Abstract
Ghrelin is a gut peptide hormone associated with feeding behavior and energy homeostasis. Acylated ghrelin binds to the growth hormone secretagogue receptor 1a subtype (GHS-R1a) in the hippocampus, leading to GH release from the anterior pituitary. However, in recent years, ghrelin and its receptor have also been implicated in other processes, including the regulation of cardiomyocyte function, muscle trophism, and bone metabolism. Moreover, GHS-R1a is distributed throughout the brain and is expressed in brain areas that regulate the stress response and emotional behavior. Consistently, a growing body of evidence supports the role of ghrelin in regulating stress response and mood. Stress has consistently been shown to increase ghrelin levels, and despite some inconsistencies, both human and rodent studies suggested antidepressant effects of ghrelin. Nevertheless, the precise mechanism by which ghrelin influences stress response and mood remains largely unknown. Intriguingly, ghrelin and GHS-R1a were consistently reported to exert anti-inflammatory, antioxidant, and neurotrophic effects both in vivo and in vitro, although this has never been directly assessed in relation to psychopathology. In the present review we will discuss available literature linking ghrelin with the stress response and depressive-like behavior in animal models as well as evidence describing the interplay between ghrelin and neuroinflammation/oxidative stress. Although further studies are required to understand the mechanisms involved in the action of ghrelin on mood, we hypothesize that the anti-inflammatory and anti-oxidative properties of ghrelin may give a key contribution.
Keywords: Ghrelin, inflammation, stress, oxidative stress, antidepressant, gut-brain axis, mood disorders, GHS-R1a
1. INTRODUCTION
Mood disorders, including Major Depressive Disorder (MDD), are the most common mental diseases worldwide [1]. Available antidepressants, classically acting by increasing monoamine bioavailability, retain significant limitations, including a delay of several weeks in the onset of therapeutic effect and a high percentage of treatment-resistant patients [2]. Our understanding of antidepressant pharmacotherapy has dramatically changed in recent years with the discovery that subanesthetic ketamine exerts antidepressant effects in a matter of minutes. This has paved the way for the development of rapid-acting antidepressants, including classic serotonergic psychedelics. Although the clinical benefits of ketamine and psychedelics are impressive, their potential for abuse limits their use [3, 4]. The study of etiopathogenetic mechanisms of depression is essential for the identification of new pharmacological targets that are required for the development of novel and safer therapeutic strategies. In this context, recent years have seen a growing interest in studying the gut-brain axis as a potential novel target for treating neuropsychiatric disorders [5, 6].
1.1. The Gut-brain Axis: Microbiota and Gut Peptides
The gut-brain axis is a bidirectional communication system enabling the gut to communicate with the brain and vice versa. The mechanisms mediating this communication are complex and not fully elucidated but include neural, endocrine, immune, and metabolic pathways (a detailed description is out of the scope of the present paper; for extensive reviews, please see [5-7]). In this context, increased emphasis has been given to the role of the microbiota and its metabolites in both health and disease. However, more recently, peptide hormones released from the gut, including glucagon-like peptide (GLP-1), peptide YY (PYY), cholecystokinin (CCK), corticotropin-releasing factor (CRF), oxytocin, and ghrelin [5], have also garnered attention. Indeed, the gastrointestinal tract is the largest endocrine organ in mammals, secreting dozens of different signaling molecules, including peptides. Importantly, gut peptide receptors are expressed not only in the hypothalamus, where they regulate appetite and food intake but also in cortico-limbic areas as well as on immune cells and vagus nerve terminals, thereby enabling indirect gut-brain communication. Moreover, the release of gut peptides can, in turn, be regulated by the gut microbiota, and the diversity and composition of the enteric bacteria can influence the release of gut peptides [5].
The present narrative review will focus on ghrelin, an orexigenic hormone that not only regulates food intake and energy balance but also has recognized roles in the control of different behaviors, including learning and memory, reward, and vulnerability to stress [8-11]. In detail, we will describe ghrelin's physiological functions, with a particular focus on possible implications in the pathophysiology of psychiatric disorders, including evidence regarding its functions in the regulation of depressive behavior. Although the link between ghrelin and depression has been clearly demonstrated at both preclinical and clinical levels (see section 3), the molecular mechanisms mediating its behavioral effects are still largely unknown. Here, we put forward the hypothesis that a major role could be played by the regulation of neuroinflammation and oxidative processes. Indeed, compelling evidence (reviewed in section 4) demonstrated that ghrelin not only has an anti-inflammatory effect mediated by a reduction of the secretion of inflammatory cytokines but also negatively regulates oxidative stress and mitochondrial dysfunction. Importantly, ghrelin exerts these functions both in peripheral tissues and in the central nervous system [12]. Given the recognized role of neuroinflammation and oxidative stress in the etiopathogenesis of mood disorders, we postulate that ghrelin actions at this level may give reason for its therapeutic potential in the management of these diseases. Data for this review were collected using the PubMed database.
2. GHRELIN SYNTHESIS AND REGULATION
Ghrelin is a 28 amino acid peptide hormone, synthesized by the X/A-like oxyntic gland cells in the gastric mucosa from its precursor pre-proghrelin, which is encoded by the GHRL gene, translated, and then cleaved into ghrelin. After its translation, ghrelin can be acylated by Ghrelin O-acyltransferase (GOAT), an enzyme expressed in the endoplasmic reticulum of ghrelin-producing cells. Both des-acyl-ghrelin and acyl-ghrelin are released in the bloodstream, and the latter is rapidly deacylated to des-acyl-ghrelin. Ghrelin is an orexigenic hormone and is mainly released in fasting conditions [8, 10, 13, 14]. The actions of acyl-ghrelin are mediated by binding to the growth hormone secretagogue receptor 1a (GHS-R1a), for which acyl-ghrelin is the only known ligand [15, 16]. GHS-R1a is a Gq-protein coupled receptor with strong, constitutive ligand-independent activity, while acyl-ghrelin further potentiates the receptor by stabilizing the active conformation of GHS-R1a. GHS-R1a is expressed both peripherally and in the central nervous system, where it is mostly detectable in the nuclei of the thalamus, the hippocampus, the midbrain, and the amygdala [8, 13, 14]. The GHRL gene also encodes for the GHS-R1b receptor, which contains only five transmembrane domains and whose function is still under investigation. Preliminary evidence shows that it may form heterodimers with GHS-R1a to act as negative feedback for acyl-ghrelin signaling [16, 17]. Importantly, GHS-R1a can also form heterodimers with other G-protein coupled receptors, including somatostatin 5, dopamine 1 and 3, serotonin 2C, and melanocortin 3 receptors, resulting in the activation of non-canonical signal transduction pathways. At the same time, several lines of evidence have revealed that the biological functions of des-acyl-ghrelin are mediated by a GHS-R1a-independent pathway, although the receptors for des-acyl-ghrelin remain unidentified [18].
2.1. Physiological Functions of Ghrelin
Although predominantly produced in the stomach, the majority of ghrelin functions are dependent on GHS-R1a located in the brain. The primary role of ghrelin is to regulate glucose homeostasis and stimulate food intake by activating hypothalamic neurons involved in homeostatic feeding [8, 13]. However, its functions go far beyond just regulating energy metabolism and food intake. Indeed, ghrelin signaling is also involved in the regulation of neuroendocrine and autonomic functions leading to behavioral changes. The effects of ghrelin are both direct (GHS-R1a-dependent) and indirect through the induction of growth hormone (GH) release in both the adenohypophysis and the hypothalamus. Importantly, a reciprocal relationship has been reported between ghrelin levels and the activation of the Hypothalamic-Pituitary-Adrenal (HPA) axis [19]. Indeed, ghrelin increases the release of corticotropin-releasing hormone (CRH) from the paraventricular nucleus, thus driving increased adrenocorticotropic hormone (ACTH) secretion from the pituitary and activating the stress response, while CRH decreases the expression of ghrelin [9, 19]. Ghrelin has been shown to increase vigilance and modulate mood, fear, and anxiety [10]. Indeed, ghrelin increases motivated behavior for food, and it has been hypothesized that when food is not readily available, it may decrease anxiety traits and not to hinder the animal from finding food [20]. Moreover, ghrelin has also recognized peripheral roles in the modulation of cardiac functions, muscle mass, bone metabolism, and cancer development and progression. For extensive reviews on ghrelin physiological functions, please see [8, 14, 21].
3. GHRELIN: LINK WITH STRESS AND MOOD DISORDERS
The impact of ghrelin on pathways orchestrating behaviors linked to reward, mood, anxiety, stress, and memory makes this hormone particularly interesting in the context of mood disorders. In this section, we will review the studies linking ghrelin to depression in both clinical and preclinical studies.
3.1. Ghrelin and Mood Disorders
The knowledge about ghrelin involvement in MDD comes from clinical studies that have shown alterations in the levels of this gut peptide in depressed patients and mood elevation following the administration of ghrelin either to depressed patients or healthy subjects [9-11].
Most of the studies reported higher levels of ghrelin in the plasma of MDD patients compared to healthy subjects [22-25], and this increase has been associated with the severity of depression, showing higher levels in patients with more severe symptomatology [24]. Interestingly, a sex effect was found in children with anxiety disorders, with higher plasma ghrelin levels in females compared to males [26].
Conversely, a few research groups reported no changes [27-29] or even decreased levels of both acyl- and des-acyl-ghrelin in depressed patients [30]. However, it is worth mentioning that not all the studies reported whether acyl-ghrelin, des-acyl-ghrelin, or total ghrelin were measured. Moreover, specific characteristics of the population examined, such as nutritional status, time of sample collection, age, sex, and drug treatments, might influence the results.
Further proof of the involvement of ghrelin in mood disorders is the fact that antidepressant drugs have been shown to modulate ghrelin expression, reducing its levels in the plasma of treated patients [22, 24, 25, 30, 31]. Notably, this effect was seen selectively in responder patients but not in non-responders, suggesting that ghrelin levels might be used as a predictor of treatment response [22, 25, 31]. Similarly, ghrelin serum levels were higher in non-responder subjects with panic disorder compared to responders and healthy controls [25].
The administration of ghrelin in healthy subjects, besides metabolic effects and hunger induction, was reported to elevate mood [32] and to increase plasma cortisol levels [33], while male but not female MDD patients who received acute ghrelin administration showed a trend for improvement of depressive symptoms including sleep disturbances [34].
Interestingly, allelic variants of the ghrelin gene were implicated in mechanisms of gene x environment interaction regulating the risk and symptom severity of psychiatric disorders. In an early study, the Leu72Met polymorphism in the pre-proghrelin gene was found to be less frequent in MDD patients than in healthy controls [35]. Moreover, male earthquake survivors carrying the Leu72Met polymorphism were reported to have a lower incidence of depression than male 72Leu/Leu homozygous, female 72Met allele carriers, or female 72Leu/Leu homozygotes [36]. At the same time, the Leu72Met polymorphism interacts with a polymorphism of the orexin gene to predict the risk of post-traumatic stress disorder (PTSD) in a sex-dependent manner [37]. For females, the lack of the pre-proghrelin 72Met allele is a risk factor for developing PTSD symptoms when the rs696217 genotype of the orexin gene is present; in contrast, the lack of the rs696217 of the orexin gene can be a risk factor for males only when the 72Met allele is present.
Since the Leu72Met polymorphism is located outside the coding region of mature ghrelin, it cannot change protein sequence but may instead influence mRNA stability or protein processing. However, the effects of the Leu72Met polymorphism on ghrelin functions are still poorly understood.
3.2. Ghrelin in Animal Models of Mood Disorders
3.2.1. Ghrelin and Stress
A huge amount of literature has investigated the link between stress, ghrelin, and depressive-/anxious-like behaviors in animal models [10] (Table 1). The first observation that suggested a role for ghrelin in behavioral outcomes goes back to 2001 when Asakawa and collaborators showed that ghrelin administration had an anxiogenic effect in mice that could be prevented using a CRH receptor antagonist [38], showing for the first time the interaction of ghrelin with the HPA axis. Moreover, they observed that acute stressors, such as tail pinch or fasting, increased ghrelin gene expression in the stomach [38].
