Abstract
ARF GAP1, a 415-amino acid GTPase activating protein (GAP) for ADP-ribosylation factor (ARF) contains an amino-terminal 115-amino acid catalytic domain and no other recognizable features. Amino acids 203–334 of ARF GAP1 were sufficient to target a GFP-fusion protein to Golgi membranes in vivo. When overexpressed in COS-1 cells, this protein domain inhibited protein transport between the ER and Golgi and, in vitro, competed with the full-length ARF GAP1 for binding to membranes. Membrane binding by ARF GAP1 in vitro was increased by a factor in cytosol and this increase was inhibited by IC261, an inhibitor selective for casein kinase Iδ (CKIδ), or when cytosol was treated with antibody to CKIδ. The noncatalytic domain of ARF GAP1 was phosphorylated both in vivo and in vitro by CKI. IC261 blocked membrane binding by ARF GAP1 in vivo and inhibited protein transport in the early secretory pathway. Overexpression of a catalytically inactive CKIδ also inhibited the binding of ARF GAP1 to membranes and interfered with protein transport. Thus, a CKI isoform is required for protein traffic through the early secretory pathway and can modulate the amount of ARF GAP1 that can bind to membranes.
INTRODUCTION
ARF1 is the founding member of a family of small GTP-binding proteins belonging to the Ras superfamily. The number of seemingly unrelated biochemical and biological activities either stimulated or inhibited by ARF1 is remarkable. ARF1 regulates the formation of several different types of coated vesicles (Stamnes and Rothman, 1993; Ooi et al., 1998), activates lipid-modifying enzymes, (Singer et al., 1997; Godi et al., 1999; Jones et al., 2000), participates in signal transduction events (Fensome et al., 1998; Shome et al., 1998), and has effects on the actin cytoskeleton (Norman et al., 1998). ARF1 is necessary for membrane traffic between the endoplasmic reticulum (ER) and Golgi complex (Dascher and Balch, 1994; Zhang et al., 1994), for the formation of secretory vesicles at the trans-Golgi Network (Barr and Huttner, 1996; Chen et al., 1997) and for the formation of vesicle coats on Golgi membranes (Serafini et al., 1991; Stamnes and Rothman, 1993) and on endosomes (Whitney et al., 1995; Gu et al., 1997; Ooi et al., 1998). In Saccharomyces cerevisiae there are two redundant ARF genes that, when deleted, can be complemented by expression of mammalian ARF1. These genes are essential for growth and for protein secretion (Stearns et al., 1990a, 1990b). The extent to which the various activities of ARF1 are related, and how each function is regulated and localized to the proper cellular site, is currently unknown.
The structural integrity and function of the Golgi complex is lost when guanine nucleotide exchange on ARF1 is inhibited by brefeldin A (Lippincott-Schwartz et al., 1989), when recombinant ARF1 mutants unable to bind nucleotide are overexpressed (Dascher and Balch, 1994) or when ARF1 function is lost through mutation (Gaynor et al., 1998). Failure to hydrolyze GTP on ARF1 also inhibits membrane traffic. The hydrolysis of GTP by ARF1 is required for the uncoating of COPI vesicle coats (Tanigawa et al., 1993) and also for the proper sorting of cargo molecules into COPI coated vesicles (Nickel et al., 1998). As purified ARF1 has essentially undetectable GTPase activity (Kahn and Gilman, 1986), the hydrolysis of GTP by ARF must be stimulated by a GTPase activating protein (GAP). The first ARF GAP to be identified, ARF GAP1, is found on Golgi membranes (Cukierman et al., 1995) and this localization is inhibited by BFA, suggesting that ARF GAP1 participates in coated vesicle formation at the Golgi complex.
Overexpression of ARF GAP1 in COS-1 cells induces a phenotype similar to cells treated with BFA or that overexpress a form of ARF1 unable to bind nucleotide (Aoe et al., 1997). Presumably this is caused by accelerated hydrolysis of GTP on ARF1, lowering the concentration of active ARF1 bound to membranes. Although the catalytic domain of ARF GAP1 is in the amino-terminal third of the protein, deletion of part of the carboxy terminus results in the loss of the phenotype caused by overexpression. Thus, the carboxyl terminal domain is required for ARF GAP1 to function in vivo (Huber et al., 1998). In addition to its GAP activity, ARF GAP1 might also function as an effector for ARF, since homologues of ARF GAP1 in S. cerevisiae have been identified as multi-copy suppressors of loss of ARF activity (Zhang et al., 1998).
Two homologues of ARF GAP1, GLO3 and GCS1, have been identified in S. cerevisiae as having overlapping but nonidentical function and their deletion results in impaired retrograde transport and inviability (Poon et al., 1999). YCK1 and YCK2, the yeast homologues of casein kinase I (CKI), and CDC55, the yeast homolog of the B regulatory subunit of protein phosphatase 2A, were isolated as multicopy suppressors of a gcs1· mutant (Wang et al., 1996). This implies that the function of GCS1 requires the proper state of phosphorylation on proteins that are yet to be identified. In S. cerevisiae, there are four casein kinase genes, designated as YCK1, 2, 3 and HRR25. YCK1 and YCK2 are functionally redundant and are required for viability. Genes encoding the subunits of clathrin adaptor proteins were identified as loss-of-function suppressors of the growth defects of a yck1− yck2–2ts yeast at nonpermissive temperature (Panek et al., 1997). These observations raise the possibility that there may be a role for CKI in molecular pathways that require ARF GAP1 homologues in yeast.
In mammalian cells, a kinase activity was coimmunoprecipitated with the ARF-regulated adaptor complex AP-3 (Faundez and Kelly, 2000). This kinase might be a CKI because it was inhibited by reagents that are selective for CKI. Inhibition of the kinase activity also reduced the recruitment of AP-3 to synaptic vesicles, providing a functional link between the kinase activity and vesicle formation. In addition, CKIα has been found associated with small synaptic vesicles and can phosphorylate one of the synaptic vesicle-associated proteins (Gross et al., 1995). There are seven isoforms of CKI in mammalian cells, designated as α, β, γ1, γ2, γ3, δ, and ε. Other than the observation that CKIδ and CKIε can inhibit their own activity by autophosphorylation of their carboxyl terminal sequences, these kinases appear to lack specific regulation. Recently, 3-[(2,4,6-trimethoxyphenyl)methylidenyl]-indolin-2-one (IC261) was identified as an ATP competitor selective for CKI (Mashhoon et al., 2000). In vitro, in the presence of 10 μM ATP, IC261 inhibits CKIδ and CKIε activity with an IC50 of 1 μM, and CKIα with an IC50 of 16 μM. It is less active on kinases outside the CKI family, such as PKA, p34cdc2 and p55fyn, all of which have an IC50 greater than 100 μM in vitro.
In this report, we investigated the functions of the carboxyl terminal noncatalytic domain of ARF GAP1. Using a variety of techniques, we demonstrate that this domain is necessary and sufficient for binding to Golgi membranes. We further demonstrate that membrane binding by ARF GAP1 is stimulated by a cytosolic factor that is inhibited by IC261 or antibody to casein kinase Iδ. Expression of a dominant negative casein kinase Iδ inhibited the binding of ARF GAP1 to membranes as well as inhibiting secretion. Our results indicate that the binding of ARF GAP1 to membranes is regulated by a casein kinase isoform, probably CKIδ.
