Abstract

The separation and purification of methane from natural gas are crucial chemical processes. Herein, we report a Zn-cluster-based MOF (SDMOF-3), enabling the efficient separation of mixed low-carbon alkanes. The unique Zn clusters in the MOF enhance its interaction with hydrogen atoms in alkanes, thus realizing the separation of saturated light alkanes. Single-component gas adsorption tests revealed that SDMOF-3 exhibits significantly higher affinity for C3H8 compared to CH4 and C2H6 at 298 K. Dynamic column penetration experiments with mixed gases demonstrated the excellent separation performance of SDMOF-3 for separating C3H8 and C2H6 from CH4.
Introduction
Natural gas is a clean and environmentally friendly energy source that is virtually free of sulfur, dust, and other hazardous substances. It produces less carbon dioxide than other fossil fuels and contributes less to the greenhouse effect.1 Methane, the primary component of natural gas (85%), is mainly used as fuel, but it is also utilized in the manufacture of acetaldehyde, acetylene, ammonia, carbon black, ethanol, and other raw materials for chemicals.2,3 However, the presence of C2H6 (9%) and C3H8 (3%) increases the risk of pipeline clogging and equipment fouling and decreases methane quality. High-purity alkanes can be generated from natural gas liquids (NGLs) that contain C2 and C3, representing high value-added products.4 Upgrading natural gas to obtain high-purity CH4, C2H6, and C3H8 offers tremendous industrial prospects. Conventional low-temperature distillation can successfully separate and recover these gases, but it consumes a significant amount of energy.5
Metal–organic frameworks (MOFs) are widely used in the field of gas separation due to their diverse topologies, tunable pore structures, and high porosity. In recent decades, the utilization of MOF materials for gas separation has been extensively explored by researchers.6−10 In order to achieve targeted and discerning recognition of gas molecules throughout the equilibrium process within MOF adsorbents, functional sites have been anchored onto the internal surface of MOFs, thus playing a pivotal role in the exploration of MOFs for gas separation.11−17 The significant role of Zn clusters in gas adsorption and separation within MOFs has been mentioned in numerous reports. For instance, Deng et al.18 reported a Zn-cluster-based MOF, Zn4O(NTB)2, with suitable cavity pockets and distinctive adsorption sites for the invert C2H6 capture from C2H6/C2H4 mixtures. The calculations displayed that C–H···O and C–H···π interactions are formed with C2H6 in the restricted space with Zn clusters and nonpolar pore surfaces. Zhu et al.19 synthesized a Zn-MOF with nonpolar channels. The wall of the channel is filled with rich nonpolar methyl groups and uncoordinated pyrazole N atoms and carboxylate O atoms of Zn clusters, which played an important role in C2H6 adsorption. However, the introduction of Zn clusters in MOF for light hydrocarbons separation has been reported less.
In this work, we report a Zn-cluster-based MOF, {[Zn4O(L)3][Zn8(μ3-OH)3(L)6(DEF)1.5(H2O)2.5](ClO4)1.5 (NH2Et2)0.5(DEF)3(H2O)7}n (SDMOF-3), features the synergistic interaction of unique Zn clusters and shows the efficient separation of light saturated aliphatic hydrocarbons. SDMOF-3 is constructed through an in-situ approach, forming a structure with Zn clusters featuring closely spaced hydroxide groups. The special pore environment facilitated strong interactions with C3H8 molecules, which have a higher number of hydrogen atoms, enabling the adsorption-based separation of C3H8 from CH4 and C2H6. Single-component gas adsorption tests and dynamic column breakthrough experiments confirmed the excellent separation performance of SDMOF-3 in CH4, C2H6, and C3H8 mixtures. A series of theoretical calculations were employed to investigate the interaction between the gas molecules and the Zn cluster. Additionally, cyclic experiments demonstrated the great recyclability of SDMOF-3. This study offers a new research viewpoint for the application of MOFs in the adsorptive separation of light aliphatic hydrocarbons.
