Abstract
SCD5 was identified as a multicopy suppressor of clathrin HC-deficient yeast. SCD5 is essential, but an scd5-Δ338 mutant, expressing Scd5p with a C-terminal truncation of 338 amino acids, is temperature sensitive for growth. Further studies here demonstrate that scd5-Δ338 affects receptor-mediated and fluid-phase endocytosis and normal actin organization. The scd5-Δ338 mutant contains larger and depolarized cortical actin patches and a prevalence of G-actin bars. scd5-Δ338 also displays synthetic negative genetic interactions with mutations in several other proteins important for cortical actin organization and endocytosis. Moreover, Scd5p colocalizes with cortical actin. Analysis has revealed that clathrin-deficient yeast also have a major defect in cortical actin organization and accumulate G-actin. Overexpression of SCD5 partially suppresses the actin defect of clathrin mutants, whereas combining scd5-Δ338 with a clathrin mutation exacerbates the actin and endocytic phenotypes. Both Scd5p and yeast clathrin physically associate with Sla2p, a homologue of the mammalian huntingtin interacting protein HIP1 and the related HIP1R. Furthermore, Sla2p localization at the cell cortex is dependent on Scd5p and clathrin function. Therefore, Scd5p and clathrin are important for actin organization and endocytosis, and Sla2p may provide a critical link between clathrin and the actin cytoskeleton in yeast, similar to HIP1(R) in animal cells.
INTRODUCTION
A major pathway for endocytosis involves the coat protein clathrin, comprised of heavy and light chains (HC and LC) and a large number of accessory factors. These proteins facilitate cargo capture, assembly of the clathrin lattice, membrane invagination, pinching off, and then uncoating of the vesicle once detached from the membrane. Many of these accessory proteins are held together by the cooperative interaction of different binding domains and appear to form a web beneath the cell surface (D'Hondt et al., 2000; Brodsky et al., 2001), although the role of the majority of these components and how they are regulated is still not completely understood.
Saccharomyces cerevisiae also contains clathrin HC and LC (Chc1p and Clc1p); however, there is only a partial dependence on clathrin for uptake at the cell surface (Payne et al., 1988; Tan et al., 1993; Huang et al., 1997). On the other hand, the actin cytoskeleton appears to be essential for endocytosis in yeast, as genetic screens to identify factors involved in internalization have repeatedly uncovered proteins important for function of the cortical actin cytoskeleton (for reviews see Baggett and Wendland, 2001; D'Hondt et al., 2000). Interestingly, many of these factors, or proteins that interact with these factors, are related to molecules that function in clathrin-mediated endocytosis in animal cells. For example, cortical actin patch components Rvs161p and Rvs167p are related to amphiphysins, which bind to clathrin HC, the heterotetrameric adaptor AP-2, and other regulatory components involved in clathrin-coated vesicle (CCV) formation in animal cells (David et al., 1996; McPherson et al., 1996; Ramjaun and McPherson, 1998; Slepnev et al., 2000). Eps15-homology (EH) domain proteins, Pan1p, End3p, and Ede1p were identified in screens for endocytosis mutants in yeast and found to be important for cortical actin structures (Raths et al., 1993; Bénédetti et al., 1994; Tang and Cai, 1996; Tang et al., 1997; Wendland and Emr, 1998; Gagny et al., 2000). Animal Eps15 has an AP-2 binding region and interacts with NPF motifs via the EH domain in proteins like epsin, which in turn has clathrin and AP-2 binding domains (Benmerah et al., 1996; Di Fiore et al., 1997; Iannolo et al., 1997; Chen et al., 1998; Drake et al., 2000). Yeast Pan1p is in a complex with End3p and another cortical actin patch component, Sla1p and has recently been shown to activate Arp2/3 complexes (Tang et al., 2000; Duncan et al., 2001; Zeng et al., 2001). This Pan1p function may couple actin polymerization to the endocytic machinery. Interestingly, Pan1p binds to NPF motifs in the yeast epsin-related proteins, Ent1/2p, and AP180/CALM-like proteins, Yap180p's, which are associated with cortical patches but also directly bind to clathrin HC in yeast (Wendland and Emr, 1998; Wendland et al., 1999). AP180/CALM promote the assembly of clathrin lattices in vitro and bind AP-2, and functional studies indicate that these proteins are also important components of the endocytic machinery in animal cells (McMahon, 1999; Traub et al., 1999).
A role for cortical actin in endocytosis in animal cells has been less clear, because actin-depolymerizing drugs, e.g., latrunculin A (Lat-A), disrupt clathrin-mediated endocytosis in some cell types or under some growth conditions but not in others (Fujimoto et al., 2000; Qualmann et al., 2000; Apodaca, 2001). Also, in some polarized epithelial cells such drug treatments inhibit internalization at the apical surface but not the basolateral membrane (Apodaca, 2001). Although actin may not be absolutely required for endocytosis in animal cells, other recent results support an important association of the actin cytoskeleton with clathrin-coated pits. Treatment of cells with Lat-A increases the lateral mobility of clathrin-coated pits at the cell surface, suggesting that cytoskeletal elements may localize the endocytic machinery to domains of the plasma membrane (Gaidarov et al., 1999; Bennett et al., 2001). In addition, a few proteins that associate with both the actin cytoskeleton and clathrin-coated pit components have been identified. Two of these factors, mAbp1p and HIP1/HIP1R, were first identified in yeast as actin-associated proteins Abp1 or Sla2p (also known as End4p) or in screens for endocytic mutants (Drubin et al., 1990; Holtzman et al., 1993; Raths et al., 1993). mAbp1 has an actin-binding region and a SH3 domain that also interacts with dynamin, and overexpression of the SH3 domain inhibits receptor-mediated endocytosis in animal cells (Kessels et al., 2001).
HIP1 was identified as a huntingtin interacting protein and is closely related to HIP1R (Kalchman et al., 1997; Wanker et al., 1997; Seki et al., 1998; Engqvist-Goldstein et al., 1999). These proteins, as well as Sla2p, contain an epsin N-terminal homology (ENTH) domain found in epsin and AP180/CALM, which may help to recruit these proteins to the plasma membrane by binding cell surface phosphoinositides (Ford et al., 2001; Itoh et al., 2001). The C terminus of the yeast and mammalian Sla2p-related proteins is homologous to the talin-like F-actin binding module (McCann and Craig, 1997). The central region of HIP1/HIP1R, which includes a coiled-coiled segment, has recently been shown to bind directly to clathrin and facilitates association with clathrin-coated pits (Engqvist-Goldstein et al., 2001; Metzler et al., 2001; Mishra et al., 2001; Waelter et al., 2001; Legendre-Guillemin et al., 2002). Although yeast Sla2p binds actin, shows partial colocalization with cortical actin patches, and is important for cortical actin organization and endocytosis (Holtzman et al., 1993; Raths et al., 1993; Wesp et al., 1997), no connection to clathrin-mediated trafficking has been reported.
In previous studies we identified the SCD5 gene as a multicopy suppressor of clathrin HC-deficient yeast (Nelson and Lemmon, 1993; Nelson et al., 1996). Here we demonstrate that Scd5p is important for actin organization and endocytosis. This finding led us to examine actin localization in cells lacking clathrin. We find that similar to scd5 mutants, loss of clathrin function causes major defects in cortical actin organization, and overexpression of SCD5 partially suppresses this defect. Moreover, two-hybrid screening with the clathrin LC has identified Sla2p as an interacting protein. Scd5p also associates with Sla2p and Sla2p's cortical localization is dependent on both clathrin and Scd5p. These data suggest that clathrin and Scd5p may play a role in organizing or stabilizing the cortical actin network, which is important for endocytosis in yeast.
MATERIALS AND METHODS
Strains, Media, and Genetic Methods
Strains used in this study are listed in Table 1. YEPD (2% yeast extract, 1% peptone, and 2% dextrose), YEP-GAL (2% yeast extract, 1% peptone, and 2% galactose), 5-fluoro-orotic acid (FOA) plates, and synthetic complete dropout media (e.g., complete-uracil [C-URA]) were prepared as described in Nelson and Lemmon (1993). Yeast mating, sporulation, and tetrad analysis were performed as described (Guthrie and Fink, 1991). Yeast were transformed using the method of Gietz et al. (1995).
Table 1.
Saccharomyces cerevisiae strains
| Strain | Genotype | Sourcea |
|---|---|---|
| BWY236 | MATa pan1-20 his3-Δ200 leu2-3,112 lys2-801 suc2-Δ9 trp1-Δ901 ura3-52 | 1 |
| DDY75 | MATa abp1Δ∷URA3 his4-619 lys2-801 ura3 | 2 |
| DDY301 | MATα sla1Δ∷URA3 his4-619 leu2-3,112 lys2-801 ura3-52 | 2 |
| DDY771 | MATα sac6Δ∷URA3 his4-619 ura3-52 | 2 |
| DDY951 | MATα srv2Δ∷HIS3 his3-Δ200 leu2-3,112 lys2-801 ura3-52 | 2 |
| MY4495 | MATα rvs161Δ∷LEU2 his3-Δ200 leu2 lys2-801 ura3-52 | 3 |
| SL13 | MATα chc1Δ∷LEU2 leu2 scd1-v trp1 ura3-52 YCp50-CHC1 [CEN, URA3, CHC1] | 4 |
| SL554 | MATa/MATα GAL1∷CHC1/GAL1∷CHC1 ade6/+ his1/+ leu2/leu2 scd1-v/scd1-i trp1/trp1 ura3-52/ura3-52 | 4 |
| SL1462 | MATa his3-Δ200 leu2 scd1-v trp1 ura3-52 | 4 |
| SL1528 | MATa/MATα his3-Δ200/his3-Δ200 leu2/leu2 trp1/trp1 ura3-52/ura3-52 scd1-v/scd1-v | 4 |
| SL1556 | MATa sla2-41 (end4-1) bar1-1 his4 leu2 ura3 | 5 |
| SL1637 | MATα clc1Δ∷HIS3 his3-Δ200 leu2 scd1-v trp1 ura3-52 pRS424-CLC1 [2μ, TRP1, CLC1] | 4 |
| SL2726 | MATα chc1-521 his3-Δ200 leu2 scd1-v trp1 ura3-52 | |
| SL3004 | MATα ade2-101 gal4 gal80 his3 leu2 lys2-801 trp1-901 ura3-52 | |
| SL3416 | MATα yap1801Δ∷URA3 his3-Δ200 leu2 scd1-v trp1 ura3-52 | 4 |
| SL3423 | MATa yap1802Δ∷KMX bar1-1 his3-Δ200 leu2 ura3-52 | 4 |
| SL3429 | MATa yap1801Δ∷URA3 yap1802Δ∷KMX bar1-1 his3-Δ200 leu2 ura3-52 | 4 |
| SL3519 | MATα scd5-Δ338 gal2 his1 leu2 scd1-i trp1 ura3-52 | |
| SL3521 | MATa scd5-Δ338 gal2 his1 leu2 scd1-i trp1 ura3-52 | |
| SL3593 | MATa chc1Δ∷LEU2 bar1-1 leu2 trp1 ura3-52 | |
| SL3739 | MATa scd5-Δ338 gal2 his3-Δ200 leu2 trp1 ura3-52 | |
| SL3740 | MATα scd5-Δ338 gal2 his3-Δ200 leu2 trp1 ura3-52 | |
| SL3849 | MATa rvs167Δ∷TRP1 scd5-Δ338 His-(his1 and/or his4) leu2 trp1Δ∷URA3 ura3-52 | |
| SL3855 | MATα rvs161Δ∷LEU2 scd5-Δ338 his3-Δ200 leu2 ura3-52 | |
| SL3863 | MATα scd5-Δ338 srv2Δ∷HIS3 his3-Δ200 leu2 ura3-52 | |
| SL3898 | MATα sac6Δ∷URA3 scd5-Δ338 His- (his4 and/or his3) ura3-52 | |
| SL3908 | MATa abp1Δ∷URA3 scd5-Δ338 his3-Δ200 his4-619 leu2 lys2-801 ura3 | |
| SL3920 | MATa/MATα scd5-Δ338/scd5-Δ338 his3-Δ200/his3-Δ200 leu2/leu2 trp1/trp1 ura3-52/ura3-52 | |
| SL3921 | MATa scd5Δ∷TRP1 bar1-1 leu2 trp1 ura3-52 pCC545 [CEN, LEU2, SCD5] | |
| SL3993 | MATa scd5Δ∷TRP1 bar1-1 leu2 trp1 ura3-52 pJSC3 [CEN, LEU2, scd5-Δ338] | |
| SL4001 | MATa ent1Δ∷LEU2 his3-Δ200 leu2 lys2-801 trp1 ura3-52 | 5 |
| SL4002 | MATα ent2Δ∷HIS3 his3-Δ200 leu2 suc2-Δ9 trp1 ura3-52 | |
| SL4049 | MATa sla2-41(end4-1) his3-Δ200 leu2 trp1 ura3-52 | |
| SL4091 | MATa ent1Δ∷LEU2 scd5-Δ338 his3-Δ200 leu2 trp1 ura3-52 | |
| SL4097 | MATa ent2Δ∷HIS3 scd5-Δ338 his3-Δ200 leu2 trp1 ura3-52 | |
| SL4101 | MATα scd5-Δ338 yap1801Δ∷URA3 his3-Δ200 leu2 trp1 ura3-52 | |
| SL4105 | MATa scd5-Δ338 yap1802Δ∷KMX his3-Δ200 leu2 trp1 ura3-52 | |
| SL4117 | MATa/MATα end3Δ∷HIS3-MX/+ scd5-Δ338/+ his3-Δ200/his3-Δ200 leu2/leu2 trp1/+ ura3-52/ura3-52 | |
| SL4162 | MATa sla2-Δ∷LEU2 bar1-1 his3-Δ200 leu2 lys2 ura3-52 | |
| SL4173 | MATa chc1-521 sla2-41(end4-1) his3-Δ200 leu2 trp1 ura3-52 | |
| SL4182 | MATa scd5-Δ338 his3-Δ200 leu2 scd1-v trp1 ura3-52 | |
| SL4212 | MATa scd5-Δ338 yap1801Δ∷URA3 yap1802Δ∷KMX bar1-1 his3-Δ200 leu2 ura3-52 | |
| SL4260 | MATa chc1-521 sla2-Δ∷LEU2 bar1-1 his3-Δ200 leu2 ura3-52 | |
| SL4292 | MATa clc1-Δ∷HIS3 sla2-41(end4-1) his3-Δ200 leu2 trp1 ura3-52 | |
| SL4300 | MATa/MATα scd5Δ∷TRP1/scd5Δ∷TRP1 his3-Δ200/his3-Δ200 leu2/leu2 trp1/trp1 ura3-52/ura3-52 pKRH21 [CEN, LEU2, GFP-SCD5] | |
| SL4301 | MATa/MATα scd5Δ∷TRP1/scd5Δ∷TRP1 his3-Δ200/his3-Δ200 leu2/leu2 trp1/trp1 ura3-52/ura3-52 pJSC3 [CEN, LEU2, scd5-Δ338] | |
| SL4302 | MATa/MATα scd5Δ∷TRP1/scd5Δ∷TRP1 his3-Δ200/his3-Δ200 leu2/leu2 trp1/trp1 ura3-52/ura3-52 pCC545 [CEN, LEU2, SCD5] | |
| SL4304 | MATα chc1-521 scd5-Δ338 his3-Δ200 leu2 trp1 ura3-52 | |
| SL4340 | MATa chc1-Δ∷LEU2 sla2-41(end4-1) his3-Δ200 leu2 scd1-v trp1 ura3-52 | |
| RH2951 | MATα rvs167-Δ∷TRP1 bar1-1 his4 leu2 trp1-Δ∷URA3 ura3-52 | 5 |
| YPJ96-4 | MATa ade2 gal4-Δ gal80-Δ GAL2-ADE2 his3-Δ200 leu2-3,112 LYS2∷GAL1-HIS3 met2∷GAL7-lacZ trp1-901 ura3-52 | 6 |
Strains were generated for this study except where indicated. Several strains were derived from crosses to strains from other sources (not indicated). 1, Beverly Wendland; 2, David Drubin; 3, Mark Rose; 4, S. Lemmon (generated for previous studies); 5, Howard Riezman; 6, Phillip James.