MATERIALS AND METHODS
DNA and Plasmid Constructions
We cloned a cDNA encoding ARF GAP1 from rat brain total RNA (kindly provided by Stefan Andersson, UT Southwestern) by RT-PCR based on published sequence information (Cukierman et al., 1995). Sequences encoding various C-terminal fragments of ARF GAP1 were obtained by PCR on a plasmid containing the cDNA for rARF GAP1 and were subcloned into plasmid pEGFP-C3 (Clontech). Full-length GAP cDNA was subcloned into vector pQE60 for protein expression in bacteria (Qiagen, Valencia CA). Sequences encoding 58 amino acids from carboxy terminus of ARF GAP1 were deleted by digesting the plasmid with SacI and BamHI. These sites were blunted and ligated to produce the GAP359 expression construct.
Antibodies
Rabbit antiserum to rat ARF GAP1 was prepared using GAP359 protein as the antigen. Monoclonal antibodies were prepared as culture supernatants from hybridomas M3A5, specific for β-COP (provided by T. Kreis, U. Geneva), hybridoma BW, specific for the cytoplasmic tail of VSV G protein (provided by W. Balch, Scripps Institute) and hybridoma FC 125, specific for the influenza virus hemagglutinin (provided by T. Braciale, U. Virginia). Rabbit antimannosidase II serum was purchased from The Complex Carbohydrate Center (U. Georgia). Goat anti-CKIδ was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Rabbit anti-green fluorescent protein (GFP) antibody was purchased from Clontech (Palo Alto, CA).
Cell Culture and Transfections
Chinese hamster ovary (CHO) K1 cells were cultured as described (Ktistakis et al., 1995). Stable transfectants expressing GFP fused at the amino-terminus of fragments of ARF GAP1 were obtained by transfecting plasmids into CHO K1 cells with FUGENE 6 (Roche Molecular Biochemicals, Indianapolis, IN). Cell clones resistant to G418 were selected and screened for uniform high expression of GFP fluorescence. For some experiments, GFP fusion proteins were transiently expressed with an influenza virus HA in COS-1 cells by cotransfecting the cells with FUGENE 6 complexed with either 1 μg of vector pKL1, (expressing HA) and 0.25 μg of plasmids expressing GFP fusion constructs, or 0.2 μg of GAP273-C3 and 0.5 μg of plasmids encoding CKIδ or CKIδ (DN).
Pulse-chase Experiments Using HA
The rate of transport of influenza virus HA from the endoplasmic reticulum to the Golgi complex of COS cells cotransfected with plasmids expressing various forms of GFP-GAP, and HA was measured by pulse-chase experiments as described (Ktistakis et al., 1995).
Indirect Immunofluorescence and GFP Studies
GFP fluorescence was imaged on cells that had been fixed with formaldehyde (Figure 1; see also Figure 6A). For the immunofluorescence studies, CHO cells stably expressing GFP-GAP273 were fixed in 3.7% formaldehyde for 15 min at room temperature and permeabilized on ice for 5 min in 100% methanol that had been prechilled at −20°C. The cells were then stained with the appropriate antibody and Texas Red–conjugated goat anti-rabbit IgG (for Man II) or Alexa 568–conjugated goat anti-mouse IgG (Molecular Probes; for 72 A or VSV). Images were collected separately using light at 488 nm to excite the GFP and 568 nm to excite the fluorophores on secondary antibodies. For the experiments shown in Figure 5, CHO cells were infected with vesicular stomatitis virus ts O45 at 32°C for 30 min. The innoculum was replaced with CHO culture medium, and the infected cells were incubated at 39°C for 3.5 h. At the end of this incubation, CHO culture medium containing 50 μM of IC261 or equal volume of DMSO carrier was applied to the cells for 5 min. The cells were shifted to 32°C to allow the transport of VSV from the ER to Golgi. At 10-min intervals after the shift to 32°C samples were fixed in methanol and stained with mAb to the G glycoprotein. For indirect immunofluorescence shown in Figure 6, cells were first incubated with IC261 at the indicated concentration for 30 min (see Figure 6A) or in 200 μM IC261 for various intervals (see Figure 6B) and then fixed and stained with anti-Man II as described above. For the experiment shown in Figure 9, COS-1 cells were transfected with a plasmid expressing CKIδ and, after 18 h, were infected with tsO45 VSV and cultured as described above. The cells were shifted from 39°C to 32°C and samples were fixed after 10, 15, and 20 min with methanol and stained with monoclonal anti VSV G and goat anti-CKIδ.
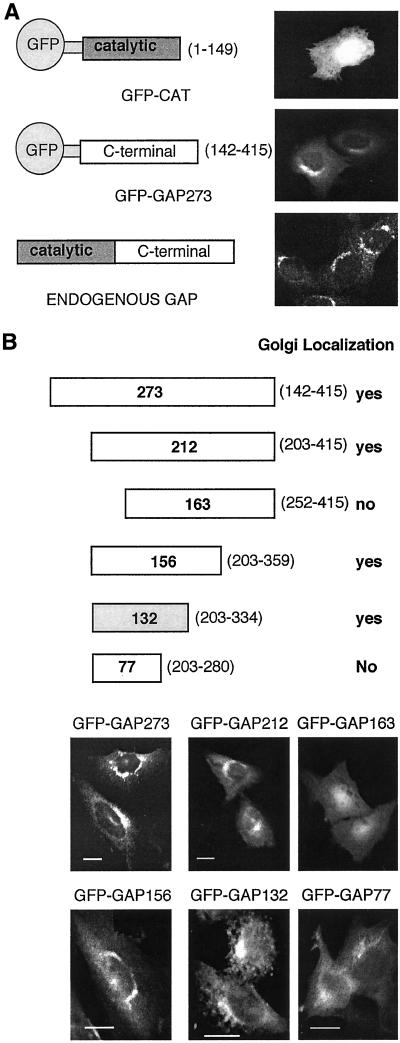
Figure 1.
Noncatalytic sequences of ARF GAP1 are sufficient for membrane binding. (A) The structures of proteins in which GFP is fused to either the catalytic domain or noncatalytic portion of ARF GAP1 are shown with fluorescence micrographs demonstrating the intracellular distribution of the fusion protein. For comparison, immunofluorescence labeling of endogenous ARF GAP1 is shown. (B) Deletion mapping of the membrane-binding domain of ARF GAP1. Various deletions of the noncatalytic domain of ARF GAP1 were fused to GFP and named by the number of ARF GAP1 amino acids remaining. The numbers in parentheses designate the starting and ending amino acids of the ARF GAP1 sequence for each construct. Images were taken of CHO cells stably transfected with the indicated GFP fusion constructs. Scale bar, 10 μm.
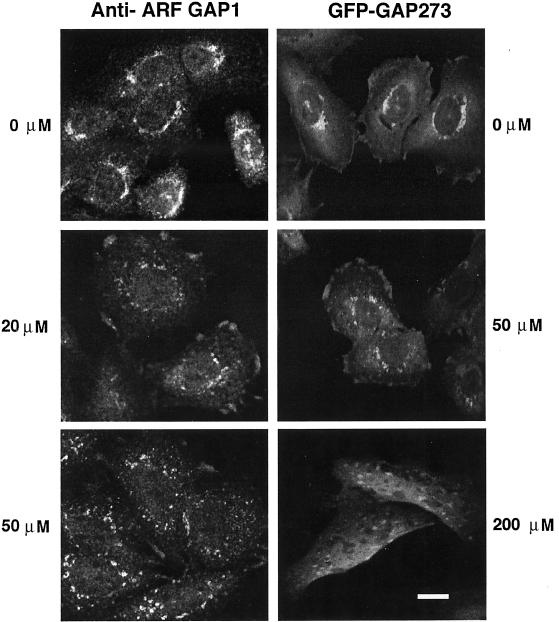
Figure 6.
