Abstract
Mitogen-activated protein kinase (MAPK) cascade is a ubiquitous signaling module that transmits extracellular stimuli through the cytoplasm to the nucleus; in response to activating stimuli, MAPKs translocate into the nucleus. Mammalian MEK MAPK kinases (MAPKKs) have in their N termini an MAPK-docking site and a nuclear export signal (NES) sequence, which are known to play critical roles in maintaining ERK MAPKs in the cytoplasm of unstimulated cells. Herein, we show that the Wis1 MAPKK of the stress-activated Spc1 MAPK cascade in fission yeast also has a MAPK-docking site and an NES sequence in its N-terminal domain. Unexpectedly, an inactivating mutation to the NES of chromosomal wis1+ does not affect the subcellular localization of Spc1 MAPK, whereas this NES mutation disturbs the cytoplasmic localization of Wis1. However, when Wis1 is targeted to the nucleus by fusing to a nuclear localization signal sequence, stress-induced nuclear translocation of Spc1 is abrogated, indicating that cytoplasmic Wis1 is required for nuclear transport of Spc1 upon stress. Moreover, we have observed that a fraction of Wis1 translocates into the nucleus in response to stress. These results suggest that cytoplasmic localization of Wis1 MAPKK by its NES is important for stress signaling to the nucleus.
INTRODUCTION
Mitogen-activated protein kinase (MAPK) cascades represent an evolutionarily conserved signaling mechanism in eukaryotes. Diverse signal transduction pathways use MAPK cascades to regulate a variety of cellular functions, including gene expression, cellular homeostasis, and differentiation in response to different extracellular stimuli (Cobb and Goldsmith, 1995; Herskowitz, 1995; Marshall, 1995; Schaeffer and Weber, 1999). MAPK cascades have an architecture of three-tiered kinases, MAPK, MAPK kinase (MAPKK), and MAPKK kinase (MAPKKK). Within an MAPK cascade, signals are transmitted by sequential activation of MAPKKK, MAPKK, and finally MAPK.
Classical MAPKs in mammalian cells, also known as extracellular signal-regulated kinases (ERKs), are activated by growth factors and translocate from the cytoplasm to the nucleus (Chen et al., 1992; Gonzalez et al., 1993; Lenormand et al., 1993) where MAPKs phosphorylate and regulate target transcription factors (Hill and Treisman, 1995; Karin and Hunter, 1995). It has been experimentally demonstrated that nuclear translocation of MAPK is crucial for proper regulation of the target genes (Brunet et al., 1999). MAPKKs, on the other hand, are mostly localized in the cytoplasm before and after stimuli (Lenormand et al., 1993; Zheng and Guan, 1994; Moriguchi et al., 1995). Recent studies of Xenopus MAPKK demonstrated that its N-terminal, noncatalytic domain contains an MAPK-docking site as well as a nuclear export signal (NES) sequence responsible for cytoplasmic localization of MAPKK. It was proposed that in the cytoplasm of quiescent cells MAPKK tethers MAPK through its MAPK-docking site (Fukuda et al., 1997b), and that upon stimulation, phosphorylated MAPK dissociates from MAPKK to move into the nucleus (Adachi et al., 1999). MAPKK also promotes export of nuclear MAPK by diffusing into the nucleus and forming a complex with MAPK before rapid export by its NES (Adachi et al., 2000).
In addition to MAPKs that are activated by mitogenic stimuli, eukaryotic organisms from yeast to human have MAPKs that are specifically responsive to environmental stress (Widmann et al., 1999; Kyriakis and Avruch, 2001). These MAPKs are also known as stress-activated protein kinases, with Hog1 MAPK in Saccharomyces cerevisiae being the prototype (Brewster et al., 1993). When budding yeast cells are exposed to high osmolarity stress, Hog1 is activated by Pbs2 MAPKK, which is in turn activated by the redundant Ssk2, Ssk22, and Ste11 MAPKKKs (Maeda et al., 1995; Posas and Saito, 1997). Whereas neither an MAPK-docking site nor an NES sequence has been identified in Pbs2 MAPKK, Pbs2 has a long N-terminal, noncatalytic region with a proline-rich sequence, PLPPLP94–99, which can bind a Src homology 3 (SH3) domain. Interaction of Pbs2 with an SH3-domain protein Sho1 at the plasma membrane is essential for Pbs2 activation by Ste11 MAPKKK in response to osmostress (Maeda et al., 1995; Posas and Saito, 1997; Raitt et al., 2000).