Table 1. Changes of ghrelin and its receptor GHSR in stress models of depression.
| Model | Species | Strain | Sex | Effect | Tissue |
mRNA/
Protein |
References |
|---|---|---|---|---|---|---|---|
| Tail pinch Fasting |
Mouse | ddY | M | ↑ Ghrelin | Stomach | mRNA | [38] |
| Restraint, predator scent, FST, OF, noise stress, social stress, novel aversive environment | Mouse | C57BL/J6 and DBA | M | ↑ Acyl-ghrelin | Plasma | Protein | [39] |
| CSDS | Mouse | C57BL/6J | M | ↑ Acyl-ghrelin ↑ GHSR |
Plasma HPC |
Protein | [40] |
| Maternal separation | Mouse | CD1 | M/F | ↑ ghrelin | Plasma | Protein | [41] |
| Restraint stress | Mouse | ICR | M | ↑ Des-acyl-ghrelin | Plasma | Protein | [42] |
| Water avoidance | Rat | Wistar Sprague-Dawley |
F | ↑ Octonoylated/des-octonoylated ghrelin | Plasma | Protein | [43] |
| Repeated restraint stress | Rat | Sprague-Dawley | M | ↑ Acyl-ghrelin | Plasma | mRNA, Protein | [44] |
| Chronic Social Isolation | Rat | Wistar | M | ↑ Acyl-ghrelin | Serum | Protein | [45] |
| Chronic Social Stress | Mouse | C57BL/6J | M | ↑ Acyl-ghrelin ↑ GHSR |
Plasma VMH |
Protein | [46] |
| CUMS | Rat | Wistar | M | ↑ Ghrelin | Serum | Protein | [47] |
| Postpartum maternal separation and immobilization stress | Mouse | ICR | F | ↑ Ghrelin/acyl-ghrelin ↓ Ghrelin and GHSR ↓ GHSR |
Serum HPC, PFC HPC, PFC |
Protein mRNA Protein |
[48] |
| CSDS | Mouse | C57BL/6J | M | ↑ Acyl-ghrelin | Plasma | Protein | [49] |
| CUMS | Rat | Sprague-Dawley | M | ↑ Ghrelin ↑ GHSR |
PFC | mRNA | [50] |
| Social stress Social isolation |
Rat | Wistar | M | = ghrelin ↑ ghrelin |
Plasma | Protein | [51] |
| Immobilization stress | Rat | Sprague-Dawley | M/F | ↑ Ghrelin | Serum | Protein | [52] |
| Social isolation | Mouse | C57BL/6 | M/F | ↑ Acyl-ghrelin (M) = Acyl-ghrelin (F) |
Plasma | Protein | [53] |
| Immobilization stress | Rat | Long Evans | M | ↑ Acyl-ghrelin | Plasma | Protein | [54] |
| CUMS | Rat | Sprague-Dawley | M | ↓ Ghrelin | Serum | Protein | [55] |
| LPS | Rat | Sprague-Dawley | M | ↓ Acyl-ghrelin ↓ Des-acyl-ghrelin |
Plasma | Protein | [56] |
| Novelty | Mouse | C57BL/6J | M | ↓ Acyl ghrelin | Plasma | Protein | [57] |
| CSDS | Mouse | CD1 | M | ↓ Ghrelin | Plasma | Protein | [58] |
| Early life social stress | Rat | n.d. | F | ↑ GHSR | PVN | mRNA | [59] |
| PNS | Rat | Sprague-Dawley | M/F | ↑ GHSR | vHPC PFC |
mRNA | [60] |
| CUMS | Mouse | C57BL/6J | M | ↑ Pre-proghrelin ↑ GHSR |
HPC | mRNA mRNA, Protein |
[61] |
| Foot shock | Rat | Long Evans | M | = Acyl-ghrelin | Plasma | Protein | [64] |
| Foot shock | Rat | Wistar | M | = ghrelin | Plasma | Protein | [65] |
| Water cage | Rat | Wistar | M | = ghrelin | Plasma | Protein | [66] |
| Water cage | Rat | Wistar | M | = ghrelin = acyl-ghrelin = des-acyl-ghrelin |
Plasma | Protein | [67] |
Abbreviations: CSDS: chronic social defeat stress, CUMS: chronic unpredictable mild stress, HPC: hippocampus, vHPC: ventral hippocampus, PFC: prefrontal cortex, PVN: paraventricular nucleus of the hypothalamus, VMH: ventral medial hypothalamus.
Most of the following studies agreed to report increased levels of ghrelin in the plasma or serum of rodents exposed to different protocols of acute or chronic stress [39-54].
Only a few groups reported opposite results, showing a decrease in ghrelin expression in the serum of rats exposed to 5 weeks of chronic unpredictable mild stress (CUMS) [55] or both in the plasma of rats acutely injected with bacterial lipopolysaccharide [56] and mice exposed to chronic social defeat (CSDS) and Novelty stress [57, 58]. Of note, differences may depend on the specific form of ghrelin measured (acyl- vs. des-acyl-ghrelin, or total ghrelin), the species and strain used, as well as the feeding conditions and time of day in which the samples were collected.
Remarkably, also the expression of the ghrelin receptor GHS-R1a was consistently shown to increase under stress exposure, as demonstrated in the PVN of rats exposed to social stress in early life [59], in the hypothalamus of mice after chronic social stress [46], in the ventral hippocampus and prefrontal cortex of adult rats after postnatal stress [60], and in the hippocampus of both defeated and CUMS mice [40, 61]. Interestingly, one study suggested sex differences in the ghrelin axis with higher ghrelin and GHSR expression in the hippocampus and amygdala of female rats compared to males, which was associated with a stronger anxiolytic response to ghrelin [62].
To the best of our knowledge, only one study reported decreased levels of GHS-R1a mRNA and protein in the hippocampus and prefrontal cortex (despite increased blood levels of ghrelin) in female mice subjected to postpartum immobilization stress [48].
Interestingly, it has been proposed that the activation of ghrelin signaling in response to stress may be a homeostatic adaptation helping to cope with stress, but at the expense of increased caloric intake (Table 1) [63-67].
3.2.2. Antidepressant/Anxiolytic Properties of Ghrelin in Animal Models
Other groups studied the effect of exogenous ghrelin administration on depressive- and anxious-like behaviors in animal models (Table 2).
Table 2. Effects of exogenous administration of ghrelin, GHSR agonists and antagonists on mood in preclinical models.
| Model | Species | Strain | Sex | Treatment | Administration | Behavioural Effect | References |
|---|---|---|---|---|---|---|---|
| Tail pinch Fasting |
Mouse | ddY | M | Ghrelin | i.c.v. | Anxiogenic | [38] |
| CSDS | Mouse | C57BL/6J | M | Ghrelin | HPC micro-injection i.p. (10 days) |
Antidepressant anxiolytic | [40] |
| CSDS | Mouse | C57BL/6J | M | Ghrelin | s.c. | Antidepressant anxiolytic | [49] |
| CUMS | Mouse Rat |
C57BL/6J Sprague-Dawley |
M M |
Acyl-ghrelin ghrelin GHRP-6 |
i.p. i.c.v. |
Antidepressant anxiolytic Antidepressant |
[61] |
| Fasting | Rat | Sprague-Dawley | M/F | Acyl-ghrelin JMV2959 |
i.p. | Anxiolytic (F) | [62] |
| Olfactory Bulbectomy |
Mouse | Albino Swiss | F | Ghrelin | i.c.v. | Antidepressant anxiolytic | [68] |
| Naive | Mouse | C57BL/6J | M | Ghrelin L-692,585 YIL781 |
dCA1 infusion | Impaired long-term memory acquisition | [69] |
| CSDS | Mouse | C57BL/6N | M | Acyl-ghrelin | s.c. (acute and osmotic pump) |
No effect on social avoidance | [70] |
Abbreviations: CSDS: chronic social defeat stress, CUMS: chronic unpredictable mild stress, s.c.: subcutaneous injection, i.c.v.: intracerebroventricular injection, i.p.: intraperitoneal injection.
Lutter and collaborators found that the subcutaneous administration of ghrelin, as well as increased endogenous ghrelin levels obtained by caloric restriction, significantly reduced anxious- and depressive-like behaviors induced by CSDS in C57BL/6J mice [49]. Similarly, the direct administration of ghrelin in the lateral ventricle of Albino Swiss mice that underwent olfactory bulbectomy induced a significant decrease in depressive-like behavior, as shown by a reduced immobility time in the tail suspension test [68]. Intracerebroventricular ghrelin administration was also shown to exert an anxiolytic effect in mice receiving tail pinch [38]. Two-week i.p. administration of acyl-ghrelin or intracerebroventricular injection of either ghrelin or of a GHS-R1a agonist improved depressive- and anhedonic-like behavior in mice and rats, respectively [61]. Repeated low-dose microinjections of ghrelin in the hippocampus were shown to alleviate depressive- and anxiety-like behaviors induced by CSDS in C57BL/6J mice [40], while the direct infusion in the dorsal hippocampus of male mice impaired memory acquisition [69]. Interestingly, Borchers and collaborators found sex differences in the effect of intraperitoneal acyl-ghrelin administration, with an anxiolytic effect being reported only in female rats [62]. Instead, the subcutaneous injection of a GHS-R1a antagonist in a mouse model of early-life stress limited the activation of the HPA axis induced by maternal separation in both male and female pups [41].
Interestingly, Gupta and collaborators showed that the subcutaneous injection of acyl-ghrelin or a ghrelin receptor agonist, both chronically during stress and acutely after stress, did not reverse social avoidance in C57BL/6N mice after ten days of CSDS [70].
3.2.3. Genetic Manipulation of Ghrelin and Ghrelin Receptor
Another approach used to study the involvement of the ghrelin system in depressive-like behavior is the generation of transgenic lines in which the expression of ghrelin or GHS-R1a were manipulated (Table 3) [39, 40, 49, 71-76]. Walker and collaborators demonstrated that GHSR-null mice (GHSR-/-) are more vulnerable to CSDS and develop a stronger depressive-like phenotype compared to controls [71]. Moreover, this behavior was paralleled by reduced cell proliferation and survival in the ventral dentate gyrus subgranular zone, suggesting the involvement of hippocampal neurogenesis in the effects of ghrelin on mood [71]. Similarly, GHSR -/- mice are more anxious after acute restraint stress, compared to wild-type [72] and the selective knockdown of GHSR in the hippocampus of male C57BL/6J mice exacerbated depressive- and anxious-like behaviors induced by CSDS [40].
Table 3. Transgenic models of ghrelin.
| Genotype | Species | Strain | Sex | Stress Paradigm | Ghrelin Level | Effect on Mood | References |
|---|---|---|---|---|---|---|---|
| GHSR knockout | Mouse | C57BL/6J and DBA | M | Restraint, predator scent, FST, OF, social stress, noise stress, novel aversive environment |
↑ Acyl-ghrelin | - | [39] |
| GHSR knockdown (AAV-shRNA) |
Mouse | C57BL/6J | M | CSDS | - | ↑ Depression- and anxiety-like behaviour | [40] |
| GHSR knockout | Mouse | C57BL/6J | M | CSDS | ↑ Ghrelin | ↑ Social avoidance | [49] |
| GHSR knockout | Mouse | C57BL/6J | M | CSDS | - | ↑ Depressive-like behaviour | [71] |
| GHR knockout | Mouse | C57BL/6 | M | Acute restraint stress | - | ↑ Anxiety-like behaviour | [72] |
| GHSR knockout GHR knockout |
Mouse | C57BL/6J | M | CRS | - | = Anxiety-like behavior ↑ Anxiety-like behaviour |
[73] |
| GHSR knockout | Mouse | C57BL/6 | M | CSDS | ↑ Ghrelin | = Anxiety and despair-like behaviour | [74] |
| GHSR knockout | Mouse | C57BL/6J | M | - | - | = Fear acquisition, extinction and extinction retention ↓ Saccarine preference |
[75] |
| GHSR knockout | Mouse | C57BL/6J | M | CSDS | ↑ Acyl-ghrelin = des-acyl-ghrelin |
↑ Social isolation | [76] |
Abbreviations: CSDS: chronic social defeat stress, FST: forced swim, OF: open field.
Mice lacking both acyl- and des-acyl-ghrelin showed higher anxiety-like behaviors, whereas the anxious phenotype was attenuated in mice lacking acyl-ghrelin but expressing des-acyl-ghrelin. In the same study, the loss of GHS-R1a did not affect anxiety-like behavior, further suggesting that the effects of acyl- and des-acyl-ghrelin are not exclusively mediated by the canonical GHS-R1a signaling pathway [73]. However, in another study, both wild-type and GHSR-/- mice showed a similar reduction in sociability after CSDS exposure, suggesting no effect of the genetic deletion on the anxious phenotype [74].
3.2.4. Ghrelin and Antidepressant Treatments
A few antidepressant drugs have been reported to affect the ghrelin system. Treatment with Selective Serotonin Reuptake Inhibitors (SSRIs), including fluoxetine and paroxetine, decreased acyl-ghrelin levels in the plasma of rats through mechanisms involving the serotonin 2c receptor [77]. Moreover, both fluoxetine and clomipramine were shown to reduce short- and long-term memory retention promoted by ghrelin administration in rats [78]. The levels of serotonin appear to be critical in allowing or blunting the physiological activity of ghrelin in specific brain regions, including the hippocampus [78]. This interplay is supported by data showing ghrelin ability to inhibit depolarization-evoked serotonin release from hypothalamic synaptoneurosomes [79].