IC261 alters the intracellular location of endogenous ARF GAP1 and GFP-GAP273. IC261 was applied to untransfected CHO cells and stably transfected CHO cells expressing GFP-GAP273 for 30 min at the indicated concentrations. Endogenous ARF GAP1 was identified by staining fixed CHO cells with anti–ARF GAP1 rabbit antiserum. GFP-GAP273 was identified by intrinsic GFP fluorescence. Endogenous ARF GAP1 and GFP-GAP273, which was expressed at much higher levels than the endogenous protein, lose their perinuclear location in cells treated with 20 and 50 μM IC261, respectively. At 200 μM IC261, the GFP-GAP273 signal is largely cytosolic with punctate concentrations of fluorescence. Scale bar, 10 μm.
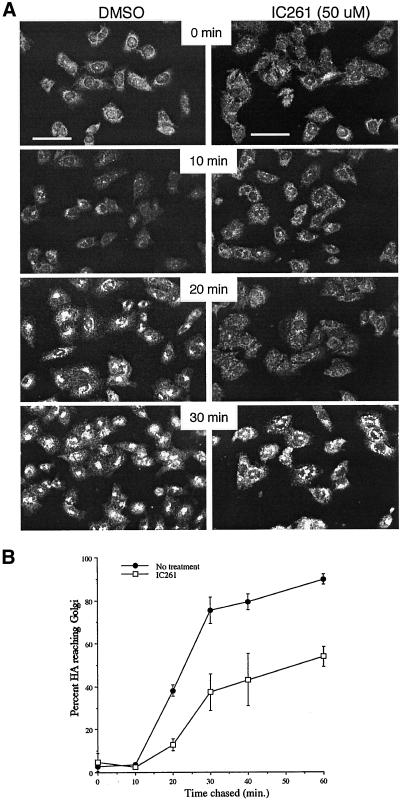
Figure 5.
IC261 inhibits protein transport in the early secretory pathway. (A) CHO cells were infected with VSV tsO45 virus and cultured at 39°C to retain the G glycoprotein in the endoplasmic reticulum. IC261 (50 μM) or an equal concentration of DMSO carrier was added to the cells for 10 min, and then the cultures were shifted to permissive temperature for the intervals shown. Cells were fixed and stained with monoclonal anti-G antibody. The bright, punctate fluorescence indicates that G protein is becoming concentrated in the Golgi and indicates that the G protein has been transported from the ER to the Golgi complex. Scale bars, 50 μm. (B) CHO cells were infected with influenza virus for 4 h and then pulse-labeled with 35S amino acids and chased in the presence or absence of 50 μM IC261. The fraction of HA reaching the Golgi as a function of chase time is shown. Error bars indicate the range of values about the averages from two (no inhibitor) or three (plus IC261) experiments.
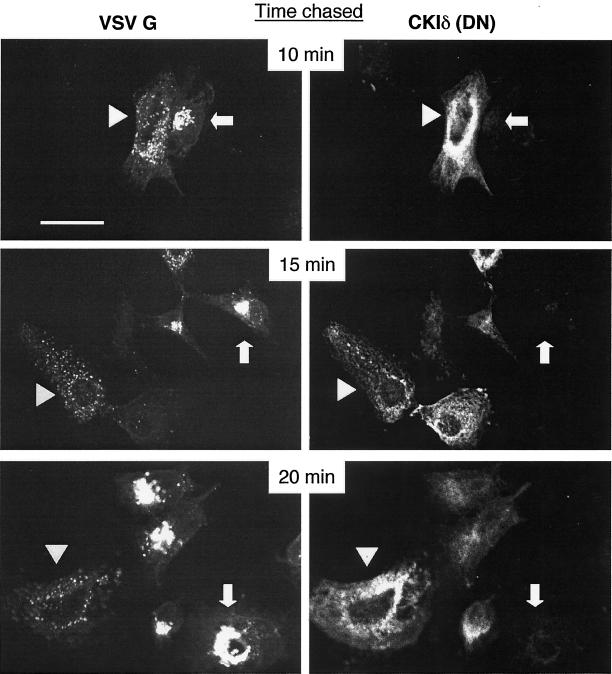
Figure 9.
The movement of VSV G glycoprotein from ER to Golgi is inhibited in cells transfected with a dominant negative CKI. COS-1 cells were transfected with a plasmid expressing CKIδ (DN) and were subsequently infected with VSV tsO45 virus. The tsO45 G protein was expressed at 39°C and then cells were shifted to 32°C to allow the G protein to move to the Golgi. The presence of transfected CKIδ (DN) and the G protein was monitored by immunofluorescence. The expression of VSV G glycoprotein is shown on the left panels. The expression of the transfected CKIδ (DN) is shown on the right panels. Arrowheads indicate cells expressing CKIδ (DN) at levels that inhibit transport of VSV G tsO45. Arrows indicate cells expressing tsO45 and little or no CKIδ (DN). Scale bar, 25 μm. The images shown are representative of two experiments.
Protein Expression and Purification
Casein Kinase I (CKIδ; Cat. no. 6030), cAMP-dependent protein kinase (PKA; Cat. no. 6000), and Casein Kinase II (CKII; Cat. no. 6011) were purchased from New England Biolab (Beverly, MA). A hexahistidine-tagged ARF GAP1 protein lacking 58 amino acids at the carboxy terminus (GAP359) was purified from bacteria by chromatography through a nickel resin column. Although the majority of the GAP359 protein formed inclusion bodies upon overexpression, protein remaining in the soluble fraction was more active than GAP359 protein that was purified under denaturing conditions, followed by dialysis to allow renaturation. GST-GAP132 and GST-GAP were overexpressed and purified from Sf9 or Sf900 cell cytosol by chromatography on glutathione sepharose 2 d after infection with recombinant baculovirus. The proteins were concentrated and frozen in 10% glycerol with protease inhibitors (Complete; Pierce Chemical Company, Rockford, IL). Myristoylated recombinant ARF was purified from bacteria according the method of Franco (Franco et al., 1995).
Binding of GAP359 or GST-GAP132 to Golgi-enriched Membranes
Golgi-enriched light membranes were isolated by sucrose step gradients as previously described (Balch et al., 1983; Ktistakis et al., 1996). Membranes were incubated with ARF GAP1 for 10 min and then centrifuged 7 min at 20,000 × g. The membrane pellet was analyzed by PAGE, and GAP proteins were detected by immunoblotting with anti–ARF GAP1 rabbit serum followed by enhanced chemiluminescence (Renaissance; NEN, Boston, MA). In reactions using cytosol, 10 μg of cytosolic protein was added. For binding reactions shown in Figure 9, 2 μl of IC261 stock solution at various concentrations in DMSO (at 100×) was added to a 200-μl reaction to give the indicated final concentration. Equal volumes of DMSO (carrier solvent) were added to reactions that contained no IC261. For the experiment shown in Figure 9B, 2 μg of the indicated antibody was first incubated with 10 μg of cytosol on ice for 15 min before this mixture was added to the binding reaction mixture. In the reaction in which a blocking peptide for CKIδ was used, the peptide was first incubated with the antibody against CKIδ for 15 min and then 10 μg of cytosol was added.
In Vitro Kinase Assay
The assay for CKI kinase activity contained 40 μl of 50 mM Tris, pH 7.5, 10 mM MgCl2, 5 mM DTT, 20 μM ATP (1 μCi/nmol [γ-32P]ATP), 0.125 mg/ml GAP359, and 10 U of CKIδ. After 10 min at 30°C, 40 μl of SDS-PAGE sample buffer was added to stop the reaction. Samples were boiled and analyzed by SDS-PAGE and phosphor imaging (Molecular Dynamics, Sunnyvale, CA). Identical procedures and units of the appropriate kinases were used in assays of PKA or CKII, with the exceptions that neither assay contained DTT, and in the CKII assay, the reaction buffer contained 20 mM Tris, pH 7.5 and, 50 mM KCl.
