Abstract
Protein folding and quality control in the early secretory pathway function as posttranslational checkpoints in eukaryote gene expression. Herein, an aberrant form of the hepatic secretory protein α1-antitrypsin was stably expressed in a human embryonic kidney cell line to elucidate the mechanisms by which glycoprotein endoplasmic reticulum-associated degradation (GERAD) is administered in cells from higher eukaryotes. After biosynthesis, genetic variant PI Z underwent alternative phases of secretion and degradation, the latter of which was mediated by the proteasome. Degradation required release from calnexin- and asparagine-linked oligosaccharide modification by endoplasmic reticulum mannosidase I, the latter of which occurred as PI Z was bound to the molecular chaperone grp78/BiP. That a distinct GERAD program operates in human embryonic kidney cells was supported by the extent of PI Z secretion, apparent lack of polymerization, inability of calnexin to participate in the degradation process, and sequestration of the glycoprotein folding sensor UDP-glucose:glycoprotein glucosyltransferase in the Golgi complex. Because UDP-glucose:glycoprotein glucosyltransferase sustains calnexin binding, its altered distribution is consistent with a GERAD program that hinders the reentry of substrates into the calnexin cycle, allowing grp78/BiP to partner with a lectin, other than calnexin, in the recognition of a two-component GERAD signal to facilitate substrate recruitment. How the processing of a mutant protein, rather than the mutation itself, can contribute to disease pathogenesis, is discussed.
INTRODUCTION
Numerous checkpoints exist in the eukaryote to maintain the integrity of genomic information (reviewed by Hartwell and Weinert, 1989; Zhou and Elledge, 2000). Importantly, these are not limited to the nucleus or restricted to the surveillance of DNA. Rather, these systems extend to compartments of the cell in which the conformational maturation of expressed gene products is facilitated to ensure the structural fidelity of the proteome (Pandey and Mann, 2000), which is, by definition, the expressed cellular genome.
In the eukaryote, secretory and cell surface proteins are transported through a series of membranous organelles before their final deployment (reviewed by Ellgaard and Helenius, 2001). The first of these compartments is the endoplasmic reticulum (ER) where nascent polypeptides rely on molecular chaperones to facilitate conformational maturation (reviewed by Gething and Sambrook, 1992), the latter of which is essential for biological activity. As a rule, protein delivery to the Golgi is tightly coupled to the acquisition of native protein structure (reviewed by Rothman, 1987; Klausner and Sitia, 1990). Misfolded polypeptides and unassembled protein subunits are usually subjected to ER-associated degradation (ERAD) (McCracken and Brodsky, 1996; Sommer and Wolf, 1997; Fewell et al., 2001), which concludes with the retro-translocation of substrates into the cytoplasm before elimination by the multicatalytic proteasome (reviewed by Bonifacino and Weissman, 1998). Although ERAD likely contributes to the molecular pathogenesis and phenotypic variation associated with many loss- and gain-of-toxic function disorders (reviewed by Thomas et al., 1995; Choudhury et al., 1997; Cabral et al., 2001), the exact mechanisms by which the entire process is orchestrated, especially at the earliest steps, is only now becoming clear (Ellgaard et al., 1999).
Protein folding and quality control is best understood for those molecules to which Glc3Man9GlcNAc2 is covalently attached (reviewed by Helenius, 1994) during translocation into the ER (reviewed by Kornfeld and Kornfeld, 1985). The hydrolysis of two terminal glucose units by glucosidase I and glucosidase II (Hammond et al., 1994) promotes cotranslational association with the ER lectins calnexin and calreticulin (Hammond and Helenius, 1995), both of which bind high-mannose monoglucosylated oligosaccharides (reviewed by Ellgard et al., 1999; Parodi, 2000). The eventual removal of the remaining glucose by glucosidase II dissociates the glycoprotein–lectin complexes (Hebert et al., 1995; VanLeeuwen and Kearse, 1996). Reentry into the calnexin cycle, which can facilitate additional folding (Hammond et al., 1994), requires oligosaccharide reglucosylation by the glycoprotein folding sensor UDP-glucose:glycoprotein glucosyltransferase (UGT) (Zapun et al., 1997), an ER resident protein in rat liver hepatocytes (Trombetta et al., 1991). Conformational maturation abolishes recognition by UGT (Sousa et al., 1992), ensuring that native glycoproteins are released from the calnexin cycle and transported to the Golgi complex (Hammond et al., 1994).
A picture recently emerged in which the modification of asparagine-linked Man9GlcNAc2 by an ER-situated α-1,2 mannosidase (i.e., ER mannosidase I) (Gonzalez et al., 1999; Tremblay and Herscovics, 1999) plays a central role in generating a common signal that targets a diverse set of aberrant and unassembled glycoproteins for clearance from the ER (reviewed by Frigerio and Lord, 2000), by a process recently coined glycoprotein ERAD (GERAD) (reviewed by Cabral et al.,2001). Because the glycan modification does not target correctly folded glycoproteins for degradation, nonnative protein structure likely functions as an inherent aglycone (i.e., noncarbohydrate) GERAD signal component (Cabral et al., 2001). Although asparagine-linked glycosylation and the earliest asparagine-linked glycan-processing events are conserved from yeast to mammals (Gonzalez and Jordan, 2000; Parodi, 2000; Herscovics, 2001), examples exist in which a given glycoprotein substrate is handled differently in distinct animal cell lines (Sitia et al., 1990; Qu et al., 1996; Novoradovskaya et al., 1998; Cabral et al., 2000). These observations, plus the apparent discrepancy as to whether calnexin promotes, or prevents, the degradation of bound glycoprotein substrates (Ayalon-Soffer et al., 1999), suggests that different strategies might orchestrate GERAD in distinct animal cells (reviewed by Cabral et al., 2001).
Serum α1-antitrypsin (AAT) deficiency has provided a medically relevant paradigm to investigate ER quality control as a posttranslational checkpoint in eukaryote genome expression (reviewed by Sifers et al., 1992). The spontaneous loop-sheet polymerization (Lomas et al., 1992) of a late folding intermediate (Yu et al., 1995) is responsible for hindering the secretion of genetic variant PI Z from liver hepatocytes (Sifers et al., 1992). Chronic obstructive pulmonary disease and liver cirrhosis are the associated loss- and gain-of-toxic function disorders, respectively (reviewed by Perlmutter and Pierce, 1989; Kopito and Ron, 2000; Carrell and Lomas, 2002), and the latter has been linked to a hindered rate of PI Z polymer degradation (Wu et al., 1994), possibly augmenting the accumulation of insoluble polymers in the hepatocyte ER (Carlson et al., 1988; Graham et al., 1990; Volpert et al., 2000). In the murine hepatoma cell line Hepa1a, modification by ER mannosidase I leads to the proteasomal degradation of terminally misfolded AAT by abrogating its dissociation from calnexin (Liu et al., 1999), resulting from the attenuated rate in which glucose is removed by glucosidase II in the absence of the full compliment of mannose units (Grinna and Robbins, 1980). That calnexin can function as a bona fide participant in GERAD, at least in Hepa1a, was established by several additional lines of evidence (Liu et al., 1999), including degradation by a coexisting nonproteasomal pathway in response to the spontaneous formation of variant PI Z loop-sheet polymers, which prevents posttranslational physical engagement with the ER lectin (Cabral et al., 2000). In the present study, variant PI Z was used as a reporter protein to characterize the administration of GERAD in the human embryonic kidney (HEK) 293 cell line as a first step toward elucidating the specific organizational differences in quality control systems among cells in higher eukaryotes. Alternative phases of secretion and degradation were detected after biosynthesis. Degradation was mediated by the ubiquitin–proteasome system, and required the modification of asparagine-linked glycans by ER mannosidase I, but only after release from calnexin and assembly with grp78/BiP. Unlike its reticular distribution in Hepa1a, the bulk of UGT is sequestered downstream of the ER in HEK293, providing a mechanism by which the reentry of PI Z into the calnexin cycle is eventually blocked, and leads to physical interaction with grp78/BiP. Taken together, the data uncover a posttranslational mechanism that mediates the transfer of an aberrant glycoprotein into an alternate folding system before degradation, and identify the existence of distinct GERAD programs that diverge in the manner by which a two-component GERAD signal is recognized.
MATERIALS AND METHODS
Stable Cell Transfection, Selection, and Expansion
The recombinant human α1-antitrypsin PI Z variant cDNA (Le et al., 1990) was subcloned into the unique EcoRI site of pCDNA3.1/Zeo(+) (Invitrogen, Carlsbad, CA) to generate an appropriate mammalian expression vector. The LipofectAMINE-mediated transfection (Invitrogen) of the cell line HEK293 (peak; Edge Biosystems, Gaithersburg, MD) led to the isolation and expansion of the representative stable cell line HEK/Z-1 after zeocin (Invitrogen) selection.