The Hog1 orthologs in other organisms, such as Spc1 (also known as Sty1) in the fission yeast Schizosaccharomyces pombe and p38 MAPK in vertebrates, are activated by diverse forms of stress, including osmostress, oxidative stress, and heat shock (Widmann et al., 1999; Kyriakis and Avruch, 2001). In S. pombe, Spc1 is activated by Wis1 MAPKK in response to stress (Millar et al., 1995; Shiozaki and Russell, 1995), and like mammalian ERK MAPKs, phosphorylated Spc1 translocates into the nucleus (Gaits et al., 1998). Spc1 phosphorylates a nuclear transcription factor, Atf1, which regulates genes required for cellular resistance against various forms of stress (Shiozaki and Russell, 1996; Wilkinson et al., 1996).
Wis1 is the only MAPKK responsible for the phosphorylation and activation of Spc1 MAPK under various stress conditions (Degols et al., 1996). Wis1 is a 605-amino acid protein with a C-terminal kinase catalytic domain (Figure 1; Warbrick and Fantes, 1991). Phosphorylation of Ser-469 and Thr-473 in the activation loop of the Wis1 kinase domain by two MAPKKKs, Wis4 (Samejima et al., 1997; Shieh et al., 1997; Shiozaki et al., 1997) and Win1 (Samejima et al., 1998), is essential for stress-induced activation of Wis1 (Samejima et al., 1997; Shieh et al., 1998; Shiozaki et al., 1998). On the other hand, no function has been assigned to the N-terminal, noncatalytic domain of Wis1; this ∼300-amino acid region shows a limited sequence similarity to that of Pbs2 MAPKK in budding yeast, including a proline-rich sequence PSDPPLP77–83 of Wis1.
Figure 1.
Schematic structure of Wis1 MAPKK. Wis1 is a 605-amino acid protein with a kinase catalytic domain at residues 326–567 (hatched box). Two MAPKKK phosphorylation sites, Ser-469 and Thr-473, in the catalytic domain are shown as S and T, respectively. Two functional domains have been identified in the N-terminal region of Wis1 during this study; a MAPK-docking region (shaded box) and an NES sequence (filled box).
In this report, we set out to define the functions of the N-terminal region of Wis1 MAPKK. We have found that the proline-rich sequence PSDPPLP77–83 is dispensable for stress signaling to Spc1 MAPK. We have also demonstrated that, like Xenopus MAPKK and mammalian MEKs, the N-terminal, noncatalytic domain of Wis1 contains an MAPK-docking region and a NES sequence. Aiming to test the significance of these sequence elements for the in vivo Wis1 function, we have constructed S. pombe strains of which chromosomal wis1 gene carries specific mutations in the MAPK-docking region and the NES. The MAPK-docking region immediately N terminal to the kinase domain in Wis1 plays a critical role in binding and phosphorylating Spc1 MAPK. In contrast to the NES function proposed for vertebrate MAPKKs, an inactivating mutation in the Wis1 NES sequence leads to no apparent defect in cellular localization of Spc1 MAPK. However, when Wis1 is targeted to the nucleus by replacing the NES with a nuclear localization signal (NLS), Spc1 fails to accumulate in the nucleus even after significant phosphorylation induced by stress. Thus, cytoplasmic localization of Wis1 seems to be necessary for nuclear transport of Spc1 MAPK in response to stress stimuli. Moreover, we have also found that Wis1 MAPKK transiently translocates into the nucleus upon stress. These results suggest that cytoplasmic localization of Wis1 by its NES is important for nucleocytoplasmic stress signaling by the Spc1 MAPK cascade.
