Abstract
Background
Restricting the placement of active ingredients (AIs) to specific panels on insecticide-treated nets (ITNs) has the potential to reduce the amount of AI required to treat a net. If the restricted placement of the AIs can exploit mosquito behaviour, particularly where they interact with the bed net interface, and not impact the net’s effectiveness, then the reduction in AI could result in cost reductions.
Methods
Nine individual experimental hut trials were conducted to compare the efficacy of three different partially-treated net relative to fully treated nets; roof-only treated nets, side-only treated nets, and nets with treated roof and pyrethroid-only side panels. These trials were conducted on a range of net products with different AIs, across a range of geographies in Africa (East and West), vector species (Anopheles gambiae, Anopheles coluzzii, Anopheles arabiensis, and Anopheles funestus), hut designs (East and West African style) and hosts (cows and humans). The combined data from these trials were analysed in a meta-analysis, and odds ratios for the effect of the different net designs on mortality and blood-feeding were estimated using mixed effects logistic regression.
Results
The results of this meta-analysis demonstrated that fully treated nets provide greater mosquito killing and reduction in blood-feeding effects than any configuration of insecticide treatment restricted to specific panels.
Conclusions
This meta-analysis showed that partially-treated net that restrict the insecticide treatment to specific panels of an ITN do not give equivalency or superiority in either mortality or blood-feeding inhibition to fully treated nets. The implications of these findings are discussed.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12936-025-05264-2.
Keywords: Malaria, Mosquito, Anopheles, Experimental hut study, Insecticide-treated net, Hybrid net, Partially-treated net
Background
Malaria control gains achieved in Africa over the past few decades, which are largely due to the scale-up of insecticide-treated bed nets (ITNs) and indoor residual spraying (IRS) [1], have recently stalled [2]. The reasons for this are multi-faceted, but a major challenge for malaria control programmes is the increase in the intensity and distribution of resistance, in the dominant malaria vectors, to the insecticide classes currently used. A push for innovation in this area, with the World Health Organization (WHO) encouraging the development of long-lasting insecticidal nets (LLINs), which target resistant mosquitoes [3], has led to the development and pre-qualification (PQ) of several next-generation ITNs [4], which contain pyrethroid insecticides plus an additional non-pyrethroid active ingredient (AI) or insecticide synergist. Optimizing the use of these AIs and novel AIs in development is vital in maintaining the long-term efficacy of new vector control tools.
Previous research has suggested the way Anopheles mosquitoes interact with bed nets is not uniform, with activity concentrated in key areas, such as the bed net roof and space above the bed net [5–8]. This is likely due to CO2, odour and heat convection plumes radiating from a host under the bed net. If this mosquito behaviour can be exploited, it could be possible to design nets to optimize AI placement and reduce the amount of AI needed per bed net. Some PQ-listed bed net products already have differing AIs on the panels, such as PermaNet 3.0 and Tsara Plus (both deltamethrin-only sides, deltamethrin + PBO roof). Further understanding of the effect of differentially treated bed net panels could potentially lead to improved cost-effectiveness, an expanded range of AIs suitable for bed nets previously deemed prohibitively expensive or, depending on placement, reduced insecticide exposure for ITN users.
A limited number of previous studies have been conducted on differentially treated nets, with varying results. A semi-field study of pyrethroid (lambdacyhalothrin 18 mg/m2) treated nets showed no significant difference in mortality between fully treated, roof-only treated, or side-only treated designs [9]. Mbewe et al. [10] observed that fully treated Interceptor®G2 (IG2) (alphacypermethrin + chlorfenapyr) bed nets were more efficacious against Anopheles arabiensis in a semi-field hut trial compared to roof-only IG2 treatment arms. This study was one of the trials included in this meta-analysis and will be discussed in further detail. A recent study [11] underlined the conclusions drawn by Murray et al. [12], and observed that insecticidal roof barriers mounted on untreated bed nets can be as effective against Anopheles gambiae sensu lato (s.l.) as insecticidal bednets.
There are limited data on how different net designs, particularly dual AI ITNs, perform against mosquito vectors. Therefore, this study aimed to systematically compare the entomological performance of nets with different combinations of treated and untreated panels against fully treated nets in several small-scale experimental hut studies against free-flying malaria vector mosquitoes. Several factors can influence mosquito interaction with an insecticide-treated net, including properties of the active ingredient on the net, the hut design and where mosquitoes enter, the host (human/animal), and the mosquito species. Therefore, several experimental hut studies were conducted in different geographical locations across Africa, with different dominant mosquito species, hosts, and active ingredients. The data synthesis aimed to explore whether it is possible to restrict the insecticide treatment to specific panels of an ITN without reducing protective efficacy.
