Abstract
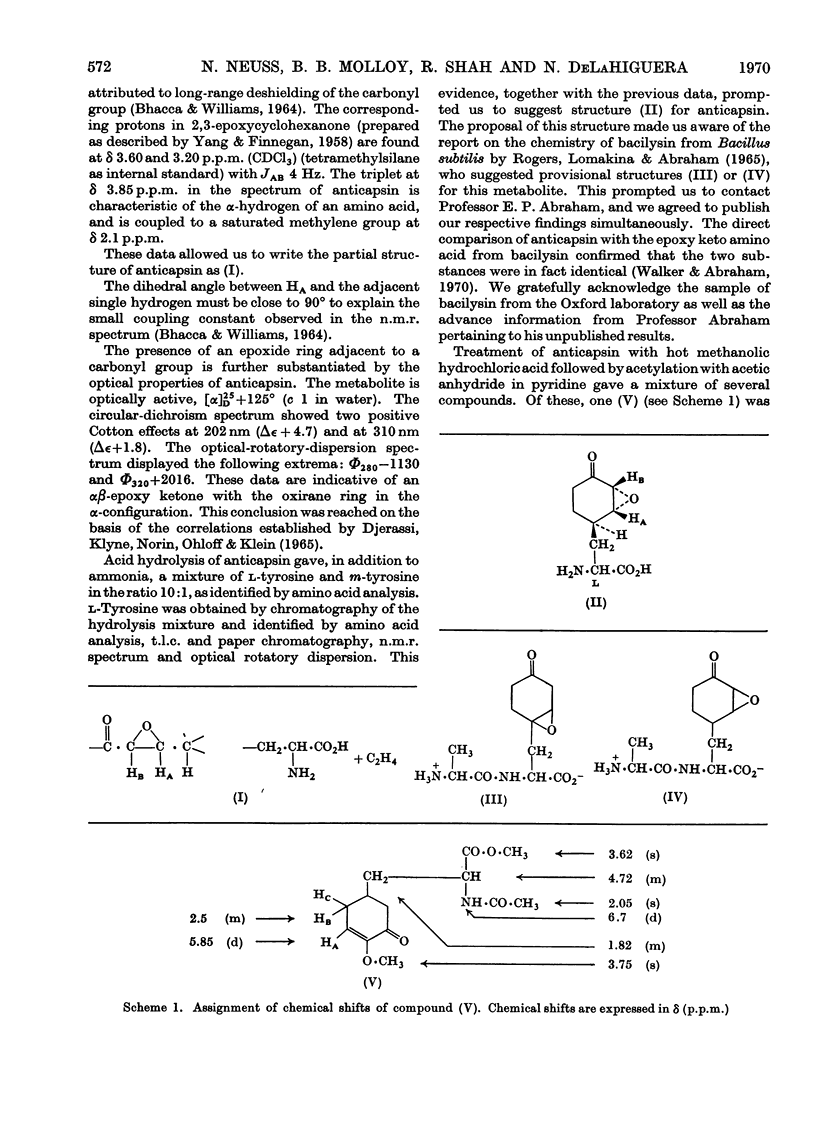
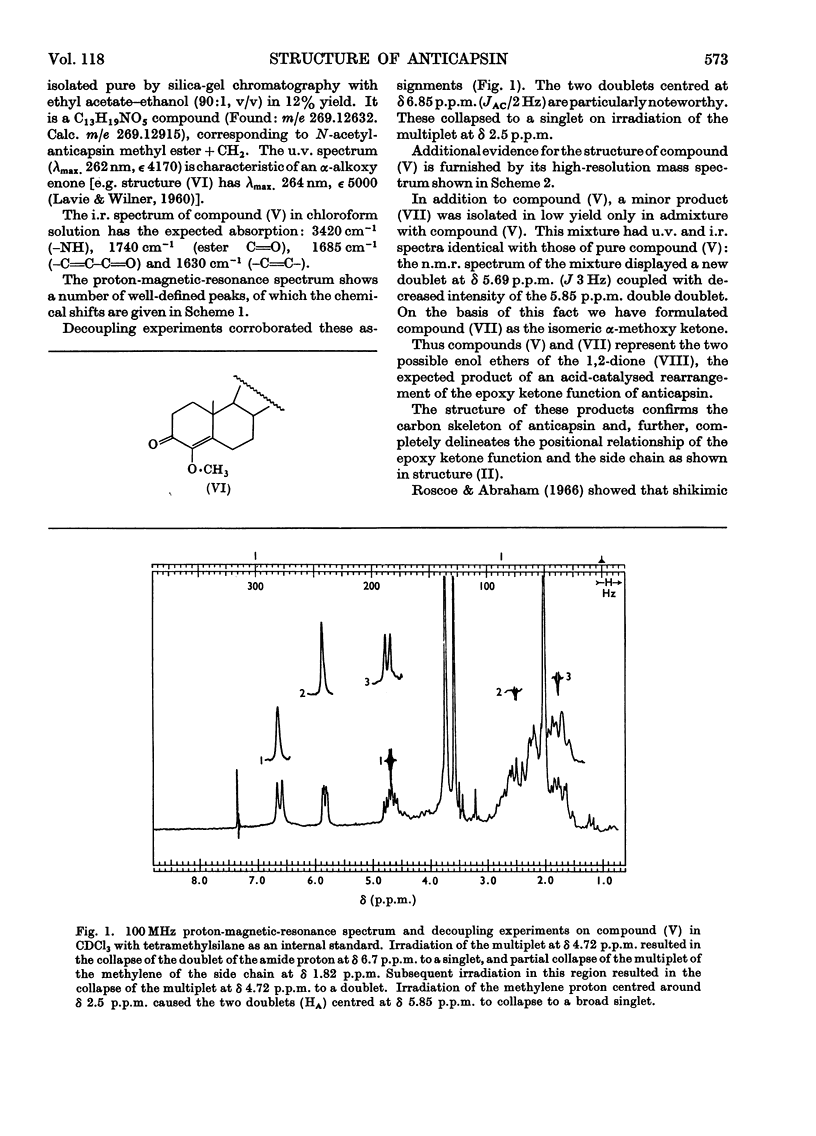
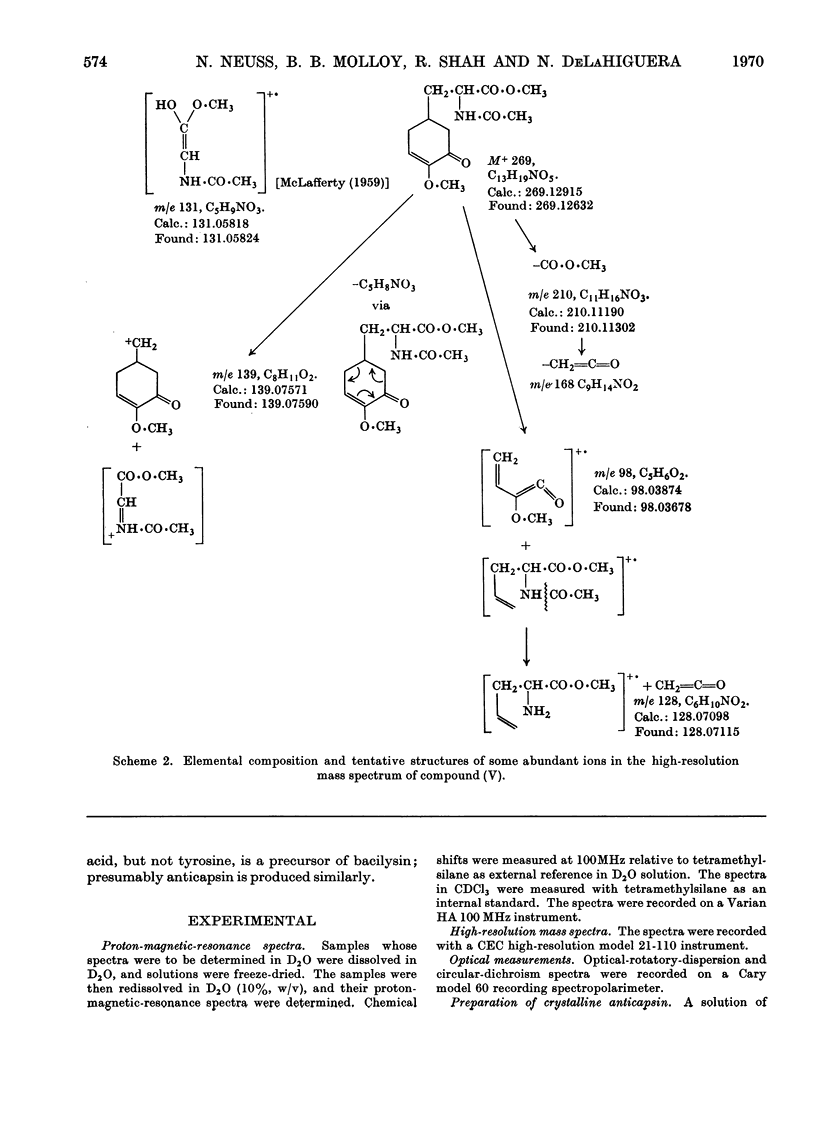
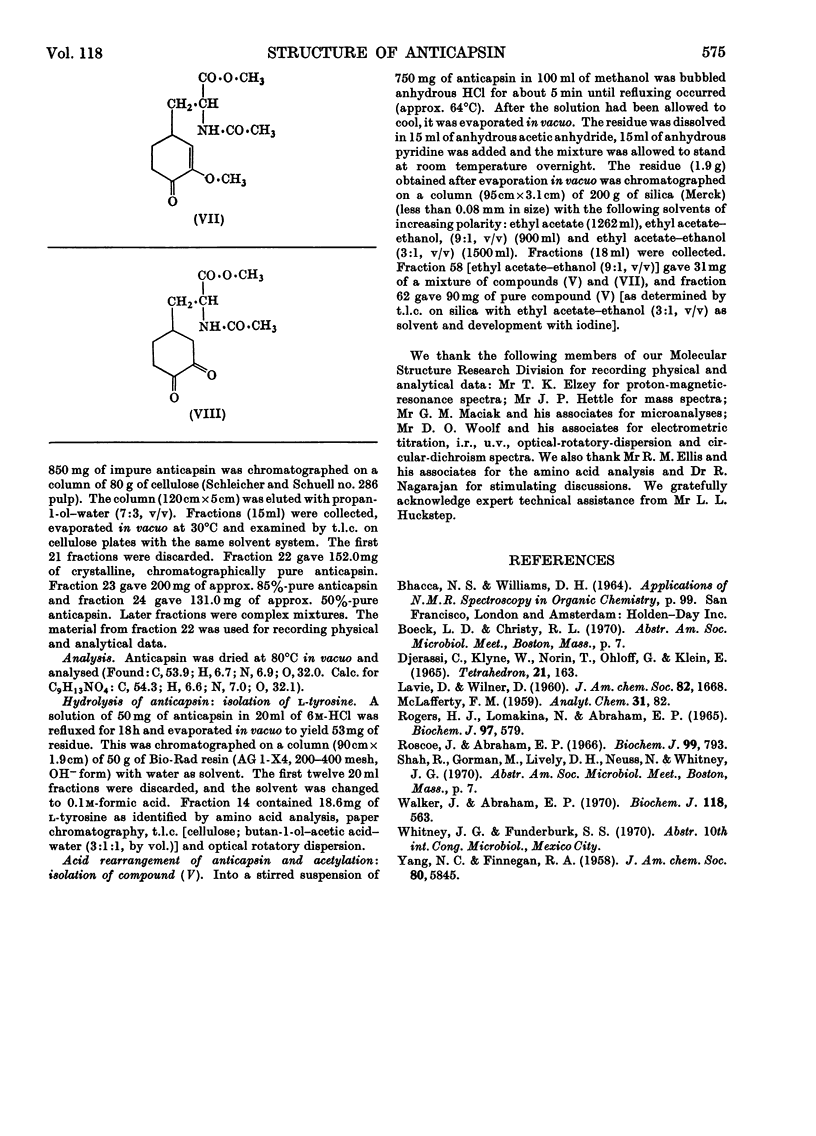
1. Physical and analytical data obtained on crystalline anticapsin indicated the empirical formula C9H13NO4. Spectral data (u.v., i.r. and proton magnetic resonance) and formation of l-tyrosine on hydrolysis revealed the functionalities and carbon skeleton of the new epoxy keto amino acid. 2. The optical properties of anticapsin (optical rotatory dispersion and circular dichroism) permitted assignment of absolute configuration to the new metabolite. 3. Treatment of anticapsin with hot methanolic hydrochloric acid followed by acetylation gave C18H19NO5, the α-alkoxycyclohexenone derivative. Analysis of the nuclear-magnetic-resonance and mass spectra of the latter allowed its structure to be determined and confirmed the assigned structure of anticapsin.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Rogers H. J., Lomakina N., Abraham E. P. Observations on the structure of bacilysin. Biochem J. 1965 Nov;97(2):579–586. doi: 10.1042/bj0970579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscoe J., Abraham E. P. Experiments relating to the biosynthesis of bacilysin. Biochem J. 1966 Jun;99(3):793–800. doi: 10.1042/bj0990793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. E., Abraham E. P. The structure of bacilysin and other products of Bacillus subtilis. Biochem J. 1970 Jul;118(4):563–570. doi: 10.1042/bj1180563. [DOI] [PMC free article] [PubMed] [Google Scholar]