Plasmids
Plasmids were propagated in Escherichia coli DH5α. Important plasmids used in this study are listed in Table 2. A clone containing the scd5-Δ338 truncation mutation under its own promoter was generated in two steps. A 4.6-kb BamHI-ClaI SCD5 fragment was cloned into pBluescript SK+ (Stratagene, La Jolla, CA). This plasmid was subjected to site directed mutagenesis by the dut− ung− method (Kunkel et al., 1987; McClary et al., 1989) to generate pAC8. The primer 5′- GAGGAAGAGTAATTTACTAAGAATTCAG-3′ used for mutagenesis created two stop codons at positions 535 and 536 of the Scd5p coding sequence. The mutation was verified by DNA sequencing. pAC10 was made by cloning the scd5-Δ338 BamHI-ClaI fragment from pAC8 into YIp5 (Botstein et al., 1979). pJSC3 was generated by gap repair and contains scd5-Δ338 from pAC8 in pRS315 (Sikorski and Hieter, 1989). The GFP-SCD5 construct contains SCD5 with the coding sequence for green fluorescent protein (GFP) fused in frame between codons 80 and 81 at a unique SacI site. A PCR product for gap repair was made using primers 5′-CAGAAATAATCACTCAAATACAGCAGCTGATAATGCCACTAACGTGAGCTCTGGTGCTGGTGCTGGTGCTGC-3′ and 5′-CAGAAAGTGTTCGTACCTCCCCATTAGGTGGATTATCCTTTGAGGAGAGCTCACTTTGTACAATTCATCCATACC-3′ and a modified YGFP3 coding sequence as a template. The modified YGFP3 contains the coding sequences for an N-terminal 5 × GlyAla linker followed by yeast enhanced GFP-S65G, S72A (gift of C. Schaerer-Brodbeck). The PCR product was cotransformed into SL1462 with YEp24-SCD5, which was cut with SacI. The final CEN, LEU2, GFP-SCD5 plasmid, pKRH21, was made by gap repairing a 2.2-kb fragment from YEpGFP-SCD5 into pCC545 gapped with XbaI.
Table 2.
Important plasmids used in this study
| Name | Features | Description |
|---|---|---|
| p31-10 | 2μ, LEU2, GAD-SLA2-292-968 | Two hybrid prey fusing codons 292–968 of SLA2 to the GAL4 activation domain coding sequence. |
| p31-2 | 2μ, LEU2, GAD-SLA2-292-520 | Two hybrid prey fusing codons 292–520 of SLA2 to the GAL4 activation domain coding sequence. |
| pAC10 | URA3, scd5-Δ338 | scd5-Δ338 cloned into YIp5. |
| pCC545 | CEN, LEU2, SCD5 | SCD5 in pRS315 (from Clarence Chan). |
| pGAD | 2μ, LEU2, GAD | Two hybrid prey for expression of the GAL4 activation domain under control of the ADH1 promoter (James et al., 1996). |
| pGBDU | 2μ, URA3, GBD | Two hybrid bait for expression of the GAL4 DNA binding domain under control of the ADH1 promoter (James et al., 1996). |
| pJC4 | CEN, URA3, GFP:ABP1 | GFP:ABP1 expression vector (from David Drubin). |
| pJSC3 | CEN, LEU2, scd5-Δ338 | scd5-Δ338 in pRS315. |
| pKH19 | 2μ, LEU2, GAD-CLC1 | Two hybrid prey fusing the CLC1 ORF to the GAL4 activation domain coding sequence. |
| pKH24 | 2μ, LEU2, GAD-CHC1-655-1653 | Two hybrid prey fusing codons 655–1653 of CHC1 to the GAL4 activation domain coding sequence. |
| pKH31 | 2μ, URA3, GBD-CLC1 | Two hybrid bait fusing the CLC1 ORF to the GAL4 DNA binding domain coding sequence. |
| pKH47 | 2μ, URA3, GBD-SLA2-292-501 | Two hybrid bait fusing codons 292–501 of SLA2 to the GAL4 DNA binding domain coding sequence. |
| pKRH20 | 2μ, URA3, GBD-scd5-Δ645 | Two hybrid bait fusing codons 1–227 of SCD5 to the GAL4 DNA binding domain coding sequence. |
| pKRH21 | CEN, LEU2, GFP-SCD5 | Vector for expression of GFP-tagged Scd5p. |
| pNT1 | 2μ, URA3, GBD-scd5-Δ338 | Two hybrid bait fusing codons 1–534 of SCD5 to the GAL4 DNA binding domain coding sequence. |
| YCp50-CHC1 | CEN, URA3, CHC1 | CHC1 in YCp50 (Lemmon and Jones, 1987). |
| YEp24-SCD5 | 2μ, URA3, SCD5 | SCD5 in YEp24 (Nelson et al., 1996). |
pKH19 was created by engineering a CLC1 PCR product with a 5′ NcoI site at the CLC1 start codon and a PvuII site downstream of the stop codon using primers 5′-CCATGGCAGAGAAATTC-3′ and 5′-GCGCAGCTGATCATTTAAGCACCG-3′, respectively. This was transferred by subcloning into pAS2 (Harper et al., 1993) to generate pKH15. The CLC1 ORF in pKH15 was then subcloned as a NcoI-BamHI fragment into pACT2 (Harper et al., 1993) to yield pKH19. pKH24 was generated by cloning a 5.0-kb ClaI-SalI fragment containing CHC1 into the SmaI-XhoI sites of pACT2. This fused codons 655-1653 of CHC1 to the GAL4 activation domain (GAD) coding sequence. In pKH31, a BamHI fragment containing the CLC1 ORF was cloned into pGBDU-C3 (James et al., 1996) to generate a full-length CLC1 ORF fused in frame to the coding region of the GAL4 binding domain (GBD). pGAD-SLA2-(292–520) (p31-2) and pGAD-SLA2-(292–968) (p31-10) were isolated from a two-hybrid screen using a LC bait (pKH31). pKH47 was generated by moving SLA2-(292–520) from p31-2 as an EcoRI-PstI fragment into pGBDU-C2 (James et al., 1996). To generate pKRH20, a 0.7-kb SCD5 PCR product was made using primers 5′-CGGGATCCATGTCGTTTGATTGGCTTA-3′and 5′-CGAGATCTACACCTCCTCG-3′. This produced a BamHI site at the SCD5 start codon and a stop at codon 227 followed by a BglII site. This product was cloned into pGBDU-C1 (James et al., 1996) fusing SCD5 codons 1–227 to the GBD coding region. pNT1 was made by cloning a XbaI-SalI fragment containing the scd5-Δ338 mutation into the XbaI-SalI sites of pKRH20. This resulted in a construct coding for residues 1–534 of Scd5p fused to the GBD (GBD-scd5-Δ338).
scd5-Δ338 Integration and END3 Gene Deletion
To integrate the scd5-Δ338 allele at the SCD5 chromosomal locus, pAC10 (URA3, scd5-Δ338) was linearized by BlpI and transformed into scd5Δ::TRP1/SCD5 strains, selecting on C-URA. YIp5-scd5-Δ338 could integrate adjacent to SCD5 or scd5Δ::TRP1. Several integrants strains were plated on FOA to select for recombinants that lost the vector and adjacent sequence. FOAR colonies were then screened for the loss of TRP1 by plating replica plating on C-TRP. The Trp− candidates represented events where integration occurred adjacent to the null allele and recombination excised scd5Δ::TRP1 retaining the scd5-Δ338 allele. The scd5-Δ338 mutation generates a new DdeI site. The integration of scd5-Δ338 was confirmed by heteroduplex analysis (Cotton, 1992) and PCR analysis combined with restriction enzyme digestion using DdeI. Tetrads were dissected to generate haploid progeny (SL3519, SL3521, and SL4182). The scd5-Δ338 segregants were temperature sensitive for growth and expressed the truncated protein, as expected.
To generate a null allele of END3 the complete ORF was replaced by HIS3-MX6 using a PCR-based one-step gene-replacement method. The disruption PCR fragment was created with primers 5′-AGTTAGTGGGTATTGGAAAGGCCGGTAAAGATAACAGGG-ATCTCTGAAAACGGATCCCCGGGTTAATTAA-3′ and 5′-AACAAACAGTAAATATTACACATTCATGTACATAAAATTAA TTATCGGTGGAATTCGAGCTCGTTTAAAC-3′ using pFA6a-HIS3MX6 (Wach, 1996) as the template. This PCR product was transformed into a scd5-Δ338/SCD5 heterozygous diploid generating SL4117. Colony PCR was used to verify the disruption.
Two-hybrid Interaction Analysis
For the two-hybrid screen with CLC1 as a bait, a yeast genomic two-hybrid library (James et al., 1996) was transformed into SL3004. YPJ96-4A (James et al., 1996) was transformed with pKH31 (pGBD-CLC1). The library strain and YPJ96-4A carrying pKH31 were mated in liquid culture for 4 h and plated on YEPD overnight. The cells were scraped from the plate and replated on synthetic medium lacking leucine, adenine, and uracil (C-ADE-LEU-URA) to select for mated zygotes and to monitor expression of the GAL2-ADE2 reporter from YPJ96-4A. Colonies appeared after 3–5 d and were further screened for false positives by testing against an empty bait vector and nonrelevant baits. Plasmids that passed these tests were sequenced from both ends of the insert.
Scd5p two-hybrid interactions studies used the mating method described above to generate strains expressing GAD and GBD plasmids. For clathrin two-hybrid interaction studies, YPJ96-4A was directly transformed with both GAD and GBD plasmids. For analysis of two-hybrid interactions, log phase cultures were diluted to 5 × 106 cells/ml, and equal volumes of cells were spotted on C-ADE-LEU-URA and C-LEU-URA plates. The plates were grown for 3–5 d at 30°C.
Other Assays
The alpha-factor endocytosis assay was performed as described previously (Dulic et al., 1991). Lucifer yellow (LY) uptake was carried out by methods described in Munn et al. (1995). The latrunculin-A (Lat-A) halo sensitivity assay was performed as described by Ayscough et al. (1997), comparing a scd5-Δ338 strain (SL4301) to an isogenic wild-type control (SL4302).
Microscopy
Yeast cells were grown to early exponential phase in YEPD or YEP-GAL. For F-actin, DNA, and chitin staining, cells were fixed with 3.7% formaldehyde and then stained with Alexa-568-phalloidin (Molecular Probes, Eugene, OR), 4′6′-diamidino-2-phenylindole (DAPI; Sigma, St. Louis, MO), or calcofluor white (Sigma), respectively, as described in Adams and Pringle (1991) and Pringle (1991). For simultaneous localization of GFP-Scd5p and actin, cells were fixed for 10 min with 2.0% formaldehyde and washed three times with PBS. The cells were stained with Alexa-594-phalloidin (Molecular Probes) as described in Adams and Pringle (1991) and visualized immediately. For GFP-Abp1p and actin colocalization, cells were fixed and processed as described in Heil-Chapdelaine et al. (1998).
For immunofluorescence, cells were prepared using a methanol/acetone fixation as described previously (Pringle et al., 1991). Affinity-purified rabbit anti–C-terminal Sla2p antiserum (Yang et al., 1999) was used at 1:75 dilution, and guinea pig anti-actin antiserum (Mulholland et al., 1994) was used at 1:2000 dilution. For fluorescent visualization, FITC-conjugated goat anti-rabbit IgG and Alexa-594–conjugated goat anti-guinea pig IgG were used at 1:800 dilution.
