Abstract
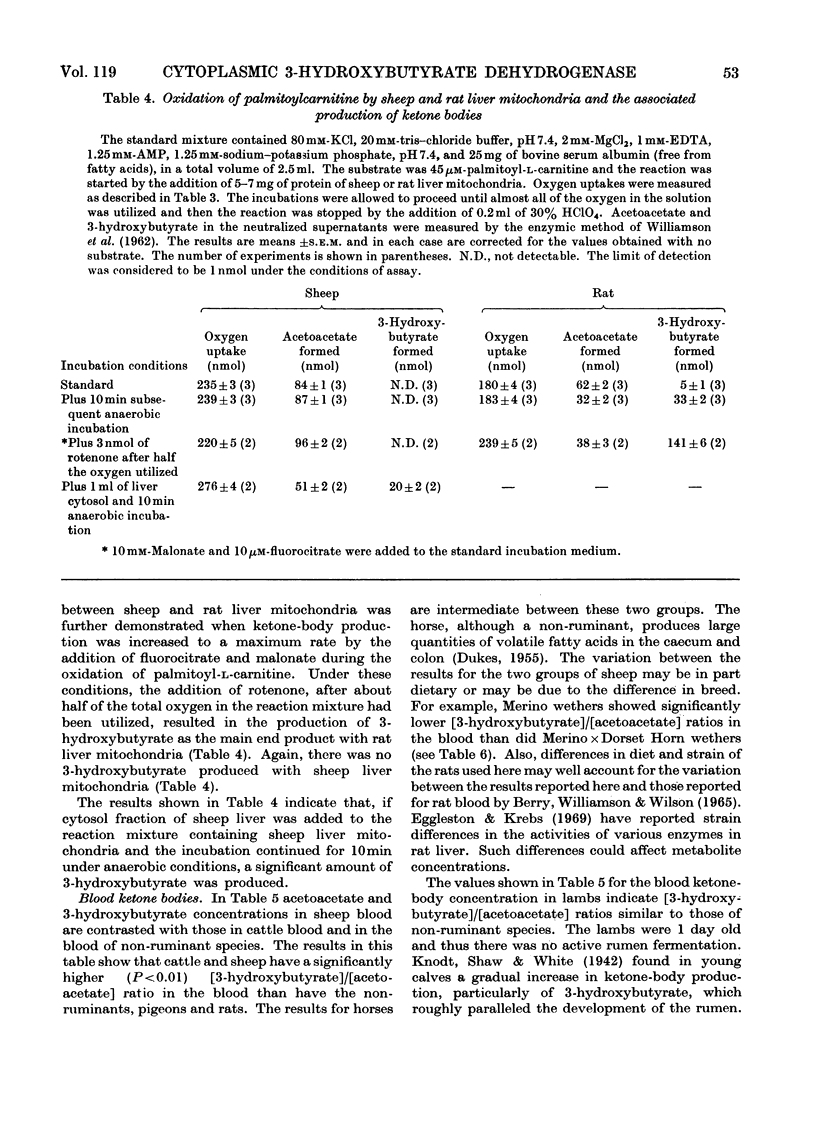
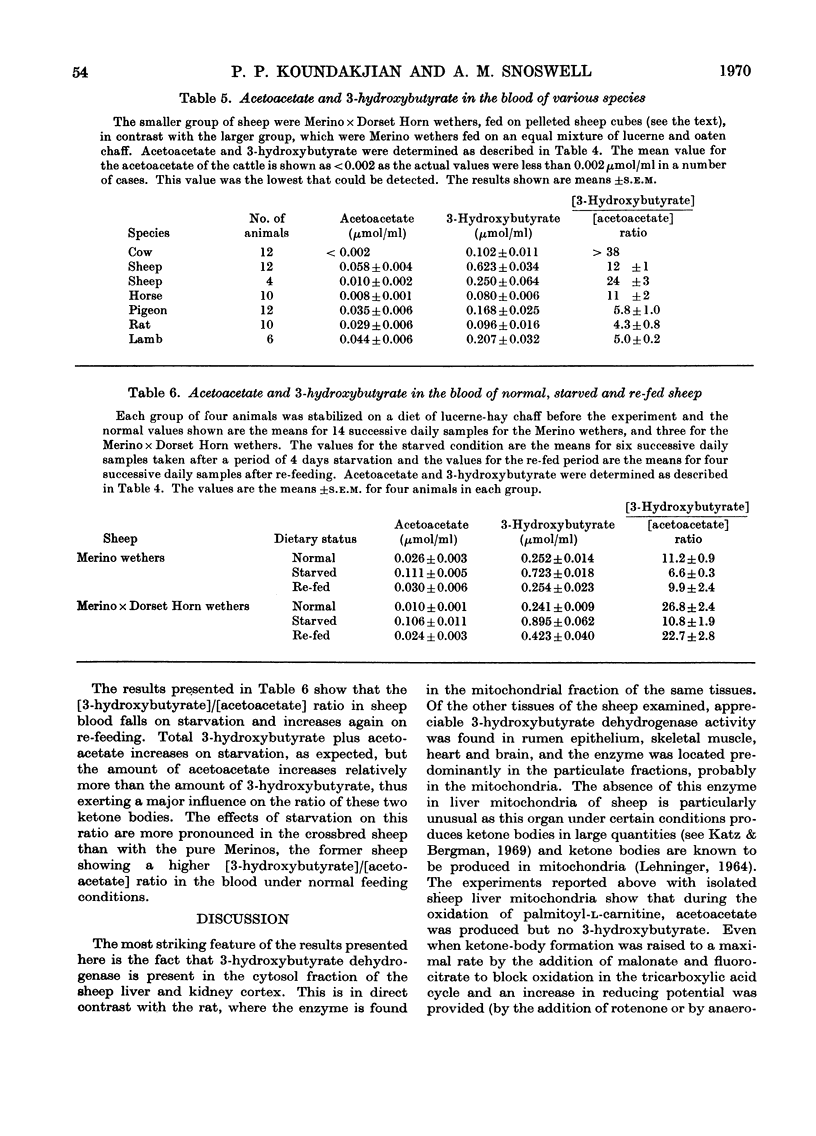
1. 3-Hydroxybutyrate dehydrogenase (EC 1.1.1.30) activities in sheep kidney cortex, rumen epithelium, skeletal muscle, brain, heart and liver were 177, 41, 38, 33, 27 and 17μmol/h per g of tissue respectively, and in rat liver and kidney cortex the values were 1150 and 170 respectively. 2. In sheep liver and kidney cortex the 3-hydroxybutyrate dehydrogenase was located predominantly in the cytosol fractions. In contrast, the enzyme was found in the mitochondria in rat liver and kidney cortex. 3. Laurate, myristate, palmitate and stearate were not oxidized by sheep liver mitochondria, whereas the l-carnitine esters were oxidized at appreciable rates. The free acids were readily oxidized by rat liver mitochondria. 4. During oxidation of palmitoyl-l-carnitine by sheep liver mitochondria, acetoacetate production accounted for 63% of the oxygen uptake. No 3-hydroxybutyrate was formed, even after 10min anaerobic incubation, except when sheep liver cytosol was added. With rat liver mitochondria, half of the preformed acetoacetate was converted into 3-hydroxybutyrate after anaerobic incubation. 5. Measurement of ketone bodies by using specific enzymic methods (Williamson, Mellanby & Krebs, 1962) showed that blood of normal sheep and cattle has a high [3-hydroxybutyrate]/[acetoacetate] ratio, in contrast with that of non-ruminants (rats and pigeons). This ratio in the blood of lambs was similar to that of non-ruminants. The ratio in sheep blood decreased on starvation and rose again on re-feeding. 