Abstract
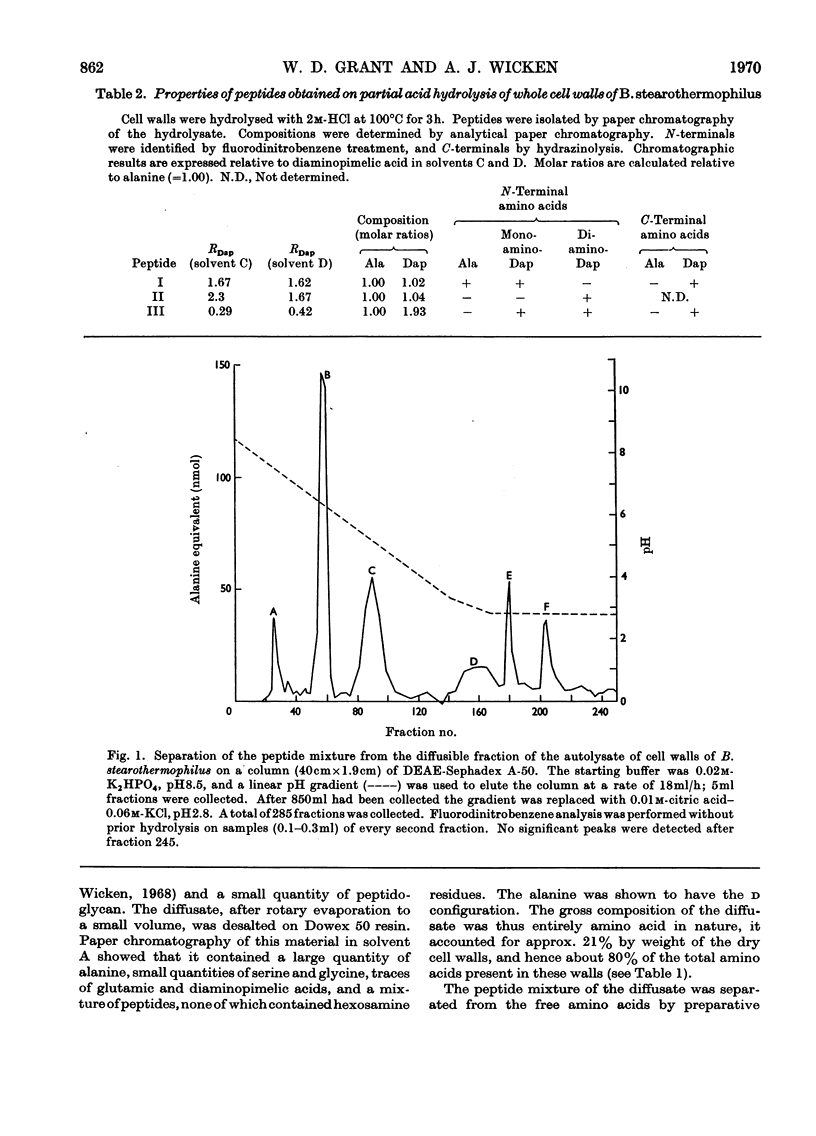
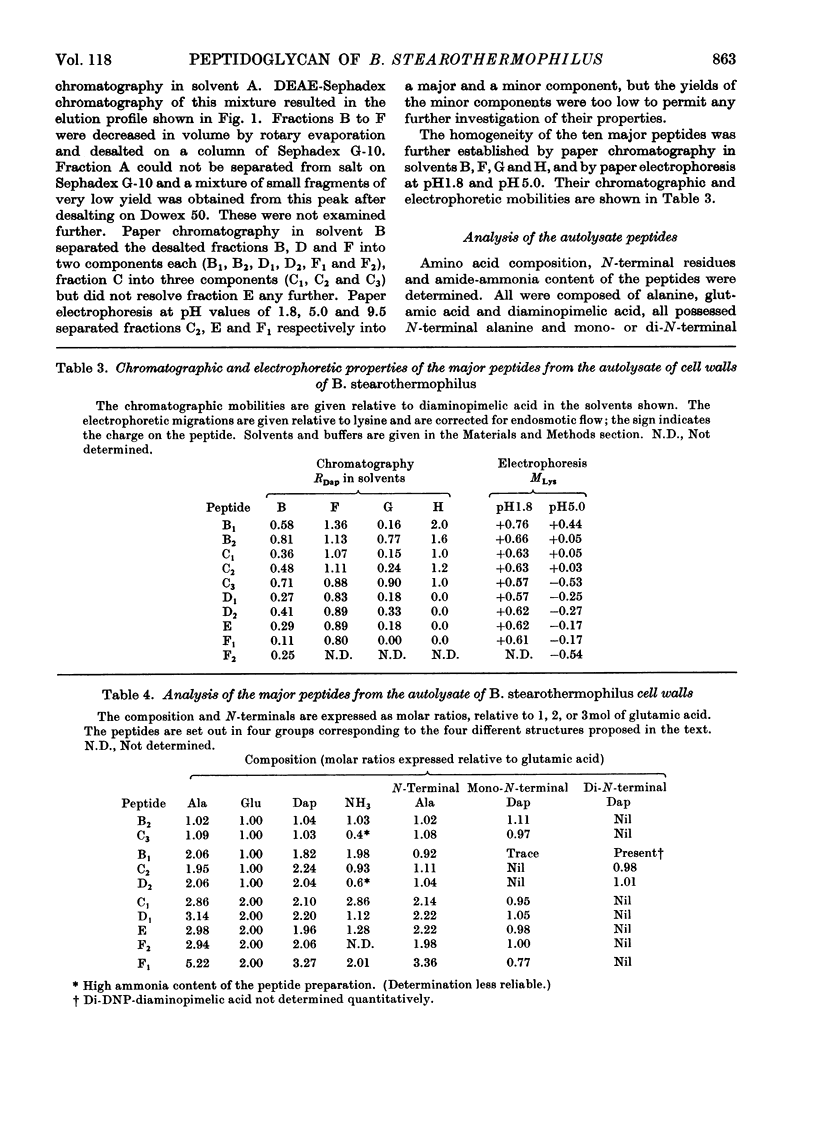
1. The cell walls of Bacillus stearothermophilus B65 contain glucosamine, muramic acid, alanine, α∈-diaminopimelic acid (Dap), glutamic acid, aspartic acid, glycine, and serine in the molecular proportions 0.60:0.64:2.30:0.85:1.00:0.11:0.13:0.31. 2. Both d- and l-alanine are present, but glutamic acid and diaminopimelic acid are present only as the d- and meso-isomers respectively. 3. The peptide fragments Ala-Dap, Dap-Ala, and Dap-Ala-Dap have been isolated from a partial acid hydrolysate of the cell walls. 4. The major products of autolysis of the cell wall were d-alanine, a peptide mixture, peptidoglycan material and a peptidoglycan–teichoic acid complex. 5. Separation of the peptide mixture into ten major peptides was achieved by DEAE-Sephadex and paper chromatography, and paper electrophoresis. 6. The structures of these peptides have been determined and they fall into four groups, the individual members of each group differing only in number or position of carboxamide substituents. 7. The structures are I, a tripeptide l-Ala–d-Glu-meso-Dap; II, a pentapeptide made up by the tripeptide (I) linked through the ∈-amino group of its diaminopimelic acid residue to the carboxyterminal of the dipeptide meso-Dap-d-Ala; III, a heptapeptide made up by a similar linkage between the tripeptide (I) and the tetrapeptide l-Ala-d-Glu-meso-Dap-d-Ala; IV, a possible undecapeptide made up by a further tetrapeptide similarly linked to the heptapeptide (III) structure. 8. The structure of the peptidoglycan and the actions of the autolytic enzymes are discussed in terms of these peptide structures.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACKBURN S., LOWTHER A. G. The separation of N-2:4-dinitrophenly amino-acids on paper chromatograms. Biochem J. 1951 Jan;48(1):126–128. doi: 10.1042/bj0480126. [DOI] [PMC free article] [PubMed] [Google Scholar]
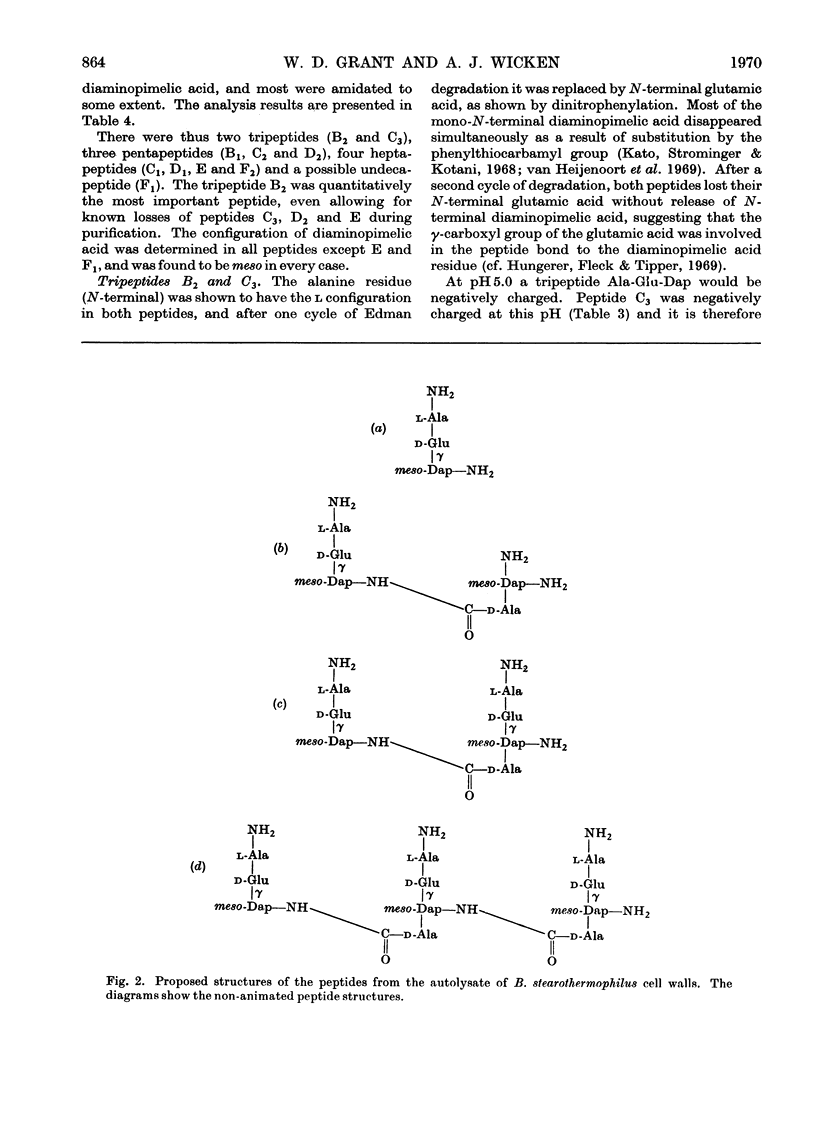