MATERIALS AND METHODS
Yeast Strains and General Techniques
S. pombe strains used in this study are listed in Table 1. Growth media as well as basic techniques for S. pombe have been described previously (Moreno et al., 1991; Alfa et al., 1993). S. pombe cells were grown in yeast extract medium YES and synthetic minimal medium EMM2. Stress treatments of S. pombe cultures have been described previously (Shiozaki et al., 1997).
Table 1.
S. pombe strains used in this study
| Strains | Genotype | Source or reference |
|---|---|---|
| PR109 | h− | Laboratory stock |
| JM544 | h− wis1::ura4+ | Laboratory stock |
| KS1376 | h− spc1:HA6H(ura4+) | Shiozaki and Russell (1995) |
| CA121 | h+ his7 spc1:HA6H(ura4+) | Laboratory stock |
| CA465 | h− wis1:HA6H(ura4+) | Laboratory stock |
| CA795 | h− his7 wis1::his7+ spc1:HA6H(ura4+) | Laboratory stock |
| CA797 | h+ his7 spc1:myc(ura4+) | Laboratory stock |
| CA839 | h− his7 wis1::his7+ spc1:myc(ura4+) | Laboratory stock |
| CA894 | h− his7 wis1::his7+ | Laboratory stock |
| CA1000 | h− his7 crm1-809 | Adachi and Yanagida (1989) |
| CA1068 | h− his7 wis1::his7+ crm1-809 | This study |
| CA1155 | h− his7 wis1:GFP(ura4+):his7+ | This study |
| CA1157 | h− his7 wis1LA:GFP(ura4+):his7+ | This study |
| CA1170 | h− his7 wis1LA:GFP(ura4+):his7+ spc1:myc(ura4+) | This study |
| CA1174 | h− his7 wis1:GFP(ura4+):his7+ spc1:HA6H(ura4+) | This study |
| CA1175 | h− his7 wis1LA:GFP(ura4+):his7+ spc1:HA6H(ura4+) | This study |
| CA1207 | h− his7 wis1NLS*:GFP(ura4+):his7+ spc1:HA6H(ura4+) | This study |
| CA1212 | h− his7 wis1NLS:GFP(ura4+):his7+ spc1:myc(ura4+) | This study |
| CA1225 | h− his7 wis1NLS:GFP(ura4+):his7+ spc1:HA6H(ura4+) | This study |
| CA1282 | h− his7 wis1:GFP(ura4+):his7+ spc1:myc(ura4+) | This study |
| CA1286 | h− his7 wis1:GFP(ura4+):his7+ spc1::ura4+ | This study |
| CA1296 | h− his7 wis1NLS*:GFP(ura4+):his7+ spc1:myc(ura4+) | This study |
| CA1310 | h− his7 wis1-2RE:GFP(ura4+):his7+ spc1:HA6H(ura4+) | This study |
| CA1314 | h− his7 wis1-4RE:GFP(ura4+):his7+ spc1:HA6H(ura4+) | This study |
All strains are leu1-32 ura4-D18. The his7 allele is his7-366.
Plasmid Construction
Sequences of the DNA primers used in this study are available upon request. All polymerase chain reaction (PCR) fragments were confirmed by sequencing.
The wis1+, wis1NLS, wis1ΔN18, wis1ΔN100, wis1ΔN200, and wis1ΔN300 fragments were amplified by PCR with S. pombe genomic DNA as template. These fragments were cloned into the pREP1 (Maundrell, 1990) or pREP41 (Basi et al., 1993) vectors together with the C-terminal HA6H or green fluorescent protein (GFP) tags, respectively. The HA6H sequence encodes two copies of the hemagglutinin (HA) epitope and six consecutive histidine residues (Shiozaki and Russell, 1997), whereas the GFP sequence encodes for the S65T mutant green fluorescent protein (Heim et al., 1995).