Results and Discussion
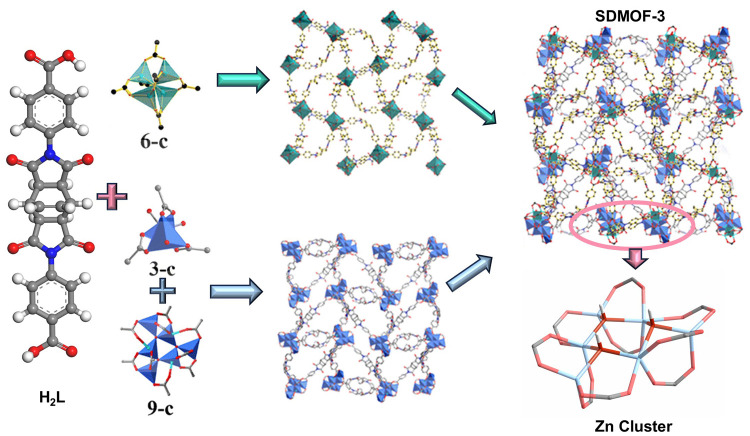
SDMOF-3 was synthesized by referring to our previous literature.20 The SCXRD analysis revealed that SDMOF-3 crystallized in the cubic system with space group P213 and the asymmetric unit (Figure 1). One structure in the asymmetric unit comprises three Zn atoms connected by an O atom, three-quarters of the ligand, and a carboxyl group attached to a partial benzene ring. The other structure in the asymmetric unit consists of five Zn atoms, four of which are distributed at the ends of the ligand, along with a μ3-OH group surrounded by three Zn atoms and coordinated to an incomplete ligand and a carboxyl group attached to a benzene ring. The central μ3-OH group connects three Zn atoms, forming a triangular [Zn3(μ4-O)]4+ unit. Three [Zn3(μ4-O)]4+ units interconnect through Zn atoms located at the vertices, creating an irregular hexagonal ring with three Zn atoms and three O atoms alternately distributed. One [Zn3(μ4-O)]4+ unit bridges the diagonal of this irregular hexagonal ring, then is enveloped by nine carboxylate groups from nine ligands, forming a heptanuclear [Zn6(μ3-OH)3(O2C−)9] metal cluster. The experimental PXRD pattern of SDMOF-3 demonstrates remarkable concordance with the simulated pattern derived from single-crystal X-ray diffraction (SCXRD) data, proving the phase purity (Figure S3). The N2 sorption isotherm measurement of activated SDMOF-3 at 77 K indicates that the Brunauer–Emmett–Teller (BET) surface area of SDMOF-3 is 1060 m2 g–1 (Figure S5). The Zn clusters in the SDMOF-3 structure are shown in Figure 1. The special pore environment produces a multiple weak interaction with the more hydrogen-containing saturated alkane molecule, leading to the stronger the interaction with the propane.
Figure 1.
Two reticular asymmetric units in SDMOF-3 and Zn cluster with adjacent hydroxide groups.
Single-component adsorption isotherms for light saturated hydrocarbons (CH4, C2H6, C3H8) were collected at 273 and 298 K. As demonstrated in Figure 2, the maximum uptake amounts of SDMOF-3 were 0.52 mmol g–1 for CH4, 2.91 mmol g–1 for C2H6, and 4.14 mmol g–1 for C3H8 at 298 K under 100 kPa, respectively. The results showed that the adsorption amounts of SDMOF-3 on different gases increase with the number of carbon atoms in the alkane molecule. Remarkably, the C3H8 uptake amount for SDMOF-3 reaches 1.61 mmol g–1 at 5 kPa, which is dramatically higher than those of C2H6 (0.61 mmol g–1) and CH4(0.06 mmol g–1). The value of C3H8 uptake amount at low pressure (5 kPa) is lower than HOF-ZJU-201a (2.23 mmol g–1),4 MIL-142A (1.84 mmol g–1),21 and Ni(TMBDC)(DABCO)0.5 (3.37 mmol g–1)22 but higher than many high-performance MOFs such as MOF-808-INO (0.89 mmol g–1),23 BSF-1 (1.22 mmol g–1),24 BSF-2 (0.89 mmol g–1),25 HOF-ZJU-202a (1.50 mmol g–1),4 and most of the Zn-cluster-based MOFs (Figure 3c).26−33 This showed that SDMOF-3 has a stronger effect on C3H8 at low pressure. To assess material reusability, adsorption cycle experiments were performed on SDMOF-3. Remarkably, the experimental results indicated that the uptake amount of C3H8 by SDMOF-3 remained stable after five cycles.
