Abstract
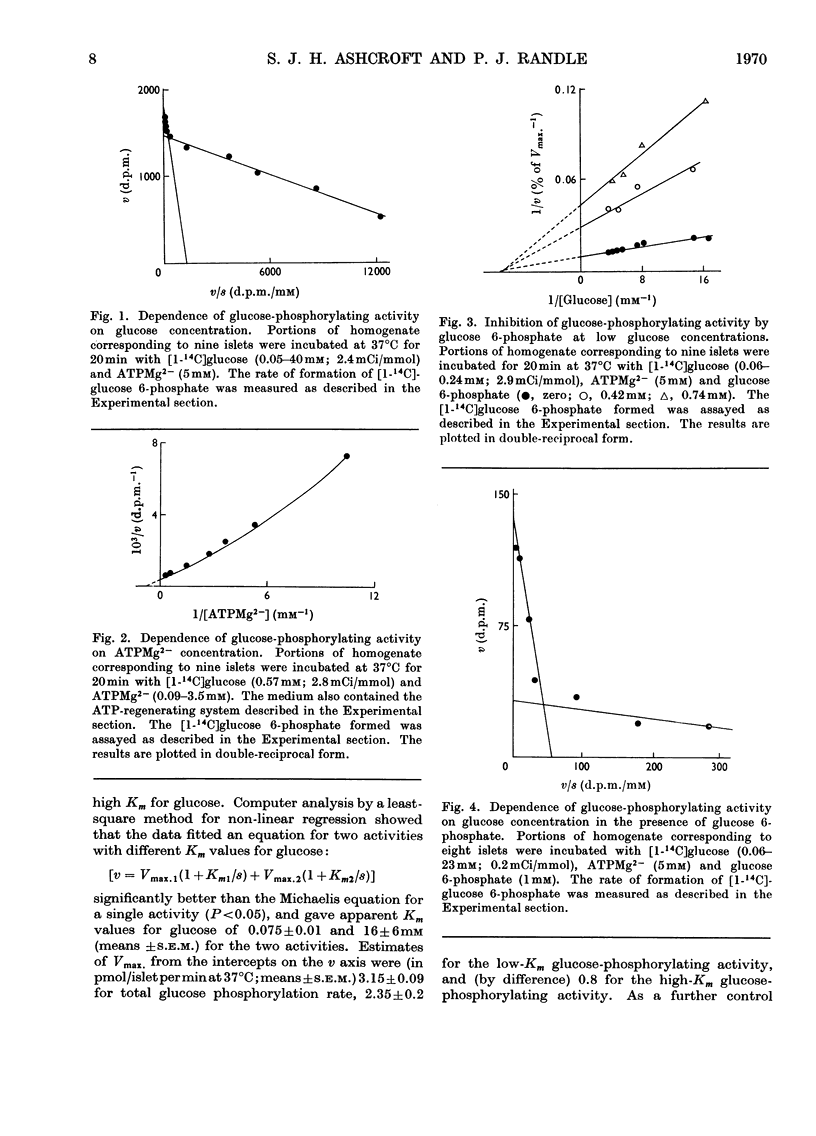
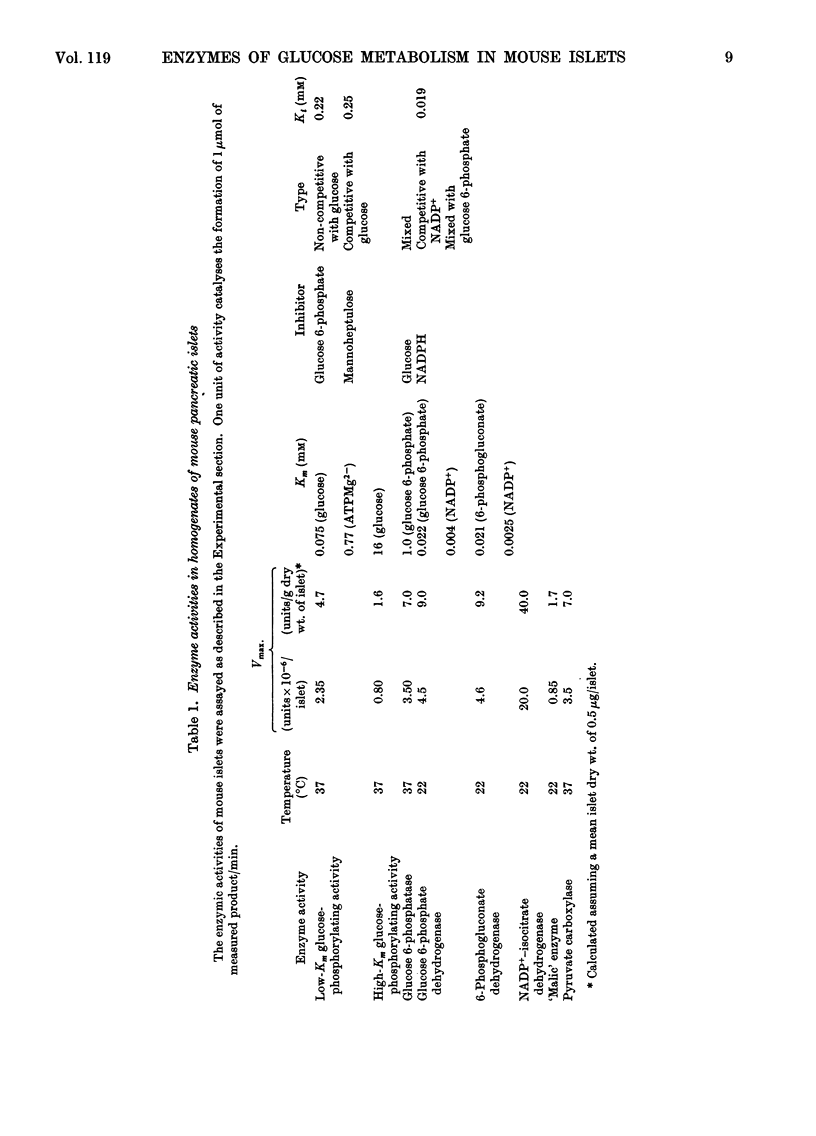
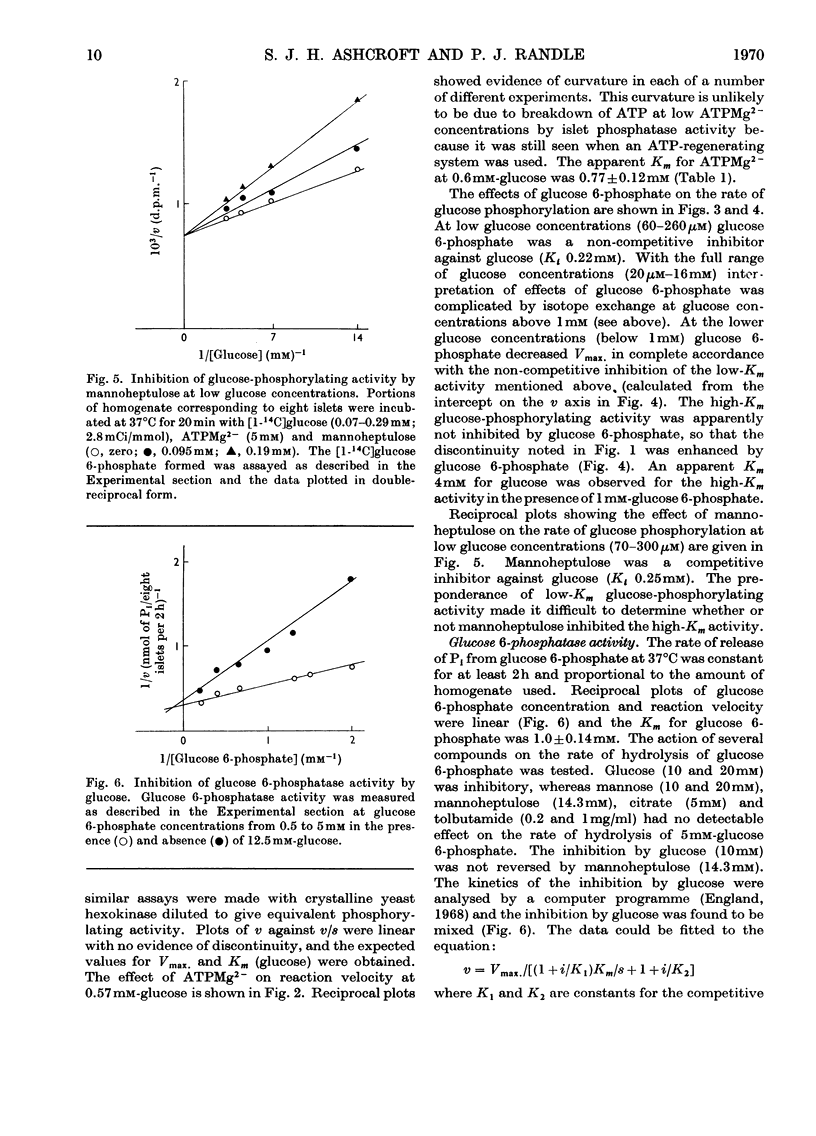
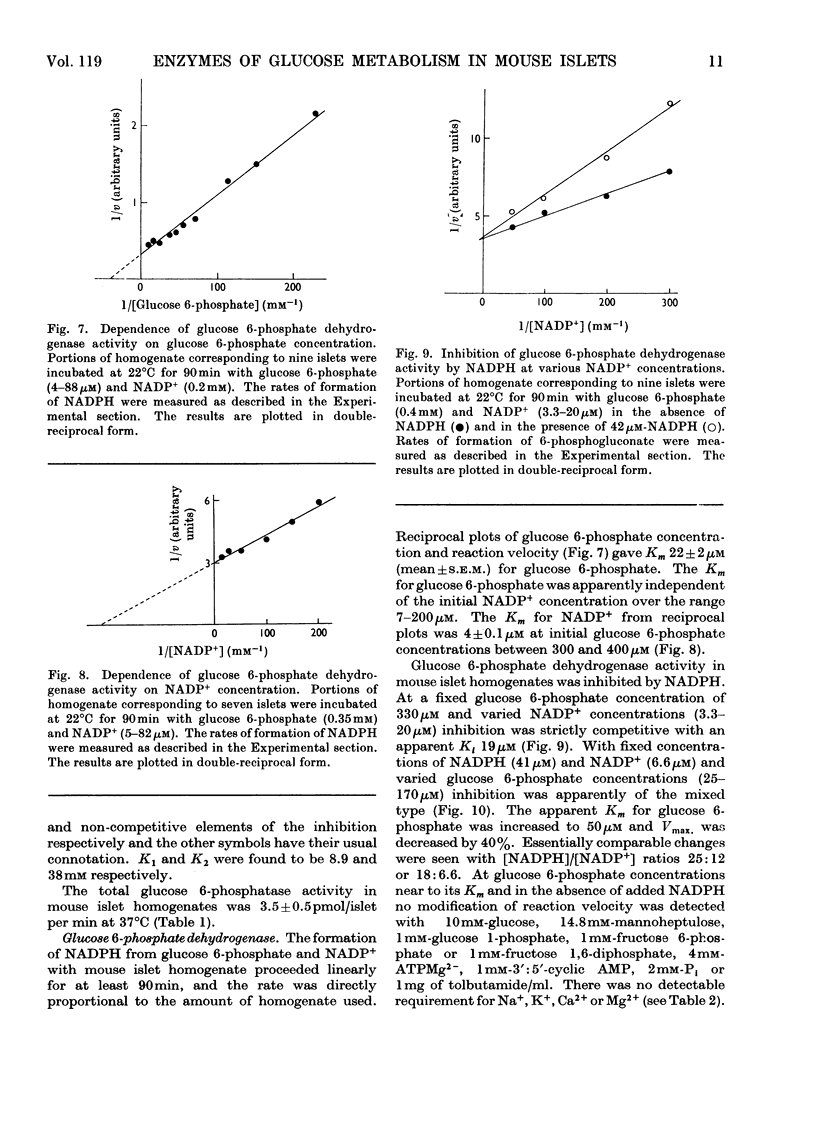
1. Glucose-phosphorylating and glucose 6-phosphatase activities, glucose 6-phosphate dehydrogenase, 6-phosphogluconate dehydrogenase, NADP+-linked isocitrate dehydrogenase, `malic' enzyme and pyruvate carboxylase were assayed in homogenates of normal mouse islets. 2. Two glucose-phosphorylating activities were detected; the major activity had Km 0.075mm for glucose and was inhibited by glucose 6-phosphate (non-competitive with glucose) and mannoheptulose (competitive with glucose). The other (minor) activity had a high Km for glucose (mean value 16mm) and was apparently not inhibited by glucose 6-phosphate. 3. Glucose 6-phosphatase activity was present in amounts comparable with the total glucose-phosphorylating activity, with Km 1mm for glucose 6-phosphate. Glucose was an inhibitor and the inhibition showed mixed kinetics. No inhibition of glucose 6-phosphate hydrolysis was observed with mannose, citrate or tolbutamide. The inhibition by glucose was not reversed by mannoheptulose. 4. 6-Phosphogluconate dehydrogenase had Km values of 2.5 and 21μm for NADP+ and 6-phosphogluconate respectively. 5. Glucose 6-phosphate dehydrogenase had Km values of 4 and 22μm for NADP+ and glucose 6-phosphate. The Km for glucose 6-phosphate was considerably below the intra-islet concentration of glucose 6-phosphate at physiological extracellular glucose concentrations. The enzyme had no apparent requirement for cations. Of a number of possible modifiers of glucose 6-phosphate dehydrogenase, only NADPH was inhibitory. The inhibition by NADPH was competitive with NADP+ and apparently mixed with respect to glucose 6-phosphate. 