Notably, the ghrelin receptor seems to be required for the antidepressant activity of natural active compounds such as Meranzin hydrate and Paeoniflorin [80, 81]. Indeed, the deletion of GHS-R1a in rats prevented the ability of Meranzin hydrate to ameliorate depressive-like behaviors [81]. Similarly, mice lacking GHS-R1a and treated with Paeoniflorin showed a reduced rescue of depressive-like behaviors induced by CMS [80].
3.2.5. The Paradox of Ghrelin
Taking together the clinical and preclinical evidence regarding the involvement of ghrelin in the modulation of mood, the picture appears somewhat paradoxical [9, 10]. Indeed, while most of the studies reported increased levels of active ghrelin in the plasma of patients with mood disorders and in stressed-based animal models of depression, exogenous ghrelin administration has been shown to exert antidepressant and anxiolytic effects. Moreover, the genetic silencing of ghrelin or GHS-R1a exacerbates the stress response in rodents exposed to different stress protocols and blunts the antidepressant effect of various treatments. Overall, experimental evidence suggests that ghrelin acts to modulate behavior and HPA axis function in a context-dependent fashion. One explanation might be that the increase of ghrelin following stress exposure could be a coping mechanism, but more investigations are required to better understand the association between ghrelin levels, GHS-R1a function, and depressive behavior.
4. GHRELIN, NEUROINFLAMMATION, AND OXIDATIVE STRESS
A wide body of literature has demonstrated the immunomodulatory and antioxidant properties of ghrelin [12].
The dynamic balance of pro- and anti-inflammatory signals has been reported to be crucial in controlling pathological risk and disease progression. Importantly, a persistent challenge to the immune system, referred to as low-grade neuroinflammation, is recognized as an etiological factor for a number of pathophysiologic processes and adverse health outcomes, including cardiovascular diseases, neurodegeneration, and mood disorders [82].
The brain possesses specialized immune cells called microglia that carry out macrophage-like functions and have the primary role of maintaining brain homeostasis and providing rapid responses to damage or infection. However, excess or prolonged inflammatory cytokine activity perturbs multiple neuronal functions, including neurotransmitter signaling and neuroplasticity processes, ultimately leading to structural, functional, and behavioral changes [83, 84]. Importantly, in conditions that weaken the blood-brain barrier, peripheral proinflammatory mediators can infiltrate the brain and contribute to the activation of microglial cells.
The redox balance is a finely tuned process that protects from excessive production of reactive oxygen species (ROS) and free radicals [85]. Conversely, the imbalance between ROS generation and the antioxidant system leads to oxidative stress and mitochondrial dysfunction, which, in turn, may induce neurotoxicity. The brain is vulnerable to oxidative stress because of its higher oxygen consumption, higher lipid content, and weaker antioxidative defense, and oxidative stress has been implicated in the pathogenesis of mood disorders [86].
Even though oxidative stress and neuroinflammation are two totally different pathological events, they are linked and affect one another [87]. Indeed, inflammatory cells secrete ROS, while some ROS can further promote intracellular signaling cascades, leading to increased expression of pro-inflammatory genes.
In the following paragraphs, we will describe the evidence showing the effects of ghrelin or ghrelin analogues on neuroinflammation and oxidative stress in the brain.
4.1. Ghrelin and Microglia Activation
Ghrelin has been extensively studied in the context of neuroinflammation using a wide range of different preclinical models [88], although models of mood disorders have never been considered. Particular attention has been given to its activity on microglia cells and on its putative anti-inflammatory properties [88, 89].
Indeed, treatment with ghrelin has been shown to exert anti-inflammatory effects in vitro and in vivo by blunting microglia activation and suppressing the p38 MAPK-JNK signaling pathway, resulting in a decreased production of inflammatory markers such as TNF-α, IL-6, and IL-1β [90, 91]. By preventing pro-inflammatory responses, ghrelin was reported to ameliorate cell survival in specific brain areas such as the hippocampus and the substantia nigra [92, 93]. Moreover, exogenous ghrelin has been associated with a reduced activation of NF-kB and nod-like receptor protein 3 (NLRP3) inflammasome signaling pathways [94].
Interestingly, contrasting results have been reported as to whether the effect of ghrelin is dependent or not on the activation of its receptor GHS-R1a [92, 93]. In fact, ghrelin anti-inflammatory properties have been observed in tissues expressing either GHS-R1a or GHS-R1b isoforms or neither of them, leading to the hypothesis of the existence of unknown ghrelin receptors or of heterodimeric forms with other G-protein coupled receptors, that may mediate its activity [95].
4.2. Ghrelin, Oxidative Stress, and Mitochondrial Dysfunctions
Ghrelin has been shown to act as an endogenous antioxidant and a free radical scavenger [96]. Indeed, ghrelin was shown to inhibit ROS formation and, on the other hand, to induce the production of antioxidant enzymes, thus supporting the redox balance [97]. This ability was reported in in vitro and in vivo models of several diseases [98-101] but never evaluated in models of mood disorders. For example, the systemic injection of ghrelin in a mouse model of Alzheimer’s Disease was associated with reduced ROS production and mitochondrial dysfunction in the hippocampus, contributing to the amelioration of cognitive impairment [101]. Furthermore, ghrelin has been reported to protect spinal cord motor neurons from apoptosis in cellular and animal models of Amyotrophic Lateral Sclerosis (ALS) [102-105]. Similarly, ghrelin was able to reduce apoptosis of dopaminergic neurons caused by the treatment with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in models of Parkinson’s Disease [106, 107].
Apart from ghrelin, synthetic molecules belonging to the family of growth hormone secretagogues (GHS) have also been shown to modulate microglia function and oxidative stress. Among them, the hexapeptides hexarelin and EP80317 showed protective effects toward different inflammatory stimuli [108-110], preserving neuronal activity, preventing apoptosis induced by hydrogen peroxide, and activating the peroxisome proliferator-activated receptor-γ (PPAR-γ) to mediate the internalization of low-density oxidized lipoproteins [111, 112].
5. NEUROINFLAMMATION AND OXIDATIVE STRESS: A THERAPEUTIC TARGET FOR MOOD DISORDERS?
Mood disorders, including MDD, have been associated with central and peripheral inflammation [113, 114]. Indeed, beyond the well-known increase of pro-inflammatory cytokines and chemokines in the blood of MDD patients, similar changes have also been found in the cerebrospinal fluid (CSF), and in postmortem brains. Accordingly, increased activated microglia were reported in postmortem brains of suicidal MDD patients [115].
Overall, these data suggested that dysregulated inflammatory processes in the brain might take part in MDD onset and development. Interestingly, preclinical models of depression also reported alterations of inflammatory markers, both peripherally and in the brain, strengthening the association between neuroinflammation and depressive-like behavior [116]. At the same time, both preclinical and clinical studies reported mitochondrial dysfunctions linked to increased oxidative stress in mood disorders [117, 118].
5.1. Neuroinflammation and Oxidative Stress as Targets of Antidepressants
Targeting neuroinflammation may represent a promising tool in the treatment of MDD. To date, clinical evidence has been mostly reporting the antiinflammatory effects of antidepressants on peripheral markers of inflammation. Indeed, the therapeutic effect of classical antidepressants was reported to be accompanied by a reduction of the peripheral levels of inflammatory markers in depressed patients [119]. Notably, ketamine, a glutamatergic drug with rapid-acting antidepressant properties, was also shown to rapidly modulate peripheral proinflammatory cytokines [120]. On the other hand, anti-inflammatory drugs such as non-steroid anti-inflammatory drugs or minocycline were reported to improve depressive symptoms in MDD patients [121, 122], corroborating the hypothesis that targeting inflammation may help to obtain an antidepressant effect.
Similarly, in stress-based animal models of depression, antidepressants were reported to rescue depressive-like behaviors together with decreasing the activation of microglial cells and the expression of pro-inflammatory cytokines in specific brain areas (especially the hippocampus, cortex and amygdala) (for a detailed review, see [123]). Moreover, ketamine rescued LPS-induced depressive-like behavior by reducing the expression of cytokines in the prefrontal cortex of rats [124] and inhibited microglia inflammation in LPS-treated BV2 cells in vitro [125].
Further, classical antidepressants have been shown to modulate the oxidative status in MDD patients, decreasing blood levels of critical enzymes in the production of free radicals such as malondialdehyde and superoxide dismutase while, on the other hand, increasing the levels of the antioxidant ascorbic acid [126, 127].
5.2. Putative Antidepressant Mechanisms of Ghrelin
Taken together, previous literature suggests that 1) ghrelin may exert antidepressant properties in both MDD patients and animal models of depression; 2) ghrelin can regulate neuroinflammation and oxidative stress in the brain, inducing neuroprotective effects in models of neurodegenerative diseases; 3) neuroinflammation and oxidative stress play a key role in the pathophysiology and progression of mood disorders; 4) the therapeutic effects of antidepressants are associated with reduced neuroinflammation and oxidative stress; 5) drugs with anti-inflammatory and antioxidant properties can exert antidepressant effects.
Thus, we may speculate that the behavioral antidepressant and anxiolytic effects of ghrelin might be dependent on its capability to regulate both neuroinflammation and oxidative stress, thus promoting neuroprotection and neuroplasticity. Indeed, disruptions in neuroplasticity pathways are considered a crucial factor in the pathophysiology of mood disorders, while stimulating molecular and cellular mechanisms of neuroplasticity may improve depressive symptoms. Importantly, GHS-R1a is highly expressed in the hippocampus and previous studies reported that elevated ghrelin levels induced by fasting enhance hippocampal neurogenesis and memory [128, 129]. Intriguingly, the stimulatory effect of ghrelin on adult neurogenesis seems to be mediated by increased levels of brain-derived neurotrophic factor (BDNF) [130].
We thus speculate that antidepressant and procognitive effects of ghrelin might be mediated, on the one hand, by direct activation of neurotrophic intracellular signaling pathways downstream of GHS-R1a activation [109, 110, 131] and, on the other hand, indirectly through anti-inflammatory and anti-oxidative mechanisms (Fig. 1). More studies are needed to understand how the modulation of the ghrelin system, which is intriguingly positioned at the interface between feeding circuitry, metabolism, and the HPA axis, can be exploited for the treatment of mood disorders. The potential role of ghrelin modulation in psychiatric diseases is further underscored by the notion that mood disorders are often associated with metabolic dysfunction and also show a high level of comorbidity with eating and metabolic disorders [86, 132, 133]. Thus, strategies targeting the ghrelin system could be of particular interest in depressed patients with appetite alterations or presenting comorbidity with eating or metabolic disorders. An interesting and never-tested hypothesis is that the combination of drugs targeting the ghrelin system with traditional antidepressants could potentially improve the therapeutic outcome in some patients and help in the personalization of treatment. Future research should explore the therapeutic potential of the modulation of the ghrelin system in psychiatric and metabolic disorders.
Fig. (1).
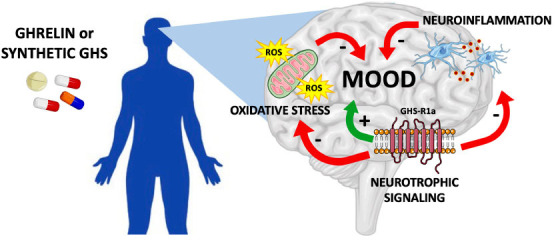
Putative pathways involved in the antidepressant and procognitive effects of ghrelin. Administration of ghrelin or its synthetic analogues may represent a promising therapeutical approach for mood disorders by dampening neuroinflammation and oxidative stress and promoting neurotrophic signaling downstream GHS-R1a activation. GHS: growth hormone secretagogue.
CONCLUSION AND FUTURE PERSPECTIVES
Overall, the studies conducted to date demonstrate that the ghrelin system has the intriguing potential of orchestrating feeding, reward, and mood behaviors at the same time, thus potentially representing a novel therapeutic target for mood and eating disorders. Most of the reports concur in attributing psychotropic and procognitive functions to ghrelin, further confirming the relevance of the gut-brain axis in regulating mood and cognition. Nevertheless, the use of ghrelin, a peptide hormone, as a therapeutic drug would not be suitable due to the poor pharmacokinetics and limited oral activity of the molecule. However, in recent years, efforts have been made on the development of synthetic compounds to find selective and highly potent GHS-R1a ligands [134], and GHS-R1a agonists have attracted attention for their potential use in several pathologies [134-136]. This could represent an innovative therapeutic strategy to alleviate the suffering of patients with mood disorders and fight against treatment-resistant depression.