Figure 2.
(a) C3H8, (b) C2H6, and (c) CH4 adsorption isotherms for SDMOF-3 at 273 and 298 K. (d) Five cycles of C3H8 adsorption for SDMOF-3 at 298 K.
Figure 3.
(a) The Qst of C3H8, C2H6, and CH4 for SDMOF-3. (b) Predicted IAST selectivity for C2H6/CH4 (v/v,15/85) and C3H8/CH4 (v/v,15/85) mixtures of SDMOF-3 at 298 K. (c) Comparison of C3H8 uptake amount at 5 kPa of the Zn-cluster-based MOFs.26−33
The Qst of C3H8, C2H6, and CH4 in the SDMOF-3 were calculated based on the data of the gas adsorption isotherms at 273 and 298 K to confirm the interaction strength between the gases and the framework. The C3H8, C2H6, and CH4 isotherm data at two different temperatures were fitted to the dual-site Langmuir–Freundlich (DSLF) equation, and the Qst values were computed by the Clausius–Clapeyron equation. As revealed in Figure 3a, the zero-coverage Qst value for C3H8 in SDMOF-3 (27.6 kJ mol–1) is significantly higher than C2H6 (22.3 kJ mol–1) and CH4 (13.4 kJ mol–1) which confirmed that SDMOF-3 has a stronger effect on C3H8 than C2H6 and CH4.
The C3H8/CH4 and C2H6/CH4 selectivities were calculated using ideal adsorbed solution theory (IAST) to evaluate the potential of SDMOF-3 for separating C3H8/C2H6/CH4 mixtures at 298 K. As displayed in Figure 3b, the calculated C3H8/CH4 (15/85, v/v) selectivity for SDMOF-3 is up to 49.4 at 298 K and 100 kPa, while the C2H6/CH4 (15/85, v/v) selectivity is 10.1. This demonstrated the ability of SDMOF-3 to effectively separate CH4 from C3H8/C2H6/CH4 mixtures.
Moreover, the interactions between alkanes and Zn clusters were elucidated through periodic density functional theory (DFT) calculations (see the Supporting Information). Each C3H8 molecule in the unit formed two C–H···O interactions with the unique Zn cluster (2.486 and 2.609 Å). C2H6 also formed two C–H···O interactions, with distances of 2.698 and 2.733 Å. However, an interaction between the Zn cluster and CH4 was observed, forming only a single C–H···O interaction (2.376 Å), which is much weaker than those with C3H8 and C2H6 (Figures S11–S13). The binding energy between SDMOF-3 and C3H8 (−59.63 kJ mol–1) was higher than that of C2H6 (−26.18 kJ mol–1) and CH4 (−12.84 kJ mol–1) (Table S5), which agreed well with the experimental Qst values. The calculation results further confirmed SDMOF-3’s outstanding propane adsorption capability and its effectiveness in light hydrocarbon separation.
