholar]
- 4.Butler JE. Pipkin Type-II fractures of the femoral head. J bone Joint Surg Am Volume. 1981;63(8):1292–6. [PubMed] [Google Scholar]
- 5.Brumback RJ, Kenzora JE, Levitt LE, Burgess AR, Poka A. Fractures of the femoral head. Hip. 1987:181-206. [PubMed]
- 6.Giannoudis PV, Kontakis G, Christoforakis Z, Akula M, Tosounidis T, Koutras C. Management, complications and clinical results of femoral head fractures. Injury. 2009;40(12):1245–51. [DOI] [PubMed] [Google Scholar]
- 7.Schönweiss T, Wagner S, Mayr E, Rüter A. [Late results after fracture of the femoral head]. Unfallchirurg. 1999;102(10):776–83. [DOI] [PubMed] [Google Scholar]
- 8.Lang-Stevenson A, Getty CJ. The Pipkin fracture-dislocation of the hip. Injury. 1987;18(4):264–9. [DOI] [PubMed] [Google Scholar]
- 9.Pipkin P G. Treatment of grade IV fracture-dislocation of the hip. J bone Joint Surg Am Volume. 1957;39–a(5):1027–42. passim. [PubMed] [Google Scholar]
- 10.Roeder LF, Jr., DeLee JC. Femoral head fractures associated with posterior hip dislocation. Clin Orthop Relat Res. 1980;147:121-130. [PubMed]
- 11.Asghar FA, Karunakar MA. Femoral head fractures: diagnosis, management, and complications. Qld Gov Min J. 2004;35(4):463–72. [DOI] [PubMed] [Google Scholar]
- 12.Behery OA, Dai AZ, McLaurin TM. Posttraumatic heterotopic ossification of the hip. J Orthop Trauma. 2018;32(Suppl 1):S18–9. [DOI] [PubMed] [Google Scholar]
- 13.Hoskinson S, Morison Z, Shahrokhi S, Schemitsch EH. Managing AVN following internal fixation: treatment options and clinical results. Injury. 2015;46(3):497–506. [DOI] [PubMed] [Google Scholar]
- 14.Ganz R, Gill TJ, Gautier E, Ganz K, Krügel N, Berlemann U. Surgical dislocation of the adult hip a technique with full access to the femoral head and acetabulum without the risk of avascular necrosis. J Bone Joint Surg Br. 2001;83(8):1119–24. [DOI] [PubMed] [Google Scholar]
- 15.Khalifa AA, Ahmed EM, Farouk OA. Surgical approaches for managing femoral Head fractures (FHFs); what and how to choose from the different options? Orthop Res Rev. 2022;14:133–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith-Petersen MN. Approach to and exposure of the hip joint for mold arthroplasty. J Bone Joint Surg Am. 1949;31a(1):40–6. [PubMed] [Google Scholar]
- 17.Gillespie WJ, Walenkamp GH. Antibiotic prophylaxis for surgery for proximal femoral and other closed long bone fractures. Cochrane Database Syst Rev. 2010;2010(3):Cd000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK, Fish DN, Napolitano LM, Sawyer RG, Slain D, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect (Larchmt). 2013;14(1):73–156. [DOI] [PubMed] [Google Scholar]
- 19.Thompson VP, Epstein HC. Traumatic dislocation of the hip; a survey of two hundred and four cases covering a period of twenty-one years. J Bone Joint Surg Am. 1951;33-A(3):746-778. [PubMed]
- 20.Stasi S, Papathanasiou G, Diochnou A, Polikreti B, Chalimourdas A, Macheras GA. Modified Harris Hip Score as patient-reported outcome measure in osteoarthritic patients: psychometric properties of the Greek version. Hip Int. 2021;31(4):516–25. [DOI] [PubMed] [Google Scholar]
- 21.Kay J, de Sa D, Shallow S, Simunovic N, Safran MR, Philippon MJ, Ayeni OR: Level of clinical evidence presented at the International Society for Hip Arthroscopy Annual Scientific Meeting over 5 years (2010–2014). J Hip Preserv Surg. 2015;2(4):332–8. [DOI] [PMC free article] [PubMed]
- 22.Schönweiss T, Wagner S, Mayr E, Rüter A: [Late results after fracture of the femoral head]. Unfallchirurg. 1999;102(10):776–83. [DOI] [PubMed]
- 23.Marchetti ME, Steinberg GG, Coumas JM. Intermediate-term experience of Pipkin fracture-dislocations of the hip. J Orthop Trauma. 1996;10(7):455–61. [DOI] [PubMed] [Google Scholar]
