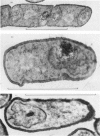
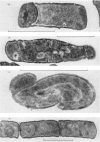
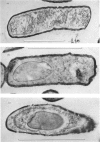
Abstract
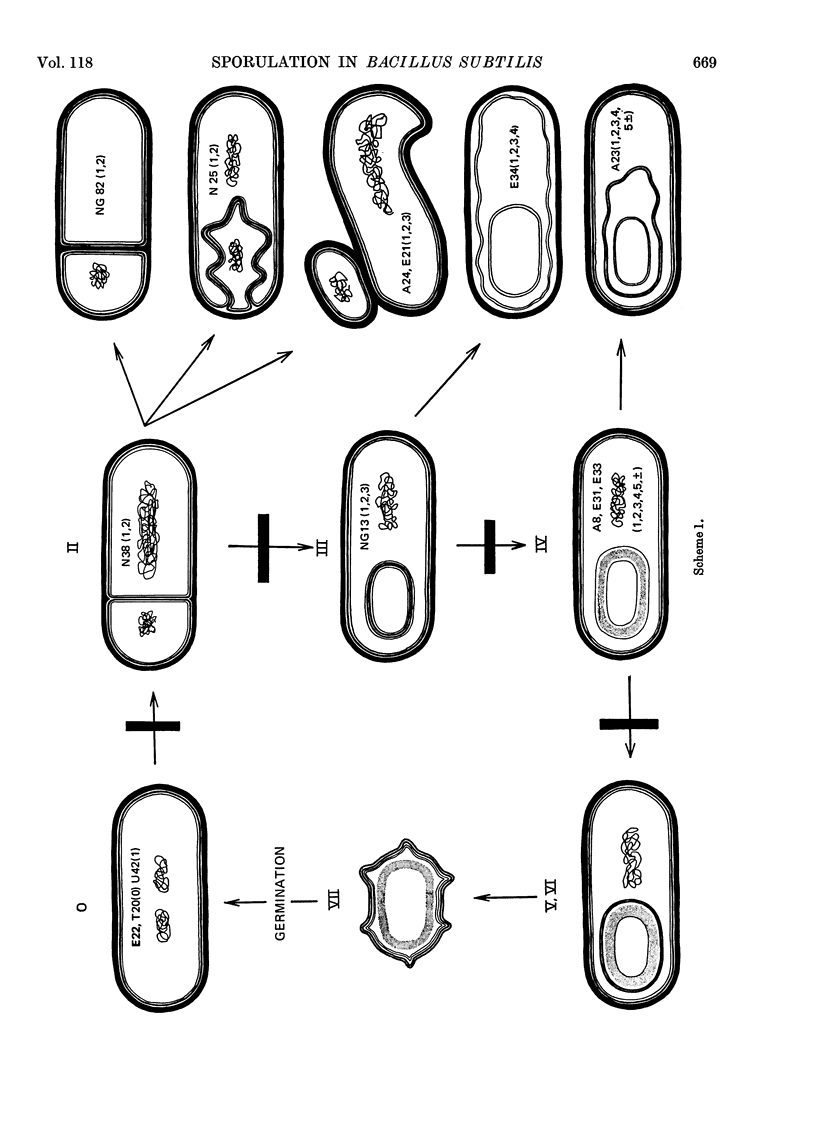
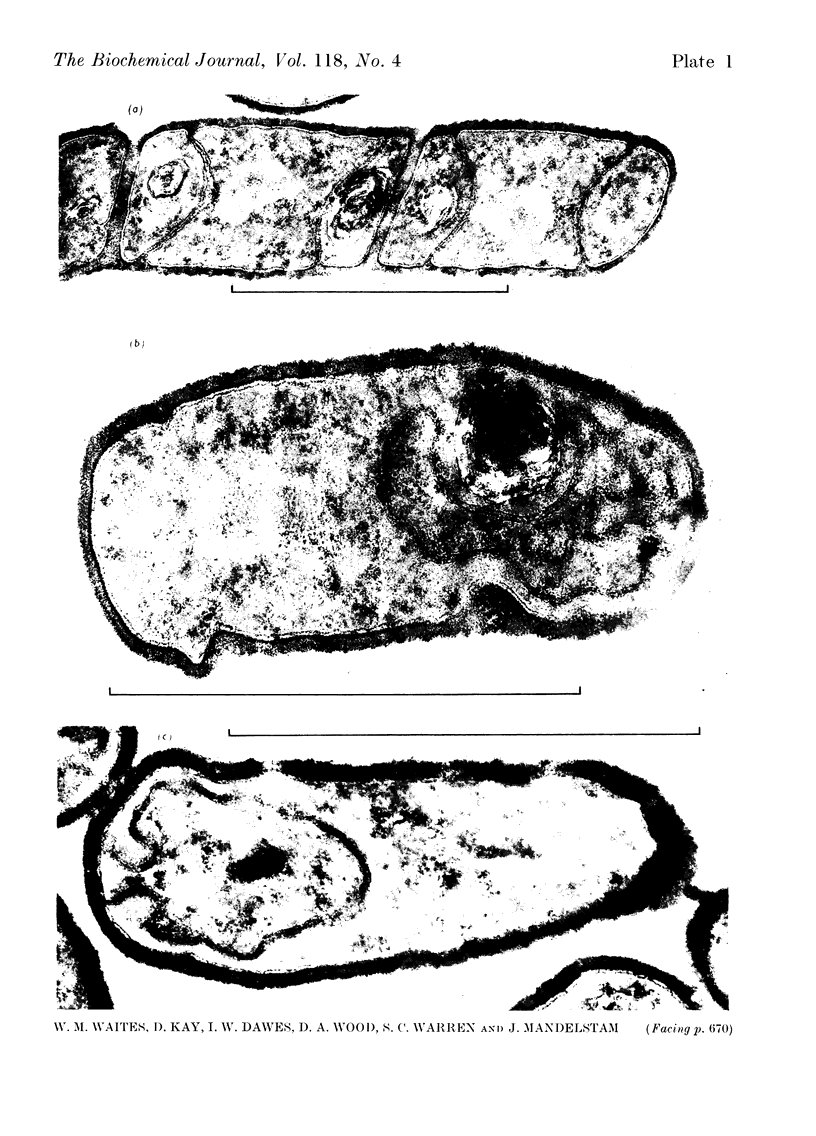
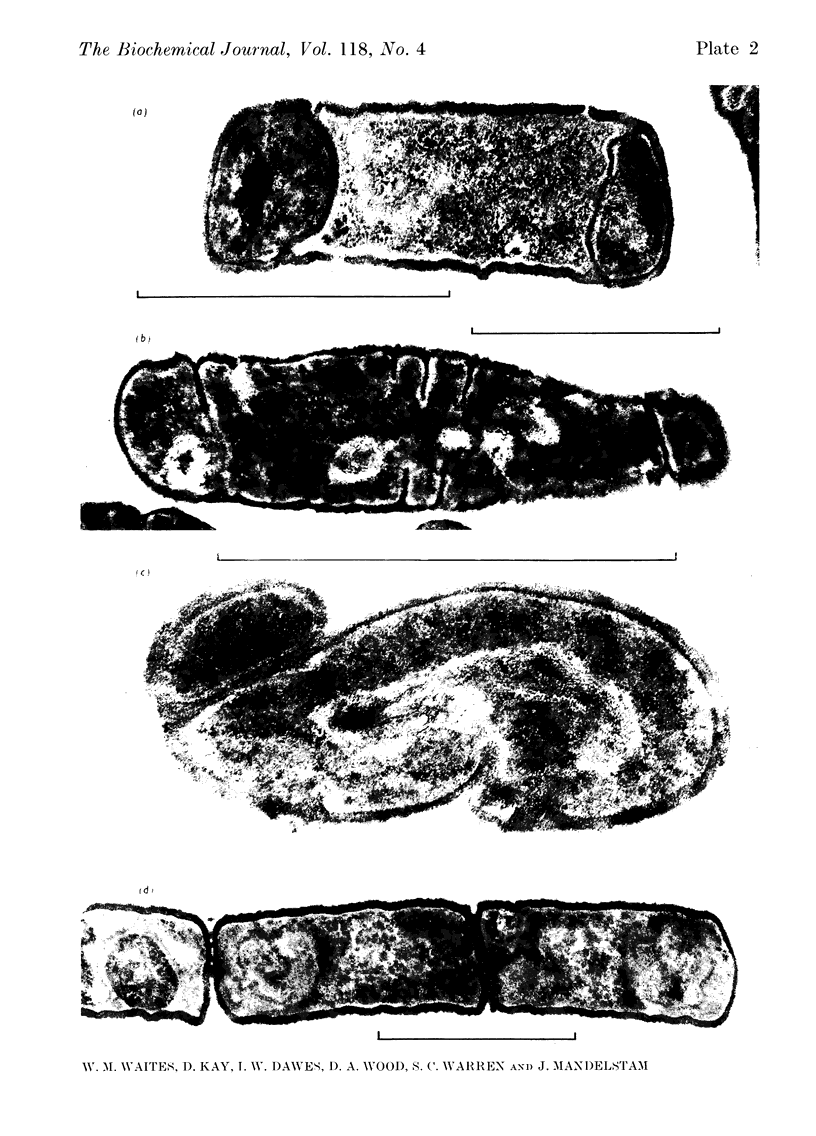
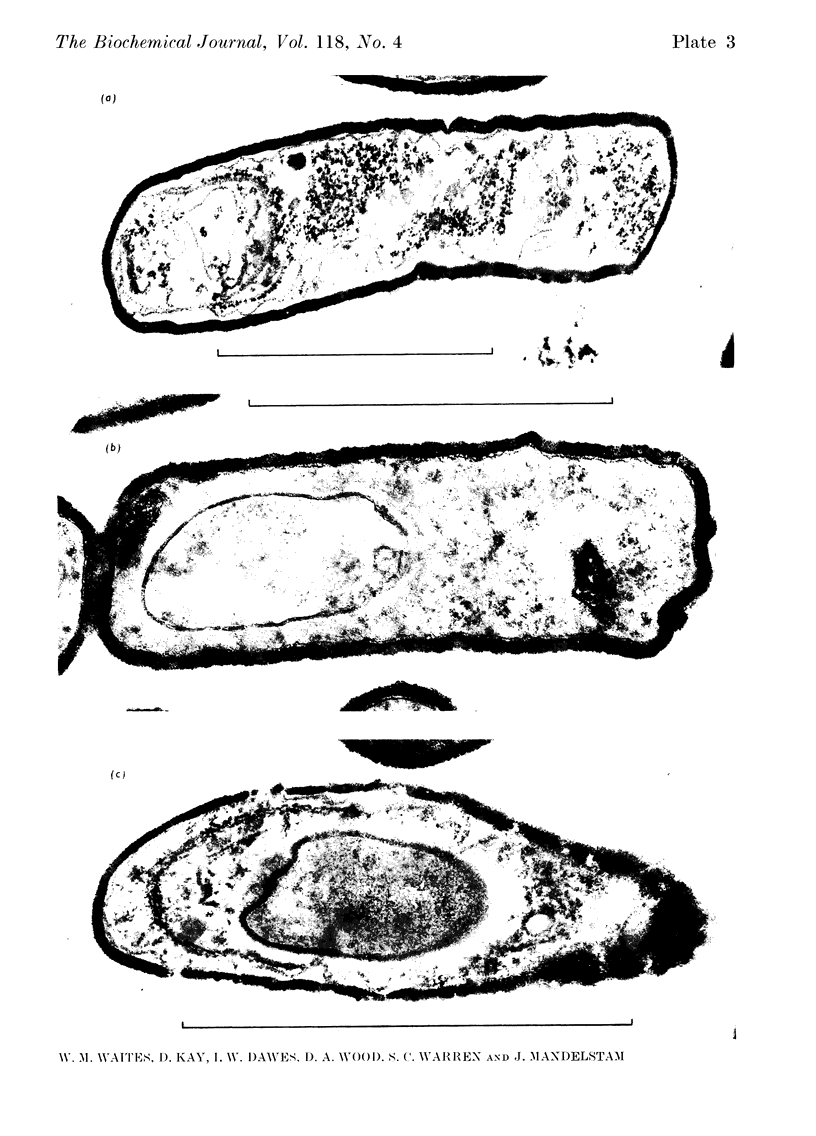
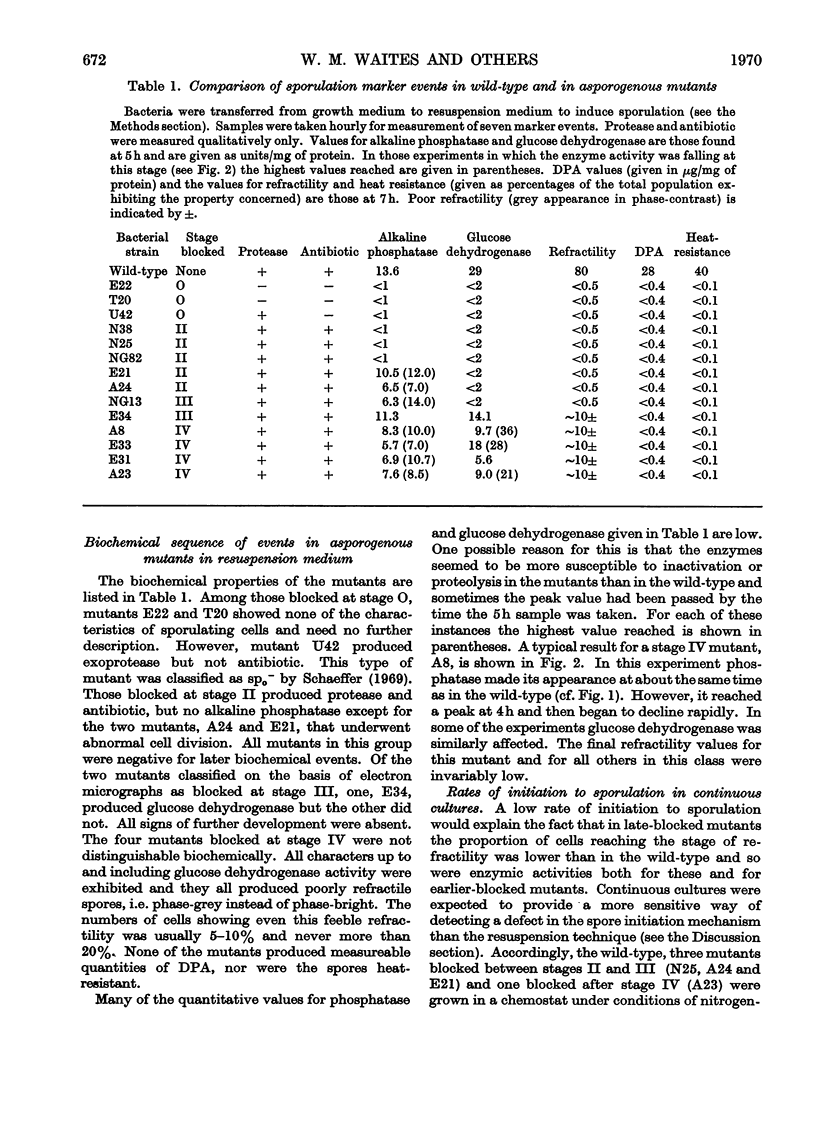
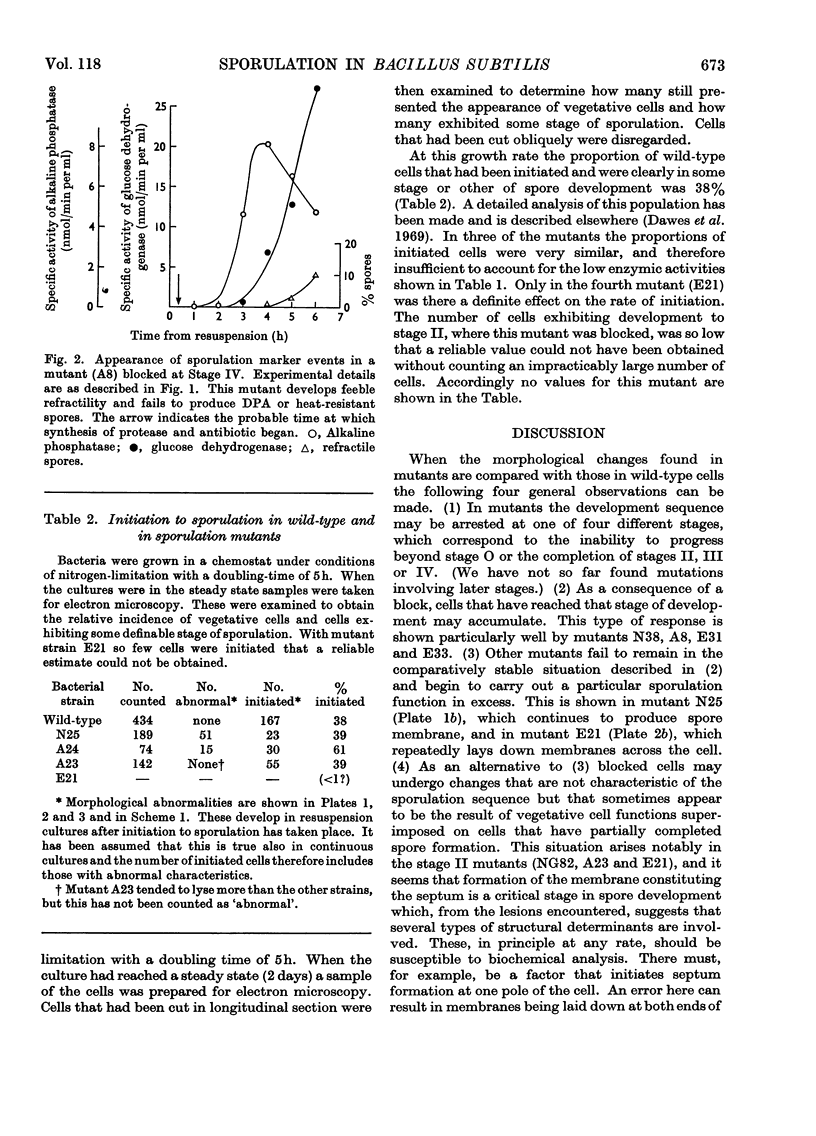
A comparison was made of morphological changes and successive, mainly biochemical, marker events for sporulation in 14 asporogenous mutants. The morphological and biochemical sequences are linked so that arrested development in one is accompanied by corresponding effects in the other. Thus mutants that fail to produce both protease and antibiotic do not