To further evaluate the C3H8/CH4 and C2H6/CH4 separation performance of SDMOF-3 in practical adsorption processes, breakthrough experiments for C3H8/CH4 and C2H6/CH4 at 298 K were conducted. The C3H8/CH4 (15/85, v/v) and C2H6/CH4 (15/85, v/v) mixture of gases flowed over a packed column of activated SDMOF-3 at a rate of 2 mL min–1 at first. Figure 4 panels a and b illustrate that, in both the C2H6/CH4 and C3H8/CH4 mixtures, CH4 undergoes rapid elution from the packed column around 5.8 min. SDMOF-3 showed the outstanding performance in purifying CH4 in C3H8/CH4 (15/85, v/v) and C2H6/CH4 (15/85, v/v) mixture, and the purity of CH4 exceeds 99.99%. The residence times of C3H8 and C2H6 within the column are recorded as 81.9 and 22.1 min. The breakthrough time for these gas components occurred in the sequence of C3H8 > C2H6 > CH4, consistent with the gas adsorption data for C3H8, C2H6, and CH4. Moreover, the imperative of exceptional reusability cannot be overstated for an efficient adsorbent given its direct implications for economic viability in industrial applications. It is noteworthy that we purged the sample column with nitrogen for 6 h at room temperature, and the gas breakthrough experiment was subsequently reexamined under identical conditions. The results indicated that SDMOF-3 retained steady separation results after three breakthrough tests (Figure 4c,d), demonstrating the excellent reusability.
Figure 4.
Experimental column breakthrough curves of SDMOF-3 for (a) C2H6/CH4 (15/85, v/v) and (b) C3H8/CH4 (15/85, v/v) gas mixtures at 298 K and 100 kPa. Multiple cycles of breakthrough tests of SDMOF-3 for (c) C2H6/CH4 (15/85, v/v) and (d) C3H8/CH4 (15/85, v/v) at 298 K and 100 kPa.
Conclusions
In this work, we constructed a Zn-cluster-based MOF, SDMOF-3, to realize the efficient separation of C3H8/C2H6/CH4. By utilization of the unique Zn clusters, a strong interaction with C3H8 molecules that contain a larger number of hydrogen atoms was achieved in SDMOF-3. This facilitated the separation of C3H8, C2H6, and CH4. Single-component gas adsorption tests demonstrated a strong affinity of SDMOF-3 toward C3H8, which was further supported by theoretical calculations. Moreover, dynamic breakthrough experiments confirmed the excellent separation performance of SDMOF-3 for C3H8, C2H6, and CH4 in mixed gas. Furthermore, single-component gas adsorption tests and breakthrough experiments conducted in cycles confirmed consistent performance over multiple iterations of SDMOF-3.
Acknowledgments
This research was made possible as a result of a generous grant from the National Natural Science Foundation of China (Grant Numbers 22001186 and 22271203), “Distinguished Professor of Jiangsu Province” Program, the Gusu Innovation Leading Talents Program (Grant Number ZXL2021459).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/cbe.3c00092.
Experimental procedures, NMR spectrum, optical microscopy image, PXRD patterns, IR spectrum, N2 isotherm, pore size distribution, TGA measurements, gas adsorption data, and DFT optimized positions, Langmuir–Freundlich equation parameters, gas uptake amounts, adsorption energies (PDF)
Author Contributions
# Y.W., Y.L., and J.L. contributed equally.
The authors declare no competing financial interest.
Special Issue
Published as part of Chem & Bio Engineeringvirtual special issue “Advanced Separation Materials and Processes”.