The QuikChange XL site-directed mutagenesis kit (Stratagene, La Jolla, CA) was used to introduce point mutations into the wis1+ gene to construct the wis1LA, wis1NLS*, wis1-2RE, and wis1-4RE alleles. The pGEX-KG vector (Guan and Dixon, 1991) was used for bacterial expression of the wild-type and mutant Wis1 proteins as glutathione S-transferase (GST)-fusions.
To construct pREP1-GST:NES, two complementary oligo DNAs encoding the NES sequence of Wis1 (residues 7–20) were synthesized and cloned into the pREP1-KZ vector (Shiozaki and Russell, 1997) after hybridization.
Purification and Detection of Spc1 and Wis1 with HA6H Tag
For low-level expression of the N-terminally truncated Wis1 proteins (Figure 2B), the Δwis1 spc1HA6H strain (CA795) was transformed with pREP1, pREP1-wis1:HA6H, pREP1-wis1ΔN200:HA6H, or pREP1-wis1ΔN300:HA6H and grown to mid-log phase at 30°C for 18 h in EMM2 medium with 0, 1.0, 0.0265, or 0 μM thiamine, respectively. At these thiamine concentrations, the expression levels of the Wis1 proteins were similar to that of endogenous Wis1 in strain CA465 (our unpublished data), except highly overexpressed ΔN300. Mutant Wis1HA6H proteins and Spc1HA6H were purified by Ni2+-nitrilotriacetic acid chromatography and analyzed by immunoblotting with anti-HA (12CA5; Roche Applied Science, Indianapolis, IN) and anti-phospho-38 MAPK (New England Biolabs, Beverly, MA) antibodies (Shiozaki and Russell, 1997). Quantification was performed using the ECL Plus Reagent (Amersham Biosciences, Piscataway, NJ) and the Storm System (Molecular Dynamics, Sunnyvale, CA).
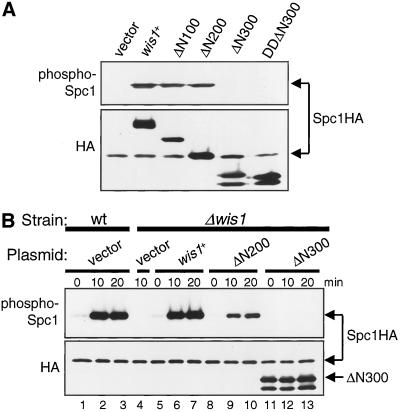
Figure 2.
Residues 201–300 in the noncatalytic domain of Wis1 MAPKK are essential for Spc1 MAPK activation. (A) Overexpression of Wis1ΔN100 and Wis1ΔN200, but not Wis1ΔN300, induces Spc1 activation. A Δwis1 strain carrying chromosomal spc1+ tagged with the HA6H sequence (CA795) was transformed with pREP1 (vector), pREP1-wis1:HA6H, pREP1-wis1ΔN100:HA6H, pREP1-wis1ΔN200:HA6H, pREP1-wis1ΔN300:HA6H, or pREP1-wis1DDΔN300:HA6H. Transformants were grown to mid-log phase at 30°C for 18 h in EMM2 medium without thiamine. Spc1HA6H and various Wis1HA6H proteins were purified by Ni2+-nitrilotriacetic acid chromatography, followed by immunoblotting with anti-phospho-p38 MAPK to detect phosphorylated Spc1 as well as with anti-HA antibodies. (B) Wis1ΔN200, but not Wis1ΔN300, can mediate stress-induced Spc1 activation. Wild-type (KS1376) cells (wt) transformed with the pREP1 vector or Δwis1 (CA795) cells transformed with the plasmids described in A were cultured to mid-log phase at 30°C for 18 h in EMM2 medium supplemented with appropriate amounts of thiamine (see MATERIALS AND METHODS). The transformants were exposed to 0.6 M KCl and activation of Spc1 was analyzed.
