Abstract
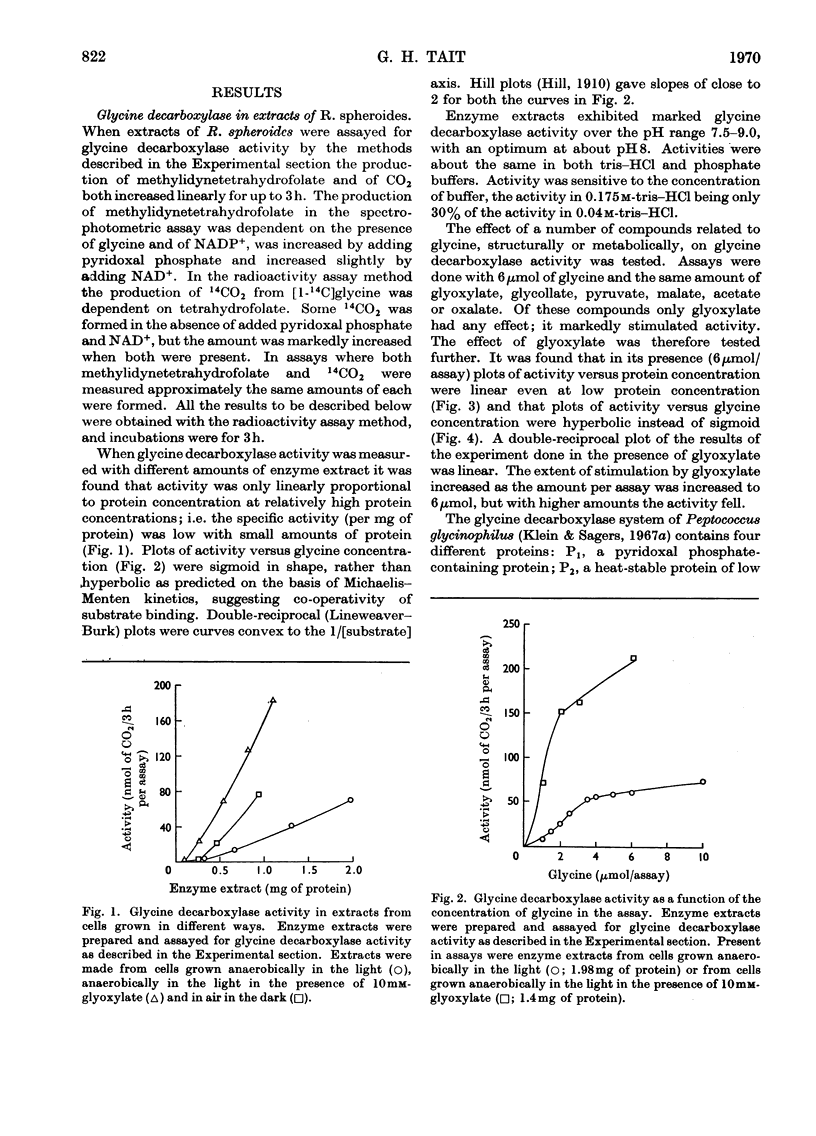
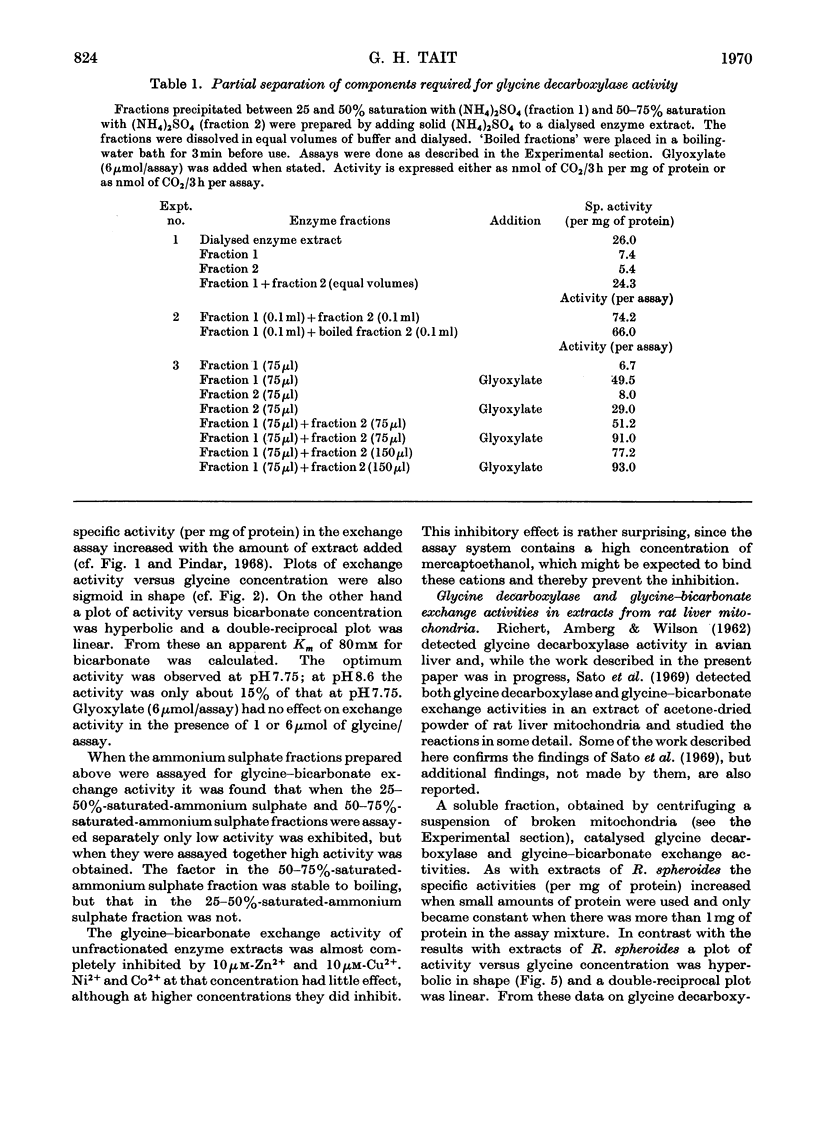
1. Glycine decarboxylase and glycine–bicarbonate exchange activities were detected in extracts of Rhodopseudomonas spheroides and in rat liver mitochondria and their properties were studied. 2. The glycine decarboxylase activity from both sources is stimulated when glyoxylate is added to the assay system. 3. Several proteins participate in these reactions and a heat-stable low-molecular-weight protein was purified from both sources. 4. These enzyme activities increase markedly when R. spheroides is grown in the presence of glycine, glyoxylate, glycollate, oxalate or serine. 5. All the enzymes required to catalyse the conversion of glycine into acetyl-CoA via serine and pyruvate were detected in extracts of R. spheroides; of these glycine decarboxylase has the lowest activity. 6. The increase in the activity of glycine decarboxylase on illumination of R. spheroides in a medium containing glycine, and the greater increase when ATP is also present in the medium, probably accounts for the increased incorporation of the methylene carbon atom of glycine into fatty acids found previously under these conditions (Gajdos, Gajdos-Török, Gorchein, Neuberger & Tait, 1968). 7. The results are compared with those obtained by other workers on the glycine decarboxylase and glycine–bicarbonate exchange activities in other systems.