Metabolic Radiolabeling
Semiconfluent monolayers of HEK/Z-1 were grown at 37°C in a humidified CO2 atmosphere to equal cell density in 100-mm dishes precoated at room temperature with poly-d-lysine (0.025 mg/ml). Metabolic radiolabeling was preceded by a 30-min methionine starvation at 37°C in methionine-free DMEM (ICN Biomedical, Inc., Aurora, OH) containing 10% fetal calf serum before a 15-min pulse with [35S]methionine (Easy Tag Express Mix; PerkinElmer Life Sciences, Boston, MA) as described previously (Liu et al., 1997). Monolayers were washed with Dulbecco's phosphate-buffered saline (Invitrogen) to remove unincorporated radiolabel and then chased for the desired period at 37°C with methionine starvation medium supplemented with a 10-fold excess of unlabeled methionine. Unless otherwise stated, the inhibition of specific glycosidase activities was accomplished by a 60-min incubation of cells at 37°C in normal growth medium containing the desired compound before methionine starvation. The specified compound was also included in the media used for methionine starvation, pulse radiolabeling, and the chase. Proteasomal activity was inhibited with 0.25 mM lactacystin (E.J. Corey Laboratories, Harvard Medical School, Cambridge, MA). ER mannosidase I was inhibited with 0.1 mM kifunensine (Toronto Research Chemicals) or 1 mM 1-deoxymannojirimycin (Roche Applied Science, Indianapolis, IN). ER mannosidase II was inhibited with 0.1 mM swainsonine (Sigma-Aldrich, St. Louis, MO). Glucosidases I and II were inhibited with 0.2 mg/ml castanospermine (Roche Applied Science). Routine buffers and salts were procured from Sigma-Aldrich.
Cell Lysis and Immunoabsorption
Equal cell monolayers grown to semiconfluence in 100-mm dishes were lysed by scraping in buffered NP-40 detergent (Calbiochem, San Diego, CA) at 4°C (Liu et al., 1997) either immediately after the pulse or after the chase as described previously (Le et al., 1990). Cell media were collected at each time point and adjusted to 0.5% NP-50. Centrifugation of all samples (3000 × g, 5 min, 4°C) was used for the removal of NP-40 insoluble material before the addition of an IgG fraction of goat anti-human α1-antitrypsin (ICN Biomedical Research Products), which was preimmobilized to protein G-agarose (Calbiochem, Toronto, ON) as described previously (Le et al., 1994). The immunoabsorption of variant PI Z was accomplished during a 2-h incubation, with constant rotation, at 4°C, and then washed as described previously (Liu et al., 1999). For the detection of radiolabeled PI Z bound to either calnexin or grp78/BiP, cell lysates were incubated with a rabbit polyclonal rabbit antibody raised against the cytoplasmic tail of canine calnexin (StressGen, Victoria, British Columbia, Canada) or the KDEL retrieval motif (StressGen), respectively. In all procedures, equal aliquots of radiolabeled PI Z immunoprecipitated from the cell lysate and medium were resolved by SDS-PAGE and then detected by fluorographic enhancement of the vacuum-dried gels before quantitation by liquid scintillation counting of excised gel pieces (Le et al., 1994). The percentage of pulse-radiolabeled PI Z subjected to degradation was determined as that amount lost from the initial pulse and not recovered in either the cell lysate or medium. Lithium dodecyl sulfate extraction of the insoluble NP-40 cell lysate pellet from each time point, in combination with immunoprecipitation with α1-antitrypsin antiserum as described previously (Graham et al., 1990), was used to detect insoluble PI Z polymers.
Enhanced Chemiluminescence (ECL) Western Blotting
After resolution by SDS-PAGE, immunoprecipitated proteins were electrophoretically transferred onto Hybond ECL nitrocellulose membranes (Amersham Biosciences, Piscataway, NJ) and blotted as described previously (Choudhury et al., 1997). Ubiquitin-conjugated PI Z was detected with a 1:500 dilution of a polyclonal rabbit antiserum against bovine ubiquitin (Calbiochem). The detection of calnexin was accomplished with a 1:1000 dilution of a polyclonal rabbit antiserum against a synthetic peptide homologous to the cytoplasmic tail of the canine homolog (StressGen). Grp78/BiP was detected with a 1:400 dilution of a purified monoclonal antibody against a synthetic peptide homologous to the six carboxyl-terminal residues of the rat homolog (KDEL retrieval motif) (StressGen). Incubation with conjugated secondary antibodies, subsequent washings, and the use of detection reagents was performed in a manner identical to that reported previously (Choudhury et al., 1997).
Indirect Immunofluorescence Microscopy
HEK293 and Hepa1a cells were grown on glass coverslips to ∼70% confluence, rinsed with phosphate-buffered saline (PBS), and fixed for at least 10 min in methanol at −20°C. The cells were then rehydrated in PBS and incubated separately with the following antibodies: rabbit polyclonal antiserum against rat UDP-glucose:glycoprotein glucosyltransferase (a generous gift from Dr. Armando Parodi, Instituto de Investigaciones Bioquimicas, Buenos Aires, Argentina), rabbit polyclonal antiserum against the catalytic domain of α-mannosidase II (Moremen et al., 1991), a polyclonal rabbit antiserum against a synthetic peptide homologous to the cytoplasmic tail of canine calnexin (StressGen), a rabbit polyclonal antiserum against recombinant human calreticulin (Affinity Bioreagents, Golden, CO), and polyclonal rabbit antiserum against the KDEL retrieval motif (StressGen). All primary antisera recognized the appropriate band(s) in ECL Western blotting of soluble NP-40 cell lysates and were incubated with cells for 1 h at 37°C in a humid chamber. After several washes in PBS, the cells were incubated with species-specific fluorescein isothiocyanate-conjugated donkey anti-rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA). In all cases, antibody specificity was again tested by incubating coverslips with each primary antibody and the cross-species secondary antibody to ensure the absence of immunofluorescence. Brefeldin A (Epicenter Technologies, Madison, WI) was added to cells 1.5 h before methanol fixation at a final concentration of 2 μg/ml where indicated to redistribute Golgi proteins into the ER (Lippincott-Schwartz et al., 1989). Samples were viewed by epifluorescence by using an Optiphot microscope equipped with a 40× fluor objective (Nikon, Melville, NY). Images were acquired using a DMX-1200 digital camera (Nikon).
RESULTS
Alternative Phases of Secretion and Disposal
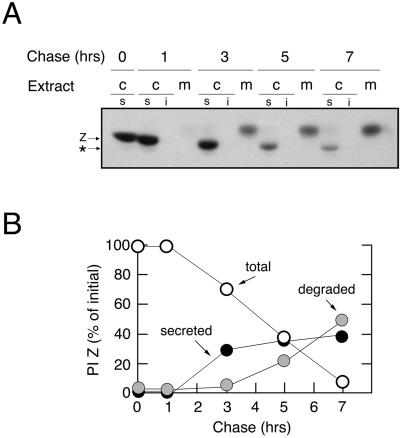
The fate of variant PI Z after stable transfection of HEK293 cells (cell line HEK/Z1) was monitored by pulse-chase radiolabeling and immunoprecipitation (see MATERIALS AND METHODS). The secretion of radiolabeled PI Z was not initiated until after the first 60 min of chase (Figure 1A) and was essentially complete by 3 h (Figure 1B) at which time 40% of the pulse-radiolabeled molecules were detected in the medium (Figure 1A). The slower migration in SDS-PAGE, relative to the intracellular species, reflects the covalent addition of sialic acid to asparagine-linked oligosaccharides during transport although the trans-Golgi network (Le et al., 1990). Only 10% of the radiolabeled molecules remained in the cell lysate at 7 h of chase (Figure 1B), and none were detected in the insoluble NP-40 cell lysate at any time point (Figure 1A), indicating that 50% had been degraded. Because degradation had not yet been initiated at 3 h of chase (Figure 1B), the fate of pulse-radiolabeled PI Z could be separated into distinct, and sequential, phases of secretion and disposal. Importantly, neither the extent of PI Z secretion nor the rate of its degradation deviated by >20% when analyzed in three additional stable transfectants (our unpublished data).
Figure 1.