progress beyond stage 0, formation of alkaline phosphatase appears to be associated with the transition from stage II to stage III and glucose dehydrogenase with that from stage III to stage IV. Stage II mutants may produce `pygmy' cells or other bizarre cell-division forms. The biochemical sequence is dependent in the sense that if the occurrence of any one event is blocked that of all the succeeding events is also blocked. This has implications for biochemical models that have been proposed to explain the temporal sequence observed in spore development.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONNELLAN J. E., Jr, NAGS E. H., LEVINSON H. S. CHEMICALLY DEFINED, SYNTHETIC MEDIA FOR SPORULATION AND FOR GERMINATION AND GROWTH OF BACILLUS SUBTILIS. J Bacteriol. 1964 Feb;87:332–336. doi: 10.1128/jb.87.2.332-336.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes I. W., Kay D., Mandelstam J. Sporulation in Bacillus subtilis. Establishment of a time scale for the morphological events. J Gen Microbiol. 1969 May;56(2):171–179. doi: 10.1099/00221287-56-2-171. [DOI] [PubMed] [Google Scholar]
- Kay D., Warren S. C. Sporulation in Bacillus subtilis. Morphological changes. Biochem J. 1968 Oct;109(5):819–824. doi: 10.1042/bj1090819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg A., Spudich J. A., Nelson D. L., Deutscher M. P. Origin of proteins in sporulation. Annu Rev Biochem. 1968;37:51–78. doi: 10.1146/annurev.bi.37.070168.000411. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Losick R., Sonenshein A. L. Change in the template specificity of RNA polymerase during sporulation of Bacillus subtilis. Nature. 1969 Oct 4;224(5214):35–37. doi: 10.1038/224035a0. [DOI] [PubMed] [Google Scholar]
- Mandelstam J., Waites W. M. Sporulation in Bacillus subtilis. The role of exoprotease. Biochem J. 1968 Oct;109(5):793–801. doi: 10.1042/bj1090793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouyard J. F., Ionesco H., Schaeffer P. Classification génétique de certains mutants de sporulation de Bacillus subtilis, Marburg. Ann Inst Pasteur (Paris) 1967 Nov;113(5):675–683. [PubMed] [Google Scholar]
- Ryter A., Szulmajster J. Action du chloramphénicol sur la sporogenèse de B. subtilis. Ann Inst Pasteur (Paris) 1965 May;108(5):640–651. [PubMed] [Google Scholar]
- STANIER R. Y. Enzymatic adaptation in bacteria. Annu Rev Microbiol. 1951;5:35–56. doi: 10.1146/annurev.mi.05.100151.000343. [DOI] [PubMed] [Google Scholar]
- SZULMAJSTER J. BIOCHIMIE DE LA SPOROG'EN'ESE CHEZ B. SUBTILIS. Bull Soc Chim Biol (Paris) 1964;46:443–481. [PubMed] [Google Scholar]
- Schaeffer P. Sporulation and the production of antibiotics, exoenzymes, and exotonins. Bacteriol Rev. 1969 Mar;33(1):48–71. doi: 10.1128/br.33.1.48-71.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterlini J. M., Mandelstam J. Commitment to sporulation in Bacillus subtilis and its relationship to development of actinomycin