LIST OF ABBREVIATIONS
- ACTH
Adrenocorticotropic Hormone
- ALS
Amyotrophic Lateral Sclerosis
- BDNF
Brain-derived Neurotrophic Factor
- CCK
Cholecystokinin
- CRF
Corticotropin-releasing Factor
- CRH
Corticotropin-releasing Hormone
- CSDS
Chronic Social Defeat Stress
- CSF
Cerebrospinal Fluid
- CUMS
Chronic Unpredictable Mild Stress
- GHS-R1a
Growth Hormone Secretagogue Receptor 1a
- GLP-1
Glucagon-like Peptide
- GOAT
Ghrelin O-acyltransferase
- HPA
Hypothalamic-pituitary-adrenal
- HPC
Hippocampus
- MDD
Major Depressive Disorder
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NLRP3
Nod-like Receptor Protein 3
- PFC
Prefrontal Cortex
- PPAR-γ
Peroxisome Proliferator-activated Receptor-γ
- PTSD
Post-Traumatic Stress Disorder
- PVN
Paraventricular Nucleus of Hypothalamus
- PYY
Peptide YY
- ROS
Reactive Oxygen Species
- SSRIs
Selective Serotonin Reuptake Inhibitors
- VMH
Ventral Medial Hypothalamus
AUTHORS’ CONTRIBUTIONS
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved the submitted version.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work was supported by the Cariplo Foundation (Biomedical Science Prog. 2019-3357).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.
REFERENCES
- 1.James S.L., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N., Abbastabar H., Abd-Allah F., Abdela J., Abdelalim A., Abdollahpour I., Abdulkader R.S., Abebe Z., Abera S.F., Abil O.Z., Abraha H.N., Abu-Raddad L.J., Abu-Rmeileh N.M.E., Accrombessi M.M.K., Acharya D., Acharya P., Ackerman I.N., Adamu A.A., Adebayo O.M., Adekanmbi V., Adetokunboh O.O., Adib M.G., Adsuar J.C., Afanvi K.A., Afarideh M., Afshin A., Agarwal G., Agesa K.M., Aggarwal R., Aghayan S.A., Agrawal S., Ahmadi A., Ahmadi M., Ahmadieh H., Ahmed M.B., Aichour A.N., Aichour I., Aichour M.T.E., Akinyemiju T., Akseer N., Al-Aly Z., Al-Eyadhy A., Al-Mekhlafi H.M., Al-Raddadi R.M., Alahdab F., Alam K., Alam T., Alashi A., Alavian S.M., Alene K.A., Alijanzadeh M., Navaei A.R., Aljunid S.M., Alkerwi A., Alla F., Allebeck P., Alouani M.M.L., Altirkawi K., Guzman A.N., Amare A.T., Aminde L.N., Ammar W., Amoako Y.A., Anber N.H., Andrei C.L., Androudi S., Animut M.D., Anjomshoa M., Ansha M.G., Antonio C.A.T., Anwari P., Arabloo J., Arauz A., Aremu O., Ariani F., Armoon B., Ärnlöv J., Arora A., Artaman A., Aryal K.K., Asayesh H., Asghar R.J., Ataro Z., Atre S.R., Ausloos M., Avila-Burgos L., Avokpaho E.F.G.A., Awasthi A., Quintanilla A.B.P., Ayer R., Azzopardi P.S., Babazadeh A., Badali H., Badawi A., Bali A.G., Ballesteros K.E., Ballew S.H., Banach M., Banoub J.A.M., Banstola A., Barac A., Barboza M.A., Barker-Collo S.L., Bärnighausen T.W., Barrero L.H., Baune B.T., Hejazi B.S., Bedi N., Beghi E., Behzadifar M., Behzadifar M., Béjot Y., Belachew A.B., Belay Y.A., Bell M.L., Bello A.K., Bensenor I.M., Bernabe E., Bernstein R.S., Beuran M., Beyranvand T., Bhala N., Bhattarai S., Bhaumik S., Bhutta Z.A., Biadgo B., Bijani A., Bikbov B., Bilano V., Bililign N., Bin Sayeed M.S., Bisanzio D., Blacker B.F., Blyth F.M., Bou-Orm I.R., Boufous S., Bourne R., Brady O.J., Brainin M., Brant L.C., Brazinova A., Breitborde N.J.K., Brenner H., Briant P.S., Briggs A.M., Briko A.N., Britton G., Brugha T., Buchbinder R., Busse R., Butt Z.A., Hurtado C.L., Cano J., Cárdenas R., Carrero J.J., Carter A., Carvalho F., Orjuela C.C.A., Rivas C.J., Castro F., López C.F., Cercy K.M., Cerin E., Chaiah Y., Chang A.R., Chang H-Y., Chang J-C., Charlson F.J., Chattopadhyay A., Chattu V.K., Chaturvedi P., Chiang P.P-C., Chin K.L., Chitheer A., Choi J-Y.J., Chowdhury R., Christensen H., Christopher D.J., Cicuttini F.M., Ciobanu L.G., Cirillo M., Claro R.M., Collado-Mateo D., Cooper C., Coresh J., Cortesi P.A., Cortinovis M., Costa M., Cousin E., Criqui M.H., Cromwell E.A., Cross M., Crump J.A., Dadi A.F., Dandona L., Dandona R., Dargan P.I., Daryani A., Das Gupta R., Das Neves J., Dasa T.T., Davey G., Davis A.C., Davitoiu D.V., De Courten B., De La Hoz F.P., De Leo D., De Neve J-W., Degefa M.G., Degenhardt L., Deiparine S., Dellavalle R.P., Demoz G.T., Deribe K., Dervenis N., Des Jarlais D.C., Dessie G.A., Dey S., Dharmaratne S.D., Dinberu M.T., Dirac M.A., Djalalinia S., Doan L., Dokova K., Doku D.T., Dorsey E.R., Doyle K.E., Driscoll T.R., Dubey M., Dubljanin E., Duken E.E., Duncan B.B., Duraes A.R., Ebrahimi H., Ebrahimpour S., Echko M.M., Edvardsson D., Effiong A., Ehrlich J.R., El Bcheraoui C., Zaki E.S.M., El-Khatib Z., Elkout H., Elyazar I.R.F., Enayati A., Endries A.Y., Er B., Erskine H.E., Eshrati B., Eskandarieh S., Esteghamati A., Esteghamati S., Fakhim H., Fallah Omrani V., Faramarzi M., Fareed M., Farhadi F., Farid T.A., Farinha C.S.E., Farioli A., Faro A., Farvid M.S., Farzadfar F., Feigin V.L., Fentahun N., Fereshtehnejad S-M., Fernandes E., Fernandes J.C., Ferrari A.J., Feyissa G.T., Filip I., Fischer F., Fitzmaurice C., Foigt N.A., Foreman K.J., Fox J., Frank T.D., Fukumoto T., Fullman N., Fürst T., Furtado J.M., Futran N.D., Gall S., Ganji M., Gankpe F.G., Basteiro G.A.L., Gardner W.M., Gebre A.K., Gebremedhin A.T., Gebremichael T.G., Gelano T.F., Geleijnse J.M., Maleras G.R., Geramo Y.C.D., Gething P.W., Gezae K.E., Ghadiri K., Falavarjani G.K., Kasman G.M., Ghimire M., Ghosh R., Ghoshal A.G., Giampaoli S., Gill P.S., Gill T.K., Ginawi I.A., Giussani G., Gnedovskaya E.V., Goldberg E.M., Goli S., Dantés G.H., Gona P.N., Gopalani S.V., Gorman T.M., Goulart A.C., Goulart B.N.G., Grada A., Grams M.E., Grosso G., Gugnani H.C., Guo Y., Gupta P.C., Gupta R., Gupta R., Gupta T., Gyawali B., Haagsma J.A., Hachinski V., Nejad H.N., Bidgoli G.H., Hagos T.B., Hailu G.B., Mirzaian H.A., Mirzaian H.A., Hamadeh R.R., Hamidi S., Handal A.J., Hankey G.J., Hao Y., Harb H.L., Harikrishnan S., Haro J.M., Hasan M., Hassankhani H., Hassen H.Y., Havmoeller R., Hawley C.N., Hay R.J., Hay S.I., Omran H.A., Heibati B., Hendrie D., Henok A., Herteliu C., Heydarpour S., Hibstu D.T., Hoang H.T., Hoek H.W., Hoffman H.J., Hole M.K., Homaie Rad E., Hoogar P., Hosgood H.D., Hosseini S.M., Hosseinzadeh M., Hostiuc M., Hostiuc S., Hotez P.J., Hoy D.G., Hsairi M., Htet A.S., Hu G., Huang J.J., Huynh C.K., Iburg K.M., Ikeda C.T., Ileanu B., Ilesanmi O.S., Iqbal U., Irvani S.S.N., Irvine C.M.S., Islam S.M.S., Islami F., Jacobsen K.H., Jahangiry L., Jahanmehr N., Jain S.K., Jakovljevic M., Javanbakht M., Jayatilleke A.U., Jeemon P., Jha R.P., Jha V., Ji J.S., Johnson C.O., Jonas J.B., Jozwiak J.J., Jungari S.B., Jürisson M., Kabir Z., Kadel R., Kahsay A., Kalani R., Kanchan T., Karami M., Karami Matin B., Karch A., Karema C., Karimi N., Karimi S.M., Kasaeian A., Kassa D.H., Kassa G.M., Kassa T.D., Kassebaum N.J., Katikireddi S.V., Kawakami N., Karyani A.K., Keighobadi M.M., Keiyoro P.N., Kemmer L., Kemp G.R., Kengne A.P., Keren A., Khader Y.S., Khafaei B., Khafaie M.A., Khajavi A., Khalil I.A., Khan E.A., Khan M.S., Khan M.A., Khang Y-H., Khazaei M., Khoja A.T., Khosravi A., Khosravi M.H., Kiadaliri A.A., Kiirithio D.N., Kim C-I., Kim D., Kim P., Kim Y-E., Kim Y.J., Kimokoti R.W., Kinfu Y., Kisa A., Skarbek K.K., Kivimäki M., Knudsen A.K.S., Kocarnik J.M., Kochhar S., Kokubo Y., Kolola T., Kopec J.A., Kosen S., Kotsakis G.A., Koul P.A., Koyanagi A., Kravchenko M.A., Krishan K., Krohn K.J., Kuate Defo B., Kucuk Bicer B., Kumar G.A., Kumar M., Kyu H.H., Lad D.P., Lad S.D., Lafranconi A., Lalloo R., Lallukka T., Lami F.H., Lansingh V.C., Latifi A., Lau K.M-M., Lazarus J.V., Leasher J.L., Ledesma J.R., Lee P.H., Leigh J., Leung J., Levi M., Lewycka S., Li S., Li Y., Liao Y., Liben M.L., Lim L-L., Lim S.S., Liu S., Lodha R., Looker K.J., Lopez A.D., Lorkowski S., Lotufo P.A., Low N., Lozano R., Lucas T.C.D., Lucchesi L.R., Lunevicius R., Lyons R.A., Ma S., Macarayan E.R.K., Mackay M.T., Madotto F., Razek M.A.E.H., Razek M.A.E.M., Maghavani D.P., Mahotra N.B., Mai H.T., Majdan M., Majdzadeh R., Majeed A., Malekzadeh R., Malta D.C., Mamun A.A., Manda A-L., Manguerra H., Manhertz T., Mansournia M.A., Mantovani L.G., Mapoma C.C., Maravilla J.C., Marcenes W., Marks A., Martins-Melo F.R., Martopullo I., März W., Marzan M.B., Thompson M.T.P., Massenburg B.B., Mathur M.R., Matsushita K., Maulik P.K., Mazidi M., McAlinden C., McGrath J.J., McKee M., Mehndiratta M.M., Mehrotra R., Mehta K.M., Mehta V., Rodriguez M.F., Mekonen T., Melese A., Melku M., Meltzer M., Memiah P.T.N., Memish Z.A., Mendoza W., Mengistu D.T., Mengistu G., Mensah G.A., Mereta S.T., Meretoja A., Meretoja T.J., Mestrovic T., Mezerji N.M.G., Miazgowski B., Miazgowski T., Millear A.I., Miller T.R., Miltz B., Mini G.K., Mirarefin M., Mirrakhimov E.M., Misganaw A.T., Mitchell P.B., Mitiku H., Moazen B., Mohajer B., Mohammad K.A., Mohammadifard N., Afrouzi M.M., Mohammed M.A., Mohammed S., Mohebi F., Moitra M., Mokdad A.H., Molokhia M., Monasta L., Moodley Y., Moosazadeh M., Moradi G., Moradi-Lakeh M., Moradinazar M., Moraga P., Morawska L., Velásquez M.I., Da-Costa M.J., Morrison S.D., Moschos M.M., Venning M.W.C., Mousavi S.M., Mruts K.B., Muche A.A., Muchie K.F., Mueller U.O., Muhammed O.S., Mukhopadhyay S., Muller K., Mumford J.E., Murhekar M., Musa J., Musa K.I., Mustafa G., Nabhan A.F., Nagata C., Naghavi M., Naheed A., Nahvijou A., Naik G., Naik N., Najafi F., Naldi L., Nam H.S., Nangia V., Nansseu J.R., Nascimento B.R., Natarajan G., Neamati N., Negoi I., Negoi R.I., Neupane S., Newton C.R.J., Ngunjiri J.W., Nguyen A.Q., Nguyen H.T., Nguyen H.L.T., Nguyen H.T., Nguyen L.H., Nguyen M., Nguyen N.B., Nguyen S.H., Nichols E., Ningrum D.N.A., Nixon M.R., Nolutshungu N., Nomura S., Norheim O.F., Noroozi M., Norrving B., Noubiap J.J., Nouri H.R., Shiadeh N.M., Nowroozi M.R., Nsoesie E.O., Nyasulu P.S., Odell C.M., Ofori-Asenso R., Ogbo F.A., Oh I-H., Oladimeji O., Olagunju A.T., Olagunju T.O., Olivares P.R., Olsen H.E., Olusanya B.O., Ong K.L., Ong S.K., Oren E., Ortiz A., Ota E., Otstavnov S.S., Øverland S., Owolabi M.O., P A M., Pacella R., Pakpour A.H., Pana A., Panda-Jonas S., Parisi A., Park E-K., Parry C.D.H., Patel S., Pati S., Patil S.T., Patle A., Patton G.C., Paturi V.R., Paulson K.R., Pearce N., Pereira D.M., Perico N., Pesudovs K., Pham H.Q., Phillips M.R., Pigott D.M., Pillay J.D., Piradov M.A., Pirsaheb M., Pishgar F., Plana-Ripoll O., Plass D., Polinder S., Popova S., Postma M.J., Pourshams A., Poustchi H., Prabhakaran D., Prakash S., Prakash V., Purcell C.A., Purwar M.B., Qorbani M., Quistberg D.A., Radfar A., Rafay A., Rafiei A., Rahim F., Rahimi K., Rahimi-Movaghar A., Rahimi-Movaghar V., Rahman M., Rahman M.H., Rahman M.A., Rahman S.U., Rai R.K., Rajati