Most of the microscopy was performed using a Zeiss Axioplan-2 microscope (Thornwood, NY) equipped with Nomarski differential interference contrast (DIC) optics, a plan-Neofluor 100× objective (numerical aperture 1.3), and a Hamamatsu C4742–95 cooled CCD camera (Bridgewater, NJ) using QED acquisition software. A LSM 410 confocal microscope equipped with a plan-Apochromat 100× objective (numerical aperture 1.4) was used for analysis of cells stained with Alexa-568-phalloidin in Figures 2 and 6. Images were manipulated in Adobe Photoshop (San Jose, CA).
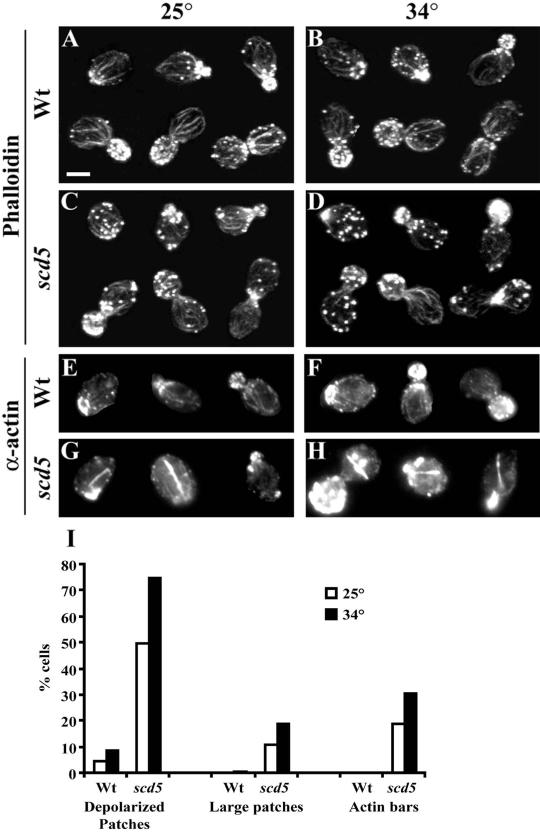
Figure 2.
Actin organization is defective in scd5-Δ338 cells. Wild-type (SL1528, A, B, E, and F) and scd5-Δ338 (SL3920, C, D, G, and H) strains were grown on YEPD to log phase at 25°C. Cells were immediately harvested (A, C, E, and G) or shifted to 34°C (B, D, F, and H) for 3 h before fixation, staining, and microscopic visualization as described in MATERIALS AND METHODS (Bar, 5 μm). (A–D) Stained with Alexa-568-phalloidin to visualize filamentous actin; (E–H) indirect immunofluorescence with anti-actin antibodies. Examples of cells through the cell cycle are shown in A–D. (I) Bar graph showing percent of cells with depolarized patches, large patches, or actin bars from the same experiments as shown in A–H. Quantification was determined as described in MATERIALS AND METHODS.
Figure 6.
Overexpression of SCD5 partially suppresses the actin organization defect of clathrin-deficient yeast. A GAL1:: CHC1 strain (SL554) was transformed with YEp24 (A, B, E, and G) or YEp24-SCD5 (C, D, F, and H). Precultures were grown on selective C-URA + 2% galactose. Then cells were washed and resuspended in medium containing galactose (A, C, E, and F) or glucose to deplete clathrin HC (B, D, G and H) for 15 h at 30°C. Cells were then fixed and stained with Alexa-568 phalloidin (A–D) or anti-actin antibodies (E–H). Bar, 5 μm. (I) Graph showing the percentage of cells from the above cultures with large patches or actin bars.
For quantification of microscopy, 100–500 cells were counted for each condition. Cells were scored as having depolarized actin patches if there were ≥10 patches in the mother cell of cells with buds. Actin patches were scored “large” (or aggregates of actin) if they were ≥0.5 μm diameter. Normal patches seen in wild-type cells were typically 0.1–0.2 μm diameter. Large patches were usually delocalized to the mother cell, but each was counted as a single patch. G-actin “bars” were scored by their unusual stick-like or bar morphology and were only detected with anti-actin antibodies. Actin bar structures were never observed in wild-type cells.
RESULTS
Endocytosis Is Defective in a scd5 Truncation Mutant
SCD5 was originally isolated as a multicopy suppressor of the lethality of a clathrin HC-deficient strain (Nelson and Lemmon, 1993). Previously we studied a temperature-sensitive scd5-Δ338 mutant, expressing a 338-amino acid COOH-terminal truncation of Scd5p (Nelson et al., 1996). This strain exhibits a partial post-Golgi secretion defect at the nonpermissive temperature but no obvious defects in sorting/retention of trans-Golgi proteins typical of clathrin mutants (Nelson et al., 1996). Because clathrin mutants also display slowed receptor-mediated endocytosis (Payne et al., 1988; Tan et al., 1993; Huang et al., 1997), we examined whether Scd5p function is required for internalization of α-factor by its receptor, Ste2p (Figure 1A). At 25°C, internalization of 35S-labeled α-factor was nearly normal compared with an isogenic wild-type strain. Preshifting scd5-Δ338 cells to 37°C for 15 min resulted in a rapid and complete block in internalization of α-factor.
Figure 1.
Endocytosis is defective in scd5-Δ338 cells at 37°C. (A) Receptor-mediated internalization of radiolabeled α-factor. Cultures were grown at 25°C to mid log phase in YEPD, pelleted, and resuspended at 1 × 109 cells/ml in fresh YEPD. Cells were preincubated at 25 (●, ▪) or 37°C (○, □) for 15 min before addition of 35S-radiolabeled α-factor. Samples were harvested at times indicated and processed for determination of internalized α-factor. Strains are isogenic wild-type (SL3921; ●, ○) and scd5-Δ338 (SL3993; ▪, □). (B) Lucifer yellow (LY) uptake. Wild-type (SL1462; a and c) and scd5-Δ338 (SL3739; b and d) cells were incubated at 25°C (a and b) or preshifted to 37°C (c and d) for 15 min. Then LY was added, and incubation was continued for 60 min. Cells were photographed using DIC (to visualize the vacuole) and fluorescence microscopy. Bar, 5 μm.
We also examined fluid-phase endocytosis in the scd5 mutant by visualizing accumulation of the fluorescent dye lucifer yellow (LY) in the vacuole (Figure 1B). At 25°C to 35–55% of scd5-Δ338 cells, depending on the isolate, showed vacuolar staining with LY. When cells were shifted to 37°C, uptake to the vacuole was completely blocked. Nearly 100% of wild-type cells internalized LY at either temperature. We conclude that Scd5p is required for both fluid-phase and receptor-mediated endocytosis.
Scd5p Is Important for Actin Organization
The endocytic defects of scd5-Δ338 are much more severe than observed in clathrin mutants (Payne et al., 1988; Tan et al., 1993; Huang et al., 1997) but resemble the internalization phenotypes of a number of mutants with a perturbed cortical actin cytoskeleton (e.g., see Kübler and Riezman, 1993; Bénédetti et al., 1994; Munn et al., 1995; Wesp et al., 1997; Madania et al., 1999; Schaerer-Brodbeck and Riezman, 2000). Moreover, some of these mutants, e.g., sla2/end4, rvs161, rvs167, and act1, also display partial post-Golgi secretory defects similar to those of scd5-Δ338 (Novick and Botstein, 1985; Nelson et al., 1996; Mulholland et al., 1997; Breton et al., 2001). Therefore, we examined actin organization in the scd5-Δ338 mutant.
Two major actin filament-based (F-actin) structures are seen in yeast cells: actin cables and cortical actin patches (Adams and Pringle, 1984; Pruyne and Bretscher, 2000a). The cables, which are bundles of actin filaments, are responsible for polarized growth and delivery of secretory vesicles, cell wall material, organelles, and other proteins into the growing daughter cell. Cortical actin patches appear at the site of bud emergence and then localize primarily to the growing bud. Late in the cell cycle, patches first redistribute across mother and daughter cells. Finally, cables reorient toward the mother/daughter cell neck and patches concentrate at the bud neck where they are involved in septum formation and cytokinesis (See Figure 2, A and B).
To examine actin organization, wild-type and scd5-Δ338 cells were grown at 25°C or shifted to 34°C for 3 h and stained with Alexa-568-phalloidin to label F-actin. Many scd5-Δ338 mutant cells displayed aberrant patch organization, which was more severe at 34°C (Figure 2, C and D). Often these patches were depolarized to the mother cell, and some patches appeared larger than normal cortical actin structures (see Figure 2I for quantification of patch morphology). In addition, there were fewer or thinner cables in some scd5-Δ338 cells, particularly in those that had larger patches, and in some cells the cables seemed to be misoriented.
There is very little nonpolymerized globular actin (G-actin) and it is not aggregated in normal yeast cells; however, G-actin aggregates or actin bars are observed in the presence of some mutations affecting the actin cytoskeleton (e.g., srv2Δ, act1-2, or sla1Δ; Novick and Botstein, 1985; Holtzman et al., 1993; Cope et al., 1999). Therefore we stained SCD5 and scd5-Δ338 cells with anti-actin antibodies, which detect both F- and G-actin. The staining of wild-type cells resembled the phalloidin pattern (Figure 2, E and F). In contrast, many scd5-Δ338 cells accumulated actin bars and other large aggregates of actin, presumably G-actin, that were not observed by phalloidin staining (examples shown in Figure 2, G and H, and quantification in I).
Latrunculin A (Lat-A) is an inhibitor of actin assembly, and many mutations affecting actin organization are hypersensitive to the drug (Ayscough et al., 1997). In addition, sla1Δ and end3Δ are slightly resistant to Lat-A (Ayscough et al., 1997). We found that scd5-Δ338 also confers mild resistance to this inhibitor. In a Lat-A halo assay the scd5-Δ338 mutant (SL4301) gave a diameter ratio of 0.8 ± 0.05 compared with an isogenic wild-type strain (SL4302; average of three experiments), although the observed effect could actually be stronger because scd5-Δ338 grows slightly slower than wild type.
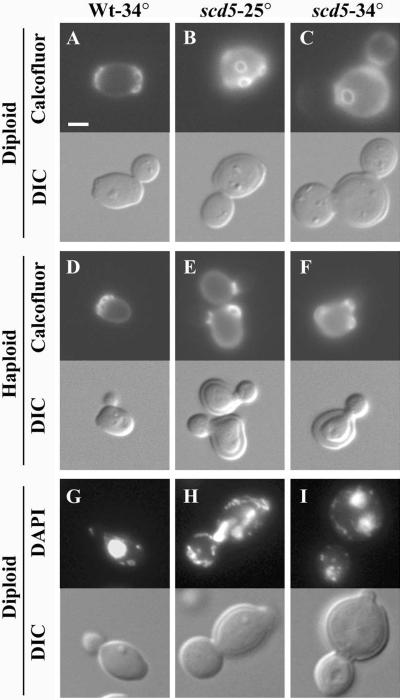
The scd5-Δ338 mutant was examined for other phenotypes that are often associated with perturbation of the actin cytoskeleton. Mutations affecting actin can cause defects in bud site pattern selection and depolarized chitin deposition (Novick and Botstein, 1985; Yang et al., 1997). Also, multinucleated cells and aberrant morphologies, including multibudded cells, are sometimes seen because of the polarity defects in actin mutants (Novick and Botstein, 1985; Yang et al., 1997). To examine budding pattern, cells were stained with calcofluor white, which labels chitin in bud scars left on mother cells after cytokinesis (Pringle, 1991). In the scd5-Δ338 cells chitin was delocalized both at 25°C and after shift to 34°C for 3 h (Figure 3, B and C, and 3, E and F). Also, the majority of scd5-Δ338 diploids (>75%) displayed random bud scar placement at 25 and 34°C, whereas >93% of wild-type cells showed the normal bipolar budding pattern (Figure 3, A–C). Haploid scd5-Δ338 cells also showed random budding (72% at 34°C), rather than the normal axial budding pattern (Figure 3, E–F). Haploid and diploid mutants were heterogeneous in size and shape but were generally larger and rounder than wild-type cells (Figures 1 and 3). Cell surface rims were observed in DIC profiles of scd5-Δ338 mutant cells, indicating the mutation results in thickened cell walls (Figure 3). EM analysis also showed thickening of the mother cell wall, similar to other cortical actin mutants. In addition, mutant cells (17 and 33% of diploids at 25 and 34°C, respectively) were often multibudded, mostly appearing to have a small aborted bud on the mother cell (see Figure 3I). Similar numbers were observed in haploids. The concentrated patches of F-actin sometimes found in mother cells (for example, see Figure 2C) were often positioned at these sites of aborted budding. A significant number of scd5-Δ338 cells were multinucleated (up to 14% at 34°C), compared with <1% seen in wild-type cells (Figure 3, G and H). From these data we conclude that scd5-Δ338 causes cell polarity defects typical of many mutations affecting the actin cytoskeleton.