Secretion, degradation, and asparagine-linked oligosaccharide processing for synthesized variant PI Z in HEK/Z1. (A) Fluorographic detection after SDS-PAGE of variant PI Z (Z) immunoprecipitated from the NP-40-soluble (s) and insoluble (i) cell lysates (c) and medium (m) after a 15-min pulse of HEK/Z1 with [35S]methionine, and chase for up to 7 h. The discrete mobility shift (*) reflects asparagine-linked oligosaccharide modification during intracellular retention. (B) Results from the pulse-chase experiment are depicted as the percentage of radiolabeled variant PI Z remaining in the cells (open circles), the percentage secreted into the medium (closed circles), and the percentage degraded (shaded circles) at each time point.
Degradation by Proteasome
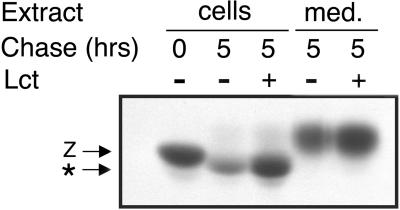
The proteasome has been implicated in mediating the degradation of numerous aberrant proteins from the ER (reviewed by Bonifacino and Weissman, 1998). To determine whether the multicatalytic system contributes to the turnover of PI Z in HEK/Z1, the pulse-radiolabeled molecules were subjected to a 5-h chase in medium containing 0.025 mM lactacystin, an irreversible covalent inhibitor of the proteasome (Fenteany et al., 1995). PI Z turnover was completely arrested under these conditions (Figure 2A, compare lanes 2 and 3), and quantitative analysis of the immunoprecipitated material revealed that the percentage of radiolabeled molecules secreted into the medium was enhanced ∼50% (Figure 2, compare lanes 4 and 5). The latter observation did not result from the saturation of ER retention machinery, because none of the secretion-incompetent AAT variant null(Hong Kong) (Sifers et al., 1989; Le et al., 1990) was secreted under an identical set of conditions (our unpublished data). The data indicate that the elimination of secretion-impaired PI Z is mediated by the proteasome in HEK293.
Figure 2.
Proteasome-mediated degradation of PI Z in HEK/Z1. Fluorographic detection after SDS-PAGE of variant PI Z (Z) immunoprecipitated from the cell lysate (cells) and medium (med.) from HEK/Z1 after a 15-min pulse with [35S]methionine and a 5-h chase with (±) or without (−) media supplemented with 0.025 mM lactacystin (Lct). The discrete mobility shift (*) reflects asparagine-linked oligosaccharide modification during intracellular retention.
Sequential Interaction with Molecular Chaperones Calnexin and grp78/BiP
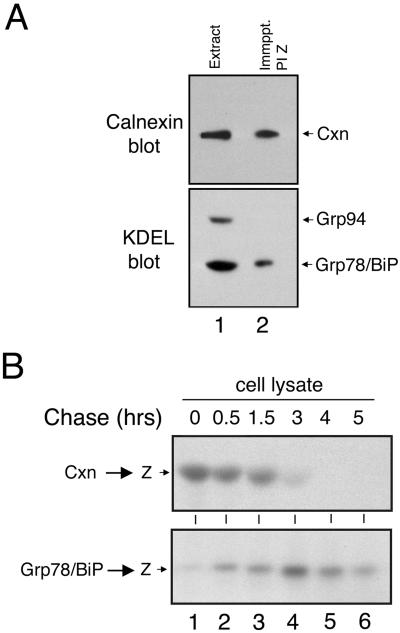
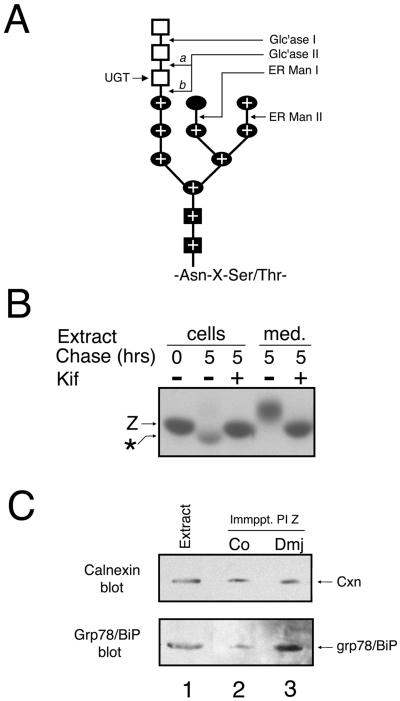
A transient interaction with calnexin, resulting from loop-sheet polymerization, ablates the secretion of all but ∼10–15% of pulse-radiolabeled PI Z from the hepatoma cell line Hepa1a (Le et al., 1992; Cabral et al., 2000). Considering the extent of PI Z secretion from HEK/Z-1, coimmunoprecipitation in combination with ECL Western blotting was a means to detect and identify any molecular chaperones that might bind to variant PI Z as an alternate ER retention mechanism, because these interactions are often responsible for both facilitating protein folding and retaining misfolded proteins in the early secretory pathway (reviewed by Gething and Sambrook, 1992). First, we asked whether a physical association with calnexin, which facilitates the folding of numerous glycoproteins, including AAT in hepatoma cells (Ou et al., 1993), was detectable. Consistent with this prediction, immunoreactive calnexin was detected in PI Z immunoprecipitates generated from HEK/Z-1 cells under steady-state conditions (Figure 3A, lane 2). Importantly, no signal was detected when the samples were incubated with protein G-agarose alone (our unpublished data). Blotting with the anti-KDEL antiserum, which recognizes a common epitope for ER retention (Vaux et al., 1990), resulted in the detection of coimmunoprecipitated grp78/BiP (Figure 3A, lane 2). The specificity of the interaction was established by the absence of coimmunoprecipitating grp94, which is one of the two most abundant KDEL-bearing proteins (Koch et al., 1986), as confirmed by our analysis of an NP-40 HEK/Z1 cell extract (Figure 3A, lane 1). Importantly, no interaction with calreticulin, a soluble homolog of calnexin (Danilczyk et al., 2000), or additional ER chaperones, was detected under numerous conditions (our unpublished data).
Figure 3.
Newly synthesized variant PI Z undergoes sequential physical interaction with calnexin and grp78/BiP. (A) Calnexin and KDEL immunoblots of variant PI Z (PI Z) immunoprecipitates (Immppt.) generated under steady-state conditions from HEK/Z1 (lane 2) and from the total cell extract (Extract) (lane1), the latter of which was used merely to show the relative migration of the endogenous immunoreactive protein. The immunological detection of calnexin (Cxn), grp78/BiP and grp94 is shown. (B) SDS-PAGE and fluorographic detection of immunoprecipitated variant PI Z (Z) released from a calnexin (Cxn → Z) or grp78/BiP (Grp78/BiP → Z) immunoprecipitate after a 15-min pulse of HEK/Z1 with [35S]methionine and chase.
Coimmunoprecipitation with the chaperone antisera, in combination with pulse-chase radiolabeling allowed us to elucidate the time course in which PI Z bound calnexin and grp78/BiP after a 15-min pulse with [35S]methionine. Radiolabeled PI Z was maximally bound to calnexin immediately after biosynthesis, with little, or no, physical interaction with grp78/BiP (Figure 3B, lane 1). Physical interaction with the ER lectin gradually diminished until it was almost absent at 3 h of chase (Figure 3B, lane 4). Conversely, physical interaction with grp78/BiP increased until maximal binding was detected at 3 h (Figure 3B, lane 4) and diminished thereafter (Figure 3B, lanes 4–6).
Because the initiation of degradation did not occur until after 3 h of chase (Figure 1B), a time point in which the intracellular fraction of PI Z was no longer bound to calnexin (Figure 3B, lane 4), we asked whether physical interaction with the ER lectin was even necessary for intracellular turnover. HEK/Z1 cells were incubated with castanospermine, an inhibitor of ER glucosidases (Elbein, 1991), before the 15-min pulse with [35S]methionine to arrest the removal of all three terminal glucose units from the asparagine-linked Glc3Man9GlcNAc2 precursor (Kornfeld and Kornfeld, 1985), and block cotranslational assembly between newly synthesized PI Z and calnexin. Under these conditions physical interaction with calnexin was prevented, but interaction with grp78/BiP was observed (our unpublished data). Importantly, PI Z turnover did not deviate by >16% during a 5-h chase compared with control (Table 1). Furthermore, degradation was almost completely arrested under these conditions when cells were coincubated with lactacystin (Table 1) and ruled out the possibility that intracellular clearance had been accomplished by an alternate nonproteasomal disposal pathway, as occurs in Hepa1a where coexisting proteolytic systems operate (Cabral et al., 2000).
Table 1.