- 24.Stannard JP, Harris HW, Volgas DA, Alonso JE. Functional outcome of patients with femoral head fractures associated with hip dislocations. Clin Orthop Relat Res. 2000;377:44–56. [DOI] [PubMed] [Google Scholar]
- 25.Mostafa MM. Femoral head fractures. Int Orthop. 2001;25(1):51–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMurtry IA, Quaile A. Closed reduction of the traumatically dislocated hip: a new technique. Injury. 2001;32(2):162–4. [DOI] [PubMed] [Google Scholar]
- 27.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–43. [DOI] [PubMed] [Google Scholar]
- 28.Dowd GS, Johnson R. Successful conservative treatment of a fracture-dislocation of the femoral head. A case report. J bone Joint Surg Am Volume. 1979;61(8):1244–6. [PubMed] [Google Scholar]
- 29.Mostafa MF, El-Adl W, El-Sayed MA. Operative treatment of displaced Pipkin type I and II femoral head fractures. Arch Orthop Trauma Surg. 2014;134(5):637–44. [DOI] [PubMed] [Google Scholar]
- 30.Jiang YQ, Huang J, Guo WK, Lai B, Wang J, Liang CX, Liu SL, Lin WM. [Treatment of Pipkin type I and II femoral head fractures through modified Smith-Peterson approach and modified Hardinge approach-a case-control studies]. Zhongguo Gu Shang. 2017;30(7):616–21. [DOI] [PubMed] [Google Scholar]
- 31.Khan OH, Malviya A, Subramanian P, Agolley D, Witt JD. Minimally invasive periacetabular osteotomy using a modified Smith-Petersen approach: technique and early outcomes. Bone Joint J. 2017;99-B(1):22-28. [DOI] [PubMed]
- 32.Hosny H, Mousa S, Salama W. Management of femoral head fracture by Ganz surgical dislocation of the hip. J Orthop Traumatol. 2022;23(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molnar RB, Routt ML Jr. Open reduction of intracapsular hip fractures using a modified Smith-Petersen surgical exposure. J Orthop Trauma. 2007;21(7):490–4. [DOI] [PubMed] [Google Scholar]
- 34.Gavaskar AS, Tummala NC. Ganz surgical dislocation of the hip is a safe technique for operative treatment of Pipkin fractures. Results of a prospective trial. J Orthop Trauma. 2015;29(12):544–8. [DOI] [PubMed] [Google Scholar]
- 35.Jiang Y-Q, Huang J, Guo W-K, Lai B, Wang J, Liang C-X, Liu S-L, Lin W-M. Treatment of Pipkin type I and II femoral head fractures through modified Smith-Peterson approach and modified Hardinge approach-a case-control studies. Zhongguo Gu shang = China J Orthop Traumatol. 2017;30(7):616–21. [DOI] [PubMed] [Google Scholar]
- 36.Lichstein PM, Kleimeyer JP, Githens M, Vorhies JS, Gardner MJ, Bellino M, Bishop J. Does the Watson-Jones or Modified Smith-Petersen Approach provide Superior exposure for femoral Neck fracture fixation? Clin Orthop Relat Res. 2018;476(7):1468–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khalifa AA, Haridy MA, Fergany A. Safety and efficacy of surgical hip dislocation in managing femoral head fractures: a systematic review and meta-analysis. World J Orthop. 2021;12(8):604–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khalifa AA, Refai O, Farouk O, Abdelnasser MK. Management of femoral head fractures through surgical hip dislocation (SHD): a demanding but safe technique. Arch Orthop Trauma Surg. 2021;141(10):1701–10. [DOI] [PubMed] [Google Scholar]
- 39.Abdelazeem A, Fahmy M, Abdelazeem H. Modified Ludloff’s medial approach for management of Pipkin’s type I femoral head fracture. Int Orthop. 2021;45(6):1591–8. [DOI] [PubMed] [Google Scholar]