Figure 3.
scd5-Δ338 cells exhibit cell polarity defects. Cells were grown in YEPD to log phase at 25°C or shifted to 34°C for 3 h before fixation and visualization. Top panels: (A–F) calcofluor white staining of chitin; (G–I) cells stained with DAPI to visualize DNA. Bottom panels: DIC images. Strains are as follows: wild-type diploid (SL1528) at 34°C (A and G); scd5-Δ338 diploid (SL3920) at 25 (B and H) or 34°C (C and I); wild-type haploid (SL1462) at 34°C (D); scd5-Δ338 haploid (SL3739) at 25 (E) or 34°C (F). Bar, 5 μm.
scd5-Δ338 Exhibits Genetic Interactions with Mutations in Genes Encoding Other Proteins that Modulate the Actin Cytoskeleton and/or Endocytosis
Many mutations affecting actin cytoskeletal organization and/or endocytosis cause synergistic phenotypes when combined, and many of the normal gene products are physically associated or directly interact (Botstein et al., 1997; D'Hondt et al., 2000). Therefore, we examined genetic interactions between scd5-Δ338 and mutations in genes important for these processes (Table 3). Haploids carrying mutations in genes to be tested were crossed to scd5-Δ338 and subjected to segregation analysis. Then wild-type, single, and combination mutant progeny from tetrads were analyzed for synthetic growth phenotypes. We found sla2-41(end4-1), pan1-20, and end3Δ were synthetically lethal with scd5-Δ338. abp1Δ, and rvs161Δ, and rvs167Δ showed strong negative synergistic growth defects with scd5-Δ338, as the double mutants were inviable at 30°C and often grew very slowly at 25°C, whereas the single mutants grew relatively well or like wild-type cells at these temperatures. A weaker negative genetic interaction was observed with sac6Δ, where the sac6Δ scd5-Δ338 double mutant grew at 30°C but not at 32°C. These genetic interactions are likely to be specific because ent1Δ, ent2Δ, sla1Δ, srv2Δ, yap1801Δ, yap1802Δ, and yap1801Δ yap1802Δ had no effect when combined with scd5-Δ338. In these cases, growth of the multiple mutants was similar to that of the scd5-Δ338 mutant alone.
Table 3.
Genetic interactions between scd5-Δ338 and mutations in other genes affecting endocytosis and/or actin cytoskeletal organization
| Relevant genotype | Growth rangea | Actinb |
|---|---|---|
| A. WT | 25–37°C | Normal |
| scd5-Δ338 | 25–34°C | Depolarized cables and patches, fewer or thinner cables |
| B. Synthetic lethal | ||
| end3Δ | 25–34°C | NDc |
| endΔ scd5-Δ338 | Lethal | ND |
| sla2-41 | 25–34°C | ND |
| sla2-41 scd5-Δ338 | Lethal | ND |
| pan1-20 | 25–34°C | ND |
| pan1-20 scd5-Δ338 | Lethal | ND |
| C. Strong negative interaction | ||
| abp1Δ | 25–37°C | Normal |
| abp1Δ scd5-Δ338 | 25°C | Depolarized cables and patches, fewer cables, larger patches |
| rvs161Δ | 25–34°C | Depolarized cables and patches |
| rvs161Δ scd5-Δ338 | 25°C | Depolarized cables and patches, increased patches, fewer cables |
| rvs167Δ | 25–34°C | Depolarized patches and cables |
| rvs167Δ scd5-Δ338 | 25°C | Depolarized patches and cables, increased patches, fewer cables |
| D. Weak negative interactions | ||
| sac6Δ | 25–34°C | Fewer cables, depolarized patches |
| sac6Δ scd5-Δ338 | 25–30°C | Depolarized cables and patches, fewer cables and larger patches |
| E. No observed interactionsd | ||
| ent1Δ, ent2Δ, sla1Δ, srv2Δ, yap1801Δ, yap1802Δ, yap1801Δ yap1802Δ |
Strains were replica plated from patches or streaked onto YEPD plates and grown for 3 days at 25, 30, 32, 34, and 37°C. Note that the scd5-Δ338 mutant grows very slowly at 34°C.
Actin was visualized by staining with Alexa-phalloidin after growth at 25°C.
ND, not determined.
The scd5-Δ338 mutation was not combined with ent1Δ ent2Δ, because the double ent mutant is inviable.
The double mutants were also analyzed for enhanced defects in actin cytoskeleton organization at 25°C, compared with the single mutants, by staining with Alexa-568-phalloidin (summarized in Table 3). Generally the results paralleled the growth phenotypes. The mutations that exacerbated the growth of scd5-Δ338 the most caused more severe actin defects. For example abp1Δ scd5-Δ338 double mutants had fewer cables and fewer, but larger and depolarized, patches than scd5-Δ338 alone, even although the actin cytoskeleton in abp1Δ single mutants was normal at 25°C (Drubin et al., 1990). In addition, disruptions in RVS161 and RVS167, which exhibit some defects in the actin cytoskeleton on their own (Bauer et al., 1993; Sivadon et al., 1995), resulted in the appearance of more and depolarized patches and fewer actin cables than the single mutants. sac6Δ, which also perturbs actin (Adams et al., 1991), resulted in the appearance of fewer cables and a few larger patches in combination with scd5-Δ338. This weaker synergistic effect on actin is consistent with the more limited effect of sac6Δ on growth of the scd5 mutant. The scd5-Δ338 double mutants that contained sla1Δ, ent1Δ, ent2Δ, yap1801Δ, yap1802Δ, or srv2Δ did not show an exaggerated actin phenotype. Instead, these double mutants resembled the scd5-Δ338 mutant, despite the fact that some of these mutations (sla1Δ and srv2Δ; Vojtek et al., 1991; Holtzman et al., 1993; Lila and Drubin, 1997) cause actin phenotypes on their own. The noninteracting mutations also did not exacerbate the LY internalization defect of scd5-Δ338 at 25°C. We note that most of the mutations causing enhanced growth and actin cytoskeleton phenotypes with scd5-Δ338 also had major endocytic defects on their own, so the LY analysis was uninformative. Overall this analysis indicates that mutations in several genes affecting actin organization and endocytosis also genetically interact with scd5-Δ338, further supporting a role for Scd5p in actin function.
Scd5p Colocalizes with Cortical Actin
Because scd5-Δ338 causes several phenotypes observed in other mutants with defects in cortical actin organization, we examined localization of Scd5p using a GFP-tagged protein in cells where this was the only source of the protein. The plasmid containing GFP-SCD5 (pKRH21) complements scd5Δ completely. We found that GFP-Scd5p colocalized with cortical actin patches visualized with Alexa-594-phalloidin. This was most easily observed in budding cells, where there was a striking polarized distribution of Scd5p to the bud (Figure 4). GFP-Scd5p could also be seen in mother cell actin patches. However, there was not complete overlap of Scd5p and cortical patches, as has been observed for other actin patch proteins like Sla2p (Yang et al., 1999). Overall, the localization results further support a role for Scd5p in cortical actin function.
Figure 4.
Scd5p localizes with cortical actin. SL4300 (scd5Δ/scd5Δ + pKRH21 [CEN, LEU2, GFP-SCD5]) was grown at 30°C to log phase. Cells were prepared for visualization of F-actin (left) and GFP-Scd5p (right) as described in MATERIALS AND METHODS. Arrows indicate examples of mother cell actin patches that also show GFP-Scd5p fluorescence.
Clathrin Deficiency Causes Defects in Cortical Actin Organization
Because Scd5p is important for endocytosis and the gene was isolated by its ability to rescue inviable strains of clathrin HC- (Chc1p) deficient yeast (Nelson and Lemmon, 1993; Nelson et al., 1996), we considered the possibility that this interaction is associated with endocytic defects seen in viable clathrin mutants. To investigate this, we transformed a viable clathrin HC null strain with YEp24, YEp24-SCD5, or a CHC1 complementing plasmid (YCp50-CHC1) and tested for suppression of the chc1Δ growth and receptor-mediated endocytosis defects. SCD5 overexpression suppressed the temperature sensitivity of the viable chc1Δ strain quite well at 34°C, although not at 37°C (Figure 5A). However, the slowed α-factor internalization seen in chc1Δ mutants was minimally affected by overexpression of SCD5 (Figure 5B), even at 30°C, a permissive growth temperature.
Figure 5.
Overexpression of SCD5 partially suppresses the temperature-sensitive growth phenotype, but not the endocytic defect of chc1Δ scd1-v cells. (A) A chc1Δ scd1-v strain (SL3593) transformed with YEp24, YEp24-SCD5, or YCp50-CHC1 was streaked onto YEPD and incubated for 3 d at 30, 34, or 37°C. (B) Cells were grown to log phase in selective medium (C-URA). Radiolabeled α-factor was prebound to cells at 4°C in YEPD. Cells were pelleted, and then endocytosis was initiated by resuspending in prewarmed YEPD at 30°C. Samples were taken at the indicated times and cells were processed to determine percentage of α-factor internalized. Strains are chc1Δ scd1-v (SL3593) transformed with YEp24 (○), YEp24-SCD5 (▵) or YCp50-CHC1 (□).
Because scd5-Δ338 causes actin defects, we next examined whether clathrin deficiency affects actin organization. We made use of a strain expressing CHC1 under control of the repressible GAL1 promoter as its only source of clathrin HC. GAL1::CHC1 cells grown in galactose medium showed the normal pattern of actin localization by phalloidin staining (Figure 6A). Shifting to glucose for 15 h, which completely depletes Chc1p (Nelson and Lemmon, 1993), caused accumulation of larger actin patches. These large patches were usually delocalized to the mother cell during bud growth and were observed in 70% of the cells (Figure 6, B and I). The clathrin-depleted cells also displayed fewer actin patches at the mother-daughter neck during cytokinesis, when compared with cells expressing clathrin HC (Figure 6B). Furthermore, 35% of HC-depleted cells contained actin bars, whereas none of the cells grown on galactose contained G-actin bars (Figure 6, E, G, and I). Similar actin phenotypes were seen in chc1Δ and clathrin LC-deficient (clc1Δ) strains.
We next tested whether overexpression of SCD5 could suppress the actin organization defect of Chc1p-depleted cells. On galactose medium GAL1:CHC1 cells transformed with YEp-SCD5 had normal actin organization (Figure 6, C and F), although during cytokinesis more depolarized patches were observed in the mother and daughter cells (see Figure 6C, 2nd row, 3rd cell). However, in total, <10% of cells with YEpSCD5 had depolarized patches when grown on galactose, and no large patches or bars were observed (Figure 6F). On glucose medium the number of Chc− cells with large patches was significantly reduced by overexpression of SCD5 (Figure 6, D and I). Thus, extra Scd5p prevented aggregation of cortical actin. However, these normal size patches were still delocalized in the majority (83%) of the clathrin-deficient cells (Figure 6D). The actin bar defect of the Chc− cells was also partially suppressed by increased Scd5p (Figure 6, H and I). In addition, SCD5 overexpression partially suppressed the enlarged size and morphological defects observed in chc1Δ and clc1Δ mutants.
Genetic Interactions of scd5-Δ338 and chc1 Mutations
Because clathrin-deficient yeast have actin phenotypes and overexpression of SCD5 partially rescues this defect, we examined whether mutations in CHC1 and SCD5 cause synthetic phenotypes. We made use of a chc1-ts mutant (chc1-521), which exhibits TGN sorting and endocytic defects at 37°C, but is normal at 25°C (Seeger and Payne, 1992a, 1992b; Tan et al., 1993). The chc1-ts mutant was crossed to a scd5-Δ338 strain, and haploid progeny from tetrad dissection were analyzed. We found that although both the chc1-ts and scd5-Δ338 spore progeny grew up to 32 or 34°C, the chc1-ts scd5-Δ338 double mutants grew very poorly at 25°C and were completely inviable at 30°C or higher (Figure 7A). Also, fluid-phase endocytosis of LY in the double mutant was almost completely blocked at 25°C (only 7% of cells had vacuolar staining), although 98% of the chc1-ts and 39% of the scd5-Δ338 single mutants internalized the dye at this temperature (Figure 7B). Furthermore, although the chc1-ts mutant showed no actin phenotype at 25°C, it exacerbated the scd5-Δ338 cortical actin defect causing appearance of more and larger actin patches than were observed in the scd5 mutant alone (Figure 7C). There were no large patches in the chc1-ts mutant and <10% of scd5-Δ338 mutant had large patches, whereas 50% of the double mutant cells contained large patches of F-actin.
Figure 7.
Synthetic phenotypes are caused by combining scd5-Δ338 and chc1-521. (A) Growth of scd5-Δ338 chc1-521 double mutant. SL4182 (scd5-Δ338) was crossed to SL2726 (chc1-521) and subjected to tetrad analysis. Resulting spore clones were streaked onto YEPD plates and incubated for 3 d at 25, 32, and 37°C. An example of a tetratype tetrad is shown. (B) Lucifer yellow uptake defect in scd5-Δ338 chc1-521 mutant. Cells were grown to log phase in liquid YEPD at 25°C and incubated for 60 min with LY at 25°C. Top panels: LY fluorescence; bottom panels: location of vacuoles revealed by DIC imaging. Strains are as follows: (a) scd5-Δ338, SL3740; (b) chc1-521, SL2726; and (c) scd5-Δ338 chc1-521, SL4304. Bar, 5 μm. (C) Actin phenotype is exacerbated by combining scd5-Δ338 with chc1-521. Cells were grown to log phase at 25°C in liquid YEPD, stained with Alexa-568-phalloidin, and photographed as described in MATERIALS AND METHODS. Strains are the same as shown in B.