Supplementary Material
References
- Tagliabue M.; Farrusseng D.; Valencia S.; Aguado S.; Ravon U.; Rizzo C.; Corma A.; Mirodatos C. Natural gas treating by selective adsorption: Material science and chemical engineering interplay. Chem. Eng. J. 2009, 155, 553–566. 10.1016/j.cej.2009.09.010. [DOI] [Google Scholar]
- He Y. B.; Zhou W.; Qian G. D.; Chen B. L. Methane storage in metal–organic frameworks. Chem. Soc. Rev. 2014, 43, 5657–5678. 10.1039/C4CS00032C. [DOI] [PubMed] [Google Scholar]
- Niu Z.; Cui X. L.; Pham T.; Lan P. C.; Xing H. B.; Forrest K. A.; Wojtas L.; Space B.; Ma S. Q. A Metal–Organic Framework Based Methane Nano-trap for the Capture of Coal-Mine Methane. Angew. Chem. Int. Ed. 2019, 58, 10138–10141. 10.1002/anie.201904507. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Xu Q. Q.; Chen L. H.; Song C. H.; Yang Q. W.; Zhang Z. G.; Lu D.; Yang Y. W.; Ren Q. L.; Bao Z. B. Hydrogen-bonded metal-nucleobase frameworks for highly selective capture of ethane/propane from methane and methane/nitrogen separation. Nano Res. 2022, 15, 7695–7702. 10.1007/s12274-022-4352-0. [DOI] [Google Scholar]
- Natural Gas. In Industrial Gases Processing, 7th ed.; Häring H. W., Ed.; Wiley-VCH, 2007; pp 217–238, 10.1002/9783527621248.fmatter. [DOI] [Google Scholar]
- Wang J. X.; Liang C. C.; Gu X. W.; Wen H. M.; Jiang C. h.; Li B.; Qian G. D.; Chen B. L. Recent advances in microporous metal–organic frameworks as promising adsorbents for gas separation. J. Mater. Chem. A 2022, 10, 17878–17916. 10.1039/D2TA04835C. [DOI] [Google Scholar]
- Zhao X.; Wang Y. X.; Li D. S.; Bu X. H.; Feng P. Y. Metal–Organic Frameworks for Separation. Adv. Mater. 2018, 30, 1705189. 10.1002/adma.201705189. [DOI] [PubMed] [Google Scholar]
- Wang T.; Lin E.; Peng Y. L.; Chen Y.; Cheng P.; Zhang Z. J. Rational design and synthesis of ultramicroporous metal-organic frameworks for gas separation. Coord. Chem. Rev. 2020, 423, 213485. 10.1016/j.ccr.2020.213485. [DOI] [Google Scholar]
- Wang J. H.; Lian X.; Zhang Z. Y.; Liu X. L.; Zhao Q.; Xu J.; Cao X. C.; Li B. Y.; Bu X. H. Thiazole functionalized covalent triazine frameworks for C2H6/C2H4 separation with remarkable ethane uptake. Chem. Commun. 2023, 59, 11240–11243. 10.1039/D3CC02880A. [DOI] [PubMed] [Google Scholar]
- Shui F.; Lei Q.; Dong X. L.; Pan T. T.; Zhang Z. Y.; Li J. L.; Yi M.; Zhang L. Y.; Liu X. L.; You Z. F.; Yang S. Q.; Yang R. F.; Zhang H. B.; Li J. X.; Shi Z.; Yin J.; Li B. Y.; Bu X. H. Iodine nanotrap for highly efficient iodine capture under high temperature. Chem. Eng. J. 2023, 468, 143525. 10.1016/j.cej.2023.143525. [DOI] [Google Scholar]
- Lin R. B.; Xiang S. C.; Zhou W.; Chen B. L. Microporous Metal-Organic Framework Materials for Gas Separation. Chem. 2020, 6, 337–363. 10.1016/j.chempr.2019.10.012. [DOI] [Google Scholar]
- Li L.; Lin R. B.; Krishna R.; Wang X. Q.; Li B.; Wu H.; Li J. P.; Zhou W.; Chen B. L. Flexible–Robust Metal–Organic Framework for Efficient Removal of Propyne from Propylene. J. Am. Chem. Soc. 2017, 139, 7733–7736. 10.1021/jacs.7b04268. [DOI] [PubMed] [Google Scholar]