In Vivo 32P Labeling of GFP-GAP273
CHO cells overexpressing the GFP-GAP273 were grown to 70% confluency, washed with DMEM lacking phosphate and pyruvate three times and then labeled with 200 μCi of inorganic 32P phosphates in 0.75 ml of DMEM lacking phosphate and pyruvate, supplemented with FBS that had been dialyzed to remove phosphates. For the experiments shown in Figure 4 cells were labeled 4 h. For the inhibition of phosphorylation activity by IC261, the drug was added at the indicated concentration 10 min before addition of the radioactive phosphate. Dephosphorylation of some samples of GFP-GAP273 was achieved by resuspending the immunoprecipitate in 200 μl of 10 mM Tris, pH 8.0, containing 300 U of bacterial alkaline phosphatase (Life Technologies, Rockville, MD) for 30 min at 37°C, with constant agitation before analysis by SDS-PAGE analysis.
Figure 4.
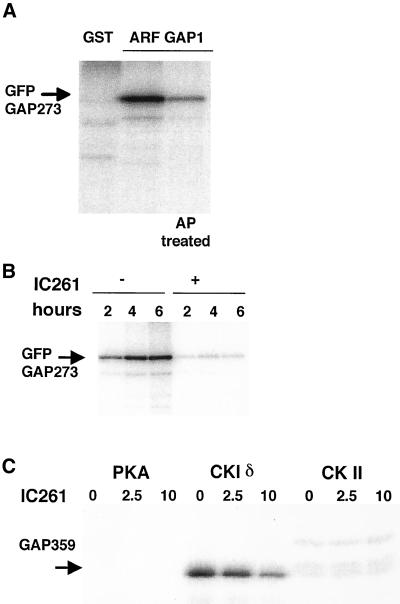
Casein kinase phosphorylates ARF GAP1. (A) CHO cells stably transfected with GFP-GAP273 were labeled with [32P]orthophosphate and GFP-GAP273 protein was purified from the cell lysate by immunoprecipitation. An irrelevant antibody, rabbit IgG against GST, was used to serve as control (lane 1). IgG against ARF GAP1 was added to samples run in lanes 2 and 3. The immunoprecipitate was treated with bacterial alkaline phosphatase (lane 3). (B) IC261 (200 μM) was added to the cells 5 min before the addition of labeling medium. Samples were labeled for the indicated time, then precipitated with anti–ARF GAP1 serum and analyzed by PAGE. A phosphorimage of the dried gel is shown. (C) Purified GAP359 was incubated with either protein kinase A, casein kinase Iδ, or casein kinase II in 0–10 μM IC261. Only CKIδ phosphorylated GAP359, and this was inhibited by IC261. Representatives of two or more experiments are shown.
RESULTS
We made fusion proteins in which either the small amino terminal catalytic domain of ARF GAP1 or sequences encoding 273 carboxyl-terminal noncatalytic amino acids of ARF GAP1 were joined to the carboxyl-terminus of GFP. These were expressed transiently in CHO cells, and their intracellular location was determined by imaging GFP fluorescence. Although the amino terminal catalytic domain (GFP-CAT), consisting of ∼150 amino acids, has been shown to bind to ARF and might therefore be expected to associate with ARF on membranes, GFP-CAT was present diffusely throughout the cell, suggesting that it was cytosolic. In contrast, the majority of the overexpressed protein containing the noncatalytic domain of ARF GAP1, GFP-GAP273, was concentrated on one side of the nucleus, in a pattern similar to that of endogenous ARF GAP1 (Figure 1A). Thus, the carboxyl-terminal noncatalytic domain of ARF GAP1 contains information to target the protein to the locations where ARF GAP1 functions. A deletion mapping experiment was conducted to identify a limited region of ARF GAP1 that was sufficient for membrane localization. A series of amino- and carboxyl-terminal truncations of the noncatalytic domain of ARF GAP1 were fused to GFP and expressed in stably transfected CHO cells. The patterns of fluorescence produced by these fusion proteins are shown in Figure 1B. Each of these proteins expressed at comparable levels in the transfected cell lines as measured by immunofluorescence (Figure 1) and by immunoblotting. A region of 132 amino acids from amino acid 203–334 was sufficient to target a fusion protein to membranes. By immunoblotting with antibody specific for GFP, we confirmed that the inability of GFP-GAP163 or GFP-GAP77 to produce a concentrated, perinuclear fluorescence was not due to detectable degradation of the GAP portion of the fusion proteins (unpublished results).
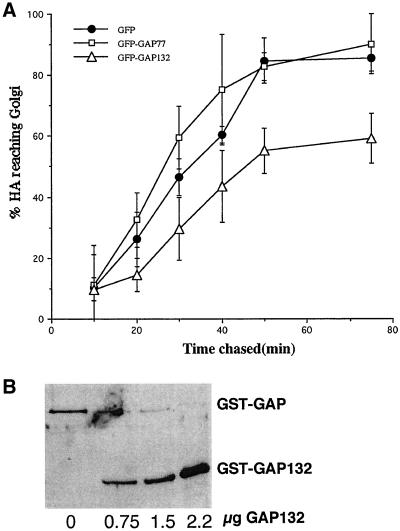
To determine if the Golgi binding of the GFP fusion proteins had functional consequences for the cell, we investigated the effect of overexpressing GFP-GAP132 in COS-1 cells on the ability of a reporter protein, the influenza virus hemagglutinin (HA), to move from the ER to the Golgi complex. Cotransfection of vectors expressing HA and GFP-GAP132 significantly slowed the rate of conversion of the high-mannose, ER form of HA to its complex-glycosylated, Golgi form (Figure 2A). In cells expressing GFP-GAP132, 50% of HA had not reached the Golgi complex even 75 min after synthesis. This effect was limited to expression of a fusion protein capable of binding to membranes. No inhibition of secretion was observed in cells coexpressing HA and GFP-GAP77, which cannot bind to membranes, compared with control cells coexpressing HA and unmodified GFP. Inhibition of HA transport by GFP-GAP132 or the larger GFP-GAP273 proteins required very high levels of overexpression in COS1 cells. A likely mechanism for this inhibition is competition between GFP-GAP132 and endogenous ARF GAP1 for membrane binding sites. We did not observe inhibition of HA transport in CHO cells stably expressing GFP-GAP132 or GFP-GAP273 at levels ∼1/10th that achieved in COS1 cells (unpublished data). It is possible that CHO cells expressing GFP-GAP proteins at inhibitory levels were selected against during the period when the transfected cells were expanded into colonies.
Figure 2.
GAP132 can compete with ARF GAP1 for binding to membranes. (A) Expression of GFP-GAP132 in COS-1 cells inhibits transport of the influenza virus HA into the Golgi as measured by a pulse-chase assay. The smaller GFP-GAP77 does not. Error bars designate the range of experimental values from two or three independent experiments. (B) GST-GAP132 competes with full-length GST-GAP for binding to membranes in vitro. Golgi-enriched membranes were incubated with 1.8 μg of GST-GAP and increasing amount of GST-GAP132. The amount of each protein that bound to membranes was determined by immunoblotting with anti-GST antibody.