Clathrin and Scd5p Interact with Sla2p by Two-hybrid Analysis
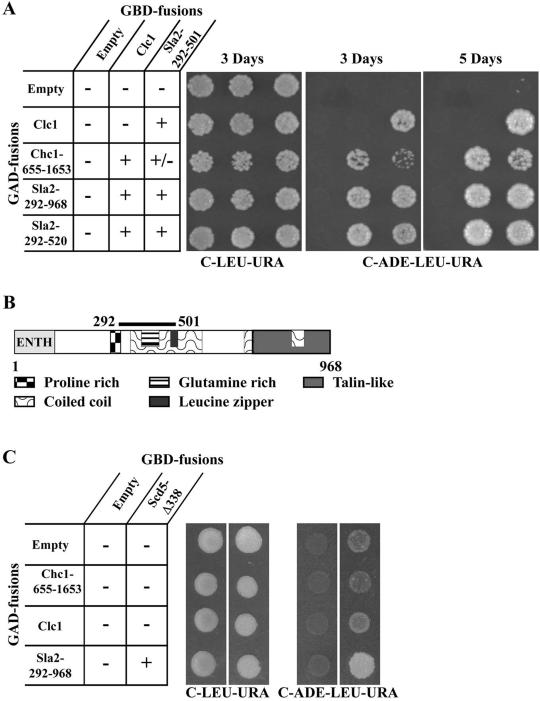
A yeast two-hybrid screen was carried out to identify clathrin LC interacting proteins (see MATERIALS AND METHODS). Thirteen positive clones were obtained. Two of the clones contained C-terminal fragments of Chc1p including the LC binding domain (Liu et al., 1995), as would be expected. The other 11 clones (representing five distinct isolates) contained various fragments of Sla2p coding sequences. Figure 8A shows two-hybrid interaction results with two of the LC interacting Sla2p clones. The smallest Sla2p prey coded for amino acids 292–520, which includes part of the predicted central coil-coiled domain as well as the glutamine-rich region, the leucine zipper, and a portion of the proline-rich region (Figure 8B; Wesp et al., 1997; Yang et al., 1999). A slightly smaller fragment (residues 292–501) also interacted with LC (Figure 8A). Interestingly, similar regions in the related mammalian proteins, HIP1 and HIP1R, were recently shown to interact with clathrin (Engqvist-Goldstein et al., 2001; Metzler et al., 2001; Mishra et al., 2001; Waelter et al., 2001). Chc1p-655–1653, which binds LC, also interacted with Sla2p-292–501. However, this association was somewhat weaker than the interaction of LC with HC or LC with the Sla2p coil domain, as the pGAD-CHC1-655–1653/pGBD-SLA2-292–501 transformant grew consistently more slowly on the C-adenine reporter plates. This suggests that the interaction of HC with Sla2p might be through LC. In addition, confirming previous studies (Wesp et al., 1997; Yang et al., 1999), we found that the coil-coiled domain of Sla2p can interact with itself (Figure 8A).
Figure 8.
Clathrin and Scd5p interact with Sla2p by two-hybrid analysis. (A) Yeast strain YPJ96-4 was transformed with GAL4 binding domain (GBD) bait plasmids pGBDU (empty), pKH19 (GBD-CLC1), or pKH47 (GBD-SLA2-292–501) in combination with GAL4 activation domain (GAD) prey plasmids pGAD (empty), pKH24 (GAD-CHC1-655–1653), pKH19 (GAD-CLC1), p31-10 (GAD-SLA2-292–968), or p31-2 (GAD-SLA2-292–520). Equal numbers of cells from transformants were spotted on complete synthetic medium lacking leucine and uracil (C-LEU-URA) and medium lacking adenine, leucine, and uracil (C-ADE-LEU-URA) and grown for 3 and 5 d. Interactions of baits and preys were scored for activation of the GAL2:: ADE2 reporter by growth on C-ADE-LEU-URA. (B) Model of Sla2p. Sla2p domains and region that associates with clathrin are indicated. (C) YPJ96-4 was transformed with GAL4 binding domain bait plasmids pGBDU (empty) or pNT1 (GBD-scd5-Δ338). SL3004 was transformed with GAL activation domain (GAD) plasmids pGAD (empty), pKH24 (GAD-CHC1-655–1653), pKH19 (GAD-CLC1), p31-10 (GAD-SLA2-292–968). Bait and prey plasmids were combined by the mating method. Cells were spotted on plates as described in A and grown for 3 d.
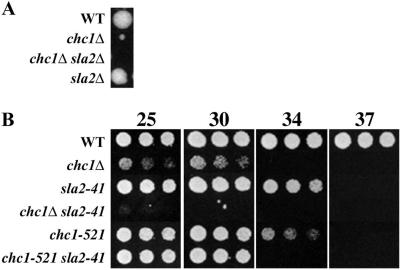
Genetic interactions were also observed between clathrin mutations and sla2, supporting the two-hybrid physical association results. Crosses of chc1 mutants to sla2 mutants resulted in strong synergistic growth phenotypes in double mutant spore progeny (Figure 9). In fact, chc1Δ was synthetically lethal with sla2Δ (Figure 9A). Similar results were observed when clc1Δ was combined with sla2 mutations. Overall these results suggest that clathrin and Sla2p function in related pathways.
Figure 9.
Clathrin mutants display negative synergistic growth defects with sla2. (A) sla2Δ and chc1Δ are synthetically lethal. SL4162 (sla2Δ) was crossed to SL13 (chc1Δ scd1-v YCp50-CHC1). The CHC1 plasmid was dropped, and the diploid was sporulated and dissected for tetrad analysis. An example of a tetratype tetrad is shown. (B) Tetrads were dissected from SL13 (chc1Δ scd1-v YCp50-CHC1) X SL4049 (sla2-41) that had lost YCp50-CHC1 or SL2726 (chc1-521) X SL4049. Overnight cultures of spore segregants were diluted to 107 cells/ml, and serial fourfold dilutions were made in YEPD. Equal volumes of cells were spotted on YEPD and plates were grown for 2 d at the indicated temperatures.
Because scd5-Δ338 shows synthetic lethality with sla2-41 (end4-1) (Table 3) and strong genetic interactions with chc1-521 (Figure 7), we tested whether Scd5p interacts with clathrin or Sla2p by two-hybrid analysis. When full-length Scd5p was fused with the GAL4 DNA binding domain (GBD), the fusion protein activated reporter genes even in the absence of the GAL4 activation domain (GAD) preys. However, the GBD-Scd5-Δ338 truncation only weakly autoactivated, allowing tests for interaction with Sla2p and clathrin preys. We found that Scd5-Δ338p could not interact with full-length LC or Chc1p-655–1653 (Figure 8C). However, a two-hybrid interaction was observed with the longer Sla2p-292–968 clone (Figure 8C) but not the Sla2p 292–520 fragment. Thus, the N-terminal region of Scd5p appears to associate with Sla2p, but this interaction depends on sequences C-terminal to the minimal region that binds clathrin LC.
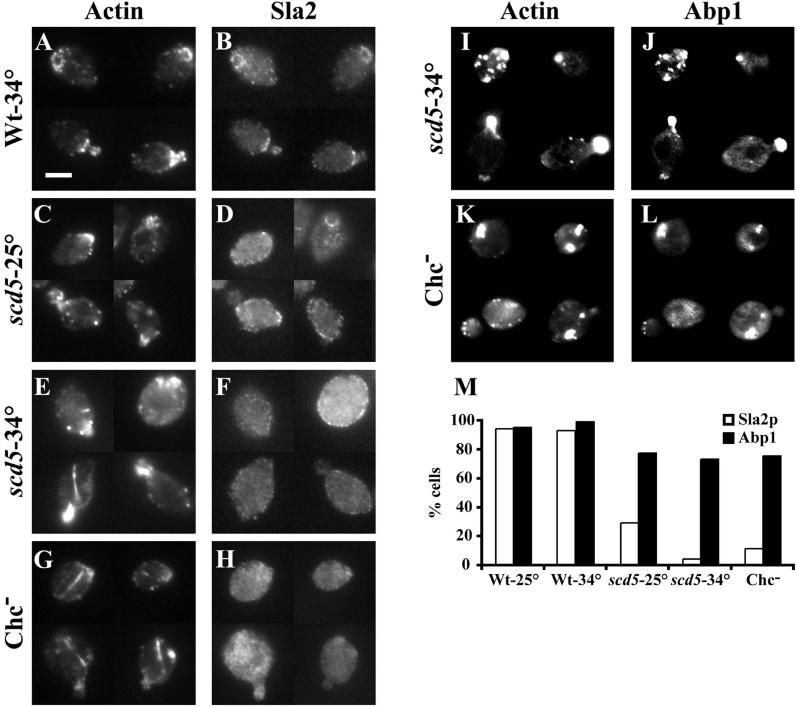
Clathrin and Scd5p Are Required for Normal Sla2p Localization
In wild-type cells, Sla2p partially colocalizes with cortical actin patches (Yang et al., 1999). This is most easily observed in unbudded and small budded cells where actin is highly polarized. Interestingly, not all actin patches stain with anti-Sla2p nor does every Sla2p patch have associated actin (Yang et al., 1999; Figure 10, A and B). Because Scd5p and Chc1p interact with Sla2p by two-hybrid analysis, we tested whether Sla2p localization is affected in scd5-Δ338 or Chc1p-depleted cells. In scd5-Δ338 cells grown at 25°C, Sla2p was partially delocalized to the cytoplasm, but there was still some residual cortical staining of Sla2p (Figure 10, C and D). This remaining cortical Sla2p was much less polarized than in wild-type cells, and less was found in actin patches (Figure 10, C, D, and M). After a 3-h shift to 34°C, nearly all of the Sla2p was cytoplasmic, although faint cortical rim staining was observed in some scd5-Δ338 cells (Figure 10F). However, these small patches at the cell periphery rarely colocalized with actin (Figure 10M). When GAL1::CHC1 cells were depleted of clathrin by growth on glucose, Sla2p was completely cytoplasmic in most cells (Figure 10, H and M), whereas cells grown on galactose showed the normal Sla2p staining pattern.
Figure 10.
Sla2p is mislocalized in scd5-Δ338 and clathrin-depleted cells. Wild-type (SL1528; A and B) and scd5-Δ338 (SL3920; C–F) strains were grown on YEPD to log phase at 25°C (C and D) or shifted to 34°C (A, B, E, F) for 3 h. The GAL1:: CHC1 strain (SL554; G and H) was grown to log phase in YEP-GAL and then shifted to YEPD for 15 h at 30°C to deplete clathrin HC. In I and J scd5-Δ338 (SL3920) + pJC4 [pGFP-ABP1] was grown in C-URA and shifted to 34°C for 3 h. In K and L the GAL1: CHC1 strain (SL554) + pJC4 [pGFP-ABP1] was grown in C-URA+GAL and then shifted to glucose medium for 15 h to deplete clathrin HC. Cells were fixed, stained with anti-actin (A, C, E, and G) and anti-Sla2p antibodies (B, D, F, and H); or in cells for GFP-Abp1p localization (J and L) actin was visualized with Alexa-594 phalloidin (I and K). (M) Quantification of the percent of cells with Sla2p or Abp1p showing colocalization with actin patches. Note that the GAL1∷CHC1 strain grown on galactose gave similar results as wild-type cells. Bar, 5 μm.
We also localized another cortical actin patch protein, Abp1p (Drubin et al., 1988), which was tagged with GFP. Interestingly, in the majority of scd5-Δ338 cells grown at either 25 or 34°C, as well as cells depleted of clathrin, GFP-Abp1p was still associated with actin patches (Figure 10, I–M). Similar results were obtained with Rvs167p. Thus, loss of Scd5p or clathrin function specifically affects Sla2p and does not cause a general effect on all cortical patch proteins.
DISCUSSION
Scd5p Plays a Role in Actin Organization and Endocytosis
In this study we found that Scd5p is crucial for normal actin organization and endocytosis. Both fluid-phase and receptor-mediated endocytosis were completely blocked in the scd5-Δ338 mutant at the nonpermissive temperature, which is a phenotype caused by many mutations that affect the cortical actin cytoskeleton. In these cells cortical actin patches were often larger or depolarized to the mother cell, and a major accumulation of G-actin in bar structures was observed, suggesting a defect in actin assembly or destabilization of F-actin. Other phenotypes typical of mutants with actin defects were observed in scd5-Δ338 (Pruyne and Bretscher, 2000a, 2000b), including delocalized chitin deposition, bud site selection defects, thickened cell walls, and the presence of multibudded and multinucleated cells. Supporting the phenotypic analysis, Scd5p colocalized with actin patches in the cell.
In our previous work on SCD5 we showed that the scd5-Δ338 mutation caused a partial post-Golgi secretory defect at the nonpermissive temperature (Nelson et al., 1996). Based on the evidence presented here, this secretory defect could be due to the effect of loss of Scd5p function on the actin cytoskeleton and/or endocytosis. Several other actin patch mutants display similar partial secretory defects or accumulation of secretory vesicles, including act1-1, myo3Δ myo5Δ, sla2Δ, rvs161Δ, and rvs167Δ (Novick and Botstein, 1985; Goodson et al., 1996; Mulholland et al., 1997; Breton et al., 2001). Budding continues and secretory vesicles still polarize to growth sites in many of these patch mutants, including scd5-Δ338 (K. Henry, unpublished results). It has been suggested that a defect in endocytosis may affect recycling of components (e.g., v-SNAREs) required for assembly of mature fusion-competent secretory vesicles (Pruyne and Bretscher, 2000a). However, we noted that the scd5 mutant had cables that were often misoriented within the cell, so the secretion phenotypes could be due to a partial effect on polarization of actin cables. This polarization defect might be caused indirectly by effects on cortical actin patches, which may contribute to anchoring of cables in the bud. Alternatively endocytic defects might affect localization of important membrane proteins that provide cues needed for cable polarization. A more complex function for Scd5p is suggested by the scd5-Δ338 budding pattern defect. Unlike most cortical patch mutants, which show defects only in the diploid bipolar budding pattern (Yang et al., 1997; Pruyne and Bretscher, 2000a), scd5-Δ338 was defective in both haploid and diploid specific budding. Also SCD5 is an essential gene, whereas many cortical actin patch components that affect endocytosis are not. This suggests that Scd5p could play a broader role in actin organization and cell polarization or may function in other cellular processes unrelated to actin.
scd5-Δ338 Shows Genetic Interactions with Mutations in Genes Encoding Other Cortical Actin Patch Components
We found that the scd5-Δ338 mutation caused more severe growth and actin organization phenotypes when combined with a number of mutations in cortical actin patch components, supporting the idea that Scd5p is important for cortical actin function. Interestingly, scd5-Δ338 was synthetically lethal with pan1-20 and end3Δ but phenotypes were not exacerbated with sla1Δ, even although Pan1p, End3p, and Sla1p exist in a complex (Tang et al., 2000; Zeng et al., 2001). This difference could be explained by the fact that pan1 and end3Δ mutants have severe endocytic phenotypes (Bénédetti et al., 1994; Tang et al., 1997; Duncan et al., 2001), whereas sla1Δ is only modestly affected (Ayscough et al., 1999; K. Henry, unpublished results). Because interaction of End3p with Pan1p is not dependent on the presence of Sla1p (Tang et al., 2000), perhaps Pan1p and End3p association are most critical for function of this complex. Also, END3, but not SLA1, overexpression rescues the temperature sensitivity of pan1-4, and overexpression of SLA1 prevents END3 overexpression from rescuing the pan1 mutant at 37°C (Tang et al., 2000). Thus, Sla1p may act antagonistically to End3p, which could explain the difference in genetic interactions with scd5-Δ338.
scd5-Δ338 had a strong genetic interaction with abp1Δ and rvs167Δ, but not srv2Δ. Interestingly both Srv2p (adenylyl cyclase–associated protein) and Rvs167p interact with Abp1p (Freeman et al., 1996; Lila and Drubin, 1997; Drees et al., 2001; Fazi et al., 2001). However, rvs167Δ, but not srv2Δ, shows a pattern of genetic interactions similar to abp1Δ when combined with other mutations in genes encoding actin cytoskeleton components (Lila and Drubin, 1997). Thus, the results with scd5-Δ338 further support the idea that Abp1p and Rvs167p functions are closely related.