Contributions of molecular chaperones and mannosidase activity toward proteasomal degradation in HEK/Z1
| Treatmenta | Percent degradation |
|---|---|
| Proteasome inhibition | |
| Control | 42.0 |
| Lct (0.025 mM) | 8.3 |
| Altered interaction with calnexin | |
| Control | 58.1 |
| Cst (0.2 mg/ml) | 41.8 |
| Cst (0.2 mg/ml) + Lct (0.025 mM) | 5.1 |
| Cst (0.2 mg/ml) + Kif (0.1 mM) | 17.8 |
| Post-Cst (0.2 mg/ml) | 6.3 |
| Mannosidase inhibition | |
| Control | 43.5 |
| Kif (0.1 mM) | 6.0 |
| Swn (0.1 mM) | 54.6 |
| Dmj (1 mM) | 8.8 |
Monolayers of HEK/Z1 were pulse-radiolabeled with [35S]methionine for 15 min and chased for 5 hrs. Variant PI Z immunoprecipitated from NP-40 cell lysates was resolved by SDS-PAGE, and protein degradation was determined as described in MATERIALS AND METHODS.
Cells were treated with lactacystin (Lct), kifunensine (Kif), swainsonine (Swn), deoxymannojirimycin (Dmj), or castanospermine (Cst) as described in MATERIALS AND METHODS, except for the case of post-CST in which the compound was added immediately after metabolic radiolabeling to allow cotranslational assembly with calnexin but to prevent dissociation. The data are separated into distinct sets of experiments, and the control used in each is designated.
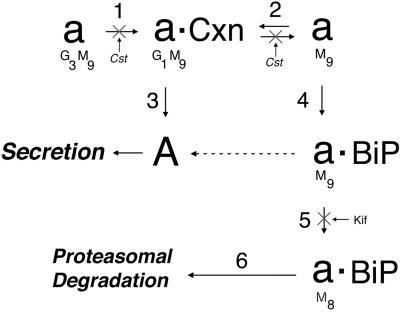
Next, the fate of pulse-radiolabeled PI Z was examined in cells in which castanospermine was added immediately after pulse radiolabeling (Figure 4, step 2) to prevent the posttranslational removal of glucose by ER glucosidase I, which dissociates glycoprotein–calnexin complexes (Hebert et al., 1995). Under these conditions, physical interaction with grp78/BiP was entirely blocked (our unpublished data), and variant PI Z degradation was arrested (Table 1). These results indicated that the molecules of PI Z eventually bound to grp78/BiP were first engaged with calnexin, and confirms that release from the ER lectin is necessary for degradation. Taken together, these findings indicate that PI Z secretion coincides with a period in which all the newly synthesized molecules are undergoing physical interaction with calnexin, and that degradation by the proteasome is preceded by physical engagement with grp78/BiP, and requires release from the ER lectin (Figure 4).
Figure 4.
Proposed order of events that coincide with the secretion, or intracellular retention and degradation of newly synthesized variant PI Z in HEK/Z1. A model is depicted in which partial deglucosylation by glucosidases I and II (step 1) leads to the assembly of newly synthesized and unfolded AAT (a) with calnexin (Cxn) (step 2) before conformational maturation (A) (step 3) and secretion. The remaining nonnative population of molecules eventually fails to bind calnexin and assemble with grp78/BiP (BiP) (step 4) before degradation by the proteasome, which requires asparagine-linked oligosacharide modification by ER mannosidase I (step 5) and recognition by a lectin (step 6). In the absence of proteasomal degradation, a significant fraction of nonnative molecules can attain conformational maturation (dashed arrow), and are secreted. The steps inhibited with castanospermine (Cst) or kifunensine (Kif) are shown, as are the predicted number of glucose (G) and mannose (M) units.
Requirement for Modification by ER Mannosidase I
Next, we asked whether the modification of asparagine-linked oligosaccharides by ER mannosidase I (Figure 5A) generates a signal that mediates the recognition of secretion-impaired variant PI Z by GERAD machinery for degradation by the proteasome. Because α1-antitrypsin contains three asparagine-linked oligosaccharides (Long et al., 1984), the glycan modification is indirectly detected by the accelerated mobility of the ER-retained radiolabeled molecules in SDS-PAGE (Le et al., 1992; Liu et al., 1997). The aberrant mobility was maximally detected at 3 h of chase (Figure 1A), at which time the secretion-impaired fraction had been released from calnexin and was bound to grp78/BiP (Figure 3B), before the onset of intracellular degradation (Figure 1B). To determine whether modification by ER mannosidase I was responsible for the electrophoretic anomaly, and was required for proteasomal degradation in HEK293, we examined the effect of kifunensine, an inhibitor of the processing enzyme (Weng and Spiro, 1993). During a 5-h chase, PI Z degradation was completely arrested (Table 1), as was the altered electrophoretic mobility in SDS-PAGE (Figure 5B, compare lanes 2 and 3). In contrast, incubation with swainsonine (Weng and Spiro, 1996), an inhibitor of ER mannosidase II (Figure 5A), had no detectable effect (Table 1).
Figure 5.
Kifunensine-sensitive degradation of variant PI Z in HEK/Z1. (A) Structural organization of the 14-unit asparagine-linked oligosaccharide precursor consisting of glucose (open squares), mannose (closed ovals), and N-acetylglucosamine (closed squares), attached to the Asn-x-Ser/Thr concensus sequence, is shown. The sites of hydrolysis by glucosidase I (Glc'ase I), glucosidase II (Glc'ase II), ER mannosidase I (ER ManI), and ER mannosidase II (ER Man II) are depicted, as is the glucose transferred by UDP-UGT. The two hydrolytic sites for glucosidase II are shown (a and b), and the combinations of sugars that constitute the Man8B isomer are depicted with a plus (±). (B) Fluorographic detection after SDS-PAGE of variant PI Z (Z) immunoprecipitated from the cell lysates (cells) and medium (med.) from HEK/Z1 after a 15-min pulse with [35S]methionine and 5-h chase with (±) or without (−) media supplemented with 0.1 mM kifunensine (Kif). The discrete mobility shift (*) reflects asparagine-linked oligosaccharide modification during intracellular retention. Also, the band intensities in lanes 4 and 5 are misleading, possibly resulting from changes in band density caused by altered oligosaccharide modification and mobility. (C) Calnexin and KDEL immunoblots of variant PI Z (PI Z) immunoprecipitates (Immppt.) generated under steady-state conditions from HEK/Z1 under control conditions (lane 2) or treated with deoxymannojirimycin (+Dmj) (lane 3), as well as from a HEK/Z1 cell extract (Extract) (lane 1), the latter of which was used merely to show the relative migration of the endogenous immunoreactive protein. The immunological detection of calnexin (Cxn) and grp78/BiP is shown by the arrows.
In cells treated with kifunensine, the percentage of radiolabeled molecules secreted into the medium increased almost 50% (Figure 5B, compare lanes 4 and 5), similar to that observed when degradation was arrested with lactacystin (Figure 2A). As before, the altered electrophoretic mobility of the secreted fraction (Figure 5B, lane 5) reflects the absence of sialic acid addition to asparagine-linked oligosaccharides in the late Golgi complex, which occurs in response to the arrested removal of mannose (Sifers et al., 1989). That enhanced secretion of PI Z did not result from the saturation of the general ER retention machinery was confirmed by the absence of terminally misfolded α1-antitrypsin variant null(Hong Kong) secretion (Le et al., 1990) under an identical set of conditions (our unpublished data). Furthermore, treatment with kifunensine arrested the proteasome-mediated degradation of PI Z in HEK/Z-1 preincubated with castanospermine (Table 1), which prevented physical assembly with calnexin (Figure 4, step 1). Taken together, these data indicate that glycan modification by ER mannosidase I plays a central role in tagging variant PI Z for elimination by the proteasome in HEK293, after release from calnexin and engagement with grp78/BiP.