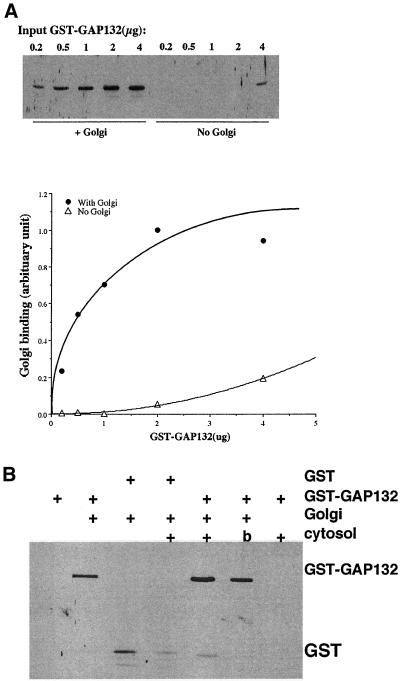
The shorter GST-GAP132 was capable of binding to Golgi-enriched membranes in vitro with characteristics similar to those of the full-length GAP. When a constant amount of GST-GAP (GST fused with the entire ARF GAP1) was coincubated with increasing amount GST-GAP132, GST-GAP132 competed for the binding sites on Golgi-enriched membranes (Figure 2B). When increasing amounts of GST-GAP132 were incubated with Golgi-enriched membranes, the membrane binding started to plateau at ∼1 μg of input GST-GAP132 protein, indicating that the binding of GST-GAP132 to membranes was saturable (Figures 3A). Moreover, when cytosol was included in the binding reaction, more GST-GAP132 bound to membranes (Figures 3B). Boiling cytosol before use prevented the stimulation of binding of GST-GAP132 to membranes, and cytosol did not cause GST-GAP132 to sediment in the absence of membranes. Cytosol did not increase the binding of GST alone to membranes (Figure 3B), indicating that the factor in cytosol acted on the ARF GAP1 portion of the chimeric protein. We consistently observed a two- to threefold enhancement of binding of GST-GAP132 to membranes in the presence of cytosol compared with binding in the absence of cytosol (unpublished data; see Figure 9B).
Figure 3.
Membrane binding by GST-GAP132 is saturable and enhanced by cytosol. (A) Golgi-enriched membranes were incubated with increasing amounts of GST-GAP132, and the protein that bound was quantified immunoblotting and densitometry. The image of a blot and the graph of the results of densitometry of the same blot are shown. (B) Cytosol stimulates the binding of GST-GAP132, but not GST, to membranes. b, boiled cytosol.
An important technical point in these experiments is that the cytosol preparation that we used was not dialyzed to remove small molecules, such as ATP. The carboxy terminus of ARF GAP1 is rich in serine and threonine residues, making it a good substrate for protein phosphorylation. Because genetic data from yeast suggested that a homolog of ARF GAP1 interacts in some way with yeast casein kinases, we tested the hypothesis that CKI could regulate the Golgi binding activity of ARF GAP1 by phosphorylation. When CHO cells overexpressing GFP-GAP273 were labeled with 32P orthophosphates, the immunopurified GFP-GAP273 protein was phosphorylated in vivo (Figure 4A). When an inhibitor of CKI was added to the cells before the addition of 32P label, the level of 32P label on GFP-GAP273 protein was significantly reduced (Figure 4B), suggesting that the activity of a CKI is required for at least some phosphorylation of GFP-GAP273 in vivo. To test if CKI can directly phosphorylate ARF GAP1, we performed an in vitro kinase assay using several commercially available kinases purified from bacteria (see MATERIAL AND METHODS) with recombinant GAP359 purified from bacteria as substrate. CKIδ could phosphorylate GAP359 in vitro, and its kinase activity could be inhibited by IC261 (Figure 4C) with an efficacy similar to reported values (Mashhoon et al., 2000). Despite the presence of numerous serine and threonine residues in the molecule, neither protein kinase A nor casein kinase II could phosphorylate GAP359 (Figure 4C). These results suggest that ARF GAP1 is a specific substrate of CKI.
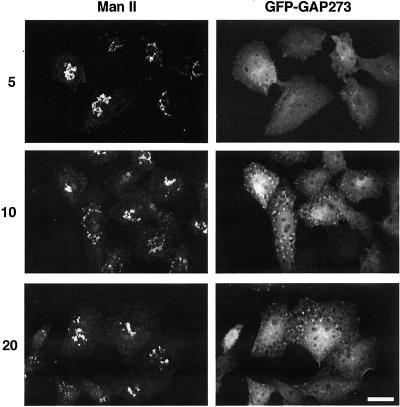
We tested the effect of inhibiting CKI on membrane traffic and on the subcellular localization of GFP-GAP273 in vivo. CHO cells were infected with either influenza virus or with VSV tsO45, which expresses a temperature-sensitive glycoprotein that remains in the endoplasmic reticulum at 39°C but folds and is exported to the Golgi at 32°C. After an interval to allow the viral glycoproteins to be made, the cells were treated with 50 μM of IC261 or with an equal concentration of DMSO carrier. In the presence of IC261, transport of both viral glycoproteins from the ER to the Golgi complex was inhibited (Figure 5, A and B). This result is consistent with the possibility that CKI activity is required for membrane protein traffic. Under the same conditions, both the endogenous ARF GAP1 and GFP-GAP273 started to dissociate from the Golgi complex (Figure 6). At higher concentration (200 μM), GFP-GAP273 fluorescence was diffusely present throughout the cells with some punctate fluorescence (Figure 6). The nature of this more concentrated fluorescence is unknown but is not due to fragmentation of the Golgi. The Golgi-like fluorescence pattern of GFP-GAP273 rapidly became cytosolic when cells were treated with 200 μM IC261 for 5 min (Figure 7). At longer intervals (10 and 20 min), widely dispersed concentrated foci of GFP-GAP273 fluorescence appeared; however, the immunofluorescence pattern of a Golgi enzyme, mannosidase II, remained concentrated near the nucleus (Figure 7). Thus, CKI activity is required to maintain the Golgi localization of GFP-GAP273 in vivo. It is important to note that in vivo intracellular ATP concentrations are ∼3 mM. In experiments in vitro, 1 μM IC261 was effective in competing with 10 μM ATP for inhibiting CKIδ (Mashhoon et al., 2000). Thus, the concentrations at which IC261 caused changes in the intracellular location of GFP-GAP273 are those expected to be needed to inhibit CKIδ in living cells.
Figure 7.
A time course of the effect of IC261. IC261, 200 μM, was applied to CHO cells stably transfected with GFP-GAP273 for 5, 10, or 20 min. The addition of IC261 released GFP-GAP273 from the Golgi to the cytosol within 5 min. At 10 and 20 min GFP-GAP273 began to collect in fluorescent foci. IC261 did not appear to fragment the Golgi complex as staining of Golgi marker Mannosidase II remained perinuclear. The images shown are representative of three experiments. Scale bars, 10 μm.
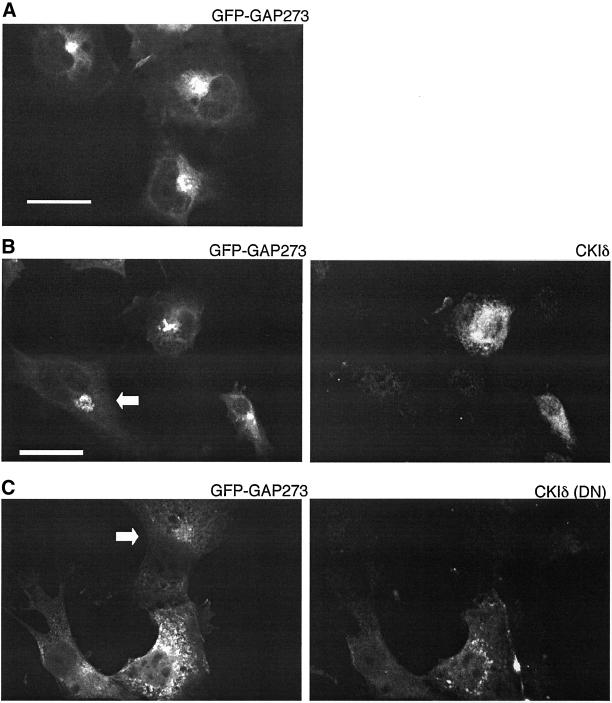
As a more specific test of the requirement for CKIδ activity for membrane binding of ARF GAP1, we cotransfected COS1 cells with GFP-GAP273 and either wild-type CKIδ or an catalytically inactive, dominant-negative mutant of CKIδ, CKIδ(DN) (McKay et al., 2001b). When expressed alone, GFP-GAP273 fluorescence was concentrated in a perinuclear, ribbon-like pattern characteristic of the labeling of Golgi membranes (Figure 8A). GFP-GAP273 fluorescence remained concentrated and perinuclear when that protein was coexpressed with wild-type CKIδ (Figure 8B) but became dispersed when coexpressed with the inactive mutant CKIδ (Figure 8C). Thus CKIδ kinase activity is required to maintain ARF GAP1 on Golgi membranes.