Sla2p Localization Is Dependent on Scd5p
Scd5p is also physically associated with components of the cortical actin cytoskeleton. In addition to showing overlapping localization with cortical actin patches, Scd5p interacts with Sla2p by two-hybrid analysis. In preliminary studies, we find that it also interacts with Rvs167p, but not Rvs161p, by two-hybrid screening (J. Chang, unpublished results). Thus far we have not been able to demonstrate association of Sla2p and Scd5p by coprecipitation, but their interaction may be transient or only occur in the context of the plasma membrane or when assembled with cortical actin structures. However, based on the synthetic lethality caused by combining sla2-41 (end4-1) with scd5-Δ338, we believe the physical interactions detected by two-hybrid analysis are functionally important. As further support for this, we found that Sla2p dissociated from cortical structures and accumulated in the cytoplasm in scd5-Δ338 cells. The delocalization of Sla2p seen in scd5-Δ338 appears to be specific, because other actin patch-associated components, Abp1p and Rvs167p, localize in cortical sites relatively normally in these cells. Also, scd5-Δ338 cells still have phalloidin-staining actin patch-like structures, indicating the effect of scd5-Δ338 on Sla2p localization is not merely caused by actin depolymerization.
It is interesting to note that although Scd5-Δ338p associates with Sla2p by two-hybrid interaction, the same truncation mutation causes delocalization of Sla2p. Possibly the C-terminal region of Scd5p is important for its localization with cortical actin, and perturbation of Scd5p localization thereby affects Sla2p's association with actin patches.
Scd5p has also been shown to interact with Glc7p (Tu et al., 1996; Uetz et al., 2000; Venturi et al., 2000; JiSuk Chang, unpublished results), which is yeast PP1, a broad specificity Ser/Thr phosphatase with many cellular functions (Stark, 1996). PP1's activity is restricted toward physiological substrates in vivo by different regulatory or targeting subunits (Stark, 1996), so it is possible that Scd5p targets PP1 to dephosphorylate specific actin-associated components. It could play a major role in regulating assembly, disassembly, or specific organization of actin-associated factors needed for endocytosis. Supporting this idea, genetic interactions with scd5-Δ338 were observed with mutations in a number of actin-associated proteins that are known to be phosphorylated. One of these proteins is Sla2p, which interacts with Ark1p, an actin regulating kinase (ARK; Cope et al., 1999). Pan1p and Sla1p are phosphorylated by Prk1p (another ARK), as well as Ark1p (Zeng and Cai, 1999; Zeng et al., 2001). Recent evidence indicates that Prk1p phosphorylation disrupts association of Pan1p with Sla1p and End3p (Zeng et al., 2001). Also, in ark1Δ prk1Δ double mutants cortical F-actin patches, and many patch components, including Sla2p, are found in large aggregates (Cope et al., 1999). Because we find that Sla2p dissociates from the cortex in the scd5-Δ338 mutant, dephosphorylation of Sla2p or other patch components may be important for assembly in actin patches, and Scd5p/PP1 may regulate this.
Clathrin Is Important for Organization of Actin in Yeast
In previous studies we identified SCD5 as a multicopy suppressor of clathrin-deficient yeast, but scd5-Δ338 cells do not show the Golgi retention defects that lead to CPY sorting and alpha factor processing phenotypes seen in clathrin mutants (Nelson et al., 1996). Also, overexpression of SCD5 could not suppress these clathrin mutant phenotypes (Nelson and Lemmon, 1993). Here we report our discovery that clathrin mutants, like scd5-Δ338 cells, have major cortical actin defects and accumulate significant nonfilamentous G-actin. SCD5 overexpression partially suppressed these actin defects, and combining scd5-Δ338 with a chc1-ts mutation exacerbated growth, actin and endocytic phenotypes. Furthermore, clathrin mutations genetically interact with sla2 mutations, and Sla2p association with the cortex is dependent on the presence of clathrin. Previous studies are also consistent with a connection between clathrin and actin in yeast. Clathrin-deficient yeast become polyploid at high frequency, and aberrant nuclear divisions within the mother cell are observed (Lemmon and Jones, 1987; Lemmon et al., 1990). Like scd5-Δ338 and other actin patch mutants, clathrin mutants are enlarged and round in appearance, they have delocalized chitin deposition, and their cell walls are thickened (Lemmon and Jones, 1987; Lemmon et al., 1990; S.K. Lemmon, unpublished results). Moreover, clathrin interacts with other cortical patch components, such as Ent1/2p (Wendland et al., 1999), in addition to Sla2p. Therefore, we suggest that clathrin plays a role in cortical actin function in yeast.
Clathrin Interacts with Sla2p
Clathrin is a trimeric molecule with three HC's joined at the C terminus and a LC bound to each leg in the hub region adjacent to the triskelion vertex. Each HC contains an N-terminal globular terminal domain (TD) that interacts with a number of proteins containing a signature clathrin binding motif (“clathrin box”) related to LLDLD in amphiphysin (Dell'Angelica, 2001). This interaction is thought to facilitate the recruitment of clathrin and many of its associated factors to sites of clathrin-mediated transport. The yeast epsins (Ent1/2p) and AP180-like proteins (Yap1801p/Yap1802p) both bind to the Chc1p TD via this type of clathrin binding motif (Wendland and Emr, 1998; Wendland et al., 1999). Interestingly, we identified Sla2p in a two-hybrid screen with the clathrin LC. Although Sla2p has some sequences distantly related to the LLDLD motif, we found that the clathrin LC interacted with a portion of the Sla2p central coiled-coil domain, which does not contain potential clathrin TD-binding sequences. A fragment of clathrin HC including the LC binding region, but not the TD, also interacted with Sla2p, albeit more weakly than LC. In preliminary studies we find that LC interacts with Sla2p by two-hybrid analysis in a chc1Δ strain, indicating that this association is not dependent on HC (T. Newpher, unpublished results). We note that, because Sla2p dimerizes via the central coiled-coil domain (Wesp et al., 1997; Yang et al., 1999; this study), the two-hybrid coiled-coil product could associate with endogenous Sla2p. Thus, we cannot yet exclude the possibility that the LC interacts with another portion of Sla2p or that clathrin HC interacts with other Sla2p regions, possibly via the TD. However, consistent with an importance for the LC-Sla2p coiled-coil interaction, deletion of the Sla2p central coiled-coil domain causes defects in endocytosis and actin organization that resemble those of clathrin-deficient cells (Wesp et al., 1997; Yang et al., 1999).
Recent work has shown that the mammalian Sla2p-related proteins, HIP1 and HIP1R, localize to sites of clathrin-mediated endocytosis in vivo, are enriched in clathrin-coated vesicles, and bind directly to clathrin (Engqvist-Goldstein et al., 1999, 2001; Metzler et al., 2001; Mishra et al., 2001; Waelter et al., 2001). Additional studies indicate that that HIP1R may link the actin cytoskeleton to coated pits and suggest that cortical actin helps organize or isolate regions of active clathrin-mediated internalization (Gaidarov et al., 1999; Bennett et al., 2001; Engqvist-Goldstein et al., 2001). Thus, HIP1(R) may be important for this organization of coated-pits or may be actively involved in the endocytic process itself.
The binding of clathrin to HIP1 and HIP1R appear to be distinct. In HIP1, there are clathrin N-TD-binding sequences as well as AP-2 binding motifs (DPF and FXDXF) adjacent to the central coiled-coil region (Metzler et al., 2001; Mishra et al., 2001; Waelter et al., 2001). In HIP1R the binding to clathrin appears to occur principally via the coil-coiled region and involves a direct interaction with clathrin LC (Engqvist-Goldstein et al., 2001; Legendre-Guillemin et al., 2002), similar to our findings of Sla2p and yeast LC binding. In addition, overexpression of a hub fragment of clathrin, which contains the LC binding site and inhibits clathrin-mediated endocytosis, causes dissociation of HIP1R from coated pits in HeLa cells (Bennett et al., 2001). Similarly, we found that depletion of clathrin causes dissociation of Sla2p from the cell cortex. Overall this indicates there are close connections between clathrin, Sla2p-related proteins and the actin cytoskeleton in all eukaryotes.
Why then might clathrin deficiency in yeast only cause a partial defect in endocytosis? Clathrin may help collect specific membrane proteins, localize regulatory factors and or mark/domains for endocytosis, but not be absolutely required for generating force for internalization, which may be primarily an actin-mediated process in yeast. Clathrin may be diffusely distributed in the cortical actin network, which would explain why surface-coated pits have yet to be detected by microscopic methods. Possibly, classic surface-derived CCV do not even form in yeast, although some plasma membrane proteins have been detected in enriched CCV fractions (Pishvaee et al., 2000). Still, it has not been ruled out that these vesicles are of endosomal origin or on a recycling trajectory (Valdivia et al., 2002). Recent work also indicates that clathrin may be important for formation of a subclass of secretory vesicles, so CCV could contain newly synthesized plasma membrane proteins (Gurunathan et al., 2002; Harsay and Schekman, 2002). Nonetheless, our findings suggest that the endocytic phenotype of clathrin-deficient cells may relate to a role for clathrin in actin-associated processes. This effect may only be partial because clathrin is one of several factors that facilitate organization or stabilization of the cortical actin network. Interestingly, many homologues of mammalian clathrin associated factors that are involved in endocytosis in yeast (e.g., Ent1/2p [epsins], Pan1p [Eps15-related], and Rvs161 and Rvs167 [amphiphysins]), are also important for actin organization (Munn et al., 1995; Sivadon et al., 1995; Tang and Cai, 1996; Wendland et al., 1996; Balguerie et al., 1999; Wendland et al., 1999).
A final question concerns why SCD5 was isolated as a multicopy suppressor of clathrin-deficient lethality. Mutations in both clathrin and SCD5 show genetic interactions with sla2 and both clathrin and Scd5p are required for localization of Sla2p at the cell cortex. Our data suggest that Scd5p, as a potential targeting subunit for PP1, may promote the recruitment of proteins, like Sla2p, to the cortical actin network. Thus, overexpression of SCD5 could counter the destabilization of cortical actin components that might occur in the absence of clathrin. Further studies will be directed toward determining whether Scd5p plays a regulatory role in actin organization and endocytosis and determining what are its targets.
ACKNOWLEDGMENTS
We thank David Drubin, Mark Rose, Beverly Wendland, Clarence Chan, and Scott Vande Pol for kindly providing strains, plasmids, and antibodies. The authors also thank Dan Gelperin, Jo Ann Wise, and Susann Brady-Kalnay for their advice and many helpful discussions and Maryanne Pendergast for help with confocal microscopy. K.R.H. was supported by a National Institutes of Health (NIH) training grant (T32 AG00105) and an individual National Research Service Award (NRSA) Minority Predoctoral Fellowship (F31 GM20082). K.H. was the recipient of an NRSA postdoctoral fellowship (F32 GM17370) from NIH. R.T.H. was supported by NIH training grant (T32 DK7319-23). This work was funded by the NIH (R01 GM55796) and the American Cancer Society (RPG-9403104-MBC to S.K.L.), the Canton Basel-Stadt and the Swiss National Science Foundation (H.R.), and the Roche Research Foundation (S.K.L. and H.R.). S.K.L. was the recipient of a Career Advancement Award from the National Science Foundation.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–01–0012. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–01–0012.