F., Ram U., Ranjan P., Ranta A., Rao P.C., Rawaf D.L., Rawaf S., Reddy K.S., Reiner R.C., Reinig N., Reitsma M.B., Remuzzi G., Renzaho A.M.N., Resnikoff S., Rezaei S., Rezai M.S., Ribeiro A.L.P., Roberts N.L.S., Robinson S.R., Roever L., Ronfani L., Roshandel G., Rostami A., Roth G.A., Roy A., Rubagotti E., Sachdev P.S., Sadat N., Saddik B., Sadeghi E., Moghaddam S., Safari H., Safari Y., Safari-Faramani R., Safdarian M., Safi S., Safiri S., Sagar R., Sahebkar A., Sahraian M.A., Sajadi H.S., Salam N., Salama J.S., Salamati P., Saleem K., Saleem Z., Salimi Y., Salomon J.A., Salvi S.S., Salz I., Samy A.M., Sanabria J., Sang Y., Santomauro D.F., Santos I.S., Santos J.V., Milicevic S.M.M., Sao Jose B.P., Sardana M., Sarker A.R., Sarrafzadegan N., Sartorius B., Sarvi S., Sathian B., Satpathy M., Sawant A.R., Sawhney M., Saxena S., Saylan M., Schaeffner E., Schmidt M.I., Schneider I.J.C., Schöttker B., Schwebel D.C., Schwendicke F., Scott J.G., Sekerija M., Sepanlou S.G., Serván-Mori E., Seyedmousavi S., Shabaninejad H., Shafieesabet A., Shahbazi M., Shaheen A.A., Shaikh M.A., Shams-Beyranvand M., Shamsi M., Shamsizadeh M., Sharafi H., Sharafi K., Sharif M., Sharif-Alhoseini M., Sharma M., Sharma R., She J., Sheikh A., Shi P., Shibuya K., Shigematsu M., Shiri R., Shirkoohi R., Shishani K., Shiue I., Shokraneh F., Shoman H., Shrime M.G., Si S., Siabani S., Siddiqi T.J., Sigfusdottir I.D., Sigurvinsdottir R., Silva J.P., Silveira D.G.A., Singam N.S.V., Singh J.A., Singh N.P., Singh V., Sinha D.N., Skiadaresi E., Slepak E.L.N., Sliwa K., Smith D.L., Smith M., Soares Filho A.M., Sobaih B.H., Sobhani S., Sobngwi E., Soneji S.S., Soofi M., Soosaraei M., Sorensen R.J.D., Soriano J.B., Soyiri I.N., Sposato L.A., Sreeramareddy C.T., Srinivasan V., Stanaway J.D., Stein D.J., Steiner C., Steiner T.J., Stokes M.A., Stovner L.J., Subart M.L., Sudaryanto A., Sufiyan M.B., Sunguya B.F., Sur P.J., Sutradhar I., Sykes B.L., Sylte D.O., Tabarés-Seisdedos R., Tadakamadla S.K., Tadesse B.T., Tandon N., Tassew S.G., Tavakkoli M., Taveira N., Taylor H.R., Tehrani-Banihashemi A., Tekalign T.G., Tekelemedhin S.W., Tekle M.G., Temesgen H., Temsah M-H., Temsah O., Terkawi A.S., Teweldemedhin M., Thankappan K.R., Thomas N., Tilahun B., To Q.G., Tonelli M., Topor-Madry R., Topouzis F., Torre A.E., Tortajada-Girbés M., Touvier M., Tovani-Palone M.R., Towbin J.A., Tran B.X., Tran K.B., Troeger C.E., Truelsen T.C., Tsilimbaris M.K., Tsoi D., Tudor Car L., Tuzcu E.M., Ukwaja K.N., Ullah I., Undurraga E.A., Unutzer J., Updike R.L., Usman M.S., Uthman O.A., Vaduganathan M., Vaezi A., Valdez P.R., Varughese S., Vasankari T.J., Venketasubramanian N., Villafaina S., Violante F.S., Vladimirov S.K., Vlassov V., Vollset S.E., Vosoughi K., Vujcic I.S., Wagnew F.S., Waheed Y., Waller S.G., Wang Y., Wang Y-P., Weiderpass E., Weintraub R.G., Weiss D.J., Weldegebreal F., Weldegwergs K.G., Werdecker A., West T.E., Whiteford H.A., Widecka J., Wijeratne T., Wilner L.B., Wilson S., Winkler A.S., Wiyeh A.B., Wiysonge C.S., Wolfe C.D.A., Woolf A.D., Wu S., Wu Y-C., Wyper G.M.A., Xavier D., Xu G., Yadgir S., Yadollahpour A., Yahyazadeh Jabbari S.H., Yamada T., Yan L.L., Yano Y., Yaseri M., Yasin Y.J., Yeshaneh A., Yimer E.M., Yip P., Yisma E., Yonemoto N., Yoon S-J., Yotebieng M., Younis M.Z., Yousefifard M., Yu C., Zadnik V., Zaidi Z., Zaman S.B., Zamani M., Zare Z., Zeleke A.J., Zenebe Z.M., Zhang K., Zhao Z., Zhou M., Zodpey S., Zucker I., Vos T., Murray C.J.L. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McIntyre R.S., Filteau M.J., Martin L., Patry S., Carvalho A., Cha D.S., Barakat M., Miguelez M. Treatment-resistant depression: Definitions, review of the evidence, and algorithmic approach. J. Affect. Disord. 2014;156:1–7. doi: 10.1016/j.jad.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 3.Kadriu B., Musazzi L., Henter I.D., Graves M., Popoli M., Zarate C.A. Jr Glutamatergic neurotransmission: Pathway to developing novel rapid-acting antidepressant treatments. Int. J. Neuropsychopharmacol. 2019;22(2):119–135. doi: 10.1093/ijnp/pyy094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wojtas A. The possible place for psychedelics in pharmacotherapy of mental disorders. Pharmacol. Rep. 2023;75(6):1313–1325. doi: 10.1007/s43440-023-00550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lach G., Schellekens H., Dinan T.G., Cryan J.F. Anxiety, depression, and the microbiome: A role for gut peptides. Neurotherapeutics. 2018;15(1):36–59. doi: 10.1007/s13311-017-0585-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lachmansingh D.A., Lavelle A., Cryan J.F., Clarke G. Microbiota-gut-brain axis and antidepressant treatment. Curr. Top. Behav. Neurosci. 2024;66:175–216. doi: 10.1007/7854_2023_449. [DOI] [PubMed] [Google Scholar]
- 7.Bhatt S., Kanoujia J., Lakshmi S., Patil C., Gupta G., Chellappan D.K., Dua K. Role of brain-gut-microbiota axis in depression: Emerging therapeutic avenues. CNS Neurol. Disord. Drug Targets. 2023;22(2):276–288. doi: 10.2174/1871527321666220329140804. [DOI] [PubMed] [Google Scholar]
- 8.Yanagi S., Sato T., Kangawa K., Nakazato M. The homeostatic force of ghrelin. Cell Metab. 2018;27(4):786–804. doi: 10.1016/j.cmet.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Spencer S.J., Emmerzaal T.L., Kozicz T., Andrews Z.B. Ghrelin’s role in the hypothalamic-pituitary-adrenal axis stress response: Implications for mood disorders. Biol. Psychiatry. 2015;78(1):19–27. doi: 10.1016/j.biopsych.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 10.Fritz E.M., Singewald N., De Bundel D. The good, the bad and the unknown aspects of ghrelin in stress coping and stress-related psychiatric disorders. Front. Synaptic Neurosci. 2020;12:594484. doi: 10.3389/fnsyn.2020.594484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stone L.A., Harmatz E.S., Goosens K.A. Ghrelin as a stress hormone: Implications for psychiatric illness. Biol. Psychiatry. 2020;88(7):531–540. doi: 10.1016/j.biopsych.2020.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Wang H., Dou S., Zhu J., Shao Z., Wang C., Cheng B. Regulatory effects of ghrelin on endoplasmic reticulum stress, oxidative stress, and autophagy: Therapeutic potential. Neuropeptides. 2021;85:102112. doi: 10.1016/j.npep.2020.102112. [DOI] [PubMed] [Google Scholar]
- 13.Gray S.M., Page L.C., Tong J. Ghrelin regulation of glucose metabolism. J. Neuroendocrinol. 2019;31(7):e12705. doi: 10.1111/jne.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pradhan G., Samson S.L., Sun Y. Ghrelin. Curr. Opin. Clin. Nutr. Metab. Care. 2013;16(6):619–624. doi: 10.1097/MCO.0b013e328365b9be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato T., Nakamura Y., Shiimura Y., Ohgusu H., Kangawa K., Kojima M. Structure, regulation and function of ghrelin. J. Biochem. 2012;151(2):119–128. doi: 10.1093/jb/mvr134. [DOI] [PubMed] [Google Scholar]
- 16.Delhanty P.J.D., Neggers S.J., van der Lely A.J. Mechanisms in endocrinology: Ghrelin: The differences between acyl- and des-acyl ghrelin. Eur. J. Endocrinol. 2012;167(5):601–608. doi: 10.1530/EJE-12-0456. [DOI] [PubMed] [Google Scholar]
- 17.Chow K.B.S., Sun J., Chu M.K., Cheung T.W., Cheng C.H.K., Wise H. The truncated ghrelin receptor polypeptide (GHS-R1b) is localized in the endoplasmic reticulum where it forms heterodimers with ghrelin receptors (GHS-R1a) to attenuate their cell surface expression. Mol. Cell. Endocrinol. 2012;348(1):247–254. doi: 10.1016/j.mce.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 18.Callaghan B., Furness J.B. Novel and conventional receptors for ghrelin, desacyl-ghrelin, and pharmacologically related compounds. Pharmacol. Rev. 2014;66(4):984–1001. doi: 10.1124/pr.113.008433. [DOI] [PubMed] [Google Scholar]
- 19.Bali A., Jaggi S.A. An integrative review on role and mechanisms of ghrelin in stress, anxiety and depression. Curr. Drug Targets. 2016;17(5):495–507. doi: 10.2174/1389450116666150518095650. [DOI] [PubMed] [Google Scholar]
- 20.Hansson C., Shirazi R.H., Näslund J., Vogel H., Neuber C., Holm G., Anckarsäter H., Dickson S.L., Eriksson E., Skibicka K.P. Ghrelin influences novelty seeking behavior in rodents and men. PLoS One. 2012;7(12):e50409. doi: 10.1371/journal.pone.0050409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stasi C., Milani S. Functions of ghrelin in brain, gut and liver. CNS Neurol. Disord. Drug Targets. 2016;15(8):956–963. doi: 10.2174/1871527315666160709203525. [DOI] [PubMed] [Google Scholar]
- 22.Kurt E., Guler O., Serteser M., Cansel N., Ozbulut O., Altınbaş K., Alataş G., Savaş H., Gecici O. The effects of electroconvulsive therapy on ghrelin, leptin and cholesterol levels in patients with mood disorders. Neurosci. Lett. 2007;426(1):49–53. doi: 10.1016/j.neulet.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 23.Algul S., Ozcelik O. Evaluating the levels of nesfatin-1 and ghrelin hormones in patients with moderate and severe major depressive disorders. Psychiatry Investig. 2018;15(2):214–218. doi: 10.30773/pi.2017.05.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozsoy S., Besirli A., Abdulrezzak U., Basturk M. Serum ghrelin and leptin levels in patients with depression and the effects of treatment. Psychiatry Investig. 2014;11(2):167–172. doi: 10.4306/pi.2014.11.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishitobi Y., Kohno K., Kanehisa M., Inoue A., Imanaga J., Maruyama Y., Ninomiya T., Higuma H., Okamoto S., Tanaka Y., Tsuru J., Hanada H., Isogawa K., Akiyoshi J. Serum ghrelin levels and the effects of antidepressants in major depressive disorder and panic disorder. Neuropsychobiology. 2012;66(3):185–192. doi: 10.1159/000339948. [DOI] [PubMed] [Google Scholar]
- 26.Ozmen S., Şeker A., Demirci E. Ghrelin and leptin levels in children with anxiety disorders. J. Pediatr. Endocrinol. Metab. 2019;32(10):1043–1047. doi: 10.1515/jpem-2019-0229. [DOI] [PubMed] [Google Scholar]
- 27.Schanze A., Reulbach U., Scheuchenzuber M., Gröschl M., Kornhuber J., Kraus T. Ghrelin and eating disturbances in psychiatric disorders. Neuropsychobiology. 2008;57(3):126–130. doi: 10.1159/000138915. [DOI] [PubMed] [Google Scholar]
- 28.Giménez-Palop O., Coronas R., Cobo J., Gallart L., Barbero J.D., Parra I., Fusté G., Vendrell J., Bueno M., González-Clemente J.M., Caixàs A. Fasting plasma peptide YY concentrations are increased in patients with major depression who associate weight loss. J. Endocrinol. Invest. 2012;35(7):645–648. doi: 10.3275/8180. [DOI] [PubMed] [Google Scholar]