Methods
Study sites
Data from 9 individual experimental hut trials are included in the multi-site analysis (Table 1). The trials were located in 4 countries in sub-Saharan Africa (Burkina Faso, Cameroon, Côte d’Ivoire, and Tanzania), and were conducted by 6 Trial Facilities (CRID, CSRS, IPR, IRSS, KCMUCo, and NIMR Mwanza), at 7 locations (Table 1). The ecological and geographical characteristics of each location are described below. The trials were conducted over 2 years, from 2020 to 2022. Each trial was powered to detect a difference in mosquito mortality between treatments based on the site-specific ecology (i.e., the expected number of mosquitoes entering a hut per night, the number of treatments used, and a Latin square rotation) and, therefore, the duration (collection nights) of each trial differed (Table 1).
Table 1.
Summary of individual experimental hut trials
| Country | Trial facility | Location | ITN | Start | End | Collections nights |
|---|---|---|---|---|---|---|
| Burkina Faso | IRSS | Vallée du Kou | IG2 | Sep-20 | Nov-20 | 72 |
| Cameroon | CRID | Mibellon | IG2 | Sep-21 | Nov-21 | 48 |
| Elende | IG2 | Feb-22 | April-22 | 42 | ||
| Vector Guard | Feb-22 | May-22 | 60 | |||
| Cote d’Ivoire | CSRS | Tiassalé | IG2 | Sep-21 | Mar-22 | 144 |
| Vector Guard | May-22 | Oct-22 | 128 | |||
| IPR | M’be | IG2 | Sept-21 | Dec-21 | 72 | |
| Tanzania | KCMUCo | Pasua | IG2 | Jun-21 | Sep-21 | 64 |
| NIMR Mwanza | Misungwi | IG2 | Oct-21 | Mar-22 | 108 |
Summary of the 9 individual experimental hut trials included in the multi-site analysis describing the country, trial facility, location, ITN products, start and end date, and number of collection nights
Burkina Faso
IRSS – Vallée du Kou
Vallée du Kou (between 4°24′59″ longitude west and 11°24′ latitude) is an irrigated rice growing area. The site is characterised by wooded savannah and contains seven discrete villages, with mean annual rainfall of ~1100 mm. Due to irrigation, the plain provides permanent Anopheles breeding sites. Mosquitoes are abundant all year round, with a peak in density observed from August to September during the rainy season. Anopheles coluzzii is the predominant vector and is highly resistant to pyrethroids and DDT (knockdown resistance (kdr) frequency: 0.8–0.95). The acetylcholinesterase insensitive Ace-1 [13, 14] and detoxifying enzymes and non-detoxification [15, 16] have also been documented in malaria vectors from this area.
Cameroon
CRID – Mibellon and Elende
Mibellon (6.46′N 11.70′E) is a village located in the Bankim Sub-division, of the Mayo Banyo Division, Adamawa region. It is situated near permanent water bodies, such as lakes and swamps, which provide suitable breeding sites for mosquitoes. The mosquito density is high all year round, and the local vector population consists mainly of Anopheles funestus sensu stricto (s.s.) (80%) with lower proportions of Anopheles gambiae s.s. (20%) [17]
Elende (3.41′N, 11.33′E) is a village located in the peri-urban locality of Nkolmefou I district, Centre region, near Cameroon’s capital city, Yaoundé. The vegetation around the village is predominantly made up of equatorial forest, which is being degraded for farming activities and infrastructure development. Environmental modification is creating both temporary and permanent breeding sites for malaria vectors. The local vector population consists mainly of An. funestus s.s. (85%) with lower proportions of An. gambiae s.s. (15%) [18].
Côte d’Ivoire
CSRS – Tiassalé
Tiassalé (5.54′N, 4.50′W) is a town in the Lagunes district in Southern Côte d’Ivoire. The experimental hut station is located near a large, irrigated rice field, which provides abundant mosquito breeding sites throughout the year. The major malaria vector here, An. gambiae s.l., is abundant and highly resistant to multiple insecticide classes, including pyrethroids, carbamates, organochlorines, and organophosphates [19–22].
IPR – M’bé
M’bé (7.97′N, 5.12′W) is located in central Côte d’Ivoire, ~40 km north of Bouaké city. The experimental huts are located in a large, irrigated rice valley. Anopheles gambiae s.l. is abundant all year round with An. coluzzii being the dominant vector species. The vectors are highly resistant to pyrethroids (kdr frequency: 0.8), and other insecticide classes, through multiple insecticide resistance mechanisms [23–25].
Tanzania
KCMUCo – Pasua
Pasua (3.22′S 37.20′E) is a village located in Lower Moshi, adjacent to the Lower Moshi rice irrigation scheme. The rice fields provide abundant breeding sites for the area’s dominant malaria vector, An. arabiensis. The vector population shows resistance to pyrethroids through elevated mixed-function oxidases and β-esterases [26]
NIMR Mwanza – Mwagagala
Mwagagala (3.07′S, 32.98′E) is a rural village located in Misungwi district. This locality has two rainy seasons; from October to December and from March to May. An. gambiae s.s., An. arabiensis and An. funestus s.s. are the main malaria vector species in the area. Adjacent to the experimental hut site, there is a large rain-fed rice irrigation scheme which provides potential breeding sites for malaria mosquito vectors. The vectors were resistant to deltamethrin (0.05%), and susceptible to bendiocarb (0.1%) and pirimiphos-methyl (0.25%).