- 40.Wang ZH, Li KN, Zhao P, Chen ED, Zheng J. In situ reduction and fixation of the Anterior Medial Fenestration Approach for femoral Head fracture. Orthop Surg. 2019;11(6):1163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aprato A, Buzzone M, Di Benedetto P, Massè A. Surgical hip dislocation vs arthroscopy for fixation of subfoveal femoral head fractures: a new technique for Pipkin type 1 fractures. Acta Biomed. 2021;92(S3):e2021016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gautier E, Ganz K, Krügel N, Gill T, Ganz R. Anatomy of the medial femoral circumflex artery and its surgical implications. J Bone Joint Surg Br. 2000;82(5):679–83. [DOI] [PubMed] [Google Scholar]
- 43.Sevitt S, Thompson RG, THE DISTRIBUTION AND ANASTOMOSES OF ARTERIES SUPPLYING THE HEAD AND NECK OF THE FEMUR. J Bone Joint Surgery-british Volume. 1965;47(3):560–73. [PubMed] [Google Scholar]
- 44.Trueta J, Harrison MH. The normal vascular anatomy of the femoral head in adult man. J Bone Joint Surg Br. 1953;35–B(3):442–61. [DOI] [PubMed] [Google Scholar]
- 45.Stirma GA, Uliana CS, Valenza WR, Abagge M. Surgical treatment of femoral head fractures through previously controlled hip luxation: four case series and literature review. Revista Brasileira De Ortop. 2018;53(3):337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Henle P, Kloen P, Siebenrock KA. Femoral head injuries: which treatment strategy can be recommended? Injury. 2007;38(4):478–88. [DOI] [PubMed] [Google Scholar]
- 47.Department of, Orthopedic S, Haeundae, Paik. Hospital, College, of, Medicine: outcomes of treatment for femoral head fractures with hip dislocation - review of 20 cases. J Korean Hip Soc. 2011;22(4):298–298. [Google Scholar]
- 48.Chen JW, Rosinsky PJ, Shapira J, Maldonado DR, Kyin C, Lall AC, Domb BG. Osteochondral Allograft Implantation using the Smith-Peterson (Anterior) Approach for Chondral lesions of the femoral head. Arthrosc Techniques. 2020;9(2):e239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beaulé PE, Le Duff MJ, Zaragoza E. Quality of life following femoral head-neck osteochondroplasty for femoroacetabular impingement. J Bone Joint Surg Am. 2007;89(4):773-779. [DOI] [PubMed]
- 50.Sink EL, Beaulé PE, Sucato D, Kim YJ, Millis MB, Dayton M, Trousdale RT, Sierra RJ, Zaltz I, Schoenecker P, et al. Multicenter study of complications following surgical dislocation of the hip. J Bone Joint Surg Am. 2011;93(12):1132-1136. [DOI] [PubMed]
- 51.Regenbogen S, Watrinet J, Beck M, Osten P, Stuby FM, Grützner PA, Jaecker V: Treatment and clinical outcome in patients with femoral head fractures: a long-term follow-up. Arch Orthop Trauma Surg. 2024;144(9):4491–7. [DOI] [PubMed]
- 52.Hanke MS, Keel MJB, Cullmann JL, Siebenrock KA, Bastian JD. Transfer of osteochondral shell autografts to salvage femoral head impaction injuries in hip trauma patients. Injury. 2020;51(3):711–8. [DOI] [PubMed] [Google Scholar]
- 53.Scolaro JA, Marecek G, Firoozabadi R, Krieg JC, Routt MLC. Management and radiographic outcomes of femoral head fractures. J Orthop Traumatology: Official J Italian Soc Orthop Traumatol. 2017;18(3):235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang CG, Li YM, Zhang HF, Li H, Li ZJ. Anterior approach versus posterior approach for Pipkin I and II femoral head fractures: a systemic review and meta-analysis. Int J Surg. 2016;27:176–81. [DOI] [PubMed] [Google Scholar]
- 55.Guo JJ, Tang N, Yang HL, Qin L, Leung KS. Impact of surgical approach on postoperative heterotopic ossification and avascular necrosis in femoral head fractures: a systematic review. Int Orthop. 2010;34(3):319–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shakya S, Chen J, Sun J, Xiang Z. Management and outcome of patients with femoral head fractures: the mid-term follow-up with injuries and associated prognostic factors. BMC Musculoskelet Disord. 2023;24(1):311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.