Differential Distribution of UGT in Secretory Pathway
The differential ability of calnexin to actively participate in proteasomal degradation was a definite clue that a distinct GERAD program, and not merely overlapping pathways, might operate in HEK293 and Hepa1a. Considering the duration in which PI Z binds calnexin, we concluded that multiple rounds of binding to the ER lectin likely occur. For this reason, we asked whether a distinct mechanism is responsible for eventually inhibiting the reentry of PI Z into the calnexin cycle (Figure 4, step 2), favoring engagement with grp78/BiP (Figure 4, step 4). Otherwise, modification by ER mannosidase I would be counterproductive and block PI Z turnover in response to its abrogated dissociation from a population of calnexin unable to participate in GERAD. Because Man7GlcNAc2 is a very poor substrate for UGT (Parodi et al., 1983), we asked whether processing by ER mannosidase II, in addition to ER mannosidase I (Figure 5A), might be responsible for eventually blocking the interaction with calnexin. To address this possibility, cells were treated under steady-state conditions with 1-deoxymannojirimycin, an inhibitor of α1,2-mannosidases I and II (Elbein, 1991) to prevent formation of the asparagine-linked Man7GlcNAc2 structure (Figure 5A). Although PI Z degradation was completely arrested (Table 1), likely owing to the inhibition of ER mannosidase I, grp78/BiP-bound PI Z increased threefold (Figure 5C, compare lanes 2 and 3), whereas the number of molecules bound to calnexin was similar to that of control (Figure 5C, compare lanes 2 and 3). Although the hypothesis was negated, the data are consistent with the notion that formation of the GERAD signal requires modification by ER mannosidase I when PI Z is bound to grp78/BiP (Figure 4, step 5).
Because asparagine-linked oligosaccharide reglucosylation induces glycoprotein assembly with calnexin (Zapun et al., 1977), in the next set of experiments we asked whether an elevated concentration of grp78/BiP might provide a subtle competitive advantage that eventually blocks recognition by UGT, thereby hindering the reentry of PI Z into the calnexin cycle. However, no significant difference in the total intracellular concentrations of the two proteins was detected by ECL Western blotting of soluble NP-40 cell extracts generated from HEK/Z-1, the untransfected HEK293 cell host, or Hepa1a cells (our unpublished data).
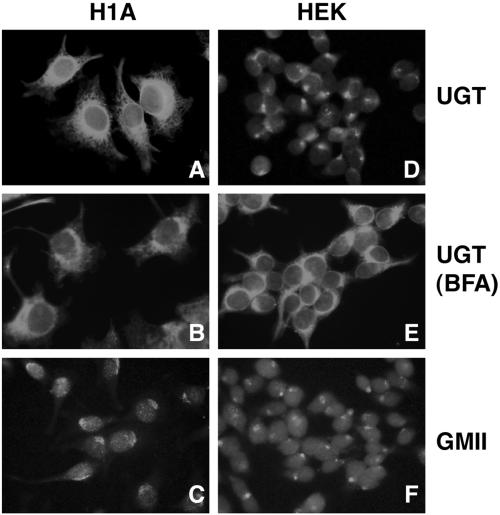
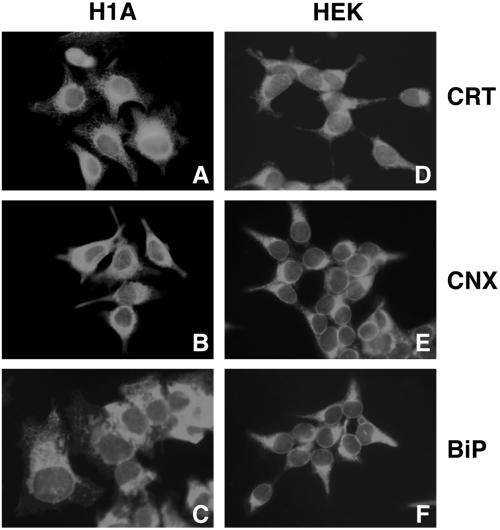
Next, indirect immunofluorescence microscopy was used to identify any differences that might exist in the distribution of UGT between Hepa1a and HEK293 cells, because a diminished concentration of the glycoprotein folding sensor in the ER could conceivably hinder the efficiency by which PI Z reenters the calnexin cycle. In Hepa1a, UGT exhibited a reticular distribution (Figure 6A), similar to that of calnexin (Figure 7B), and distinct from Golgi mannosidase II (Figure 6C). In contrast, only minimal reticular staining of UGT was detected in HEK293, and the bulk was distributed in a perinuclear manner (Figure 6D), similar to that of Golgi mannosidase II (Figure 6F). Consistent with the conclusion that the bulk of UGT was sequestered downstream of the ER, UGT staining was redistributed into a distinct reticular pattern in response to the treatment of cells with brefeldin A (Figure 6E), which induces the redistribution of proximal Golgi contents into the early secretory pathway (Lippincott-Schwartz et al., 1989). That the altered distribution of UGT in HEK293 was not representative of the general ER chaperone content was confirmed by the reticular staining of calreticulin, calnexin, and grp78/BiP in both cell lines (Figure 7). Importantly, in parallel studies, the pattern of chaperone staining was unaffected in both host cell lines following stable transfection with PI Z (our unpublished data), indicating that the selective distribution of UGT in HEK293 reflects a cell-specific organizational distinction, rather than a differential response to PI Z synthesis.
Figure 6.
Intracellular distribution of UGT by indirect immunofluorescence microscopy (see MATERIALS AND METHODS). Hepa1a (H1A) cells (A–C) and HEK293 (HEK) cells (D–F) were grown on coverslips, fixed in methanol and incubated with antibodies against UDP-UGT, calnexin (CNX), or Golgi mannosidase II (GMII), before incubation with species-specific fluorescein isothiocyanate-conjugated fluorescent secondary antibodies. In each case, the fluorescence pattern was indicative of >95% of cells. Brefeldin A (BFA) was added to H1A cells (B) and HEK cells (E) 1.5 h before methanol fixation at a final concentration of 2 μg/ml.
Figure 7.
Intracellular distribution of molecular chaperones by indirect immunofluorescence microscopy (see MATERIALS AND METHODS). Hepa1a (H1A) cells (A–C) and HEK293 (HEK) cells (D–F) were grown on coverslips, fixed in methanol, and incubated with antibodies against calreticulin (CRT), calnexin (CRT), or grp78/BiP (BiP) (see MATERIALS AND METHODS) before incubation with species-specific fluorescein isothiocyanate-conjugated fluorescent secondary antibodies. In each case, the fluorescence pattern was indicative of >95% of the cells.
DISCUSSION
Posttranslational Quality Control as a Modifier of Protein Fate
In the ER, a compartment through which all secretory and cell surface proteins must pass, folding and quality control systems work in parallel as a posttranslational checkpoint in eukaryote genome expression (reviewed by Cabral et al., 2001). Therefore, a central goal of the present study was to determine whether the mechanism by which a newly synthesized glycoprotein is handled after biosynthesis is sufficient to modify its defective secretion, and thereby potentially contribute to the phenotypic variation of disease, or even disease penetrance. Variant PI Z was chosen as a reporter protein because the spontaneous loop-sheet polymerization (Lomas et al., 1992) of a late folding intermediate (Yu et al., 1995) leads to its defective secretion from liver hepatocytes and is directly responsible for chronic obstructive pulmonary disease as a loss-of-function phenotype (reviewed by Culliton, 1989; Kopito and Ron, 2000).
In stably transfected HEK293, the degradation of secretion-impaired PI Z was mediated by the ubiquitin-proteasome system, rather than by a coexisting nonproteasomal system used in the hepatoma cell line Hepa1a (Cabral et al.,2000). Furthermore, as much as 40% of the newly synthesized molecules were eventually secreted into the medium under control conditions, which is a significant increase over the 10–15% secreted from Hepa1a (Le et al., 1990, 1992; Cabral et al., 2000). Because loop-sheet polymerization blocks formation of the secretion-competent α1-antitrypsin monomer (Yu et al., 1995), a 50% enhancement in secretion from HEK293 under conditions that stopped degradation by the proteasome suggests that polymerization plays no significant role in mediating ER retention in the foreign folding and quality control environment. Consistent with this idea, calnexin and grp78/BiP sequentially bound secretion-incompetent PI Z, although neither of these molecules is expected to persistently interact with the polymer. However, we cannot disregard the possibility that the small fraction of molecules never secreted from HEK293, under any circumstances, might consist of the polymerized material. Finally, it is noteworthy that the extent to which PI Z is secreted from HEK293 is hypothetically sufficient to prevent the elastolytic destruction of lung connective tissue, if faithfully duplicated in vivo. As such, the present study provides a proof-of-principle to support the notion that the manner in which a newly synthesized gene product is processed is potentially sufficient to modify the severity of a loss-of-function phenotype, implicating a possible role for protein biosynthetic quality control as a modifier of disease.