Experimental hut trials
Preparation of net treatments
Several net types, i.e. with different AIs, were tested (Table 2). Different trials tested different combinations of net designs, encompassing nets with AI on all panels (‘fully treated nets’), nets treated with AI only on the roof panels (‘roof-treated nets’), and nets treated with AI only on the side panels (‘side-treated nets’). Roof-treated and side-treated nets are referred to as ‘partially-treated nets’. Additionally, some studies included extra treatment arms with a dual-AI-treated roof panel and pyrethroid-only side panels. A summary of all net treatments tested in each trial is listed in Table S1 (Additional file 1. supplementary tables). In each trial, partially-treated nets were prepared by separating the roof and side panels from whole nets and then re-joining them in different combinations by either resewing them or by attaching them to wooden frames. Standard operating procedures were followed in all trials to safeguard against cross-contamination between treatment arms whilst preparing the partially-treated nets.
Table 2.
Summary of active ingredients by net treatment
| Net brand | Active ingredient(s) |
|---|---|
| Untreated | No active ingredient |
| IG1 (Interceptor) | 200 mg/m2 alpha-cypermethrin |
| IG2 (Interceptor G2) |
100 mg/m2 alpha-cypermethrin 200 mg/m2 chlorfenapyr |
| ACM + PBO net | alphacypermethrin (5.8 g/kg) + 23.2 g/kg piperonyl butoxide (PBO) |
| ACM + PBO roof, ACM sides (Vector Guard) | alphacypermethrin (5.8 g/kg) + 23.2 g/kg piperonyl butoxide (PBO) |
| ACM + PBO roof, UN sides | alphacypermethrin (5.8 g/kg) + 23.2 g/kg piperonyl butoxide (PBO) |
Nets were unwashed and intentionally holed according to the WHO method [27], except for the trial conducted at KCMUCo. In the WHO method, 6 (4 × 4 cm) holes are cut in the net side panels; 2 each on the long sides, and 1 each on the short sides. At KCMUCo, 30 (4 × 4 cm) holes are cut in the net side panels; 9 each on the long sides, and 6 each on the short sides. The increase in holes is standard practice at KCMUCo, as this has been found to increase blood-feeding rates in the control arm, improving the trial’s power to detect a difference in blood-feeding inhibition between the test and control arms. In this analysis, test nets were compared to their corresponding fully treated net for each trial; therefore, the difference in holing practice is unlikely to have impacted the results.
Hut trials
The facilities adhered to the WHO guidelines for conducting experimental hut trials [27]. Any deviations from the WHO protocol are noted below. Due to the different geographical locations, site-specific practices, and net treatments, among other reasons, the individual trials differed on several important methodological characteristics, such as hut style, host, and dominant malaria vector species (Table 3).
Table 3.
Summary of site-specific experimental factors of each hut trial
| Trial (facility/treatment) | Hut style | Host | Dominant malaria vector | Number of treatments | Number of holes per treatment |
|---|---|---|---|---|---|
| IRSS IG2 | West | Cows | An. gambiae s.l. | 3 | 6 |
| KCMUCo IG2 | East | Cows | An. gambiae s.l. | 4 | 30 |
| CRID IG2 (Mibellon) | West | Humans | An. funestus s.l. | 4 | 6 |
| CRID IG2 (Elende) | West | Humans | An. funestus s.l. | 4 | 6 |
| CSRS IG2 | West | Humans | An. gambiae s.l. | 6 | 6 |
| IPR IG2 | West | Humans | An. gambiae s.l. | 4 | 6 |
| NIMR IG2 | East | Humans | An. gambiae s.l. | 6 | 6 |
| CRID Vector Guard | West | Humans | An. funestus s.l. | 4 | 6 |
| CSRS Vector Guard | West | Humans | An. gambiae s.l. | 4 | 6 |
Huts were constructed and prepared by following WHO guidelines [27]. Whether East or West African-style huts were used depended on the location of the trial facility. Hosts were either human participants or cows, depending on the anthropophilic (humans) or zoophilic (cows) feeding preference of the local dominant malaria vector species, to increase the number of mosquitoes attracted inside the huts. Net treatments and hosts were randomly allocated to the huts and were rotated using a Latin square. The four major malaria vectors in sub-Saharan Africa (An. gambiae s.s., An. coluzzii, An. funestus and An. arabiensis) were represented across the trial locations. Mosquitoes were identified morphologically. No molecular identification was conducted for these trials.
Net treatments were hung inside huts (studies with human hosts) or secured to wooden crates (studies with cow hosts). Hosts (humans or cows) entered huts in the evening and remained under the nets until the following morning. Cows were contained within wooden crates inside the huts. Mosquitoes were collected daily from inside the nets, inside the huts, inside the veranda, and inside the window traps (East African huts only). Mosquitoes were recorded by location as dead or alive and non-blood fed or blood-fed. Where samples were coded as gravid or semi-gravid, these were excluded from data analysis as it is not possible to know what treatments these mosquitoes had been subject to over multiple days of blood digestion. This resulted in 13% excluded (N = 5184), from the NIMR 1G2 dataset only. A total of N = 33,926 mosquitoes were included in the final meta-analysis. Live mosquitoes were provided with 10% sugar solution and monitored for 72 h to record delayed mortality.