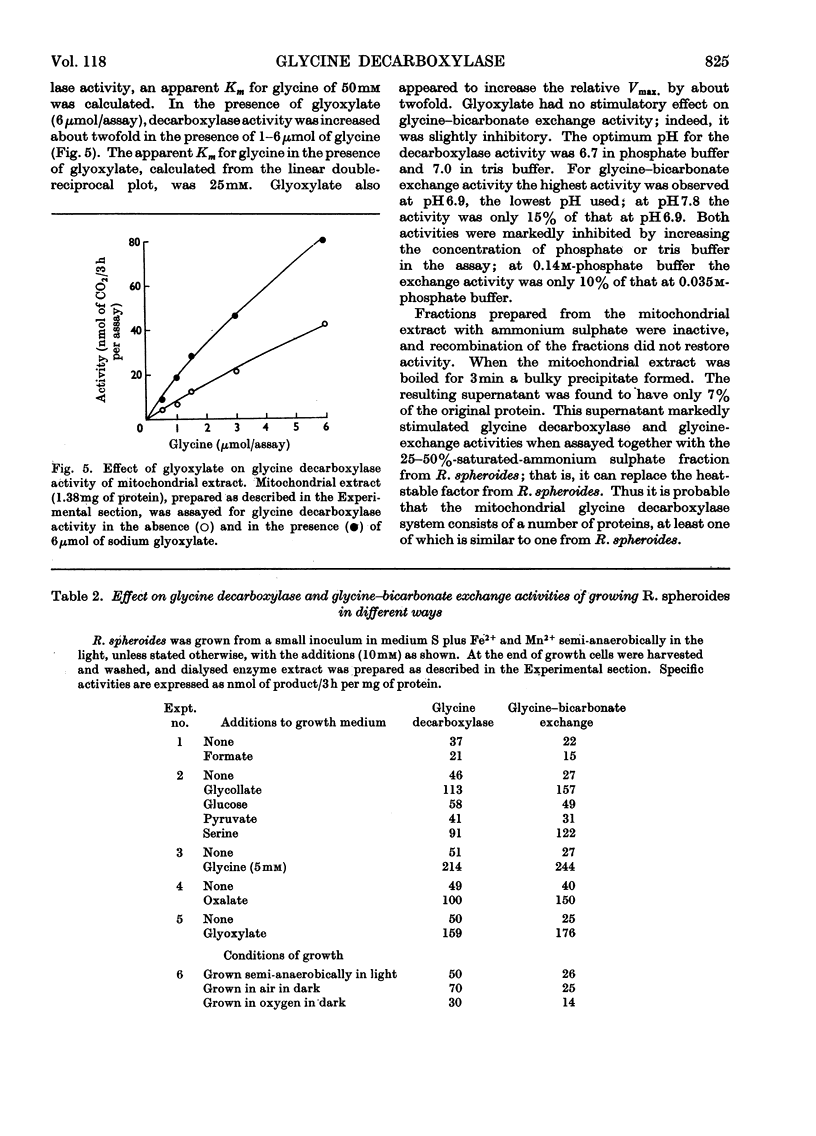
Full text
PDF


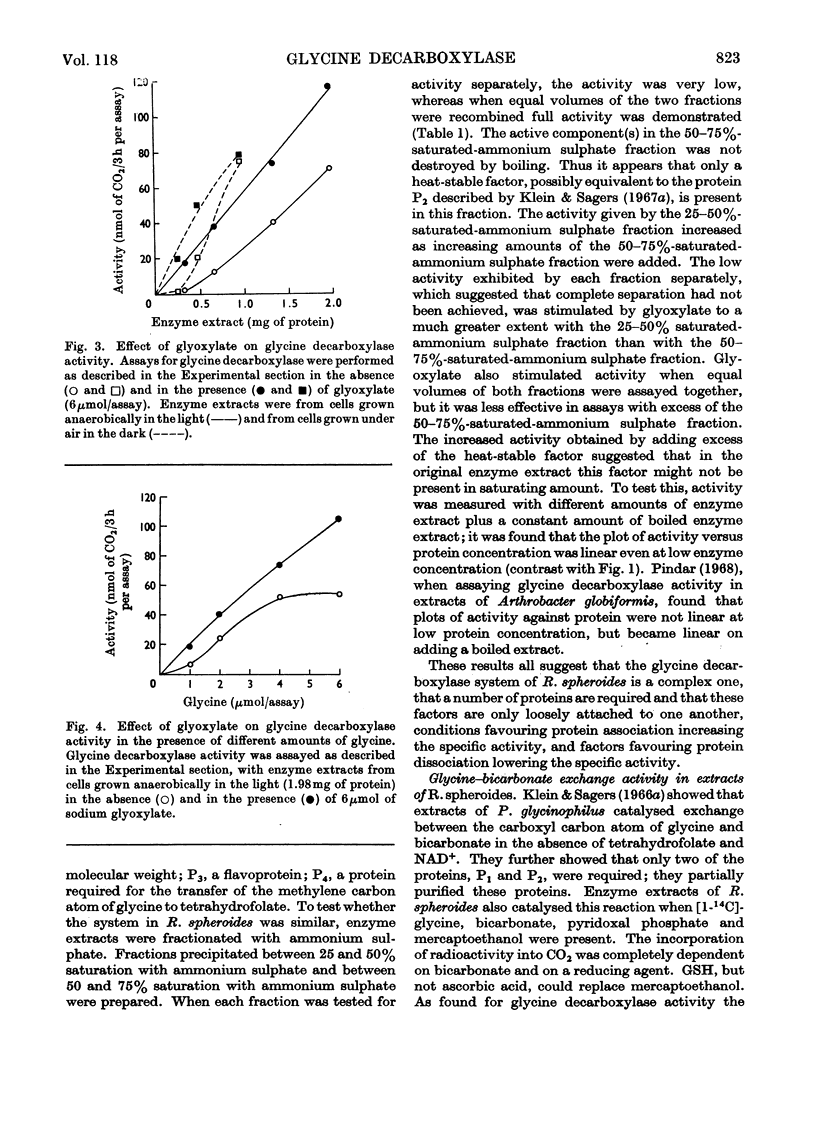




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cossins E. A., Sinha S. K. The interconversion of glycine and serine by plant tissue extracts. Biochem J. 1966 Nov;101(2):542–549. doi: 10.1042/bj1010542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON K. D., NEUBERGER A., TAIT G. H. Studies on the biosynthesis of porphyrin and bacteriochlorophyll by Rhodopseudomonas spheroides. 1. The effect of growth conditions. Biochem J. 1962 Jun;83:539–549. doi: 10.1042/bj0830539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorchein A., Neuberger A., Tait G. H. Incorporation of radioactivity from [me-14C]methionine and [2-14C]glycine into the lipids of Rhodopseudomonas spheroides. Proc R Soc Lond B Biol Sci. 1968 Jul 2;170(1020):299–310. doi: 10.1098/rspb.1968.0040. [DOI] [PubMed] [Google Scholar]
- HATEFI Y., OSBORN M. J., KAY L. D., HUENNEKENS F. M. Hydroxymethyl tetrahydrofolic dehydrogenase. J Biol Chem. 1957 Aug;227(2):637–647. [PubMed] [Google Scholar]
- Howell S. L., Taylor K. W. Potassium ions and the secretion of insulin by islets of Langerhans incubated in vitro. Biochem J. 1968 Jun;108(1):17–24. doi: 10.1042/bj1080017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S. M., Sagers R. D. Glycine metabolism. 3. A flavin-linked dehydrogenase associated with the glycine cleavage system in Peptococcus glycinophilus. J Biol Chem. 1967 Jan 25;242(2):297–300. [PubMed] [Google Scholar]