- Forrester I. T., Wicken A. J. The chemical composition of the cell walls of some thermophilic bacilli. J Gen Microbiol. 1966 Jan;42(1):147–154. doi: 10.1099/00221287-42-1-147. [DOI] [PubMed] [Google Scholar]
- Ghuysen J. M., Dierickx L., Coyette J., Leyh-Bouille M., Guinand M., Campbell J. N. An improved technique for the preparation of Streptomyces peptidases and N-acetylmuramyl-l-alanine amidase active on bacterial wall peptidoglycans. Biochemistry. 1969 Jan;8(1):213–222. doi: 10.1021/bi00829a031. [DOI] [PubMed] [Google Scholar]
- Ghuysen J. M. Use of bacteriolytic enzymes in determination of wall structure and their role in cell metabolism. Bacteriol Rev. 1968 Dec;32(4 Pt 2):425–464. [PMC free article] [PubMed] [Google Scholar]
- Grant W. D., Wicken A. J. Muramic acid phosphate and the linkage of teichoic acid to peptidoglycan in Bacillus stearothermophilus cell walls. Biochem Biophys Res Commun. 1968 Jul 26;32(2):122–128. doi: 10.1016/0006-291x(68)90356-2. [DOI] [PubMed] [Google Scholar]
- Grant W. D., Wicken A. J. Thin-layer and paper chromatography of dinitrophenyl derivatives of amino acids from bacterial cell wall peptidoglycans. J Chromatogr. 1970 Feb 18;47(1):124–126. doi: 10.1016/0021-9673(70)80019-x. [DOI] [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- HEILMANN J., BARROLLIER J., WATZKE E. Beitrag zur Aminosäurebestimmung auf Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1957;309(4-6):219–220. [PubMed] [Google Scholar]
- HEYNS K., LEGLER G. Uber Proteine und deren Abbauprodukte. XIII. Die carboxylendständigen und die aminoendständigen Aminosäuren der Gelatine. Hoppe Seylers Z Physiol Chem. 1957 Feb 5;306(4-6):165–172. [PubMed] [Google Scholar]
- Hughes R. C. The cell wall of Bacillus licheniformis N.C.T.C. 6346. Composition of the mucopeptide component. Biochem J. 1968 Jan;106(1):41–48. doi: 10.1042/bj1060041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. C. The cell wall of Bacillus licheniformis N.C.T.C. 6346. Isolation of low-molecular-weight fragments from the soluble mucopeptide. Biochem J. 1968 Jan;106(1):49–59. doi: 10.1042/bj1060049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungerer K. D., Fleck J., Tipper D. J. Structure of the cell wall peptidoglycan of Lactobacillus casei RO94. Biochemistry. 