- Niu Z.; Cui X. L.; Pham T.; Verma G.; Lan P. C.; Shan C.; Xing H. B.; Forrest K. A.; Suepaul S.; Space B.; Nafady A.; Al-Enizi A. M.; Ma S. Q. A MOF-based Ultra-Strong Acetylene Nano-trap for Highly Efficient C2H2/CO2 Separation. Angew. Chem. Int. Ed. 2021, 60, 5283–5288. 10.1002/anie.202016225. [DOI] [PubMed] [Google Scholar]
- Fan W. D.; Yuan S.; Wang W. J.; Feng L.; Liu X. P.; Zhang X. R.; Wang X.; Kang Z. X.; Dai F. N.; Yuan D. Q.; Sun D. F.; Zhou H. C. Optimizing Multivariate Metal–Organic Frameworks for Efficient C2H2/CO2 Separation. J. Am. Chem. Soc. 2020, 142, 8728–8737. 10.1021/jacs.0c00805. [DOI] [PubMed] [Google Scholar]
- Fan W. D.; Ying Y. P.; Peh S. B.; Yuan H. Y.; Yang Z. Q.; Yuan Y. D.; Shi D. C.; Yu X.; Kang C. J.; Zhao D. Multivariate Polycrystalline Metal–Organic Framework Membranes for CO2/CH4 Separation. J. Am. Chem. Soc. 2021, 143, 17716–17723. 10.1021/jacs.1c08404. [DOI] [PubMed] [Google Scholar]
- Wang Y. T.; Fu M. Y.; Zhou S. N.; Liu H. Y.; Wang X. K.; Fan W. D.; Liu Z. N.; Wang Z. K.; Li D. C.; Hao H. G.; Lu X. Q.; Hu S. Q.; Sun D. F. Guest-molecule-induced self-adaptive pore engineering facilitates purification of ethylene from ternary mixture. Chem. 2022, 8, 3263–3274. 10.1016/j.chempr.2022.08.014. [DOI] [Google Scholar]
- Fan W. D.; Wang X.; Zhang X. R.; Liu X. P.; Wang Y. T.; Kang Z. X.; Dai F. N.; Xu B.; Wang R. M.; Sun D. F. Fine-Tuning the Pore Environment of the Microporous Cu-MOF for High Propylene Storage and Efficient Separation of Light Hydrocarbons. ACS Central Sci. 2019, 5, 1261–1268. 10.1021/acscentsci.9b00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Zhou S.; Liu X.; Zhang P.; Yan Z.; Hu J.; Wei Z.; Chen L.; Wang J.; Deng S. An ethane-trapping Zn (II) cluster-based metal-organic framework with suitable pockets for efficient ethane/ethylene separation. Sep. Purif. Technol. 2022, 301, 122011. 10.1016/j.seppur.2022.122011. [DOI] [Google Scholar]
- Wang G. D.; Chen J.; Li Y. Z.; Hou L.; Wang Y. Y.; Zhu Z. H. A robust ethane-selective metal-organic framework with nonpolar pore surface for efficient C2H6/C2H4 separation. Chem. Eng. J. 2022, 433, 133786. 10.1016/j.cej.2021.133786. [DOI] [Google Scholar]
- Shi R.; Xu Z. M.; Cao C.; Niu Z.; Lang J. P. A 3D/3D hetero-interpenetrated MOF with a novel (3,9)-c net and 6-c lcy net for the fluorescence detection of carbaryl. Dalton Trans 2022, 51, 15644–15647. 10.1039/D2DT01893D. [DOI] [PubMed] [Google Scholar]
- Yuan Y. N.; Wu H. X.; Xu Y. Z.; Lv D. F.; Tu S.; Wu Y.; Li Z.; Xia Q. B. Selective extraction of methane from C1/C2/C3 on moisture-resistant MIL-142A with interpenetrated networks. Chem. Eng. J. 2020, 395, 125057. 10.1016/j.cej.2020.125057. [DOI] [Google Scholar]
- Wu Y. F.; Liu Z. W.; Peng J. J.; Wang X.; Zhou X.; Li Z. Enhancing Selective Adsorption in a Robust Pillared-Layer Metal–Organic Framework via Channel Methylation for the Recovery of C2–C3 from Natural Gas. ACS Appl. Mater. Interfaces 2020, 12, 51499–51505. 10.1021/acsami.0c15267. [DOI] [PubMed] [Google Scholar]