Figure 8.
Subcellular localization of GFP-GAP273 in COS-1 cells cotransfected with wild-type CKIδ or dominant negative CKIδ (DN). (A) COS-1 cells overexpressing only GFP-GAP273. (B) Cotransfection of GFP-GAP273 and CKIδ. GFP-GAP273 expression is shown on the left and CKIδ expression in the same cells on the right. The cell in the lower left corner was apparently transfected with GFP-GAP273 but not with CKIδ (arrow). (C) Cotransfection of GFP-GAP273 and CKIδ (DN). GFP-GAP273 expression (left panel). CKIδ (DN) expression in the same cells (right panel). The cell on the top was transfected with GFP-GAP273 but not with CKIδ (DN). Scale bar, 25 μm. The images shown are representative of two experiments.
If the dominant negative mutant CKI affected endogenous ARF GAP1 as it did GFP-GAP273, movement of proteins through the early secretory pathway should be inhibited. COS-1 cells were transfected with a plasmid encoding the dominant negative CKIδ and, after an interval to allow CKIδ to be expressed, were infected with VSV tsO45. Figure 9 shows the results of shifting cells from 39 to 32°C for various intervals and then preparing them for double immunofluorescence. After only 10 min at 32°C, cells expressing tsO45 G alone exhibited a concentrated, perinuclear labeling with antibody to G protein, showing that G had left the ER and reached the Golgi complex. In nearby cells expressing mutant CKIδ(DN), G staining was diffuse and punctate, indicating that the protein had not reached the Golgi. After 20 min, cells expressing the highest levels of mutant CKIδ(DN) still showed no labeling of Golgi with antibodies to G, whereas cells expressing less CKIδ(DN) began to show Golgi labeling. Thus, expression of a dominant negative CKIδ(DN) inhibited protein traffic in the early secretory pathway similar to the CKIδ inhibitor IC261
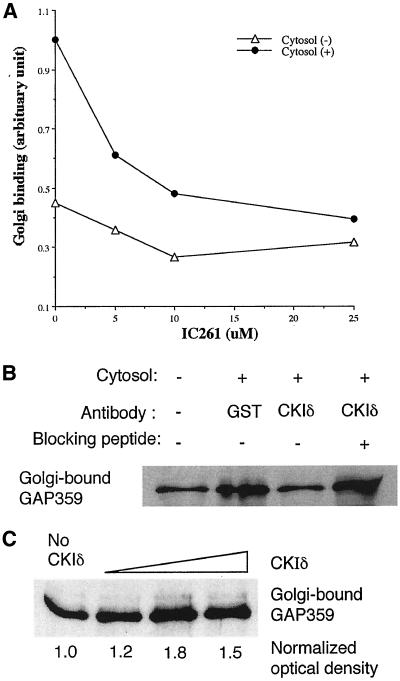
To determine if CKI contributes to the ability of cytosol to stimulate membrane binding by GST-GAP132 in vitro, an experiment similar to that presented in Figure 3A was conducted with increasing concentrations of IC261. The ability of cytosol to enhance membrane binding by GAP359 decreased with increasing concentrations of IC261 (Figure 10A). At 25 μM of IC261, the amount of GAP359 binding to membranes in the presence of cytosol was reduced to the level of that without cytosol, indicating that targets of IC261 account for most of the ability of cytosol to increase Golgi binding by GAP359. Because IC261 most potently inhibits CKIδ, we preincubated cytosol with antibody specific to CKIδ and found that this essentially eliminated the ability of cytosol to stimulate the binding of GAP359 to Golgi-enriched membranes (Figure 10B, lane 3). An irrelevant antibody raised against GST did not block the stimulation of membrane binding by GAP359 to membranes (Figure 10B, lane 2). When anti-CKIδ antibody was preincubated with a blocking peptide, the antibody no longer inhibited the ability of cytosol to stimulate membrane binding by GAP359 (Figure 10B, lane 4). Incubating GAP359 for 15 min with CKIδ before adding it to Golgi membranes increased the amount of GAP359 that bound to membranes (Figure 10C), and this increased binding also occurred when the phosphorylated GAP359 was added to membranes in the presence of inhibitory concentrations of IC261 (unpublished data).
Figure 10.
The effect of inactivating CKIδ on the binding of GAP359 to membranes. (A) Inhibition of CKIδ by IC261 reduced the ability of cytosol to stimulate the binding of GAP359 to membranes. (B) Neutralizing antibody to CKIδ decreased Golgi binding by GAP359 in the presence of cytosol. Antibody against GST did not show this effect and antibody against CKIδ that had been preincubated with CKIδ peptide was unable to block the ability of cytosol to enhance membrane binding by GAP359. (C) The phosphorylation of GAP359 by CKIδ enhanced the Golgi binding activity of GAP359. GAP359, 0.3 μg, was incubated with 0, 0.05, 0.2, and 1 U of CKIδ for 15 min, and then the Golgi binding assay was performed. Data are representative of two or more experiments.
DISCUSSION
ARF GAP1 localizes to Golgi membranes and is one of two ARF GAP proteins that have been identified to play a role in membrane traffic in the early secretory pathway (Dogic et al., 1999; Poon et al., 1999; Eugster et al., 2000). Previous work has indicated that ARF GAP1 binds to Erd2, the KDEL receptor responsible for returning escaped ER proteins to the ER (Aoe et al., 1997). ARF GAP1 will also bind to liposomes in the absence of other proteins, preferring membranes enriched in diacylglycerol (Antonny et al., 1997). In this report we document that there is another level of regulation of ARF GAP1. We have identified a region of ARF GAP1, amino acids 203–334, that is sufficient to target a fusion protein (GFP or GST) to Golgi membranes both in vivo and in vitro. Cytosol stimulated the Golgi binding activity of ARF GAP1 in vitro and a CKI-specific inhibitor, IC261, inhibited the binding of ARF GAP1 to membranes both in vivo and in vitro. Antibody specific for CKIδ antagonized the ability of cytosol to stimulate Golgi binding by GAP359, indicating that CKIδ is likely to be one of the CKI isoforms responsible. CKIδ phosphorylated ARF GAP1 in vitro, and phosphorylation increased the ability of ARF GAP1 to bind membranes. Therefore, at least one of the targets of CKIδ that regulates membrane binding of ARF GAP1 is ARF GAP1 itself. Although proteins such as ERD2, p24, and COPI probably play important roles in the incorporation of ARF GAP1 onto budding vesicles, our results suggest that the binding of ARF GAP1 to membranes is also regulated by CKIδ and presumably by an opposing protein phosphatase.