1969 Sep;8(9):3567–3577. doi: 10.1021/bi00837a012. [DOI] [PubMed] [Google Scholar]
- JUSIC D., ROY C., SCHOCHER A. J., WATSON R. W. Chromatographic separation of isomeric dinitrophenyl derivatives of alpha-epsilon diaminopimelic acid. Can J Biochem Physiol. 1963 Mar;41:817–820. [PubMed] [Google Scholar]
- Kato K., Strominger J. L., Kotani S. Structure of the cell wall of Corynebacterium diphtheriae. I. Mechanism of hydrolysis by the L-3 enzyme and the structure of the peptide. Biochemistry. 1968 Aug;7(8):2762–2773. doi: 10.1021/bi00848a010. [DOI] [PubMed] [Google Scholar]
- Kingan S. L., Ensign J. C. Isolation and characterization of three autolytic enzymes associated with sporulation of Bacillus thuringiensis var. thuringiensis. J Bacteriol. 1968 Sep;96(3):629–638. doi: 10.1128/jb.96.3.629-638.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Ensign J. C. Activity of an autolytic N-acetylmuramidase during sphere-rod morphogenesis in Arthrobacter crystallopoietes. J Bacteriol. 1968 Sep;96(3):857–859. doi: 10.1128/jb.96.3.857-859.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEUTGEB W., WEIDEL W. OLIGO-MUCOPEPTIDE AUS DER STUETZMEMBRAN VON E. COLI. Z Naturforsch B. 1963 Dec;18:1065–1069. [PubMed] [Google Scholar]
- Matsuda T., Kotani S., Kato K., Katayama T., Moriyama T. Isolation of D-alanyl-meso-alpha, alpha'-diaminopimelic acid endopeptidase from a crude preparation in Streptomyces "L-3 enzyme". Biken J. 1968 Jun;11(2):145–148. [PubMed] [Google Scholar]
- Matsuda T., Kotani S., Kato K. Structure of the cell walls of Lactobacillus plantarum, ATCC 8014. 1. Isolation and identication of the peptides released from cell wall peptidoglycans by Streptomyces L-3 enzyme. Biken J. 1968 Jun;11(2):111–126. [PubMed] [Google Scholar]