resistance. Biochem J. 1969 Jun;113(1):29–37. doi: 10.1042/bj1130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHASHI I. LOCALIZATION OF SPORE MARKERS ON THE CHROMOSOME OF BACILLUS SUBTILIS. J Bacteriol. 1965 Apr;89:1065–1067. doi: 10.1128/jb.89.4.1065-1067.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
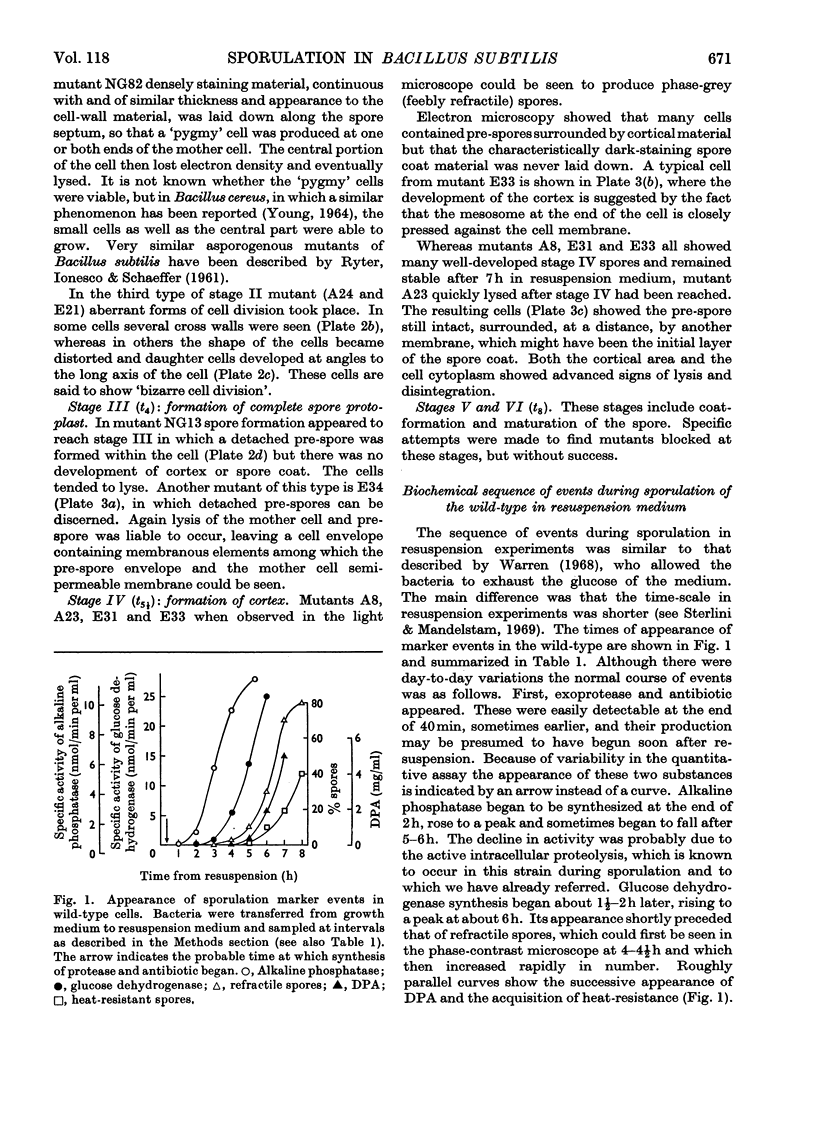
- Warren S. C. Sporulation in Bacillus subtilis. Biochemical changes. Biochem J. 1968 Oct;109(5):811–818. doi: 10.1042/bj1090811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG I. E. CHARACTERISTICS OF AN ABORTIVELY DISPORIC VARIANT OF BACILLUS CEREUS. J Bacteriol. 1964 Jul;88:242–254. doi: 10.1128/jb.88.1.242-254.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]