We do not know if the inhibition of protein traffic that occurs when cells are treated with IC261 or transfected with a dominant negative CKIδ is due entirely to an effect on ARF GAP1. In yeast, two related ARF GAP proteins, Gcs1p and Glo3p, have overlapping functions. Of these, Gcs1p is more similar in its noncatalytic sequences to ARF GAP1, but Glo3p appears to play a more major role in retrograde membrane traffic between the Golgi and ER (Dogic et al., 1999; Poon et al., 1999). The original observation of a functional link between YCK1 and YCK2 and GCS1 indicated that the kinases acted either in parallel or downstream of Gcs1p, because they suppressed the phenotype caused by a deletion of GCS1 (Wang et al., 1996). One possible mechanism for this suppression would be to increase or alter the activity of Glo3p so that it compensates for loss of Gcs1p. It will be of interest to see if the mammalian Glo3p ortholog is also phosphorylated by CKIδ and if phosphorylation also affects membrane binding.
The protein kinase A selective inhibitor H89 blocks protein traffic from the ER to the Golgi in vivo and inhibits the recruitment of COPII to ER exit sites in vitro by blocking the membrane binding of Sar1p (Aridor and Balch, 2000; Lee and Linstedt, 2000). We report that at least one other kinase, CKIδ, regulates constitutive membrane traffic between the ER and Golgi, presumably through its action on one or more ARF GAP proteins involved in the formation of COPI vesicles. The purpose of this level of regulation of constitutive membrane traffic is currently unknown and was not anticipated by current models of ARF function in secretion. We propose that this level of regulation modulates the rate of the process in response to changes in cellular physiology. Because the a role for casein kinases is conserved between yeast and mammals, at least some of this regulation must be quite basic, such as a response of the secretory pathway to varying nutrient levels, ER stress, or membrane cargo load. It is also likely that in mammalian cells regulation of constitutive secretion by kinases has expanded to additional levels of complexity. For example, all of the casein kinase isoforms can contribute to the Wnt signaling pathway (McKay et al., 2001a). It will be of interest to determine if there is an acute response of the early secretory pathway that is a component of the activation of extracellular signal transduction pathways.
ACKNOWLEDGMENTS
We thank Jon Graff (UT Southwestern) for providing CKI expression plasmids, Anthony Demaggio (ICOS Corp.) for providing IC261, and Katrina Latham and Maria Kosfiszer for their technical assistance. We thank our former colleague Dr. Kun Bi, and members of the laboratory of Paul Sternweis for providing various reagents and advice. A grant from the Texas-Affiliate of the American Heart Association, and grant GM37547 from the National Institutes of Health to M.G.R. supported this work.
Abbreviations used:
- ARF1
ADP-ribosylation factor. GAP, GTPase activating protein. CKI, casein kinase. HA, influenza virus hemagglutinin. GST, glutathione sulfo-transferase. GFP, green fluorescent protein. PKA, protein kinase A. IC261, [(2,4,6-trimethoxyphenyl)methylidenyl]-indolin-2-one)
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–04–0189. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–04–0189.
REFERENCES
- Antonny B, Huber I, Paris S, Chabre M, Cassel D. Activation of ADP-ribosylation factor 1 GTPase-activating protein by phosphatidylcholine-derived diacylglycerols. J Biol Chem. 1997;272:30848–30851. doi: 10.1074/jbc.272.49.30848. [DOI] [PubMed] [Google Scholar]
- Aoe T, Cukierman E, Lee A, Cassel D, Peters PJ, Hsu VW. The KDEL receptor, Erd2, regulates intracellular traffic by recruiting a GTPase-activating protein for Arf1. EMBO J. 1997;16:7305–7316. doi: 10.1093/emboj/16.24.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aridor M, Balch WE. Kinase signaling initiates coat complex II (COPII) recruitment and export from the mammalian endoplasmic reticulum. J Biol Chem. 2000;275:35673–35676. doi: 10.1074/jbc.C000449200. [DOI] [PubMed] [Google Scholar]
- Balch WE, Fries E, Dunphy WH, Urbani LJ, Rothman JE. Transport-coupled oligosaccharide processing in a cell-free system. Methods Enzymol. 1983;98:37–47. doi: 10.1016/0076-6879(83)98137-5. [DOI] [PubMed] [Google Scholar]
- Barr FA, Huttner WB. A role for ADP-ribosylation factor 1, but not COP I, in secretory vesicle biogenesis from the trans-Golgi network. FEBS Lett. 1996;384:65–70. doi: 10.1016/0014-5793(96)00285-2. [DOI] [PubMed] [Google Scholar]
- Chen YG, Siddhanta A, Austin CD, Hammond SM, Sung TC, Frohman MA, Morris AJ, Shields D. Phospholipase D stimulates release of nascent secretory vesicles from the trans-Golgi network. J Cell Biol. 1997;138:495–504. doi: 10.1083/jcb.138.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman E, Huber I, Rotman M, Cassel D. The ARF1 GTPase-activating protein: zinc finger motif and Golgi complex localization. Science. 1995;270:1999–2002. doi: 10.1126/science.270.5244.1999. [DOI] [PubMed] [Google Scholar]
- Dascher C, Balch WE. Dominant inhibitory mutants of ARF1 block endoplasmic reticulum to Golgi transport and trigger disassembly of the Golgi apparatus. J Biol Chem. 1994;269:1437–1448. [PubMed] [Google Scholar]
- Dogic D, de Chassey B, Pick E, Cassel D, Lefkir Y, Hennecke S, Cosson P, Letourneur F. The ADP-ribosylation factor GTPase-activating protein Glo3p is involved in ER retrieval. Eur J Cell Biol. 1999;78:305–310. doi: 10.1016/s0171-9335(99)80064-8. [DOI] [PubMed] [Google Scholar]
- Eugster A, Frigerio G, Dale M, Duden R. COP I domains required for coatomer integrity, and novel interactions with ARF and ARF-GAP. EMBO J. 2000;19:3905–3917. doi: 10.1093/emboj/19.15.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faundez VV, Kelly RB. The AP-3 complex required for endosomal synaptic vesicle biogenesis is associated with a casein kinase I alpha-like isoform. Mol Biol Cell. 2000;11:2591–2604. doi: 10.1091/mbc.11.8.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fensome A, Whatmore J, Morgan C, Jones D, Cockcroft S. ADP-ribosylation factor and Rho proteins mediate FMLP-dependent activation of phospholipase D in human neutrophils. J Biol Chem. 1998;273:13157–13164. doi: 10.1074/jbc.273.21.13157. [DOI] [PubMed] [Google Scholar]
- Franco M, Chardin P, Chabre M, Paris S. Myristoylation of ADP-ribosylation factor 1 facilitates nucleotide exchange at physiological Mg2+ levels. J Biol Chem. 1995;270:1337–1341. doi: 10.1074/jbc.270.3.1337. [DOI] [PubMed] [Google Scholar]
- Gaynor EC, Chen CY, Emr SD, Graham TR. ARF is required for maintenance of yeast Golgi and endosome structure and function. Mol Biol Cell. 1998;9:653–670. doi: 10.1091/mbc.9.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godi A, Pertile P, Meyers R, Marra P, Di Tullio G, Iurisci C, Luini A, Corda D, De Matteis MA. ARF mediates recruitment of PtdIns-4-OH kinase-beta and stimulates synthesis of PtdIns(4,5)P-2 on the Golgi complex. Nat Cell Biol. 1999;1:280–287. doi: 10.1038/12993. [DOI] [PubMed] [Google Scholar]