REFERENCES
- Adams AE, Botstein D, Drubin DG. Requirement of yeast fimbrin for actin organization and morphogenesis in vivo. Nature. 1991;354:404–408. doi: 10.1038/354404a0. [DOI] [PubMed] [Google Scholar]
- Adams AE, Pringle JR. Relationship of actin and tubulin distribution to bud growth in wild- type and morphogenetic-mutant Saccharomyces cerevisiae. J Cell Biol. 1984;98:934–945. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams AE, Pringle JR. Staining of actin with fluorochrome-conjugated phalloidin. Methods Enzymol. 1991;194:729–731. doi: 10.1016/0076-6879(91)94054-g. [DOI] [PubMed] [Google Scholar]
- Apodaca G. Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Traffic. 2001;2:149–159. doi: 10.1034/j.1600-0854.2001.020301.x. [DOI] [PubMed] [Google Scholar]
- Ayscough KR, Eby JJ, Lila T, Dewar H, Kozminski KG, Drubin DG. Sla1p is a functionally modular component of the yeast cortical actin cytoskeleton required for correct localization of both Rho1p-GTPase and Sla2p, a protein with talin homology. Mol Biol Cell. 1999;10:1061–1075. doi: 10.1091/mbc.10.4.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayscough KR, Stryker J, Pokala N, Sanders M, Crews P, Drubin DG. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J Cell Biol. 1997;137:399–416. doi: 10.1083/jcb.137.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggett JJ, Wendland B. Clathrin function in yeast endocytosis. Traffic. 2001;2:297–302. doi: 10.1034/j.1600-0854.2001.002005297.x. [DOI] [PubMed] [Google Scholar]
- Balguerie A, Sivadon P, Bonneu M, Aigle M. Rvs167p, the budding yeast homolog of amphiphysin, colocalizes with actin patches. J Cell Sci. 1999;112:2529–2537. doi: 10.1242/jcs.112.15.2529. [DOI] [PubMed] [Google Scholar]
- Bauer F, Urdaci M, Aigle M, Crouzet M. Alteration of a yeast SH3 protein leads to conditional viability with defects in cytoskeletal and budding patterns. Mol Cell Biol. 1993;13:5070–5084. doi: 10.1128/mcb.13.8.5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénédetti H, Raths S, Crausaz F, Riezman H. The END3 gene encodes a protein that is required for the internalization step of endocytosis and for actin cytoskeleton organization in yeast. Mol Biol Cell. 1994;5:1023–1037. doi: 10.1091/mbc.5.9.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmerah A, Begue B, Dautryvarsat A, Cerfbensussan N. The ear of alpha-adaptin interacts with the COOH-terminal domain of the Eps15 protein. J Biol Chem. 1996;271:12111–12116. doi: 10.1074/jbc.271.20.12111. [DOI] [PubMed] [Google Scholar]
- Bennett EM, Chen C-Y, Engqvist-Goldstein AEY, Drubin DG, Brodsky FM. Clathrin hub expression dissociates the actin binding protein Hip1R from coated pits and disrupts their alignment with the actin cytoskeleton. Traffic. 2001;2:851–858. doi: 10.1034/j.1600-0854.2001.21114.x. [DOI] [PubMed] [Google Scholar]
- Botstein D, Amberg D, Mullholland J, Huffaker T, Adams A, Drubin D, Stearns T. The yeast cytoskeleton. In: Pringle JR, Broach JR, Jones EW, editors. The Molecular and Cellular Biology of the Yeast Saccharomyces: Cell Cycle and Cell Biology. Vol. 3. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 1–90. [Google Scholar]
- Botstein D, Falco SC, Stewart SE, Brennan M, Scherer S, Stinchcomb DT, Struhl K, Davis RW. Sterile host yeasts (SHY): a eukaryotic system of biological containment for recombinant DNA experiments. Gene. 1979;8:17–24. doi: 10.1016/0378-1119(79)90004-0. [DOI] [PubMed] [Google Scholar]
- Breton AM, Schaeffer J, Aigle M. The yeast Rvs161 and Rvs167 proteins are involved in secretory vesicles targeting the plasma membrane and in cell integrity. Yeast. 2001;18:1053–1068. doi: 10.1002/yea.755. [DOI] [PubMed] [Google Scholar]
- Brodsky FM, Chen CY, Knuehl C, Towler MC, Wakeham DE. Biological basket weaving. Formation and function of clathrin-coated vesicles. Annu Rev Cell Dev Biol. 2001;17:517–568. doi: 10.1146/annurev.cellbio.17.1.517. [DOI] [PubMed] [Google Scholar]
- Chen H, Fre S, Slepnev VI, Capua MR, Takei K, Butler MH, Di Fiore PP, De Camilli P. Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature. 1998;394:793–797. doi: 10.1038/29555. [DOI] [PubMed] [Google Scholar]
- Cope MJ, Yang S, Shang C, Drubin DG. Novel protein kinases Ark1p and Prk1p associate with and regulate the cortical actin cytoskeleton in budding yeast. J Cell Biol. 1999;144:1203–1218. doi: 10.1083/jcb.144.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton RG. Detection of mutations in DNA. Curr Opin Biotechnol. 1992;3:24–30. doi: 10.1016/0958-1669(92)90121-x. [DOI] [PubMed] [Google Scholar]
- D'Hondt K, Heese-Peck A, Riezman H. Protein and lipid requirements for endocytosis. Annu Rev Genet. 2000;34:255–295. doi: 10.1146/annurev.genet.34.1.255. [DOI] [PubMed] [Google Scholar]
- David C, McPherson PS, Mundigl O, DeCamilli P. A role of amphiphysin in synaptic vesicle endocytosis suggested by its binding to dynamin in nerve terminals. Proc Natl Acad Sci USA. 1996;93:331–335. doi: 10.1073/pnas.93.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Angelica EC. Clathrin-binding proteins: got a motif? Join the network! Trends Cell Biol. 2001;11:315–318. doi: 10.1016/s0962-8924(01)02043-8. [DOI] [PubMed] [Google Scholar]
- DiFiore PP, Pelicci PG, Sorkin A. EH: a novel protein-protein interaction domain potentially involved in intracellular sorting. Trends Biochem Sci. 1997;22:411–413. doi: 10.1016/s0968-0004(97)01127-4. [DOI] [PubMed] [Google Scholar]
- Drake MT, Downs MA, Traub LM. Epsin binds to clathrin by associating directly with the clathrin-terminal domain. Evidence for cooperative binding through two discrete sites. J Biol Chem. 2000;275:6479–6489. doi: 10.1074/jbc.275.9.6479. [DOI] [PubMed] [Google Scholar]
- Drees BL, et al. A protein interaction map for cell polarity development. J Cell Biol. 2001;154:549–576. doi: 10.1083/jcb.200104057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin DG, Miller KG, Botstein D. Yeast actin-binding proteins: evidence for a role in morphogenesis. J Cell Biol. 1988;107:2551–2561. doi: 10.1083/jcb.107.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin DG, Mulholland J, Zhu ZM, Botstein D. Homology of a yeast actin-binding protein to signal transduction proteins and myosin-I. Nature. 1990;343:288–290. doi: 10.1038/343288a0. [DOI] [PubMed] [Google Scholar]
- Dulic V, Egerton M, Elguindi I, Raths S, Singer B, Riezman H. Yeast endocytosis assays. Methods Enzymol. 1991;194:697–710. doi: 10.1016/0076-6879(91)94051-d. [DOI] [PubMed] [Google Scholar]
- Duncan MC, Cope MJ, Goode BL, Wendland B, Drubin DG. Yeast Eps15-like endocytic protein, Pan1p, activates the Arp2/3 complex. Nat Cell Biol. 2001;3:687–690. doi: 10.1038/35083087. [DOI] [PubMed] [Google Scholar]
- Engqvist-Goldstein AE, Kessels MM, Chopra VS, Hayden MR, Drubin DG. An actin-binding protein of the Sla2/Huntingtin interacting protein 1 family is a novel component of clathrin-coated pits and vesicles. J Cell Biol. 1999;147:1503–1518. doi: 10.1083/jcb.147.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engqvist-Goldstein AE, Warren RA, Kessels MM, Keen JH, Heuser J, Drubin DG. The actin-binding protein Hip1R associates with clathrin during early stages of endocytosis and promotes clathrin assembly in vitro. J Cell Biol. 2001;154:1209–1224. doi: 10.1083/jcb.200106089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazi B, et al. Unusual binding properties of the SH3 domain of the yeast actin binding protein Abp1: structural and functional analysis. J Biol Chem. 2001;19:5290–5298. doi: 10.1074/jbc.M109848200. [DOI] [PubMed] [Google Scholar]
- Ford MG, Pearse BM, Higgins MK, Vallis Y, Owen DJ, Gibson A, Hopkins CR, Evans PR, McMahon HT. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science. 2001;291:1051–1055. doi: 10.1126/science.291.5506.1051. [DOI] [PubMed] [Google Scholar]
- Freeman NL, Lila T, Mintzer KA, Chen Z, Pahk AJ, Ren R, Drubin DG, Field J. A conserved proline-rich region of the Saccharomyces cerevisiae cyclase-associated protein binds SH3 domains and modulates cytoskeletal localization. Mol Cell Biol. 1996;16:548–556. doi: 10.1128/mcb.16.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto LM, Roth R, Heuser JE, Schmid SL. Actin assembly plays a variable, but not obligatory role in receptor-mediated endocytosis in mammalian cells. Traffic. 2000;1:161–171. doi: 10.1034/j.1600-0854.2000.010208.x. [DOI] [PubMed] [Google Scholar]
- Gagny B, Wiederkehr A, Dumoulin P, Winsor B, Riezman H, Haguenauer-Tsapis R. A novel EH domain protein of Saccharomyces cerevisiae, Ede1p, involved in endocytosis. J Cell Sci. 2000;113:3309–3319. doi: 10.1242/jcs.113.18.3309. [DOI] [PubMed] [Google Scholar]
- Gaidarov I, Santini F, Warren RA, Keen JH. Spatial control of coated-pit dynamics in living cells. Nat Cell Biol. 1999;1:1–7. doi: 10.1038/8971. [DOI] [PubMed] [Google Scholar]
- Gietz RD, Schiestl RH, Willems AR, Woods RA. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast. 1995;11:355–360. doi: 10.1002/yea.320110408. [DOI] [PubMed] [Google Scholar]
- Goodson HV, Anderson BL, Warrick HM, Pon LA, Spudich JA. Synthetic lethality screen identifies a novel yeast myosin I gene (MYO5): myosin I proteins are required for polarization of the actin cytoskeleton. J Cell Biol. 1996;133:1277–1291. doi: 10.1083/jcb.133.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurunathan S, David D, Gerst JE. Dynamin and clathrin are required for the biogenesis of a distinct class of secretory vesicles in yeast. EMBO J. 2002;21:602–614. doi: 10.1093/emboj/21.4.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie C, Fink GR. Guide to Yeast Genetics and Molecular Biology. San Diego: Academic Press; 1991. p. 933. pp. [Google Scholar]
- Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Harsay E, Schekman R. A subset of yeast vacuolar protein sorting mutants is blocked in one branch of the exocytic pathway. J Cell Biol. 2002;156:271–285. doi: 10.1083/jcb.200109077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil-Chapdelaine RA, Tran NK, Cooper JA. The role of Saccharomyces cerevisiae coronin in the actin and microtubule cytoskeletons. Curr Biol. 1998;8:1281–1284. doi: 10.1016/s0960-9822(07)00539-8. [DOI] [PubMed] [Google Scholar]
- Holtzman DA, Yang S, Drubin DG. Synthetic-lethal interactions identify two novel genes, SLA1 and SLA2, that control membrane cytoskeleton assembly in Saccharomyces cerevisiae. J Cell Biol. 1993;122:635–644. doi: 10.1083/jcb.122.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang KM, Gullberg L, Nelson KK, Stefan CJ, Blumer K, Lemmon SK. Novel functions of clathrin light chains: clathrin heavy chain trimerization is defective in light chain-deficient yeast. J Cell Sci. 1997;110:899–910. doi: 10.1242/jcs.110.7.899. [DOI] [PubMed] [Google Scholar]
- Iannolo G, Salcini AE, Gaidarov I, Goodman OB, Baulida J, Carpenter G, Pelicci PG, DiFiore PP, Keen JH. Mapping of the molecular determinants involved in the interaction between Eps15 and AP-2. Cancer Res. 1997;57:240–245. [PubMed] [Google Scholar]
- Itoh T, Koshiba S, Kigawa T, Kikuchi A, Yokoyama S, Takenawa T. Role of the ENTH domain in phosphatidylinositol-4,5-bisphosphate binding and endocytosis. Science. 2001;291:1047–1051. doi: 10.1126/science.291.5506.1047. [DOI] [PubMed] [Google Scholar]
- James P, Halladay J, Craig EA. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics. 1996;144:1425–1436. doi: 10.1093/genetics/144.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalchman MA, et al. HIP1, a human homologue of S. cerevisiae Sla2p, interacts with membrane-associated huntingtin in the brain. Nat Genet. 1997;16:44–53. doi: 10.1038/ng0597-44. [DOI] [PubMed] [Google Scholar]
- Kessels MM, Engqvist-Goldstein AE, Drubin DG, Qualmann B. Mammalian Abp1, a signal-responsive F-actin-binding protein, links the actin cytoskeleton to endocytosis via the GTPase dynamin. J Cell Biol. 2001;153:351–366. doi: 10.1083/jcb.153.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kübler E, Riezman H. Actin and fimbrin are required for the internalization step of endocytosis in yeast. EMBO J. 1993;12:2855–2862. doi: 10.1002/j.1460-2075.1993.tb05947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel TA, Roberts JD, Zakour RA. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Legendre-Guillemin V, Metzler M, Charbonneau M, Gan L, Chopra V, Philie J, Hayden MR, McPherson PS. HIP1 and HIP12 display differential binding to F-actin, AP2, and clathrin: identification of a novel interaction with clathrin-light chain. J Biol Chem. 2002;277:19897–19904. doi: 10.1074/jbc.M112310200. [DOI] [PubMed] [Google Scholar]
- Lemmon SK, Freund C, Conley K, Jones EW. Genetic instability of clathrin-deficient strains of S. cerevisiae. Genetics. 1990;124:27–38. doi: 10.1093/genetics/124.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon SK, Jones EW. Clathrin requirement for normal growth of yeast. Science. 1987;238:504–509. doi: 10.1126/science.3116672. [DOI] [PubMed] [Google Scholar]