- Klein S. M., Sagers R. D. Glycine metabolism. I. Properties of the system catalyzing the exchange of bicarbonate with the carboxyl group of glycine in Peptococcus glycinophilus. J Biol Chem. 1966 Jan 10;241(1):197–205. [PubMed] [Google Scholar]
- Klein S. M., Sagers R. D. Glycine metabolism. II. Kinetic and optical studies on the glycine decarboxylase system from Peptococcus glycinophilus. J Biol Chem. 1966 Jan 10;241(1):206–209. [PubMed] [Google Scholar]
- Klein S. M., Sagers R. D. Glycine metabolism. IV. Effect of borohydride reduction on the pyridoxal phosphate-containing glycine decarboxylase from Peptococcus glycinophilus. J Biol Chem. 1967 Jan 25;242(2):301–305. [PubMed] [Google Scholar]
- Kochi H., Kikuchi G. Reactions of glycine synthesis and glycine cleavage catalyzed by extracts of Arthrobacter globiformis grown on glycine. Arch Biochem Biophys. 1969 Jul;132(2):359–369. doi: 10.1016/0003-9861(69)90377-4. [DOI] [PubMed] [Google Scholar]
- LASCELLES J. The synthesis of porphyrins and bacteriochlorophyll by cell suspensions of Rhodopseudomonas spheroides. Biochem J. 1956 Jan;62(1):78–93. doi: 10.1042/bj0620078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lascelles J. The regulation of haem and chlorophyll synthesis. Biochem Soc Symp. 1968;28:49–59. [PubMed] [Google Scholar]