- Matsuda T., Kotani S., Kato K. Structure of the cell walls of Lactobacillus plantarum, ATCC 8014. 2. Cross linkage between D-alanine and alpha,alpha'-diaminopimelic acid in the cell wall peptidoglycans studied with an L-11 enzyme from Flavobacterium sp. Biken J. 1968 Jun;11(2):127–138. [PubMed] [Google Scholar]
- PELZER H. MUCOPEPTIDHYDROLASEN IN ESCHERICHIA COLI B. I. NACHWEIS UND WIRKUNGSSPEZIFITAET. Z Naturforsch B. 1963 Nov;18:950–956. [PubMed] [Google Scholar]
- PRIMOSIGH J., PELZER H., MAASS D., WEIDEL W. Chemical characterization of mucopeptides released from the E. coli B cell wall by enzymic action. Biochim Biophys Acta. 1961 Jan 1;46:68–80. doi: 10.1016/0006-3002(61)90647-3. [DOI] [PubMed] [Google Scholar]
- Partridge S. M. Filter-paper partition chromatography of sugars: 1. General description and application to the qualitative analysis of sugars in apple juice, egg white and foetal blood of sheep. with a note by R. G. Westall. Biochem J. 1948;42(2):238–250. doi: 10.1042/bj0420238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit J. F., Adam A., Wietzerbin-Falszpan J., Lederer E., Ghuysen J. M. Chemical structure of the cell wall of Mycobacterium smegmatis. I. Isolation and partial characterization of the peptidoglycan. Biochem Biophys Res Commun. 1969 May 22;35(4):478–485. doi: 10.1016/0006-291x(69)90371-4. [DOI] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Zur chemischen Zusammensetzung der Zellwand der Streptokokken. I. Die Aminosäuresequenz des Mureins von Str. thermophilus und Str. faecalis. Arch Mikrobiol. 1967 Jul 6;57(4):335–364. [PubMed] [Google Scholar]
- Shockman G. D., Thompson J. S., Conover M. J. The autolytic enzyme system of Streptococcus faecalis. II. Partial characterization of the autolysin and its substrate. Biochemistry. 1967 Apr;6(4):1054–1065. doi: 10.1021/bi00856a014. [DOI] [PubMed] [Google Scholar]
- Soda K. Microdetermination of D-amino acids and D-amino acid oxidase activity with 3,methyl-2-benzothiazolone hydrazone hydrochloride. Anal Biochem. 1968 Oct 24;25(1):228–235. doi: 10.1016/0003-2697(68)90095-x. [DOI] [PubMed] [Google Scholar]