Data analysis
The outcomes in this analysis were mortality (72 h) and blood feeding. Since the full data set was available for each study, an individual level meta-analysis was carried out (i.e. an analysis at the level of the mosquito). Odds ratios for the effect of net type were estimated using mixed effects logistic regression. Fixed effects were used for study, hut and host. In most cases where studies were combined, the active ingredient (AI) was constant within the study. Where this was not the case, a fixed effect for AI was included. A random effect for study night was included.
Results
Fully treated nets vs. roof-treated nets (all net types)
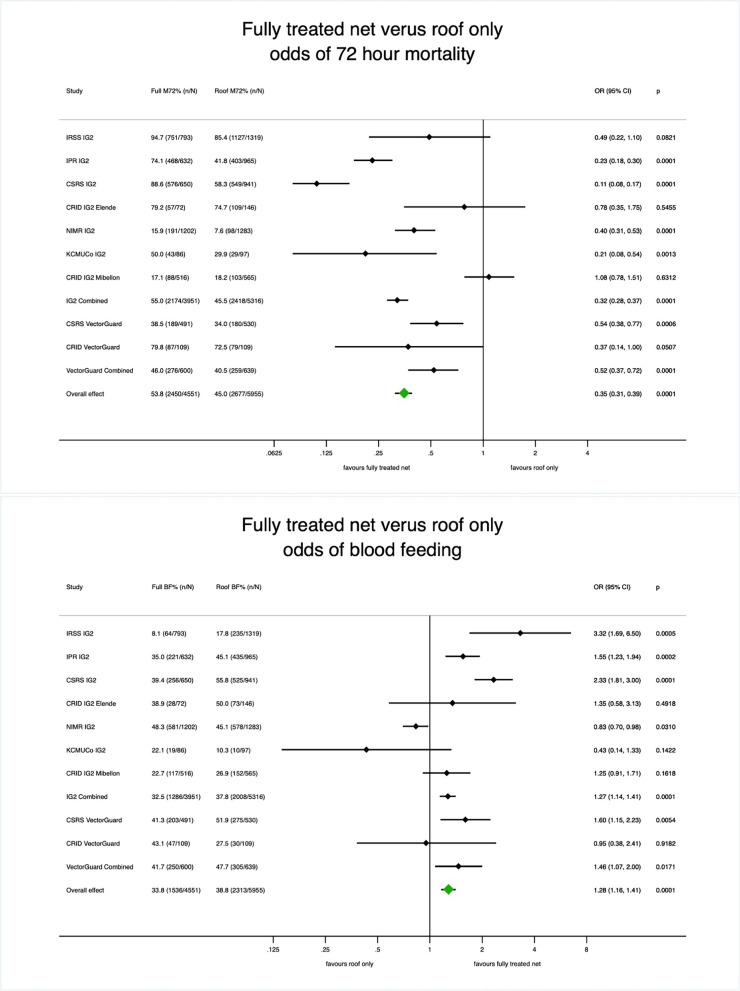
The 72-h mortality induced by the fully treated net, i.e. the positive control, ranged from 17.1% (Study; CRID Mibellon, IG2) to 94.7% (Study; IRSS, IG2). When all studies were included in the meta-analysis (Fig. 1), fully treated nets induced significantly higher 72-h mortality (53.8%, n = 4551 mosquitoes) compared to roof-treated (45.0%, n = 5955 mosquitoes) (OR = 0.35, 95% CI [0.31, 0.39]). At an individual study level, the magnitude of the effect varied by study. Fully treated nets induced significantly higher 72-h mortality relative to roof-treated when results were separated by net type for IG2 (OR = 0.32, 95% CI [0.28, 0.37]) and Vector Guard (OR = 0.52, 95% CI [0.37, 0.72]).
Fig. 1.
Mortality and blood-feeding of fully treated net vs. roof-treated net. Meta-analysis of odds ratios (OR) of the effect of net type (fully treated net vs. roof-treated net) on 72-h mortality (top) and blood feeding (bottom). Mixed effects logistic regression was used to estimate OR with the fully treated net as the reference
Blood-feeding in fully treated net arms ranged from 8.1% (Study; IRSS, IG2) to 48.3% (Study; NIMR, IG2). Fully treated nets also induced lower proportions of blood-feeding (33.8%, n = 4551 mosquitoes) compared to roof-treated (38.8%, n = 5955 mosquitoes) (OR = 1.28, 95% CI [1.16, 1.41]) when all studies were combined, although the magnitude of the effect varied at an individual study level (Fig. 1). Again, when separated by net type, lower blood feeding was observed for fully treated relative to roof-treated IG2 (OR = 1.27, 95% CI [1.14, 1.41]) and Vector Guard (OR = 1.46, 95% CI [1.07, 2.00]).