6. NADP+–isocitrate dehydrogenase was present but the islet homogenate contained little, if any, `malic' enzyme. The presence of pyruvate carboxylase was also demonstrated. 7. The results obtained are discussed with reference to glucose phosphorylation and glucose 6-phosphate oxidation in the intact mouse islet, and the possible nature of the β-cell glucoreceptor mechanism.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft S. J., Hedeskov C. J., Randle P. J. Glucose metabolism in mouse pancreatic islets. Biochem J. 1970 Jun;118(1):143–154. doi: 10.1042/bj1180143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft S. J., Randle P. J. Glucose phosphorylation in mouse pancreatic islets. Biochem J. 1968 Apr;107(4):599–600. doi: 10.1042/bj1070599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft S. J., Randle P. J. Glucose-6-phosphatase activity of mouse pancreatic islets. Nature. 1968 Aug 24;219(5156):857–858. doi: 10.1038/219857a0. [DOI] [PubMed] [Google Scholar]
- Coll-Garcia E., Gill J. R. Insulin release by isolated pancreatic islets of the mouse incubated in vitro. Diabetologia. 1969 Apr;5(2):61–66. doi: 10.1007/BF01211999. [DOI] [PubMed] [Google Scholar]
- Coore H. G., Randle P. J. Regulation of insulin secretion studied with pieces of rabbit pancreas incubated in vitro. Biochem J. 1964 Oct;93(1):66–78. doi: 10.1042/bj0930066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint A. P., Denton R. M. The role of nicotinamide-adenine dinucleotide phosphate-dependent malate dehydrogenase and isocitrate dehydrogenase in the supply of reduced nicotinamide-adenine dinucleotide phosphate for steroidogenesis in the superovulated rat ovary. Biochem J. 1970 Mar;117(1):73–83. doi: 10.1042/bj1170073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLASER L., BROWN D. H. Purification and properties of d-glucose-6-phosphate dehydrogenase. J Biol Chem. 1955 Sep;216(1):67–79. [PubMed] [Google Scholar]
- LAZARUS S. S. Acid and glucose-6-phosphatase activity of pancreatic B cells after cortisone and sulfonylureas. Proc Soc Exp Biol Med. 1959 Nov;102:303–306. doi: 10.3181/00379727-102-25227. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Lea M. A., Malaisse-Lagae F. The effect of mannoheptulose on the phosphorylation of glucose and the secretion of insulin by islets of Langerhans. Metabolism. 1968 Feb;17(2):126–132. doi: 10.1016/0026-0495(68)90138-8. [DOI] [PubMed] [Google Scholar]
- Matschinsky F. M., Ellerman J. E. Metabolism of glucose in the islets of Langerhans. J Biol Chem. 1968 May 25;243(10):2730–2736. [PubMed] [Google Scholar]
- Matschinsky F. M., Kauffman F. C., Ellerman J. E. Effect of hyperglycemia on the hexose monophosphate shunt in islets of Langerhans. Diabetes. 1968 Aug;17(8):475–480. doi: 10.2337/diab.17.8.475. [DOI] [PubMed] [Google Scholar]