Recognition of a Two-Component GERAD Signal
In the present study, variant PI Z bound grp78/BiP before its degradation in HEK293, and required oligosaccharide modification, possibly by ER mannosidase I. Because the glycan modification is not sufficient to target native glycoproteins for degradation, we (Cabral et al.,2001) recently proposed that nonnative protein structure likely participates as an additional aglycone (noncarbohydrate) GERAD signal component. If one accepts the two-component signal hypothesis, and assumes that GERAD functions in a manner similar to other multistep biological processes, then the initiation of a downstream step requires the completion of a preceding event. As such, the enhanced physical interaction with grp78/BiP under conditions that inhibit formation of the glycan-based signal component might indicate that the molecular chaperone functions, at least in HEK293, as an aglycone (noncarbohydrate) signal recognition factor.
Because interaction with grp78/BiP and subsequent modification by ER mannosidase I are completed well before the onset of proteasomal degradation (Figure 1B), it is likely that recognition of the modified oligosaccharide, functions as the rate-limiting step for substrate recruitment into GERAD. Consistent with this idea, Nagata and coworkers recently demonstrated that the elevated expression of an inactive mammalian homolog of ER mannosidase I, designated EDEM, was sufficient to accelerate the proteasomal degradation of terminally misfolded α1-antitrypsin variant null(Hong Kong) in HEK293 (Hosokawa et al., 2001). The idea is that EDEM likely functions as a glycan-based GERAD signal recognition factor by recognizing the Man8B product generated by ER mannosidase I, a role recently implicated for its yeast homologs Htm1p (Jakob et al., 2001) and Mnl1p (Nakatsukasa et al., 2001). In this manner, asparagine-linked oligosaccharide modification would play a pivotal role in the partitioning of grp78/BiP-bound PI Z between folding and degradation pathways.
In the present study, PI Z secretion was enhanced 50% after mannosidase inhibition (Figure 5B). Therefore, one can assume that a significant fraction of the molecules is maintained in a folding-competent state when bound to grp78/BiP, and can eventually attain secretion-competence, if not degraded. In the event that grp78/BiP does function as an aglycone signal recognition factor, as we suspect, its partnering in the recognition of a two-component GERAD signal provides an attractive explanation as to how a molecular chaperone can participate in both protein folding and degradation pathways, thereby addressing a central unanswered question in protein folding and quality control research. Although a direct correlation was detected between grp78/BiP binding, glycan modification, and degradation, we cannot entirely rule out the possibility that an alternate factor, or even EDEM itself, might be capable of functioning as the aglycone GERAD signal recognition factor. Finally, it should be noted that the cellular machinery that facilitates the recognition and recruitment of aberrant nonglycosylated proteins into ERAD, a close relative of GERAD, has not yet been identified, nor was this a goal of the present study.
Evidence for Distinct GERAD Programs
Our (Liu et al., 1999; Cabral et al., 2000) original finding that the proteasome-mediated degradation of aberrant AAT in the hepatoma cell line Hepa1a requires physical interaction with calnexin was met with some speculation because the interaction is known to suppress substrate degradation in many cell lines (reviewed by Ellgaard et al., 1999; Frigerio and Lord, 2000). Initially, we thought that the proteasomal degradation of PI Z in HEK293 might simply reflect its inability to divert PI Z polymers into a nonproteasomal degradation pathway, which is used in Hepa1a (Cabral et al., 2000). However, this is probably not the case because PI Z polymers are of low abundance in HEK293, if they exist at all.
Considering the distinct roles and intracellular locations of calnexin and UGT, respectively, it is unlikely that alternate branches of the same degradation system operate in the two cell lines. Rather, we propose that the data are more compatible with a model in which distinct GERAD programs are administered. Whether this difference reflects a component of cell differentiation, or merely a difference in cell adaptation mechanisms is not yet known. However, possibly favoring the former idea, a post-ER distribution for UGT has been detected in additional extrahepatic cells lines (Cannon and Helenius, 1999; Zuber et al., 2001), including that in which variant PI Z is degraded by the proteasome (Novoradovskaya et al., 1998). Although one might question the value of sequestering a glycoprotein folding sensor downstream of the ER, it was recently suggested that some newly synthesized glycoproteins might actually utilize this situation as they fold when cycling between early compartments in the secretory pathway (Cannon and Helenius, 1999). As such, the exploitation of specific steps in the GERAD program, and not the entire program itself, might explain why some proteins exhibit different fates in distinct cell lines, whereas others do not (Sitia et al., 1990; Qu et al., 1996; Novoradovskaya et al., 1998; Cabral et al., 2000).
Divergence at Level of GERAD Signal Recognition
One interpretation of our present findings is that the putative cell-specific GERAD programs converge at the earliest steps, including signal formation, but diverge in the manner by which the signals are recognized. In Hepa1a, the proteasomal degradation of aberrant AAT requires physical engagement with calnexin (Liu et al., 1999; Cabral et al., 2000), suggesting that the ER lectin might replace EDEM as the glycan-based signal recognition factor. In this scenario, it would follow that UGT replaces grp78/BiP as the partnering aglycone signal recognition factor, which results in the formation of a ligand for calnexin that, when modified by ER mannosidase I, is a poor substrate for glucosidase II (Liu et al., 1999), thereby leading to molecular capture by the ER lectin. Of course, we cannot entirely ignore the possibility that an alternative, or additional, protein such as the oxidoreductase ERp57, which is bound to calnexin and facilitates intramolecular disulfide bond formation (Oliver et al., 1997), might function, or play a role, because the aglyone-based signal recognition step. However, this seems unlikely because only a single cysteine residue exists in human AAT (Long et al., 1984).
Final Remarks
By studying early protein folding events, Helenius' group recently discovered that the site of asparagine-linked glycosylation on the nascent polypeptide contributes to the rules that govern cotranslational chaperone selection in the ER (Molinari and Helenius, 2000). In the present study, our analysis of glycoprotein disposal led to the discovery of a posttranslational mechanism capable of causing the transfer of a GERAD substrate into distinct folding pathways. Although not directly studied, it is entirely possible that the initial role for the transfer event is to provide the newly synthesized protein with an additional folding landscape to facilitate conformational maturation as a last resort, just before the onset of degradation. Whether latent engagement with grp78/BiP is responsible for hindering PI Z loop-sheet polymerization in HEK293, is not yet understood, and will require additional analysis.
Because the mechanism by which a mutant gene product is processed, rather than the mutation itself, can potentially play a profound role in the severity of a loss-of-function disorder, it is now evident that GERAD has the potential to function as an epigenetic modifier of disease, especially in the event of its inappropriate development or dysregulation. Whether the utilization of distinct GERAD programs reflects a previously unappreciated component of cell differentiation, is not yet known, but will be the focus of future studies.
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health grants HL-62553 (to R.N.S.), GM-47533 (to K.W.M.), and RR-05351 (to K.W.M.); the Alpha-1 Foundation Fernandez Liver Research Initiative (to R.N.S.); and an Alpha-1 Foundation Young Investigator training grant (to C.M.C.).
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–02–0068. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–02–0068.