Fully treated IG2 vs. side-treated IG2
Combined analysis showed that fully-treated IG2 nets performed significantly better than side-treated nets (Fig. 2), inducing greater mortality (45.1%, n = 3158 mosquitoes) compared to side-treated (43.8% n = 3244 mosquitoes, OR = 0.80, 95% CI [0.69, 0.92]). Even though at an individual study level, only one of the six trials showed a significant difference (Study: CSRS IG2), the wide confidence intervals indicate a lack of precision in the estimates and the point estimate favours the fully treated net in most studies. Blood-feeding rates were also lower with the fully treated nets in the CSRS study (OR = 1.36, 95% CI [1.08, 1.73]). However, this was not significantly different when all studies were combined (Fig. 2).
Fig. 2.
Mortality and blood-feeding of fully treated net vs. side-treated net. Meta-analysis of odds ratios (OR) of the effect of net type (fully treated net vs. side-treated net) on 72-h mortality (top) and blood feeding (bottom). Mixed effects logistic regression was used to estimate OR with the fully treated net as the reference
Fully treated IG2 vs. roof IG2, side pyrethroid-only
Two studies examined the effect of fully treated IG2 nets compared to nets with IG2 roofs and pyrethroid-only sides (Fig. 3). The IG2 nets induced significantly higher 72-h mortality (41.4%, n = 1852 mosquitoes) compared to the nets with IG2 roofs and pyrethroid-only side panels (29.1%, n = 1941) (OR = 0.36, 95% CI [0.29, 0.44]). The results for blood-feeding were inconclusive (Fig. 3). The two trials differed in the direction of effect with the trial at CSRS showing a benefit of full IG2 treatment and the trial in NIMR demonstrating lower blood feeding with IG2 roofs and pyrethroid-only side panels nets.
Fig. 3.
Mortality and blood feeding of full IG2 net vs. roof IG2+ side pyrethroid-only net. Meta-analysis of odds ratios (OR) of the effect of net type (fully treated IG2 net vs. roof IG2+ side pyrethroid-only treated net) on 72-h mortality (top) and blood feeding (bottom). Mixed effects logistic regression was used to estimate OR
Fully treated ACM + PBO net vs. Roof ACM + PBO, side ACM-only (VectorGuard)
No significant difference in 72-h mortality was observed when comparing fully treated alphacypermethrin (ACM) + PBO nets with ACM + PBO roofs and ACM-only side nets i.e., VectorGuard (Fig. 4), in both trials the confidence intervals were wide. Blood feeding was significantly higher in fully treated ACM + PBO nets (41.7%, n = 600 mosquitoes) compared to nets with ACM + PBO roofs and ACM-only sides (34.2%, n = 571) (OR = 0.55, 95% CI [0.40, 0.77]).
Fig. 4.
Mortality and blood-feeding of fully treated ACM + PBO net vs. Roof ACM + PBO, side ACM-only net. Meta-analysis of odds ratios (OR) of the effect of net type (fully treated ACM + PBO net vs. Roof ACM + PBO, side ACM-only net) on 72-h mortality and blood feeding. Mixed effects logistic regression was used to estimate OR
Discussion
This meta-analysis on multiple experimental hut trials compared the entomological efficacy of various hybrid-net designs. Nine individual experimental hut trials were conducted to compare the efficacy of different partially-treated net designs against fully treated nets; roof-treated nets, side-treated nets, and nets with dual AI-treated roof and pyrethroid-only side panels.
Due to the mode of action of some of the AI’s tested (i.e., chlorfenapyr), 72-h mortality was used as a primary outcome. While the magnitude of difference and statistical significance between individual trials varied, the meta-analysis showed a consistent trend between trials for significantly greater mortality in fully-treated nets than roof- and side-only treatments, and with IG2 roof + pyrethroid-only side net treatments. However, no significant difference in mortality was observed when comparing fully-treated ACM + PBO nets with ACM + PBO roof + ACM-only side nets (VectorGuard nets). While some individual trials measured no significant difference in mortality, the direction of effect tended to favour the fully treated net over a partially-treated net in all design options, with greater mortality induced by fully treated nets. In roof and side-treated trials, there was more likely to be a significant difference in mortality in roof-treated trials (5/9 studies), compared to side-treated trials (1/6 studies), suggesting that the mosquito mortality increases when a greater area of the comparator net was treated with AI. This underlines the hypothesis made by Mbewe et al. [10] that the total surface area of treatment, rather than specific placement, may have a greater impact on efficacy. It could also be due to the artificial holing of the nets, which due to standard practice [27] only occurred on the sides and not the roof panel. This simulates net damage under real life conditions, where holing more commonly occurs on the side panels, rather than the roof, due to normal user use [28–30]. However, this could introduce bias into the results, dependant on where mosquitoes contact the net when host-seeking and entering through holes to blood-fed. Future studies should consider (1) leaving nets unholed for measuring mortality only, or (2) holing both the roof and side panels for measuring blood-feeding, where the number of holes per panel is relative to their size.