- 29.Matsuo K., Nakano M., Nakashima M., Watanuki T., Egashira K., Matsubara T., Watanabe Y. Neural correlates of plasma acylated ghrelin level in individuals with major depressive disorder. Brain Res. 2012;1473:185–192. doi: 10.1016/j.brainres.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 30.Barim A.O., Aydin S., Colak R., Dag E., Deniz O., Sahin İ. Ghrelin, paraoxonase and arylesterase levels in depressive patients before and after citalopram treatment. Clin. Biochem. 2009;42(10-11):1076–1081. doi: 10.1016/j.clinbiochem.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 31.Ricken R., Bopp S., Schlattmann P., Himmerich H., Bschor T., Richter C., Elstner S., Stamm T.J., Schulz-Ratei B., Lingesleben A., Reischies F.M., Sterzer P., Borgwardt S., Bauer M., Heinz A., Hellweg R., Lang U.E., Adli M. Ghrelin serum concentrations are associated with treatment response during lithium augmentation of antidepressants. Int. J. Neuropsychopharmacol. 2017;20(9):692–697. doi: 10.1093/ijnp/pyw082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmid D.A., Held K., Ising M., Uhr M., Weikel J.C., Steiger A. Ghrelin stimulates appetite, imagination of food, GH, ACTH, and cortisol, but does not affect leptin in normal controls. Neuropsychopharmacology. 2005;30(6):1187–1192. doi: 10.1038/sj.npp.1300670. [DOI] [PubMed] [Google Scholar]
- 33.Lambert E., Lambert G., Ika-Sari C., Dawood T., Lee K., Chopra R., Straznicky N., Eikelis N., Drew S., Tilbrook A., Dixon J., Esler M., Schlaich M.P. Ghrelin modulates sympathetic nervous system activity and stress response in lean and overweight men. Hypertension. 2011;58(1):43–50. doi: 10.1161/HYPERTENSIONAHA.111.171025. [DOI] [PubMed] [Google Scholar]
- 34.Kluge M., Schüssler P., Dresler M., Schmidt D., Yassouridis A., Uhr M., Steiger A. Effects of ghrelin on psychopathology, sleep and secretion of cortisol and growth hormone in patients with major depression. J. Psychiatr. Res. 2011;45(3):421–426. doi: 10.1016/j.jpsychires.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Nakashima K., Akiyoshi J., Hatano K., Hanada H., Tanaka Y., Tsuru J., Matsushita H., Kodama K., Isogawa K. Ghrelin gene polymorphism is associated with depression, but not panic disorder. Psychiatr. Genet. 2008;18(5):257. doi: 10.1097/YPG.0b013e328306c979. [DOI] [PubMed] [Google Scholar]
- 36.Su M., Cao T., Feng Y., Guo Q.W., Fan M., Fang D.Z. Longitudinal changes of associations between the preproghrelin Leu72Met polymorphism with depression in Chinese Han adolescents after the Wenchuan earthquake. Psychiatr. Genet. 2017;27(5):161–168. doi: 10.1097/YPG.0000000000000180. [DOI] [PubMed] [Google Scholar]
- 37.Li G., Zhang K., Wang L., Cao C., Fang R., Liu P., Luo S., Liberzon I. The preliminary investigation of orexigenic hormone gene polymorphisms on posttraumatic stress disorder symptoms. Psychoneuroendocrinology. 2019;100:131–136. doi: 10.1016/j.psyneuen.2018.09.042. [DOI] [PubMed] [Google Scholar]
- 38.Asakawa A., Inui A., Kaga T., Yuzuriha H., Nagata T., Fujimiya M., Katsuura G., Makino S., Fujino M.A., Kasuga M. A role of ghrelin in neuroendocrine and behavioral responses to stress in mice. Neuroendocrinology. 2001;74(3):143–147. doi: 10.1159/000054680. [DOI] [PubMed] [Google Scholar]
- 39.Patterson Z.R., Ducharme R., Anisman H., Abizaid A. Altered metabolic and neurochemical responses to chronic unpredictable stressors in ghrelin receptor‐deficient mice. Eur. J. Neurosci. 2010;32(4):632–639. doi: 10.1111/j.1460-9568.2010.07310.x. [DOI] [PubMed] [Google Scholar]
- 40.Han Q.Q., Huang H.J., Wang Y.L., Yang L., Pilot A., Zhu X.C., Yu R., Wang J., Chen X.R., Liu Q., Li B., Wu G.C., Yu J. Ghrelin exhibited antidepressant and anxiolytic effect via the p38-MAPK signaling pathway in hippocampus. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2019;93:11–20. doi: 10.1016/j.pnpbp.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt M.V., Levine S., Alam S., Harbich D., Sterlemann V., Ganea K., De Kloet E.R., Holsboer F., Müller M.B. Metabolic signals modulate hypothalamic-pituitary-adrenal axis activation during maternal separation of the neonatal mouse. J. Neuroendocrinol. 2006;18(11):865–874. doi: 10.1111/j.1365-2826.2006.01482.x. [DOI] [PubMed] [Google Scholar]
- 42.Nahata M., Saegusa Y., Sadakane C., Yamada C., Nakagawa K., Okubo N., Ohnishi S., Hattori T., Sakamoto N., Takeda H. Administration of exogenous acylated ghrelin or rikkunshito, an endogenous ghrelin enhancer, improves the decrease in postprandial gastric motility in an acute restraint stress mouse model. Neurogastroenterol. Motil. 2014;26(6):821–831. doi: 10.1111/nmo.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kristenssson E., Sundqvist M., Astin M., Kjerling M., Mattsson H., de la Cour D.C., Håkanson R., Lindström E. Acute psychological stress raises plasma ghrelin in the rat. Regul. Pept. 2006;134(2-3):114–117. doi: 10.1016/j.regpep.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 44.Zheng J., Dobner A., Babygirija R., Ludwig K., Takahashi T. Effects of repeated restraint stress on gastric motility in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296(5):R1358–R1365. doi: 10.1152/ajpregu.90928.2008. [DOI] [PubMed] [Google Scholar]
- 45.Dulabi A.N., Shakerin Z., Mehranfard N., Ghasemi M. Vitamin C protects against chronic social isolation stress-induced weight gain and depressive-like behavior in adult male rats. Endocr. Regul. 2020;54(4):266–274. doi: 10.2478/enr-2020-0030. [DOI] [PubMed] [Google Scholar]
- 46.Nascimento C.S., Opacka-Juffry J., Costabile A., Boyle C.N., Herde A.M., Ametamey S.M., Sigrist H., Pryce C.R., Patterson M. Chronic social stress in mice alters energy status including higher glucose need but lower brain utilization. Psychoneuroendocrinology. 2020;119:104747. doi: 10.1016/j.psyneuen.2020.104747. [DOI] [PubMed] [Google Scholar]
- 47.Zhou X., Wang J., Lu Y., Chen C., Hu Y., Liu P., Dong X. Anti-depressive effects of Kai-Xin-San on lipid metabolism in depressed patients and CUMS rats using metabolomic analysis. J. Ethnopharmacol. 2020;252:112615. doi: 10.1016/j.jep.2020.112615. [DOI] [PubMed] [Google Scholar]
- 48.Xing J.W., Tian X.Y., Chen M.M., Peng X.H., Gao P. Expression of ghrelin or growth hormone secretagogue receptor in the brain of postpartum stress mice. Neuroreport. 2021;32(8):678–685. doi: 10.1097/WNR.0000000000001633. [DOI] [PubMed] [Google Scholar]
- 49.Lutter M., Sakata I., Lawrence O.S., Rovinsky S.A., Anderson J.G., Jung S., Birnbaum S., Yanagisawa M., Elmquist J.K., Nestler E.J., Zigman J.M. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat. Neurosci. 2008;11(7):752–753. doi: 10.1038/nn.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu W., Wang H., Wang Y., Li H., Ji L. Metabolic factors-triggered inflammatory response drives antidepressant effects of exercise in CUMS rats. Psychiatry Res. 2015;228(3):257–264. doi: 10.1016/j.psychres.2015.05.102. [DOI] [PubMed] [Google Scholar]
- 51.Radahmadi M., Izadi M.S., Ghasemi M., Rayatpour A. Effects of isolation and social subchronic stresses on food intake and levels of leptin, ghrelin, and glucose in male rats. Adv. Biomed. Res. 2018;7(1):118. doi: 10.4103/abr.abr_28_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elbassuoni E.A. Gender differences in ghrelin response to chronic immobilization stress in rats: Possible role of estrogen. Gen. Physiol. Biophys. 2014;33(1):111–120. doi: 10.4149/gpb_2013061. [DOI] [PubMed] [Google Scholar]
- 53.Yamada C., Saegusa Y., Nahata M., Sadakane C., Hattori T., Takeda H. Influence of aging and gender differences on feeding behavior and ghrelin-related factors during social isolation in mice. PLoS One. 2015;10(10):e0140094. doi: 10.1371/journal.pone.0140094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meyer R.M., Burgos-Robles A., Liu E., Correia S.S., Goosens K.A. A ghrelin–growth hormone axis drives stress-induced vulnerability to enhanced fear. Mol. Psychiatry. 2014;19(12):1284–1294. doi: 10.1038/mp.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang M., Jiang P., Li H., Liu Y., Cai H., Dang R., Zhu W., Cao L. Fish oil supplementation alleviates depressant-like behaviors and modulates lipid profiles in rats exposed to chronic unpredictable mild stress. BMC Complement. Altern. Med. 2015;15(1):239. doi: 10.1186/s12906-015-0778-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stengel A., Goebel M., Wang L., Reeve J.R., Jr, Taché Y., Lambrecht N.W.G. Lipopolysaccharide differentially decreases plasma acyl and desacyl ghrelin levels in rats: Potential role of the circulating ghrelin-acylating enzyme GOAT. Peptides. 2010;31(9):1689–1696. doi: 10.1016/j.peptides.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saegusa Y., Takeda H., Muto S., Nakagawa K., Ohnishi S., Sadakane C., Nahata M., Hattori T., Asaka M. Decreased plasma ghrelin contributes to anorexia following novelty stress. Am. J. Physiol. Endocrinol. Metab. 2011;301(4):E685–E696. doi: 10.1152/ajpendo.00121.2011. [DOI] [PubMed] [Google Scholar]
- 58.Razzoli M., Sanghez V., Bartolomucci A. Chronic subordination stress induces hyperphagia and disrupts eating behavior in mice modeling binge-eating-like disorder. Front. Nutr. 2015;1(30):1. doi: 10.3389/fnut.2014.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murgatroyd C.A., Peña C.J., Podda G., Nestler E.J., Nephew B.C. Early life social stress induced changes in depression and anxiety associated neural pathways which are correlated with impaired maternal care. Neuropeptides. 2015;52:103–111. doi: 10.1016/j.npep.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berry A., Mazzelli M., Musillo C., Riva M.A., Cattaneo A., Cirulli F. High‐fat diet during adulthood interacts with prenatal stress, affecting both brain inflammatory and neuroendocrine markers in male rats. Eur. J. Neurosci. 2022;55(9-10):2326–2340. doi: 10.1111/ejn.15181. [DOI] [PubMed] [Google Scholar]
- 61.Huang H.J., Zhu X.C., Han Q.Q., Wang Y.L., Yue N., Wang J., Yu R., Li B., Wu G.C., Liu Q., Yu J. Ghrelin alleviates anxiety- and depression-like behaviors induced by chronic unpredictable mild stress in rodents. Behav. Brain Res. 2017;326:33–43. doi: 10.1016/j.bbr.2017.02.040. [DOI] [PubMed] [Google Scholar]