REFERENCES
- Ayalon-Soffer M, Shenkman M, Lederkremer GZ. Differential role of mannose and glucose trimming in the ER degradation of asialoglycoprotein receptor subunits. J Cell Sci. 1999;112:3309–3318. doi: 10.1242/jcs.112.19.3309. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Weissman AM. Ubiquitin and the control of protein fate in the secretory and endocytic pathways. Annu Rev Cell Dev Biol. 1998;14:19–57. doi: 10.1146/annurev.cellbio.14.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral CM, Choudhury P, Liu Y, Sifers RN. Processing by endoplasmic reticulum mannosidases partitions a secretion-impaired glycoprotein into distinct disposal pathways. J Biol Chem. 2000;275:25015–25022. doi: 10.1074/jbc.M910172199. [DOI] [PubMed] [Google Scholar]
- Cabral CM, Liu Y, Sifers RN. Dissecting glycoprotein quality control in the secretory pathway. Trends Biochem Sci. 2001;26:619–624. doi: 10.1016/s0968-0004(01)01942-9. [DOI] [PubMed] [Google Scholar]
- Cannon KS, Helenius A. Trimming and re-addition of glucose to N-linked oligosaccharides determines calnexin association of a substrate glycoprotein in living cells. J Biol Chem. 1999;274:7537–7544. doi: 10.1074/jbc.274.11.7537. [DOI] [PubMed] [Google Scholar]
- Carlson JA, Rogers BB, Sifers RN, Hawkins HK, Finegold MJ, Woo SLC. The accumulation of PI Z α1-antitrypsin causes liver damage in transenic mice. J Clin Invest. 1988;82:26–36. doi: 10.1172/JCI113580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrell RW, Lomas DA. α1-Antitrypsin deficiency - a model for conformational diseases. N Engl J Med. 2002;346:45–53. doi: 10.1056/NEJMra010772. [DOI] [PubMed] [Google Scholar]
- Choudhury P, Liu Y, Sifers RN. Quality control of protein folding: participation in human disease. News Physiol Sci. 1997;12:162–165. [Google Scholar]
- Culliton BJ. A genetic shield to prevent emphysema? Science. 1989;246:750–751. doi: 10.1126/science.2814494. [DOI] [PubMed] [Google Scholar]
- Danilczyk UG, Cohen-Doyle MF, Williams DB. Functional relationship between calreticulin, calnexin, and the endoplasmic reticulum luminal domain of calnexin. J Biol Chem. 2000;275:13089–13097. doi: 10.1074/jbc.275.17.13089. [DOI] [PubMed] [Google Scholar]
- Elbein AD. Glycosidase inhibitors: inhibitors of N-linked oligosaccharide processing. FASEB. 1991;5:3055–3063. doi: 10.1096/fasebj.5.15.1743438. [DOI] [PubMed] [Google Scholar]
- Ellgaard L, Helenius A. ER quality control: towards an understanding at the molecular level. Curr Opin Cell Biol. 2001;13:431–437. doi: 10.1016/s0955-0674(00)00233-7. [DOI] [PubMed] [Google Scholar]
- Ellgaard L, Molinari M, Helenius A. Setting the standards: quality control in the secretory pathway. Science. 1999;286:1882–1888. doi: 10.1126/science.286.5446.1882. [DOI] [PubMed] [Google Scholar]
- Fenteany G, Standaert RF, Lane WS, Choi S, Corey EJ, Schreiber SL. Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science. 1995;268:726–731. doi: 10.1126/science.7732382. [DOI] [PubMed] [Google Scholar]
- Fewell SW, Travers KJ, Weissman JS, Brodsky JL. The action of molecular chaperones in the early secretory pathway. Annu Rev Genet. 2001;35:149–191. doi: 10.1146/annurev.genet.35.102401.090313. [DOI] [PubMed] [Google Scholar]
- Frigerio L, Lord JM. Glycoprotein degradation: do sugars hold the key? Curr Biol. 2000;10:R674–R677. doi: 10.1016/s0960-9822(00)00680-1. [DOI] [PubMed] [Google Scholar]
- Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Gonzalez DS, Jordan IK. The α-mannosidases: phylogeny and adaptive diversification. Mol Biol Evol. 2000;17:292–300. doi: 10.1093/oxfordjournals.molbev.a026309. [DOI] [PubMed] [Google Scholar]
- Gonzalez DS, Karaveg K, Vandersall-Nairn AS, Lal A, Moremen KW. Identification, expression, and characterization of a cDNA encoding human endoplasmic reticulum mannosidase I, the enzyme that catalyzes the first mannose trimming step in mammalian Asn-linked oligosaccharide biosynthesis. J Biol Chem. 1999;274:21375–21386. doi: 10.1074/jbc.274.30.21375. [DOI] [PubMed] [Google Scholar]
- Graham KS, Le A, Sifers RN. Accumulation of the insoluble PI Z variant of human α1-antitrypsin within the hepatic endoplasmic reticulum does not elevate the steady-state level of grp78/BiP. J Biol Chem. 1990;265:20463–20468. [PubMed] [Google Scholar]
- Grinna LS, Robbins PW. Substrate specificities of rat liver microsomal glucosidases which process glycoproteins. J Biol Chem. 1980;255:2255–2258. [PubMed] [Google Scholar]
- Hammond C, Braakman I, Helenius A. Role of N-linked oligosaccharide recognition, glucose trimming, and calnexin in glycoprotein folding and quality control. Proc Natl Acad Sci USA. 1994;91:913–917. doi: 10.1073/pnas.91.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond E, Helenius A. Quality control in the secretory pathway. Curr Opin Cell Biol. 1995;7:523–529. doi: 10.1016/0955-0674(95)80009-3. [DOI] [PubMed] [Google Scholar]
- Hartwell LH, Weinert TA. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- Hebert DN, Foellmer B, Helenius A. Glucose trimming and reglucosylation determine glycoprotein association with calnexin in the endoplasmic reticulum. Cell. 1995;81:425–433. doi: 10.1016/0092-8674(95)90395-x. [DOI] [PubMed] [Google Scholar]
- Helenius A. How N-linked oligosaccharides affect glycoprotein folding in the endoplasmic reticulum. Mol Biol Cell. 1994;5:253–265. doi: 10.1091/mbc.5.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herscovics A. Structure and function of class I α1,2-mannosidases involved in glycoprotein synthesis and endoplasmic reticulum quality control. Biochimie. 2001;83:757–762. doi: 10.1016/s0300-9084(01)01319-0. [DOI] [PubMed] [Google Scholar]
- Hosokawa N, Wada I, Hasegawa K, Yorihuzi T, Tremblay LO, Herscovics A, Nagata K. A novel ER α-mannosidase-like protein accelerates ER-associated degradation. EMBO Rep. 2001;2:415–422. doi: 10.1093/embo-reports/kve084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob CA, Bodmer D, Spririg U, Battig P, Marcil A, Dignard D, Bergeron JJM, Thomas DY, Aebi M. Htm1p, a mannosidase-like protein, is involved in glycoprotein degradation in yeast. EMBO Rep. 2001;2:423–430. doi: 10.1093/embo-reports/kve089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner RD, Sitia R. Protein degradation in the endoplasmic reticulum. Cell. 1990;62:611–614. doi: 10.1016/0092-8674(90)90104-m. [DOI] [PubMed] [Google Scholar]
- Koch G, Smith M, Macer D, Webster P, Mortara R. Endoplasmic reticulum contains a common, abundant calcium-binding glycoprotein, endoplasmin. J Cell Sci. 1986;86:217–232. doi: 10.1242/jcs.86.1.217. [DOI] [PubMed] [Google Scholar]
- Kopito RR, Ron D. Conformational disease. Nat Cell Biol. 2000;2:E207–209. doi: 10.1038/35041139. [DOI] [PubMed] [Google Scholar]
- Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Le A, Ferrell GA, Dishon DS, Le Q-Q, Sifers RN. Soluble aggregates of the human PI Z α1-antitrypsin variant are degraded within the endoplasmic reticulum by a mechanism sensitive to inhibitors of protein synthesis. J Biol Chem. 1992;267:1072–1080. [PubMed] [Google Scholar]
- Le A, Graham KS, Sifers RN. Intracellular degradation of the transport-impaired human PI Z α1-antitrypsin variant. Biochemical mapping of the degradative event among compartments of the secretory pathway. J Biol Chem. 1990;265:14001–14007. [PubMed] [Google Scholar]
- Le A, Steiner JL, Ferrell GA, Shaker JC, Sifers RN. Association between calnexin and a secretion-incompetent variant of human α1-antitrypsin. J Biol Chem. 1994;269:7514–7519. [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Yuan LC, Bonifacino JS, Klausner RD. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989;56:801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Choudhury P, Cabral CM, Sifers RN. Intracellular disposal of incompletely folded human α1-antitrypsin involves release from calnexin and post-translational trimming of asparagine-linked oligosaccharides. J Biol Chem. 1997;272:7946–7951. doi: 10.1074/jbc.272.12.7946. [DOI] [PubMed] [Google Scholar]