Previous mosquito behavioural studies have observed mosquito activity to be concentrated on the bed net roof and space above the bed net [5–8, 31], this potential behaviour has not correlated with improved bed net efficacy in this study. One reason for this could be the sleeper’s position under the net, which was not standardized in this study, and could impact the way CO2, odour, and heat convection plumes radiate from the host, subsequently impacting mosquito host-seeking behaviour. Two of the trials included also used cow hosts, resulting in different odour and heat plumes to human hosts. Future behavioural studies could investigate how sleeper position affects how mosquitoes interact with the bed net interface. Additionally, Sutcliffe et al. [32] show that changes to indoor air movement and temperature affect the way mosquitoes interact with nets, with cross-drafts and warmer air potentially impacting how these plumes radiate, being the cause of this variation.
The mortality induced by the fully treated net (positive control) ranged from 17.1 to 94.7%, showing a larger variability than expected. The lower than anticipated induced mortality in the fully treated net for some of the hut trials requires further investigation.
The results for blood-feeding were more variable, however, the meta-analysis showed that blood-feeding was significantly greater with roof-treated compared to fully treated nets, suggesting fully treated nets offer greater personal protection by reducing the chances of blood-feeding. No significant difference in blood-feeding was observed in the meta-analysis comparing fully treated nets to side-treated nets, or the fully treated IG2 nets to IG2 roof + pyrethroid-only side net. Fully-treated ACM + PBO nets showed significantly greater blood-feeding compared with ACM + PBO roof + ACM-only side nets, suggesting a reversal of personal protection. However, only two studies looked at this comparison and one had large confidence intervals; therefore, these results should be interpreted cautiously.
Although this study was designed to cover a range of vector species and AIs, results may differ for other insecticides, particularly those with novel modes of action. Therefore, future testing of other AIs and products may be beneficial. There may also be ways to increase the interaction of mosquitoes with the roof with barrier designs [12]. Additionally, other ways to reduce the cost of nets through different net designs may be more effective. For example, composite nets constructed by alternating insecticide-incorporated filaments and untreated filaments, or bicomponent yarns, which could reduce the loading of an AI through innovative yarn construction, may be alternative approaches to reducing the costs of nets without significantly impacting on entomological efficacy.
The individual trials conducted in this study covered a range of geographies (East and West Africa), vector species (An. gambiae, An. coluzzii, An. arabiensis, and An. funestus), hut designs (east and west style) and hosts (cows and humans). However, the trials were not designed and powered to detect if these factors affected mosquito mortality (i.e., no study tested both East and West-style huts in one location). Therefore, it is impossible to ascertain if the variability observed in the results is due to these factors or an artefact of the different trials i.e. different airflow, mosquito entry and exit, and ITN surface area to hut volume ratio. Further testing to look at the impact of hut design on entomological endpoints is ongoing (Moore, Unpublished) but was out of this studies scope.
Conclusion
This meta-analysis of nine different hut studies across six trial facilities, using several partially-treated net designs and net types, showed that partially-treated net strategies that restrict the insecticide treatment to specific panels of an ITN do not give equivalency or superiority in either mortality or blood-feeding inhibition to fully treated nets.
Supplementary Information
Additional file 1. Supplementary tables.
Acknowledgements
We would like to thank the volunteer sleepers and mosquito collectors for their dedication and active participation in this study. We would like to acknowledge BASF (IG2) and DCT (VectorGuard) for supplying net materials.
Abbreviations
- AI
Active ingredients
- ACM
Alphacypermethrin
- IRS
Indoor residual spraying
- ITN
Insecticide-treated nets
- kdr
Knockdown resistance
- LLIN
Long-lasting insecticidal nets
- PBO
Piperonyl-butoxide
- PQ
Pre-qualification
- WHO
World Health Organization
- CRID
Centre for Research in Infectious Diseases (Cameroon)
- CSRS
Centre Suisse de Recherches Scientifiques (Côte d’Ivoire)
- IPR
Institut Pierre Richet (Côte d’Ivoire)
- IRSS
Institut de Recherche en Sciences de la Santé (Burkina Faso)
- KCMUCo
Kilimanjaro Christian Medical University College (Tanzania)
- NIMR
National Institute for Medical Research (Tanzania)
Author contributions
JS, DN, SJM, WO, GS, AM, NM, AD, KB, RN, BK, CW design of the study. BM, CE, KB, NM, BE, JM, SJM collected the data. JS and NL supervised the project. JB curated and analysed the data. SJM prepared the graphs. NL, JS, JB and SJM interpreted the data. NL drafted the manuscript. JS, SJM, JB contributed to manuscript preparation. All authors read and approved the final manuscript.