- Lai Y. J.; Ji J. Y.; Wang H. F.; Niu Z.; Lang J. P. Enhanced selectivity of alkane mixture gas on the N-oxide group decorated metal-organic framework. Microporous Mesoporous Mater. 2023, 359, 112670. 10.1016/j.micromeso.2023.112670. [DOI] [Google Scholar]
- Zhang Y. B.; Yang L. F.; Wang L. Y.; Duttwyler S.; Xing H. B. A Microporous Metal-Organic Framework Supramolecularly Assembled from a CuII Dodecaborate Cluster Complex for Selective Gas Separation. Angew. Chem. Int. Ed. 2019, 58, 8145–8150. 10.1002/anie.201903600. [DOI] [PubMed] [Google Scholar]
- Zhang Y. B.; Yang L. F.; Wang L. Y.; Cui X. L.; Xing H. B. Pillar iodination in functional boron cage hybrid supramolecular frameworks for high performance separation of light hydrocarbons. J. Mater. Chem. A 2019, 7, 27560–27566. 10.1039/C9TA09928J. [DOI] [Google Scholar]
- Hu P.; Hu J. L.; Liu H.; Wang H.; Zhou J.; Krishna R.; Ji H. Quasi-Orthogonal Configuration of Propylene within a Scalable Metal–Organic Framework Enables Its Purification from Quinary Propane Dehydrogenation Byproducts. ACS Cent. Sci. 2022, 8, 1159–1168. 10.1021/acscentsci.2c00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q. S.; Yao S.; Liu B.; Liu X. Y.; Li G. H.; Liu X.Y.; Liu Y. L. A novel polyhedron-based metal–organic framework with high performance for gas uptake and light hydrocarbon separation. Dalton Trans 2018, 47, 5005–5010. 10.1039/C7DT04622G. [DOI] [PubMed] [Google Scholar]
- Wang X.; Wang X. K.; Zhang X. R.; Fan W. D.; Li Q.; Jiang W. F.; Dai F. N.; Sun D. F. A Stable Interpenetrated Zn-MOF with Efficient Light Hydrocarbon Adsorption/Separation Performance. Cryst. Growth Des. 2020, 20, 5670–5675. 10.1021/acs.cgd.0c00925. [DOI] [Google Scholar]
- Kan L.; Li G. H.; Liu Y. L. Highly selective separation of C3H8 and C2H2 from CH4 within two water-stable Zn5 cluster-based metal–organic frameworks. ACS Appl. Mater. Interfaces 2020, 12, 18642–18649. 10.1021/acsami.0c04538. [DOI] [PubMed] [Google Scholar]
- Sun X. D.; Yao S.; Li G. H.; Zhang L. R.; Huo Q. S.; Liu Y. L. A Flexible Doubly Interpenetrated Metal–Organic Framework with Breathing Behavior and Tunable Gate Opening Effect by Introducing Co2+ into Zn4O Clusters. Inorg. Chem. 2017, 56, 6645–6651. 10.1021/acs.inorgchem.7b00744. [DOI] [PubMed] [Google Scholar]
- Chen Y. W.; Wu H. X.; Yu L.; Tu S.; Wu Y.; Li Z.; Xia Q. B. Exploiting thermodynamic-kinetic synergetic effect for C3H6/C3H8 separation in pillar-layer MOFs. Authorea 2021, 10.22541/au.162467214.42441891/v1. [DOI] [Google Scholar]
- Kan L.; Cai J.; Jin Z. W.; Li G. H.; Liu Y. L.; Xu L. R. Two Stable Zn-Cluster-Based Metal–Organic Frameworks with Breathing Behavior: Synthesis, Structure, and Adsorption Properties. Inorg. Chem. 2019, 58, 391–396. 10.1021/acs.inorgchem.8b02507. [DOI] [PubMed] [Google Scholar]
- Ding Q.; Zhang Z. Q.; Yu C.; Zhang P. X.; Wang J.; Kong L. Y.; Cui X. L.; He C. H.; Deng S. G.; Xing H. B. Separation of propylene and propane with a microporous metal–organic framework via equilibrium-kinetic synergetic effect. AlChE J. 2021, 67, e17094 10.1002/aic.17094. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.