- 62.Börchers S., Krieger J.P., Maric I., Carl J., Abraham M., Longo F., Asker M., Richard J.E., Skibicka K.P. From an empty stomach to anxiolysis: Molecular and behavioral assessment of sex differences in the ghrelin axis of rats. Front. Endocrinol. 2022;13:901669. doi: 10.3389/fendo.2022.901669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chuang J.C., Zigman J.M. Ghrelin’s roles in stress, mood, and anxiety regulation. Int. J. Pept. 2010;2010:1–5. doi: 10.1155/2010/460549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harmatz E.S., Stone L., Lim S.H., Lee G., McGrath A., Gisabella B., Peng X., Kosoy E., Yao J., Liu E., Machado N.J., Weiner V.S., Slocum W., Cunha R.A., Goosens K.A. Central ghrelin resistance permits the overconsolidation of fear memory. Biol. Psychiatry. 2017;81(12):1003–1013. doi: 10.1016/j.biopsych.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rostamkhani F., Zardooz H., Goshadrou F., Baveisi M., Hedayati M. Stress increased ghrelin secretion from pancreatic isolated islets in male rats. Gen. Physiol. Biophys. 2016;35(1):109–117. doi: 10.4149/gpb_2015037. [DOI] [PubMed] [Google Scholar]
- 66.Gul S., Saleem D., Haleem M.A., Haleem D.J. Inhibition of hormonal and behavioral effects of stress by tryptophan in rats. Nutr. Neurosci. 2019;22(6):409–417. doi: 10.1080/1028415X.2017.1395551. [DOI] [PubMed] [Google Scholar]
- 67.Ochi M., Tominaga K., Tanaka F., Tanigawa T., Shiba M., Watanabe T., Fujiwara Y., Oshitani N., Higuchi K., Arakawa T. Effect of chronic stress on gastric emptying and plasma ghrelin levels in rats. Life Sci. 2008;82(15-16):862–868. doi: 10.1016/j.lfs.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 68.Carlini V.P., Machado D.G., Buteler F., Ghersi M., Ponzio M.F., Martini A.C., Schiöth H.B., de Cuneo M.F., Rodrigues A.L.S., de Barioglio S.R. Acute ghrelin administration reverses depressive-like behavior induced by bilateral olfactory bulbectomy in mice. Peptides. 2012;35(2):160–165. doi: 10.1016/j.peptides.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 69.Li N., Xiao K., Mi X., Li N., Guo L., Wang X., Sun Y., Li G.D., Zhou Y. Ghrelin signaling in dCA1 suppresses neuronal excitability and impairs memory acquisition via PI3K/Akt/GSK-3β cascades. Neuropharmacology. 2022;203:108871. doi: 10.1016/j.neuropharm.2021.108871. [DOI] [PubMed] [Google Scholar]
- 70.Gupta D., Chuang J.C., Mani B.K., Shankar K., Rodriguez J.A., Lawrence O.S., Metzger N.P., Zigman J.M. β1-adrenergic receptors mediate plasma acyl-ghrelin elevation and depressive-like behavior induced by chronic psychosocial stress. Neuropsychopharmacology. 2019;44(7):1319–1327. doi: 10.1038/s41386-019-0334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walker A.K., Rivera P.D., Wang Q., Chuang J-C., Tran S., Lawrence O.S., Estill S.J., Starwalt R., Huntington P., Morlock L., Naidoo J., Williams N.S., Ready J.M., Eisch A.J., Pieper A.A., Zigman J.M. The P7C3 class of neuroprotective compounds exerts antidepressant efficacy in mice by increasing hippocampal neurogenesis. Mol. Psychiatry. 2015;20(4):500–508. doi: 10.1038/mp.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spencer S.J., Xu L., Clarke M.A., Lemus M., Reichenbach A., Geenen B., Kozicz T., Andrews Z.B. Ghrelin regulates the hypothalamic-pituitary-adrenal axis and restricts anxiety after acute stress. Biol. Psychiatry. 2012;72(6):457–465. doi: 10.1016/j.biopsych.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 73.Mahbod P., Smith E.P., Fitzgerald M.E., Morano R.L., Packard B.A., Ghosal S., Scheimann J.R., Perez-Tilve D., Herman J.P., Tong J. Desacyl ghrelin decreases anxiety-like behavior in male mice. Endocrinology. 2018;159(1):388–399. doi: 10.1210/en.2017-00540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guo L., Niu M., Yang J., Li L., Liu S., Sun Y., Zhou Z., Zhou Y. GHS-R1a deficiency alleviates depression-related behaviors after chronic social defeat stress. Front. Neurosci. 2019;13:364. doi: 10.3389/fnins.2019.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pierre A., Regin Y., Van Schuerbeek A., Fritz E.M., Muylle K., Beckers T., Smolders I.J., Singewald N., De Bundel D. Effects of disrupted ghrelin receptor function on fear processing, anxiety and saccharin preference in mice. Psychoneuroendocrinology. 2019;110:104430. doi: 10.1016/j.psyneuen.2019.104430. [DOI] [PubMed] [Google Scholar]
- 76.Chuang J.C., Sakata I., Kohno D., Perello M., Osborne-Lawrence S., Repa J.J., Zigman J.M. Ghrelin directly stimulates glucagon secretion from pancreatic α-cells. Mol. Endocrinol. 2011;25(9):1600–1611. doi: 10.1210/me.2011-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fujitsuka N., Asakawa A., Hayashi M., Sameshima M., Amitani H., Kojima S., Fujimiya M., Inui A. Selective serotonin reuptake inhibitors modify physiological gastrointestinal motor activities via 5-HT2c receptor and acyl ghrelin. Biol. Psychiatry. 2009;65(9):748–759. doi: 10.1016/j.biopsych.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 78.Carlini V.P., Gaydou R.C., Schiöth H.B., de Barioglio S.R. Selective serotonin reuptake inhibitor (fluoxetine) decreases the effects of ghrelin on memory retention and food intake. Regul. Pept. 2007;140(1-2):65–73. doi: 10.1016/j.regpep.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 79.Brunetti L., Recinella L., Orlando G., Michelotto B., Nisio D.C., Vacca M. Effects of ghrelin and amylin on dopamine, norepinephrine and serotonin release in the hypothalamus. Eur. J. Pharmacol. 2002;454(2-3):189–192. doi: 10.1016/S0014-2999(02)02552-9. [DOI] [PubMed] [Google Scholar]
- 80.Zhang Y., Zhu M.Z., Qin X.H., Zeng Y.N., Zhu X.H. The ghrelin/growth hormone secretagogue receptor system is involved in the rapid and sustained antidepressant-like effect of paeoniflorin. Front. Neurosci. 2021;15:631424. doi: 10.3389/fnins.2021.631424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lin L.Y., Jun X.Y., Ran H.L. Meranzin hydrate improves depression-like behaviors and hypomotility via ghrelin and neurocircuitry. Chin. J. Integr. Med. 2023;29(6):490–499. doi: 10.1007/s11655-022-3308-2. [DOI] [PubMed] [Google Scholar]
- 82.Yeung Y.T., Aziz F., Castilla G.A., Arguelles S. Signaling pathways in inflammation and anti-inflammatory therapies. Curr. Pharm. Des. 2018;24(14):1449–1484. doi: 10.2174/1381612824666180327165604. [DOI] [PubMed] [Google Scholar]
- 83.Beurel E., Toups M., Nemeroff C.B. The bidirectional relationship of depression and inflammation: Double trouble. Neuron. 2020;107(2):234–256. doi: 10.1016/j.neuron.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maydych V. The interplay between stress, inflammation, and emotional attention: Relevance for depression. Front. Neurosci. 2019;13:384. doi: 10.3389/fnins.2019.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miller A.H., Maletic V., Raison C.L. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol. Psychiatry. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bhatt S., Nagappa A.N., Patil C.R. Role of oxidative stress in depression. Drug Discov. Today. 2020;25(7):1270–1276. doi: 10.1016/j.drudis.2020.05.001. [DOI] [PubMed] [Google Scholar]
- 87.Hassamal S. Chronic stress, neuroinflammation, and depression: an overview of pathophysiological mechanisms and emerging anti-inflammatories. Front. Psychiatry. 2023;14:1130989. doi: 10.3389/fpsyt.2023.1130989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ma Y., Zhang H., Guo W., Yu L. Potential role of ghrelin in the regulation of inflammation. FASEB J. 2022;36(9):e22508. doi: 10.1096/fj.202200634R. [DOI] [PubMed] [Google Scholar]
- 89.Russo C., Valle M.S., Russo A., Malaguarnera L. The interplay between ghrelin and microglia in neuroinflammation: Implications for obesity and neurodegenerative diseases. Int. J. Mol. Sci. 2022;23(21):13432. doi: 10.3390/ijms232113432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu C.R., Yang Q.Y., Chen Q.W., Li C.Q., He W.Y., Zhao Y.P., Wang L. Ghrelin attenuate cerebral microvascular leakage by regulating inflammation and apoptosis potentially via a p38 MAPK-JNK dependent pathway. Biochem. Biophys. Res. Commun. 2021;552:37–43. doi: 10.1016/j.bbrc.2021.03.032. [DOI] [PubMed] [Google Scholar]
- 91.Qi L., Cui X., Dong W., Barrera R., Nicastro J., Coppa G.F., Wang P., Wu R. Ghrelin attenuates brain injury after traumatic brain injury and uncontrolled hemorrhagic shock in rats. Mol. Med. 2012;18(2):186–193. doi: 10.2119/molmed.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee J.Y., Yune T.Y. Ghrelin inhibits oligodendrocyte cell death by attenuating microglial activation. Endocrinol. Metab. 2014;29(3):371–378. doi: 10.3803/EnM.2014.29.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jiao L., Du X., Jia F., Li Y., Zhu D., Tang T., Jiao Q., Jiang H. Early low-dose ghrelin intervention via miniosmotic pumps could protect against the progressive dopaminergic neuron loss in Parkinson’s disease mice. Neurobiol. Aging. 2021;101:70–78. doi: 10.1016/j.neurobiolaging.2021.01.011. [DOI] [PubMed] [Google Scholar]
- 94.Liu F., Li Z., He X., Yu H., Feng J. Ghrelin attenuates neuroinflammation and demyelination in experimental autoimmune encephalomyelitis involving NLRP3 inflammasome signaling pathway and pyroptosis. Front. Pharmacol. 2019;10:1320. doi: 10.3389/fphar.2019.01320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Carniglia L., Ramírez D., Durand D., Saba J., Turati J., Caruso C., Scimonelli T.N., Lasaga M. Neuropeptides and microglial activation in inflammation, pain, and neurodegenerative diseases. Mediators Inflamm. 2017;2017:1–23. doi: 10.1155/2017/5048616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Akki R., Raghay K., Errami M. Potentiality of ghrelin as antioxidant and protective agent. Redox Rep. 2021;26(1):71–79. doi: 10.1080/13510002.2021.1913374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.El Eter E., Al Tuwaijiri A., Hagar H., Arafa M. In vivo and in vitro antioxidant activity of ghrelin: Attenuation of gastric ischemic injury in the rat. J. Gastroenterol. Hepatol. 2007;22(11):1791–1799. doi: 10.1111/j.1440-1746.2006.04696.x. [DOI] [PubMed] [Google Scholar]
- 98.Omrani H., Alipour M.R., Mohaddes G. Ghrelin improves antioxidant defense in blood and brain in normobaric hypoxia in adult male rats. Adv. Pharm. Bull. 2015;5(2):283–288. doi: 10.15171/apb.2015.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu Y., Chen L., Xu X., Vicaut E., Sercombe R. Both ischemic preconditioning and ghrelin administration protect hippocampus from ischemia/reperfusion and upregulate uncoupling protein-2. BMC Physiol. 2009;9(1):17. doi: 10.1186/1472-6793-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Barazzoni R., Semolic A., Cattin M.R., Zanetti M., Guarnieri G. Acylated ghrelin limits fat accumulation and improves redox state and inflammation markers in the liver of high‐fat‐fed rats. Obesity. 2014;22(1):170–177. doi: 10.1002/oby.20454. [DOI] [PubMed] [Google Scholar]