Funding
One study (CRID IG2 Elende) was funded by CRID. All other studies were funded by the Bill and Melina Gates Foundation (PR078BMA). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. JB and SJM were supported as part of the “Accelerate to Eliminate Malaria” program awarded to IVCC. This study is made possible by the generous support of the American people through the United States Agency for International Development (USAID). The “Accelerate to Eliminate Malaria” program is a five-year cooperative agreement funded by the U.S. Agency for International Development under Agreement No. 7200AA23CA000025, beginning October 2023. It is implemented by IVCC. The information provided in this manuscript is not official U.S. Government information and does not necessarily represent the views or positions of the U.S. Agency for International Development.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Declarations
Ethics approval and consent to participate
Prior to the start of all trials, ethical approval was obtained from: IRSS IG2; Ethic committee of IRSS (A017-2019/CEIRES). CRID IG2 Mibellon; LSTM, UK (Ref: 21-033) and Comité national d’éthique de la recherche pour la santé humaine, Cameroon (Ref: 2021/07/1372/CE/CNERSH/SP). CRID IG2 Elende; Comité national d’éthique de la recherche pour la santé humaine, Cameroon (Ref: 2021/07/1372/CE/CNERSH/SP). CSRS IG2; LSTM, UK (Ref: 21-033) and National ethics committee of Côte d’Ivoire (Ref: 069-21/MSHP/CNESVS-km). IPR IG2; LSTM, UK (Ref: 21-033) and Ivorian Ministry of Health (N° 12/MSHP/CNESVS-kp2021). NIMR IG2; LSTM, UK (Ref: 21-033) and NatHREC, National Institute for Medical Research, Tanzania (NIMR/HQ/R.8a/Vol. IX/3763). CRID Vector Guard; LSTM, UK (Ref: 21-077) and Comité national d’éthique de la recherche pour la santé humaine, Cameroon (Ref: N_2021/07/1372/CE/CNERSH/SP). CSRS Vector Guard; LSTM, UK (Ref: 21-077) and National ethics committee of Côte d’Ivoire (Ref: 201-21/MSHPCMU/CNESVS-km). KCMUCo IG2 & IVC-5743; The use of cows in experimental hut studies has been reviewed by the LSHTM Animal Welfare and Ethical Review Board (AWERB), and Tanzania’s National Institute for Medical Research (NIMR/HQ/R.8a/Vol.IX/1656).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:7572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. World malaria report 2022. Geneva: World Health Organization; 2022.
- 3.WHO. Global technical strategy for malaria 2016-2030. Geneva: World Health Organization; 2015.
- 4.WHO. Prequalified Vector Control Products. Geneva: World Health Organization; 2023.
- 5.Sutcliffe JF, Yin S. Behavioural responses of females of two anopheline mosquito species to human-occupied, insecticide-treated and untreated bed nets. Malar J. 2014;13:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lynd A, McCall PJ. Clustering of host-seeking activity of Anopheles gambiae mosquitoes at the top surface of a human-baited bed net. Malar J. 2013;12:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parker JEA, Angarita-Jaimes N, Abe M, Towers CE, Towers D, McCall PJ. Infrared video tracking of Anopheles gambiae at insecticide-treated bed nets reveals rapid decisive impact after brief localised net contact. Sci Rep. 2015;5:13392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker JEA, Angarita Jaimes NC, Gleave K, Mashauri F, Abe M, Martine J, et al. Host-seeking activity of a Tanzanian population of Anopheles arabiensis at an insecticide treated bed net. Malar J. 2017;16:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oxborough RM, Mosha FW, Matowo J, Mndeme R, Feston E, Hemingway J, et al. Mosquitoes and bednets: testing the spatial positioning of insecticide on nets and the rationale behind combination insecticide treatments. Ann Trop Med Parasitol. 2008;102(8):717–27. [DOI] [PubMed] [Google Scholar]
- 10.Mbewe NJ, Rowland MW, Snetselaar J, Azizi S, Small G, Nimmo DD, et al. Efficacy of bednets with dual insecticide-treated netting (Interceptor® G2) on side and roof panels against Anopheles arabiensis in north-eastern Tanzania. Parasit Vectors. 2022;15:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abbott AJ, Matope A, Jones J, Voloshin V, Towers C, Towers D, et al. Insecticidal roof barriers mounted on untreated bednets can be as effective against Anopheles gambiae as regular insecticide-treated bed nets. Sci Rep. 2023;12:22080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray GPD, Lissenden N, Jones J, Voloshin V, Toé KH, Sherrard-Smith E, et al. Barrier bednets target malaria vectors and expand the range of usable insecticides. Nat Microbiol. 2020;5:40–7. [DOI] [PubMed] [Google Scholar]
- 13.Dabiré KR, Diabaté A, Namontougou M, Djogbenou L, Kengne P, Simard F, et al. Distribution of insensitive acetylcholinesterase (ace-1R) in Anopheles gambiae s.l. populations from Burkina Faso (West Africa). Trop Med Int Health. 2009;14(4):396–403. [DOI] [PubMed] [Google Scholar]