6. The physiological implications of the low activity of 3-hydroxybutyrate dehydrogenase in sheep liver and the fact that it is found in the cytoplasm in sheep liver and kidney cortex are discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird G. D., Hibbitt K. G., Hunter G. D. Biochemical aspects of bovine ketosis. Biochem J. 1968 May;107(5):683–689. doi: 10.1042/bj1070683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard F. J., Hanson R. W., Kronfeld D. S. Factors controlling the concentration of mitochondrial oxaloacetate in liver during spontaneous bovine ketosis. Biochem Biophys Res Commun. 1968 Jan 11;30(1):100–104. doi: 10.1016/0006-291x(68)90719-5. [DOI] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Eggleston L. V., Krebs H. A. Strain differences in the activities of rat liver enzymes. Biochem J. 1969 Oct;114(4):877–879. doi: 10.1042/bj1140877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greville G. D., Tubbs P. K. The catabolism of long chain fatty acids in mammalian tissues. Essays Biochem. 1968;4:155–212. [PubMed] [Google Scholar]
- Katz M. L., Bergman E. N. Hepatic and portal metabolism of glucose, free fatty acids, and ketone bodies in the sheep. Am J Physiol. 1969 Apr;216(4):953–960. doi: 10.1152/ajplegacy.1969.216.4.953. [DOI] [PubMed] [Google Scholar]
- LEHNINGER A. L., SUDDUTH H. C., WISE J. B. D-beta-Hydroxybutyric dehydrogenase of muitochondria. J Biol Chem. 1960 Aug;235:2450–2455. [PubMed] [Google Scholar]
- LENG R. A., ANNISON E. F. Metabolism of acetate, propionate and butyrate by sheep-liver slices. Biochem J. 1963 Feb;86:319–327. doi: 10.1042/bj0860319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng R. A., Annison E. F. The metabolism of D(--)-beta-hydroxybutyrate in sheep. Biochem J. 1964 Mar;90(3):464–469. doi: 10.1042/bj0900464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portenhauser R., Schäfer G., Lamprecht W. Metabolic action of palmitoylcarnitine on rat liver mitochondria. Hoppe Seylers Z Physiol Chem. 1969 May;350(5):641–654. doi: 10.1515/bchm2.1969.350.1.641. [DOI] [PubMed] [Google Scholar]