- Gross SD, Hoffman DP, Fisette PL, Baas P, Anderson RA. A phosphatidylinositol 4,5-bisphosphate-sensitive casein kinase I alpha associates with synaptic vesicles and phosphorylates a subset of vesicle proteins. J Cell Biol. 1995;130:711–724. doi: 10.1083/jcb.130.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu F, Aniento F, Parton RG, Gruenberg J. Functional dissection of COP-I subunits in the biogenesis of multivesicular endosomes. J Cell Biol. 1997;139:1183–1195. doi: 10.1083/jcb.139.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber I, Cukierman E, Rotman M, Aoe T, Hsu VW, Cassel D. Requirement for both the amino-terminal catalytic domain and a noncatalytic domain for in vivo activity of ADP-ribosylation factor GTPase-activating protein. J Biol Chem. 1998;273:24786–24791. doi: 10.1074/jbc.273.38.24786. [DOI] [PubMed] [Google Scholar]
- Jones DH, Morris JB, Morgan CP, Kondo H, Irvine RF, Cockcroft S. Type I phosphatidylinositol 4-phosphate 5-kinase directly interacts with ADP-ribosylation factor I and is responsible for phosphatidylinositol 4,5-bisphosphate synthesis in the Golgi compartment. J Biol Chem. 2000;275:13962–13966. doi: 10.1074/jbc.c901019199. [DOI] [PubMed] [Google Scholar]
- Kahn RA, Gilman AG. The protein cofactor necessary for ADP-ribosylation of Gs by cholera toxin is itself a GTP binding protein. J Biol Chem. 1986;261:7906–7911. [PubMed] [Google Scholar]
- Ktistakis NT, Brown HA, Waters MG, Sternweis PC, Roth MG. Evidence that phospholipase D mediates ADP ribosylation factor-dependent formation of Golgi coated vesicles. J Cell Biol. 1996;134:295–306. doi: 10.1083/jcb.134.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ktistakis NT, Kao CY, Wang RH, Roth MG. A fluorescent lipid analogue can be used to monitor secretory activity and for isolation of mammalian secretion mutants. Mol Biol Cell. 1995;6:135–150. doi: 10.1091/mbc.6.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TH, Linstedt AD. Potential role for protein kinases in regulation of bidirectional endoplasmic reticulum-to-Golgi transport revealed by protein kinase inhibitor H89. Mol Biol Cell. 2000;11:2577–2590. doi: 10.1091/mbc.11.8.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Yuan LC, Bonifacino JS, Klausner RD. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989;56:801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashhoon N, DeMaggio AJ, Tereshko V, Bergmeier SC, Egli M, Hoekstra MF, Kuret J. Crystal structure of a conformation-selective casein kinase-1 inhibitor. J Biol Chem. 2000;275:20052–20060. doi: 10.1074/jbc.M001713200. [DOI] [PubMed] [Google Scholar]
- McKay RM, Peters JM, Graff JM. The casein kinase I family in Wnt signaling. Dev Biol. 2001a;235:388–396. doi: 10.1006/dbio.2001.0308. [DOI] [PubMed] [Google Scholar]
- McKay RM, Peters JM, Graff JM. The casein kinase I family: roles in morphogenesis. Dev Biol. 2001b;235:378–387. doi: 10.1006/dbio.2001.0307. [DOI] [PubMed] [Google Scholar]
- Nickel W, Malsam J, Gorgas K, Ravazzola M, Jenne N, Helms JB, Wieland FT. Uptake by COPI-coated vesicles of both anterograde and retrograde cargo is inhibited by GTP-gamma-S in vitro. J Cell Sci. 1998;111:3081–3090. doi: 10.1242/jcs.111.20.3081. [DOI] [PubMed] [Google Scholar]
- Norman JC, Jones D, Barry ST, Holt MR, Cockcroft S, Critchley DR. ARF1 mediates paxillin recruitment to focal adhesions and potentiates Rho-stimulated stress fiber formation in intact and permeabilized Swiss 3T3 fibroblasts. J Cell Biol. 1998;143:1981–1995. doi: 10.1083/jcb.143.7.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi CE, Dell'Angelica EC, Bonifacino JS. ADP-ribosylation factor 1 (ARF1) regulates recruitment of the AP-3 adaptor complex to membranes. J Cell Biol. 1998;142:391–402. doi: 10.1083/jcb.142.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panek HR, Stepp JD, Engle HM, Marks KM, Tan PK, Lemmon SK, Robinson LC. Suppressors of YCK-encoded yeast casein kinase 1 deficiency define the four subunits of a novel clathrin AP-like complex. EMBO J. 1997;16:4194–4204. doi: 10.1093/emboj/16.14.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon PP, Cassel D, Spang A, Rotman M, Pick E, Singer RA, Johnston GC. Retrograde transport from the yeast Golgi is mediated by two ARF GAP proteins with overlapping function. EMBO J. 1999;18:555–564. doi: 10.1093/emboj/18.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini T, Orci L, Amherdt M, Brunner M, Kahn RA, Rothman JE. ADP-ribosylation factor is a subunit of the coat of Golgi-derived COP-coated vesicles: a novel role for a GTP-binding protein. Cell. 1991;67:239–253. doi: 10.1016/0092-8674(91)90176-y. [DOI] [PubMed] [Google Scholar]
- Shome K, Nie Y, Romero G. ADP-ribosylation factor proteins mediate agonist-induced activation of phospholipase D. J Biol Chem. 1998;273:30836–30841. doi: 10.1074/jbc.273.46.30836. [DOI] [PubMed] [Google Scholar]
- Singer WD, Brown HA, Sternweis PC. Regulation of eukaryotic phosphatidylinositol-specific phospholipase C and phospholipase D. Annu Rev Biochem. 1997;66:475–509. doi: 10.1146/annurev.biochem.66.1.475. [DOI] [PubMed] [Google Scholar]
- Stamnes MA, Rothman JE. The binding of AP-1 clathrin adaptor particles to Golgi membranes requires ADP-ribosylation factor, a small GTP-binding protein. Cell. 1993;73:999–1005. doi: 10.1016/0092-8674(93)90277-w. [DOI] [PubMed] [Google Scholar]
- Stearns T, Kahn RA, Botstein D, Hoyt MA. ADP ribosylation factor is an essential protein in Saccharomyces cerevisiae and is encoded by two genes. Mol Cell Biol. 1990a;10:6690–6699. doi: 10.1128/mcb.10.12.6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns T, Willingham MC, Botstein D, Kahn RA. ADP-ribosylation factor is functionally and physically associated with the Golgi complex. Proc Natl Acad Sci USA. 1990b;87:1238–1242. doi: 10.1073/pnas.87.3.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanigawa G, Orci L, Amherdt M, Ravazzola M, Helms JB, Rothman JE. Hydrolysis of bound GTP by ARF protein triggers uncoating of Golgi-derived COP-coated vesicles. J Cell Biol. 1993;123:1365–1371. doi: 10.1083/jcb.123.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Hoekstra MF, DeMaggio AJ, Dhillon N, Vancura A, Kuret J, Johnston GC, Singer RA. Prenylated isoforms of yeast casein kinase I, including the novel Yck3p, suppress the gcs1 blockage of cell proliferation from stationary phase. Mol Cell Biol. 1996;16:5375–5385. doi: 10.1128/mcb.16.10.5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney JA, Gomez M, Sheff D, Kreis TE, Mellman I. Cytoplasmic coat proteins involved in endosome function. Cell. 1995;83:703–713. doi: 10.1016/0092-8674(95)90183-3. [DOI] [PubMed] [Google Scholar]
- Zhang CJ, Cavenagh MM, Kahn RA. A family of Arf effectors defined as suppressors of the loss of Arf function in the yeast Saccharomyces cerevisiae. J Biol Chem. 1998;273:19792–19796. doi: 10.1074/jbc.273.31.19792. [DOI] [PubMed] [Google Scholar]
- Zhang CJ, Rosenwald AG, Willingham MC, Skuntz S, Clark J, Kahn RA. Expression of a dominant allele of human ARF1 inhibits membrane traffic in vivo. J Cell Biol. 1994;124:289–300. doi: 10.1083/jcb.124.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]