- 14.Namountougou M, Simard F, Baldet T, Diabaté A, Ouédraogo JB, Martin T, et al. Multiple insecticide resistance in Anopheles gambiae s.l. populations from Burkina Faso, West Africa. PLoS ONE. 2012;7(11):e48412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toé KH, N’Falé S, Dabiré RK, Ranson H, Jones CM. The recent escalation in strength of pyrethroid resistance in Anopheles coluzzi in West Africa is linked to increased expression of multiple gene families. BMC Genomics. 2015;16:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Namountougou M, Soma DD, Kientega M, Balboné M, Kaboré DPA, Drabo SF, et al. Insecticide resistance mechanisms in Anopheles gambiae complex populations from Burkina Faso. West Africa Acta Trop. 2019;197: 105054. [DOI] [PubMed] [Google Scholar]
- 17.Menze BD, Wondji MJ, Tchapga W, Tchoupo M, Riveron JM, Wondji CS. Bionomics and insecticides resistance profiling of malaria vectors at a selected site for experimental hut trials in central Cameroon. Malar J. 2018;17:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nkemngo FN, Mugenzi LMJ, Terence E, Niang A, Wondji MJ, Tchoupo M, et al. Multiple insecticide resistance and Plasmodium infection in the principal malaria vectors Anopheles funestus and Anopheles gambiae in a forested locality close to the Yaoundé airport, Cameroon. Wellcome Open Res. 2020;5:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edi AVC, N’Dri BP, Chouaibou M, Kouadio FB, Pignatelli P, Raso G, et al. First detection of N1575Y mutation in pyrethroid resistant Anopheles gambiae in Southern Côte d’Ivoire. Wellcome Open Res. 2017;2:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ngufor C, Chouaïbou M, Tchicaya E, Loukou B, Kesse N, N’Guessan R, et al. Combining organophosphate-treated wall linings and long-lasting insecticidal nets fails to provide additional control over long-lasting insecticidal nets alone against multiple insecticide-resistant Anopheles gambiae in Côte d’Ivoire: an experimental hut trial. Malar J. 2014;13:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edi CV, Djogbénou L, Jenkins AM, Regna K, Muskavitch MAT, Poupardin R, et al. CYP6 P450 enzymes and ACE-1 duplication produce extreme and multiple insecticide resistance in the malaria mosquito Anopheles gambiae. PLoS Genet. 2014;10(3):e1004236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ranson H, Edi CVA, Koudou BG, Jones CM, Weetman D. Multiple-insecticide resistance in Anopheles gambiae mosquitoes, Southern Côte d’Ivoire. Emerg Infect Dis. 2012;18(9):1508–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oumbouke WA, Rowland M, Koffi AA, Alou LPA, Camara S, N’guessan R. Evaluation of an alpha-cypermethrin + PBO mixture long-lasting insecticidal net VEERALIN® LN against pyrethroid resistant Anopheles gambiae s.s.: an experimental hut trial in M’bé, central Côte d’Ivoire. Parasit Vectors. 2019;12:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koffi AA, Ahoua Alou LP, Adja MA, Chandre F, Pennetier C. Insecticide resistance status of Anopheles gambiae s.s population from M’Bé: a WHOPES-labelled experimental hut station, 10 years after the political crisis in Côte d’Ivoire. Malar J. 2013;12:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camara S, Koffi AA, Ahoua Alou LP, Koffi K, Kabran JPK, Koné A, et al. Mapping insecticide resistance in Anopheles gambiae (s.l.) from Côte d’Ivoire. Parasit Vectors. 2018;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matowo J, Kulkarni MA, Mosha FW, Oxborough RM, Kitau JA, Tenu F, et al. Biochemical basis of permethrin resistance in Anopheles arabiensis from Lower Moshi, north-eastern Tanzania. Malar J. 2010;9:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO. Guidelines for laboratory and field-testing of long-lasting insecticidal nets. Geneva: World Health Organization; 2013.
- 28.Smith S, Joshi U, Grabowsky M, Selanikio J, Nobiya T, Aapore T. Evaluation of bednets after 38 months of household use in northwest Ghana. Am J Trop Med Hyg. 2007;77:243–8. [PubMed] [Google Scholar]
- 29.Boussougou-Sambe ST, Awono-Ambene P, Tasse GCT, Etang J, Binyang JA, Nouage LD, et al. Physical integrity and residual bio-efficacy of used LLINs in three cities of the South-West region of Cameroon 4 years after the first national mass-distribution campaign. Malar J. 2017;16:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minta AA, Landman KZ, Mwandama DA, Shah MP, Eng JLV, Sutcliffe JF, et al. The effect of holes in long-lasting insecticidal nets on malaria in Malawi: results from a case-control study. Malar J. 2017;16:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gleave K, Guy A, Mechan F, Emery M, Murphy A, Voloshin V, et al. Impacts of dual active-ingredient bed nets on the behavioural responses of pyrethroid resistant Anopheles gambiae determined by room-scale infrared video tracking. Malar J. 2023;22:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sutcliffe JF, Yin S. Effects of indoor air movement and ambient temperature on mosquito (Anopheles gambiae) behaviour around bed nets: implications for malaria prevention initiatives. Malar J. 2021;20:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplementary tables.
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].