- Matschinsky F. M., Rutherford C. R., Ellerman J. E. Accumulation of citrate in pancreatic islets of obese hyperglycemic mice. Biochem Biophys Res Commun. 1968 Dec 9;33(5):855–862. doi: 10.1016/0006-291x(68)90240-4. [DOI] [PubMed] [Google Scholar]
- Moellering H., Gruber W. Determination of citrate with citrate lyase. Anal Biochem. 1966 Dec;17(3):369–376. doi: 10.1016/0003-2697(66)90172-2. [DOI] [PubMed] [Google Scholar]
- Montague W., Taylor K. W. Pentitols and insulin release by isolated rat islets of Langerhans. Biochem J. 1968 Sep;109(3):333–339. doi: 10.1042/bj1090333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague W., Taylor K. W. Regulation of insulin secretion by short chain fatty acids. Nature. 1968 Mar 2;217(5131):853–853. doi: 10.1038/217853a0. [DOI] [PubMed] [Google Scholar]
- Nordlie R. C., Lygre D. G. The inhibition by citrate of inorganic pyrophosphate-glucose phosphotransferase and glucose 6-phosphatase. J Biol Chem. 1966 Jul 10;241(13):3136–3141. [PubMed] [Google Scholar]
- Ockerman P. A. Glucose-6-phosphatase assay on microgram amounts of liver tissue. Clin Chim Acta. 1967 Aug;17(2):201–206. doi: 10.1016/0009-8981(67)90119-2. [DOI] [PubMed] [Google Scholar]
- TäljedalIB Presence, induction and possible role of glucose 6-phosphatase in mammalian pancreatic islets. Biochem J. 1969 Sep;114(2):387–394. doi: 10.1042/bj1140387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veech R. L., Eggleston L. V., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide phosphate in the cytoplasm of rat liver. Biochem J. 1969 Dec;115(4):609–619. doi: 10.1042/bj1150609a. [DOI] [PMC free article] [PubMed] [Google Scholar]