- Lila T, Drubin DG. Evidence for physical and functional interactions among two S. cerevisiae SH3 domain proteins, an adenylyl cyclase-associated protein and the actin cytoskeleton. Mol Biol Cell. 1997;8:367–385. doi: 10.1091/mbc.8.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SH, Wong ML, Craik CS, Brodsky FM. Regulation of clathrin assembly and trimerization defined using recombinant triskelion hubs. Cell. 1995;83:257–267. doi: 10.1016/0092-8674(95)90167-1. [DOI] [PubMed] [Google Scholar]
- Madania A, Dumoulin P, Grava S, Kitamoto H, Scharer-Brodbeck C, Soulard A, Moreau V, Winsor B. The S. cerevisiae homologue of human Wiskott-Aldrich syndrome protein Las17p interacts with the Arp2/3 complex. Mol Biol Cell. 1999;10:3521–3538. doi: 10.1091/mbc.10.10.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann RO, Craig SW. The I/LWEQ module: a conserved sequence that signifies F-actin binding in functionally diverse proteins from yeast to mammals. Proc Natl Acad Sci USA. 1997;94:5679–5684. doi: 10.1073/pnas.94.11.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClary JA, Witney F, Geisselsoder J. Efficient site-directed in vitro mutagenesis using phagemid vectors. Biotechniques. 1989;7:282–289. [PubMed] [Google Scholar]
- McMahon HT. Endocytosis: an assembly protein for clathrin cages. Curr Biol. 1999;9:R332–R335. doi: 10.1016/s0960-9822(99)80206-1. [DOI] [PubMed] [Google Scholar]
- McPherson PS, et al. A presynaptic inositol-5-phosphatase. Nature. 1996;379:353–357. doi: 10.1038/379353a0. [DOI] [PubMed] [Google Scholar]
- Metzler M, Legendre-Guillemin V, Gan L, Chopra V, Kwok A, McPherson PS, Hayden MR. HIP1 functions in clathrin-mediated endocytosis through binding to clathrin and adaptor protein 2. J Biol Chem. 2001;276:39271–39276. doi: 10.1074/jbc.C100401200. [DOI] [PubMed] [Google Scholar]
- Mishra SK, Agostinelli NR, Brett TJ, Mizukami I, Ross TS, Traub LM. Clathrin- and AP-2-binding sites in HIP1 uncover a general assembly role for endocytic accessory proteins. J Biol Chem. 2001;276:46230–46236. doi: 10.1074/jbc.M108177200. [DOI] [PubMed] [Google Scholar]
- Mulholland J, Preuss D, Moon A, Wong A, Drubin D, Botstein D. Ultrastructure of the yeast actin cytoskeleton and its association with the plasma membrane. J Cell Biol. 1994;125:381–391. doi: 10.1083/jcb.125.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland J, Wesp A, Riezman H, Botstein D. Yeast actin cytoskeleton mutants accumulate a new class of Golgi-derived secretary vesicle. Mol Biol Cell. 1997;8:1481–1499. doi: 10.1091/mbc.8.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn AL, Stevenson BJ, Geli MI, Riezman H. end5, end6, and end7: mutations that cause actin delocalization and block the internalization step of endocytosis in S. cerevisiae. Mol Biol Cell. 1995;6:1721–1742. doi: 10.1091/mbc.6.12.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson KK, Holmer M, Lemmon SK. SCD5, a suppressor of clathrin deficiency, encodes a novel protein with a late secretory function in yeast. Mol Biol Cell. 1996;7:245–260. doi: 10.1091/mbc.7.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson KK, Lemmon SK. Suppressors of clathrin deficiency: overexpression of ubiquitin rescues lethal strains of clathrin-deficient S. cerevisiae. Mol Cell Biol. 1993;13:521–532. doi: 10.1128/mcb.13.1.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P, Botstein D. Phenotypic analysis of temperature-sensitive yeast actin mutants. Cell. 1985;40:405–416. doi: 10.1016/0092-8674(85)90154-0. [DOI] [PubMed] [Google Scholar]
- Payne GS, Baker D, van Tuinen E, Schekman R. Protein transport to the vacuole and receptor-mediated endocytosis by clathrin heavy chain-deficient yeast. J Cell Biol. 1988;106:1453–1461. doi: 10.1083/jcb.106.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pishvaee B, Costaguta G, Yeung BG, Ryazantsev S, Greener T, Greene LE, Eisenberg E, McCaffery JM, Payne GS. A yeast DNA J protein required for uncoating of clathrin-coated vesicles in vivo. Nat Cell Biol. 2000;2:958–963. doi: 10.1038/35046619. [DOI] [PubMed] [Google Scholar]
- Pringle JR. Staining of bud scars and other cell wall chitin with calcofluor. Methods Enzymol. 1991;194:732–735. doi: 10.1016/0076-6879(91)94055-h. [DOI] [PubMed] [Google Scholar]
- Pringle JR, Adams AE, Drubin DG, Haarer BK. Immunofluorescence methods for yeast. Methods Enzymol. 1991;194:565–602. doi: 10.1016/0076-6879(91)94043-c. [DOI] [PubMed] [Google Scholar]
- Pruyne D, Bretscher A. Polarization of cell growth in yeast. J Cell Sci. 2000a;113:571–585. doi: 10.1242/jcs.113.4.571. [DOI] [PubMed] [Google Scholar]
- Pruyne D, Bretscher A. Polarization of cell growth in yeast. I. Establishment and maintenance of polarity states. J Cell Sci. 2000b;113:365–375. doi: 10.1242/jcs.113.3.365. [DOI] [PubMed] [Google Scholar]
- Qualmann B, Kessels MM, Kelly RB. Molecular links between endocytosis and the actin cytoskeleton. J Cell Biol. 2000;150:F111–F116. doi: 10.1083/jcb.150.5.f111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramjaun AR, McPherson PS. Multiple amphiphysin II splice variants display differential clathrin binding: identification of two distinct clathrin-binding sites. J Neurochem. 1998;70:2369–2376. doi: 10.1046/j.1471-4159.1998.70062369.x. [DOI] [PubMed] [Google Scholar]
- Raths S, Rohrer J, Crausaz F, Riezman H. end3 and end4: two mutants defective in receptor-mediated and fluid-phase endocytosis in S. cerevisiae. J Cell Biol. 1993;120:55–65. doi: 10.1083/jcb.120.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaerer-Brodbeck C, Riezman H. Functional interactions between the p35 subunit of the Arp2/3 complex and calmodulin in yeast. Mol Biol Cell. 2000;11:1113–1127. doi: 10.1091/mbc.11.4.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger M, Payne GS. A role for clathrin in the sorting of vacuolar proteins in the Golgi complex of yeast. EMBO J. 1992a;11:2811–2818. doi: 10.1002/j.1460-2075.1992.tb05348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger M, Payne GS. Selective and immediate effects of clathrin heavy chain mutations on Golgi membrane protein retention in S. cerevisiae. J Cell Biol. 1992b;118:531–540. doi: 10.1083/jcb.118.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki N, Muramatsu M, Sugano S, Suzuki Y, Nakagawara A, Ohhira M, Hayashi A, Hori T, Saito T. Cloning, expression analysis, and chromosomal localization of HIP1R, an isolog of huntingtin interacting protein (HIP1) J Hum Genet. 1998;43:268–271. doi: 10.1007/s100380050087. [DOI] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in S. cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivadon P, Bauer F, Aigle M, Crouzet M. Actin cytoskeleton and budding pattern are altered in the yeast rvs161 mutant: the Rvs161 protein shares common domains with the brain protein amphiphysin. Mol Gen Genet. 1995;246:485–495. doi: 10.1007/BF00290452. [DOI] [PubMed] [Google Scholar]
- Slepnev VI, Ochoa GC, Butler MH, DeCamilli P. Tandem arrangement of the clathrin and AP-2 binding domains in amphiphysin 1 and disruption of clathrin coat function by amphiphysin fragments comprising these sites. J Biol Chem. 2000;275:17583–17589. doi: 10.1074/jbc.M910430199. [DOI] [PubMed] [Google Scholar]
- Stark MJR. Yeast protein serine/threonine phosphatases: multiple roles and diverse regulation. Yeast. 1996;12:1647–1675. doi: 10.1002/(SICI)1097-0061(199612)12:16%3C1647::AID-YEA71%3E3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Tan PK, Davis NG, Sprague GF, Payne GS. Clathrin facilitates the internalization of seven transmembrane segment receptors for mating pheromones in yeast. J Cell Biol. 1993;123:1707–1716. doi: 10.1083/jcb.123.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang HY, Cai M. The EH-domain-containing protein Pan1 is required for normal organization of the actin cytoskeleton in S. cerevisiae. Mol Cell Biol. 1996;16:4897–4914. doi: 10.1128/mcb.16.9.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang HY, Munn A, Cai M. EH domain proteins Pan1p and End3p are components of a complex that plays a dual role in organization of the cortical actin cytoskeleton and endocytosis in S. cerevisiae. Mol Cell Biol. 1997;17:4294–4304. doi: 10.1128/mcb.17.8.4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang HY, Xu J, Cai M. Pan1p, End3p, and S1a1p, three yeast proteins required for normal cortical actin cytoskeleton organization, associate with each other and play essential roles in cell wall morphogenesis. Mol Cell Biol. 2000;20:12–25. doi: 10.1128/mcb.20.1.12-25.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub LM, Downs MA, Westrich JL, Fremont DH. Crystal structure of the alpha appendage of AP-2 reveals a recruitment platform for clathrin-coat assembly. Proc Natl Acad Sci USA. 1999;96:8907–8912. doi: 10.1073/pnas.96.16.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu JL, Song WJ, Carlson M. Protein phosphatase type 1 interacts with proteins required for meiosis and other cellular processes in S. cerevisiae. Mol Cell Biol. 1996;16:4199–4206. doi: 10.1128/mcb.16.8.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetz P, et al. A comprehensive analysis of protein-protein interactions in S. cerevisiae. Nature. 2000;403:623–627. doi: 10.1038/35001009. [DOI] [PubMed] [Google Scholar]
- Valdivia RH, Baggott D, Chuang JS, Schekman RW. The yeast clathrin adaptor protein complex 1 is required for the efficient retention of a subset of late Golgi membrane proteins. Dev Cell. 2002;2:283–294. doi: 10.1016/s1534-5807(02)00127-2. [DOI] [PubMed] [Google Scholar]
- Venturi GM, Bloecher A, Williams-Hart T, Tatchell K. Genetic interactions between GLC7, PPZ1 and PPZ2 in S. cerevisiae. Genetics. 2000;155:69–83. doi: 10.1093/genetics/155.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojtek A, Haarer B, Field J, Gerst J, Pollard TD, Brown S, Wigler M. Evidence for a functional link between profilin and CAP in the yeast S. cerevisiae. Cell. 1991;66:497–505. doi: 10.1016/0092-8674(81)90013-1. [DOI] [PubMed] [Google Scholar]
- Wach A. PCR-synthesis of marker cassettes with long flanking homology regions for gene disruptions in S. cerevisiae. Yeast. 1996;12:259–265. doi: 10.1002/(SICI)1097-0061(19960315)12:3%3C259::AID-YEA901%3E3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Waelter S, et al. The huntingtin interacting protein HIP1 is a clathrin- and alpha-adaptin-binding protein involved in receptor-mediated endocytosis. Hum Mol Genet. 2001;10:1807–1817. doi: 10.1093/hmg/10.17.1807. [DOI] [PubMed] [Google Scholar]
- Wanker EE, Rovira C, Scherzinger E, Hasenbank R, Walter S, Tait D, Colicelli J, Lehrach H. HIP-I: a huntingtin interacting protein isolated by the yeast two-hybrid system. Hum Mol Genet. 1997;6:487–495. doi: 10.1093/hmg/6.3.487. [DOI] [PubMed] [Google Scholar]
- Wendland B, Emr SD. Pan1p, yeast Eps15, functions as a multivalent adaptor that coordinates protein-protein interactions essential for endocytosis. J Cell Biol. 1998;141:71–84. doi: 10.1083/jcb.141.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland B, McCaffery JM, Xiao Q, Emr SD. A novel fluorescence-activated cell sorter-based screen for yeast endocytosis mutants identifies a yeast homologue of mammalian eps15. J Cell Biol. 1996;135:1485–1500. doi: 10.1083/jcb.135.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland B, Steece KE, Emr SD. Yeast epsins contain an essential N-terminal ENTH domain, bind clathrin and are required for endocytosis. EMBO J. 1999;18:4383–4393. doi: 10.1093/emboj/18.16.4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesp A, Hicke L, Palecek J, Lombardi R, Aust T, Munn AL, Riezman H. End4p/Sla2p interacts with actin-associated proteins for endocytosis in S. cerevisiae. Mol Biol Cell. 1997;8:2291–2306. doi: 10.1091/mbc.8.11.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Ayscough KR, Drubin DG. A role for the actin cytoskeleton of S. cerevisiae in bipolar bud-site selection. J Cell Biol. 1997;136:111–123. doi: 10.1083/jcb.136.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Cope MJ, Drubin DG. Sla2p is associated with the yeast cortical actin cytoskeleton via redundant localization signals. Mol Biol Cell. 1999;10:2265–2283. doi: 10.1091/mbc.10.7.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng G, Cai M. Regulation of the actin cytoskeleton organization in yeast by a novel serine/threonine kinase Prk1p. J Cell Biol. 1999;144:71–82. doi: 10.1083/jcb.144.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng G, Yu X, Cai M. Regulation of yeast actin cytoskeleton-regulatory complex Pan1p/Sla1p/End3p by serine/threonine kinase Prk1p. Mol Biol Cell. 2001;12:3759–3772. doi: 10.1091/mbc.12.12.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]