- TAKEBE I. EXTENT OF CROSS LINKAGE IN THE MUREIN SACCULUS OF ESCHERICHIA COLI B CELL WALL. Biochim Biophys Acta. 1965 Mar 1;101:124–126. doi: 10.1016/0926-6534(65)90038-2. [DOI] [PubMed] [Google Scholar]
- Tinelli R. Structure de la paroi de Listeria monocytogenes. I. Fractionnement et identification partielle des produits obtenus par autolyse du glycopeptide; nature des enzymes autolytiques pariétaux. Bull Soc Chim Biol (Paris) 1969 Jun 26;51(2):283–297. [PubMed] [Google Scholar]
- Tipper D. J., Katz W., Strominger J. L., Ghuysen J. M. Substituents on the alpha-carboxyl group of D-glutamic acid in the peptidoglycan of several bacterial cell walls. Biochemistry. 1967 Mar;6(3):921–929. doi: 10.1021/bi00855a036. [DOI] [PubMed] [Google Scholar]
- Tipper D. J. Mechanism of autolysis of isolated cell walls of Staphylococcus aureus. J Bacteriol. 1969 Feb;97(2):837–847. doi: 10.1128/jb.97.2.837-847.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heijenoort J., Elbaz L., Dezélée P., Petit J. F., Bricas E., Ghuysen J. M. Structure of the meso-diaminopimelic acid containing peptidoglycans in Escherichia coli B and Bacillus megaterium KM. Biochemistry. 1969 Jan;8(1):207–213. doi: 10.1021/bi00829a030. [DOI] [PubMed] [Google Scholar]
- WICKEN A. J., BADDILEY J. Structure of intracellular teichoic acids from group D streptococci. Biochem J. 1963 Apr;87:54–62. doi: 10.1042/bj0870054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss N., Plapp R., Kandler O. Die Aminosäuresequenz des DAP-haltigen Mureins von Lactobacillus plantarum und Lactobacillus inulinus. Arch Mikrobiol. 1967;58(4):313–323. [PubMed] [Google Scholar]
- Wicken A. J. The glycerol teichoic acid from the cell wall of Bacillus stearothermophilus B65. Biochem J. 1966 Apr;99(1):108–116. doi: 10.1042/bj0990108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E. Autolytic enzyme associated with cell walls of Bacillus subtilis. J Biol Chem. 1966 Aug 10;241(15):3462–3467. [PubMed] [Google Scholar]