- Roe W. E., Bergman E. N., Kon K. Absorption of ketone bodies and other metabolites via the portal blood of sheep. Am J Vet Res. 1966 May;27(118):729–736. [PubMed] [Google Scholar]
- Schnaitman C., Erwin V. G., Greenawalt J. W. The submitochondrial localization of monoamine oxidase. An enzymatic marker for the outer membrane of rat liver mitochondria. J Cell Biol. 1967 Mar;32(3):719–735. doi: 10.1083/jcb.32.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoswell A. M., Henderson G. D. Aspects of carnitine ester metabolism in sheep liver. Biochem J. 1970 Aug;119(1):59–65. doi: 10.1042/bj1190059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoswell A. M. Respiration-dependent proton movements in rat liver mitochondria. Biochemistry. 1966 May;5(5):1660–1666. doi: 10.1021/bi00869a031. [DOI] [PubMed] [Google Scholar]
- THIN C., ROBERTSON A. The estimation of acetone bodies. Biochem J. 1952 May;51(2):218–223. doi: 10.1042/bj0510218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubbs P. K., Garland P. B. Membranes and fatty acid metabolism. Br Med Bull. 1968 May;24(2):158–164. doi: 10.1093/oxfordjournals.bmb.a070619. [DOI] [PubMed] [Google Scholar]
- Veech R. L., Eggleston L. V., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide phosphate in the cytoplasm of rat liver. Biochem J. 1969 Dec;115(4):609–619. doi: 10.1042/bj1150609a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann M. J., Krebs H. A. The fuel of respiration of rat kidney cortex. Biochem J. 1969 Apr;112(2):149–166. doi: 10.1042/bj1120149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Lund P., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967 May;103(2):514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]