- Motokawa Y., Kikuchi G. Glycine metabolism by rat liver mitochondria. IV. Isolation and characterization of hydrogen carrier protein, an essential factor for glycine metabolism. Arch Biochem Biophys. 1969 Dec;135(1):402–409. doi: 10.1016/0003-9861(69)90556-6. [DOI] [PubMed] [Google Scholar]
- NEWMAN E. B., MAGASANIK B. THE RELATION OF SERINE--GLYCINE METABOLISM TO THE FORMATION OF SINGLE-CARBON UNITS. Biochim Biophys Acta. 1963 Nov 15;78:437–448. doi: 10.1016/0006-3002(63)90905-3. [DOI] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- RICHERT D. A., AMBERG R., WILSON M. Metabolism of glycine by avian liver. J Biol Chem. 1962 Jan;237:99–103. [PubMed] [Google Scholar]
- SAGERS R. D., GUNSALUS I. C. Intermediatry metabolism of Diplococcus glycinophilus. I. Glycine cleavage and one-carbon interconversions. J Bacteriol. 1961 Apr;81:541–549. doi: 10.1128/jb.81.4.541-549.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrimgeour K. G., Vitols K. S. The reduction of folate by borohydride. Biochemistry. 1966 Apr;5(4):1438–1443. doi: 10.1021/bi00868a042. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Kikuchi G. Physiological significance of glycine cleavage system in human liver as revealed by the study of a case of hyperglycinemia. Biochem Biophys Res Commun. 1969 May 22;35(4):577–583. doi: 10.1016/0006-291x(69)90387-8. [DOI] [PubMed] [Google Scholar]