- Liu Y, Choudhury P, Cabral CM, Sifers RN. Oligosaccharide modification in the early secretory pathway directs the selection of a misfolded glycoprotein for degradation by the proteasome. J Biol Chem. 1999;274:5861–5867. doi: 10.1074/jbc.274.9.5861. [DOI] [PubMed] [Google Scholar]
- Lomas DA, Evans DLI, Finch JT, Carrell RW. The mechanism of Z α1-antitrypsin accumulation in the liver. Nature. 1992;357:605–607. doi: 10.1038/357605a0. [DOI] [PubMed] [Google Scholar]
- Long GL, Chandra T, Woo SL, Davie EW, Kurachi K. Complete sequence of the cDNA for human α1-antitrypsin and the gene for the S variant. Biochemistry. 1984;23:4828–4837. doi: 10.1021/bi00316a003. [DOI] [PubMed] [Google Scholar]
- McCracken AA, Brodsky JL. Assembly of ER-associated protein degradation in vitro: dependence on cytosol, calnexin, and ATP. J Cell Biol. 1996;132:291–298. doi: 10.1083/jcb.132.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari M, Helenius A. Chaperone selection during glycoprotein translocation into the endoplasmic reticulum. Science. 2000;288:331–333. doi: 10.1126/science.288.5464.331. [DOI] [PubMed] [Google Scholar]
- Moremen KW, Touster O, Robbins PW. Novel purification of the catalytic domain of Golgi α-mannosidase II. Characterization and comparison with the intact enzyme. J Biol Chem. 1991;266:16876–16885. [PubMed] [Google Scholar]
- Nakatsukasa K, Nishikawa S, Hosokawa N, Nagata K, Endo T. Mnl1p, an α-mannosidase-like protein in yeast Saccharomyces cerevisiae, is required for endoplasmic reticulum-associated degradation of glycoproteins. J Biol Chem. 2001;276:8635–8638. doi: 10.1074/jbc.C100023200. [DOI] [PubMed] [Google Scholar]
- Novoradovskaya N, Lee J, Yu ZX, Ferrans VJ, Brantly M. Inhibition of intracellular degradation increases secretion of a mutant form of α1-antitrypsin associated with a profound deficiency. J Clin Invest. 1998;101:2693–2701. doi: 10.1172/JCI549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver JD, van der Wal FJ, Bullied NJ, High S. Interaction of the thiol-dependent reductace ERp57 with nascent glycoproteins. Science. 1997;275:86–88. doi: 10.1126/science.275.5296.86. [DOI] [PubMed] [Google Scholar]
- Ou WJ, Camerron PH, Thomas DY, Bergeron JJM. Association of folding intermediates of glycoproteins with calnexin during protein maturation. Nature. 1993;364:771–776. doi: 10.1038/364771a0. [DOI] [PubMed] [Google Scholar]
- Pandey A, Mann M. Proteomics to study genes and genomes. Nature. 2000;405:837–846. doi: 10.1038/35015709. [DOI] [PubMed] [Google Scholar]
- Parodi A. Protein glucosylation and its role in protein folding. Annu Rev Biochem. 2000;69:69–93. doi: 10.1146/annurev.biochem.69.1.69. [DOI] [PubMed] [Google Scholar]
- Parodi AJ, Mendelzon DH, Lederkremer GZ. Transient glucosylation of protein-bound Man9GlcNAc2, Man8GlcNAc2, and Man7GlcNAc2 in calf thyroid cells. A possible recognition signal in the processing of glycoproteins. J Biol Chem. 1983;258:8260–8265. [PubMed] [Google Scholar]
- Perlmutter DH, Pierce JA. The α1-antitrypsin gene and emphysema. Am J Physiol. 1989;257:L147–L162. doi: 10.1152/ajplung.1989.257.4.L147. [DOI] [PubMed] [Google Scholar]
- Qu D, Teckman JH, Omura S, Perlmutter DH. Degradation of a mutant secretory protein, α1-antitrypsin Z, in the endoplasmic reticulum requires proteasome activity. J Biol Chem. 1996;271:22791–22795. doi: 10.1074/jbc.271.37.22791. [DOI] [PubMed] [Google Scholar]
- Rothman JE. Protein sorting by selective retention in the endoplasmic reticulum. Cell. 1987;50:521–524. doi: 10.1016/0092-8674(87)90024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifers RN, Brashears-Macatee S, Kidd VJ, Muensch H, Woo SLC. A frameshift mutation results in a truncated α1-antitrypsin that is retained within the rough endoplasmic reticulum. J Biol Chem. 1989;263:7330–7335. [PubMed] [Google Scholar]
- Sifers RN, Finegold MJ, Woo SLC. Molecular biology and genetics of α1-antirypsin deficiency. Sem Liv Dis. 1992;12:301–310. doi: 10.1055/s-2008-1040399. [DOI] [PubMed] [Google Scholar]
- Sitia R, Neuberger M, Alberini C, Bet P, Fra A, Valetti C, Williams G, Milstein C. Developmental regulation of IgM secretion: the role of the carboxy-terminal cysteine. Cell. 1990;60:781–790. doi: 10.1016/0092-8674(90)90092-s. [DOI] [PubMed] [Google Scholar]
- Sommer T, Wolf DH. Endoplasmic reticulum degradation. Reverse protein transport and its end in the proteasome. FASEB J. 1997;11:1227–1233. doi: 10.1096/fasebj.11.14.9409541. [DOI] [PubMed] [Google Scholar]
- Sousa MC, Ferro-Garcia MA, Parodi AJ. Recognition of the oligosaccharide and protein moieties of glycoproteins by the UDP-glc:glycoprotein glucosyltransferase. Biochemistry. 1992;31:97–105. doi: 10.1021/bi00116a015. [DOI] [PubMed] [Google Scholar]
- Thomas PJ, Qu B-H, Pedersen PL. Defective protein folding as a basis of human disease. Trends Biochem Sci. 1995;20:456–459. doi: 10.1016/s0968-0004(00)89100-8. [DOI] [PubMed] [Google Scholar]
- Tremblay LO, Herscovics Cloning and expression of a specific human α1,2-mannosidase that trims Man9GlcNAc2 to Man8GlcNAc2 isomer B during N-glycan biosynthesis. Glycobiology. 1999;9:1073–1078. doi: 10.1093/glycob/9.10.1073. [DOI] [PubMed] [Google Scholar]
- Trombetta SE, Ganan S, Parodi AJ. The UDP-Glc:glycoprotein glucosyltransferase is a soluble protein of the endoplasmic reticulum. Glycobiology. 1991;1:155–161. doi: 10.1093/glycob/1.2.155. [DOI] [PubMed] [Google Scholar]
- VanLeeuwen JEM, Kearse KP. Deglucosylation of N-linked glycans is an important step in the dissociation of calreticulin-class I-TAP complexes. Proc Natl Acad Sci USA. 1996;93:13997–14001. doi: 10.1073/pnas.93.24.13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux D, Tooze J, Fuller SD. Identification by anti-idiotype antibodies of an intracellular membrane protein that recognizes a mammalian endoplasmic reticulum retention signal. Nature. 1990;345:495–502. doi: 10.1038/345495a0. [DOI] [PubMed] [Google Scholar]
- Volpert D, Molleston JP, Perlmutter DH. α1-Antitrypsin deficiency-associated liver disease progresses slowly in some children. J Pediatr Gastroenterol Nutr. 2000;31:258–263. doi: 10.1097/00005176-200009000-00011. [DOI] [PubMed] [Google Scholar]
- Weng S, Spiro RG. Demonstration that a kifunensine-resistant α-mannosidase with a unique processing action on N-linked oligosaccharides occurs in rat liver endoplasmic reticulum and various cultured cells. J Biol Chem. 1993;268:25656–25663. [PubMed] [Google Scholar]
- Weng S, Spiro RG. Endoplasmic reticulum kifunensine-resistant α-mannosidase is enzymatically and immunologically related to the cytosolic α-mannosidase. Arch Biochem Biophys. 1996;325:113–123. doi: 10.1006/abbi.1996.0014. [DOI] [PubMed] [Google Scholar]
- Wu Y, Whitman I, Molmenti E, Moore K, Hippenmeyer P, Perlmutter DH. A lag in intracellular degradation of mutant α1-antitrypsin correlates with the liver disease phenotype in homozygous PiZZ α1-antitrypsin deficiency. Proc Natl Acad Sci USA. 1994;91:9014–9018. doi: 10.1073/pnas.91.19.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X, Chong E, Skach WR. Evidence that endoplasmic reticulum (ER)-associated degradation of cystic fibrosis transmembrane conductance regulator is linked to retrograde translocation from the ER membrane. J Biol Chem. 1999;274:2616–2624. doi: 10.1074/jbc.274.5.2616. [DOI] [PubMed] [Google Scholar]
- Yu MH, Lee KN, Kim J. The Z type variation of human α1-antitrypsin causes a protein folding defect. Nat Struct Biol. 1995;2:363–367. doi: 10.1038/nsb0595-363. [DOI] [PubMed] [Google Scholar]
- Zapun A, Petrescu SM, Rudd PM, Dwek RA, Thomas EY, Bergeron JJM. Conformation-independent binding of monoglucosylated ribonuclease B to calnexin. Cell. 1997;88:29–38. doi: 10.1016/s0092-8674(00)81855-3. [DOI] [PubMed] [Google Scholar]
- Zhou BB, Elledge SJ. The DNA damage response: putting checkpoints into perspective. Nature. 2000;408:433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]
- Zuber C, Fan JY, Guhl B, Parodi A, Fessler JH, Parker C, Roth J. Immunolocalization of UDP-glucose:glycoprotein glucosyltransferase indicates involvement of pre-Golgi intermediates in protein quality control. Proc Natl Acad Sci USA. 2001;98:10710–10715. doi: 10.1073/pnas.191359198. [DOI] [PMC free article] [PubMed] [Google Scholar]