- 101.Santos V.V., Stark R., Rial D., Silva H.B., Bayliss J.A., Lemus M.B., Davies J.S., Cunha R.A., Prediger R.D., Andrews Z.B. Acyl ghrelin improves cognition, synaptic plasticity deficits and neuroinflammation following amyloid β (Aβ1‐40) administration in mice. J. Neuroendocrinol. 2017;29(5):jne.12476. doi: 10.1111/jne.12476. [DOI] [PubMed] [Google Scholar]
- 102.Lee S., Kim Y., Li E., Park S. Ghrelin protects spinal cord motoneurons against chronic glutamate excitotoxicity by inhibiting microglial activation. Korean J. Physiol. Pharmacol. 2012;16(1):43–48. doi: 10.4196/kjpp.2012.16.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee J.Y., Oh T.H., Yune T.Y. Ghrelin inhibits hydrogen peroxide-induced apoptotic cell death of oligodendrocytes via ERK and p38MAPK signaling. Endocrinology. 2011;152(6):2377–2386. doi: 10.1210/en.2011-0090. [DOI] [PubMed] [Google Scholar]
- 104.Lim E., Lee S., Li E., Kim Y., Park S. Ghrelin protects spinal cord motoneurons against chronic glutamate-induced excitotoxicity via ERK1/2 and phosphatidylinositol-3-kinase/Akt/glycogen synthase kinase-3β pathways. Exp. Neurol. 2011;230(1):114–122. doi: 10.1016/j.expneurol.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 105.Meanti R., Bresciani E., Rizzi L., Coco S., Zambelli V., Dimitroulas A., Molteni L., Omeljaniuk R.J., Locatelli V., Torsello A. Potential applications for growth hormone secretagogues treatment of amyotrophic lateral sclerosis. Curr. Neuropharmacol. 2023;21(12):2376–2394. doi: 10.2174/1570159X20666220915103613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jiang H., Li L.J., Wang J., Xie J.X. Ghrelin antagonizes MPTP-induced neurotoxicity to the dopaminergic neurons in mouse substantia nigra. Exp. Neurol. 2008;212(2):532–537. doi: 10.1016/j.expneurol.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 107.Bayliss J.A., Lemus M., Santos V.V., Deo M., Elsworth J.D., Andrews Z.B. Acylated but not des‐acyl ghrelin is neuroprotective in an MPTP mouse model of Parkinson’s disease. J. Neurochem. 2016;137(3):460–471. doi: 10.1111/jnc.13576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bulgarelli I., Tamiazzo L., Bresciani E., Rapetti D., Caporali S., Lattuada D., Locatelli V., Torsello A. Desacyl‐ghrelin and synthetic GH‐secretagogues modulate the production of inflammatory cytokines in mouse microglia cells stimulated by β‐amyloid fibrils. J. Neurosci. Res. 2009;87(12):2718–2727. doi: 10.1002/jnr.22088. [DOI] [PubMed] [Google Scholar]
- 109.Meanti R., Rizzi L., Bresciani E., Molteni L., Locatelli V., Coco S., Omeljaniuk R.J., Torsello A. Hexarelin modulation of MAPK and PI3K/Akt pathways in neuro-2A cells inhibits hydrogen peroxide—induced apoptotic toxicity. Pharmaceuticals. 2021;14(5):444. doi: 10.3390/ph14050444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Meanti R., Licata M., Rizzi L., Bresciani E., Molteni L., Coco S., Locatelli V., Omeljaniuk R.J., Torsello A. Protective effects of hexarelin and JMV2894 in a human neuroblastoma cell line expressing the SOD1-G93A mutated protein. Int. J. Mol. Sci. 2023;24(2):993. doi: 10.3390/ijms24020993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Biagini G., Torsello A., Marinelli C., Gualtieri F., Vezzali R., Coco S., Bresciani E., Locatelli V. Beneficial effects of desacyl-ghrelin, hexarelin and EP-80317 in models of status epilepticus. Eur. J. Pharmacol. 2011;670(1):130–136. doi: 10.1016/j.ejphar.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 112.Giordano C., Costa A.M., Lucchi C., Leo G., Brunel L., Fehrentz J.A., Martinez J., Torsello A., Biagini G. Progressive seizure aggravation in the repeated 6-hz corneal stimulation model is accompanied by marked increase in hippocampal p-ERK1/2 immunoreactivity in neurons. Front. Cell. Neurosci. 2016;10:281. doi: 10.3389/fncel.2016.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Réus G.Z., Manosso L.M., Quevedo J., Carvalho A.F. Major depressive disorder as a neuro-immune disorder: Origin, mechanisms, and therapeutic opportunities. Neurosci. Biobehav. Rev. 2023;155:105425. doi: 10.1016/j.neubiorev.2023.105425. [DOI] [PubMed] [Google Scholar]
- 114.Afridi R., Suk K. Neuroinflammatory basis of depression: Learning from experimental models. Front. Cell. Neurosci. 2021;15:691067. doi: 10.3389/fncel.2021.691067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rahimian R., Perlman K., Canonne C., Mechawar N. Targeting microglia–oligodendrocyte crosstalk in neurodegenerative and psychiatric disorders. Drug Discov. Today. 2022;27(9):2562–2573. doi: 10.1016/j.drudis.2022.06.015. [DOI] [PubMed] [Google Scholar]
- 116.Boyle C.C., Bower J.E., Eisenberger N.I., Irwin M.R. Stress to inflammation and anhedonia: Mechanistic insights from preclinical and clinical models. Neurosci. Biobehav. Rev. 2023;152:105307. doi: 10.1016/j.neubiorev.2023.105307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Khan M., Baussan Y., Chatelain H.E. Connecting dots between mitochondrial dysfunction and depression. Biomolecules. 2023;13(4):695. doi: 10.3390/biom13040695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Alshial E.E., Abdulghaney M.I., Wadan A.H.S., Abdellatif M.A., Ramadan N.E., Suleiman A.M., Waheed N., Abdellatif M., Mohammed H.S. Mitochondrial dysfunction and neurological disorders: A narrative review and treatment overview. Life Sci. 2023;334:122257. doi: 10.1016/j.lfs.2023.122257. [DOI] [PubMed] [Google Scholar]
- 119.Wang L., Wang R., Liu L., Qiao D., Baldwin D.S., Hou R. Effects of SSRIs on peripheral inflammatory markers in patients with major depressive disorder: A systematic review and meta-analysis. Brain Behav. Immun. 2019;79:24–38. doi: 10.1016/j.bbi.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 120.Chen M.H., Li C.T., Lin W.C., Hong C.J., Tu P.C., Bai Y.M., Cheng C.M., Su T.P. Rapid inflammation modulation and antidepressant efficacy of a low-dose ketamine infusion in treatment-resistant depression: A randomized, double-blind control study. Psychiatry Res. 2018;269:207–211. doi: 10.1016/j.psychres.2018.08.078. [DOI] [PubMed] [Google Scholar]
- 121.Köhler-Forsberg O., N Lydholm C., Hjorthøj C., Nordentoft M., Mors O., Benros M.E. Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: Meta-analysis of clinical trials. Acta Psychiatr. Scand. 2019;139(5):404–419. doi: 10.1111/acps.13016. [DOI] [PubMed] [Google Scholar]
- 122.Köhler O., Benros M.E., Nordentoft M., Farkouh M.E., Iyengar R.L., Mors O., Krogh J. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: A systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71(12):1381–1391. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- 123.Wang H., He Y., Sun Z., Ren S., Liu M., Wang G., Yang J. Microglia in depression: An overview of microglia in the pathogenesis and treatment of depression. J. Neuroinflammation. 2022;19(1):132. doi: 10.1186/s12974-022-02492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang C., Shen J., Hong T., Hu T.T., Li Z.J., Zhang H.T., Zhang Y.J., Zhou Z.Q., Yang J.J. Effects of ketamine on lipopolysaccharide-induced depressive-like behavior and the expression of inflammatory cytokines in the rat prefrontal cortex. Mol. Med. Rep. 2013;8(3):887–890. doi: 10.3892/mmr.2013.1600. [DOI] [PubMed] [Google Scholar]
- 125.Lu Y., Ding X., Wu X., Huang S. Ketamine inhibits LPS‐mediated BV2 microglial inflammation via NMDA receptor blockage. Fundam. Clin. Pharmacol. 2020;34(2):229–237. doi: 10.1111/fcp.12508. [DOI] [PubMed] [Google Scholar]
- 126.Khanzode S.D., Dakhale G.N., Khanzode S.S., Saoji A., Palasodkar R. Oxidative damage and major depression: The potential antioxidant action of selective serotonin re-uptake inhibitors. Redox Rep. 2003;8(6):365–370. doi: 10.1179/135100003225003393. [DOI] [PubMed] [Google Scholar]
- 127.Bilici M., Efe H., Köroğlu M.A., Uydu H.A., Bekaroğlu M., Değer O. Antioxidative enzyme activities and lipid peroxidation in major depression: Alterations by antidepressant treatments. J. Affect. Disord. 2001;64(1):43–51. doi: 10.1016/S0165-0327(00)00199-3. [DOI] [PubMed] [Google Scholar]
- 128.Kent B.A., Beynon A.L., Hornsby A.K.E., Bekinschtein P., Bussey T.J., Davies J.S., Saksida L.M. The orexigenic hormone acyl-ghrelin increases adult hippocampal neurogenesis and enhances pattern separation. Psychoneuroendocrinology. 2015;51:431–439. doi: 10.1016/j.psyneuen.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hornsby A.K.E., Redhead Y.T., Rees D.J., Ratcliff M.S.G., Reichenbach A., Wells T., Francis L., Amstalden K., Andrews Z.B., Davies J.S. Short-term calorie restriction enhances adult hippocampal neurogenesis and remote fear memory in a Ghsr-dependent manner. Psychoneuroendocrinology. 2016;63:198–207. doi: 10.1016/j.psyneuen.2015.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bekinschtein P., Kent B.A., Oomen C.A., Clemenson G.D., Gage F.H., Saksida L.M., Bussey T.J. BDNF in the dentate gyrus is required for consolidation of “pattern-separated” memories. Cell Rep. 2013;5(3):759–768. doi: 10.1016/j.celrep.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chung H., Li E., Kim Y., Kim S., Park S. Multiple signaling pathways mediate ghrelin-induced proliferation of hippocampal neural stem cells. J. Endocrinol. 2013;218(1):49–59. doi: 10.1530/JOE-13-0045. [DOI] [PubMed] [Google Scholar]
- 132.Milaneschi Y., Simmons W.K., van Rossum E.F.C., Penninx B.W.J.H. Depression and obesity: Evidence of shared biological mechanisms. Mol. Psychiatry. 2019;24(1):18–33. doi: 10.1038/s41380-018-0017-5. [DOI] [PubMed] [Google Scholar]
- 133.Fox M.E., Lobo M.K. The molecular and cellular mechanisms of depression: a focus on reward circuitry. Mol. Psychiatry. 2019;24(12):1798–1815. doi: 10.1038/s41380-019-0415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liang Y., Yin W., Yin Y., Zhang W. Ghrelin based therapy of metabolic diseases. Curr. Med. Chem. 2021;28(13):2565–2576. doi: 10.2174/0929867327666200615152804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tesauro M., Schinzari F., Caramanti M., Lauro R., Cardillo C. Cardiovascular and metabolic effects of ghrelin. Curr. Diabetes Rev. 2010;6(4):228–235. doi: 10.2174/157339910791658871. [DOI] [PubMed] [Google Scholar]
- 136.Reich N., Hölscher C. Acylated ghrelin as a multi-targeted therapy for Alzheimer’s and Parkinson’s Disease. Front. Neurosci. 2020;14:614828. doi: 10.3389/fnins.2020.614828. [DOI] [PMC free article] [PubMed] [Google Scholar]