In Vitro Kinase Assay
The GST-Wis1ΔN300 and GST-Wis1DDΔN300 proteins were isolated from Escherichia coli DH5α strains carrying the pGEX-KG-wis1ΔN300 and pGEX-KG-wis1DDΔN300 plasmids, respectively, by glutathione (GSH)-Sepharose (Amersham Biosciences) precipitation (Shiozaki et al., 1994). Kinase reactions were performed in 50 μl of KA buffer (25 mM Tris-HCl, pH 7.2, 10 mM MgCl2, 0.1 mM EDTA, 0.1 mM Na3VO4, 1 mM 2-mercaptoethanol, and 10 mM glutathione) containing 50 μM [γ-32P]ATP at 25°C for 15 min, followed by SDS-PAGE and autoradiography.
For phosphorylation of GST-Spc1 by the mutant Wis1 proteins, GST-Spc1 from Δwis1 (JM544) cells was isolated onto GSH-beads (Shiozaki and Russell, 1995) as a substrate. Bacterially produced GST or GST-Wis1 proteins were mixed with the GST-Spc1 beads and incubated in 50 μl of KA buffer containing 50 μM ATP at 25°C for 15 min. The samples were analyzed by immunoblotting with anti-phospho-p38 MAPK antibodies.
Physical Interaction between Spc1myc and GST-Wis1 Fusion Proteins
Δwis1 spc1:myc (CA839) cells, grown to mid-log phase at 30°C in YES medium, were lysed in lysis buffer (50 mM Tris-HCl pH 7.2, 5 mM EDTA, 150 mM NaCl, 1 mM 2-mercaptoethanol, 10% glycerol, 0.1 mM Na3VO4, and 50 mM NaF) containing 0.5% Triton X-100. The soluble fraction of the lysate was mixed with GSH-beads containing the bacterially produced GST or GST-Wis1 proteins described above, for 35 min at 4°C. After extensive washes, proteins bound to the beads were analyzed by immunoblotting.
Construction of wis1:GFP Strains
The wis1:GFP, wis1LA:GFP, wis1NLS:GFP, wis1NLS*:GFP, wis1–2RE:GFP, and wis1-4RE:GFP fragments described above were cloned into the pRIP42 vector (Maundrell, 1993), to construct a series of pRIP42-wis1:GFP integration plasmids. The 1.1-kb promoter region of wis1+ was amplified by PCR from S. pombe genomic DNA and used to replace the nmt1 promoter within the pRIP42-wis1:GFP plasmids. The resulting plasmids were linearized at the BstXI site within the wis1+ promoter and used to transform a wis1::his7+ (CA894) strain, in which the wis1+ open reading frame was replaced with the his7+ marker gene. Integration of the constructs into the wis1 locus was confirmed by Southern analysis.
Fluorescence Microscopy
Indirect immunofluorescence microscopy was performed by the method described previously (Hagan and Hyams, 1988), with the primary antibody of anti-GST or anti-myc (9E10) antibodies (BabCO, Berkeley, CA) and the Cy3-conjugated secondary antibodies (Chemicon International, Temecula, CA). An Eclipse E600 microscope (Nikon, Tokyo, Japan) equipped with a 60× objective lens and digital charge-coupled device camera (Hamamatsu, Bridgewater, NJ) was used in all microscopy experiments. Images were captured by the Openlab software (Improvision) and transferred to Adobe Photoshop (Adobe Systems, Mountain View, CA) for figure preparation.
RESULTS
Residues 201–300 in Noncatalytic Domain of Wis1 MAPKK Are Required for Activation of Spc1 MAPK
Aiming to identify the function of the N-terminal, noncatalytic domain of Wis1, we constructed a series of N-terminally truncated wis1 mutant genes, ΔN100, ΔN200, and ΔN300, which encode Wis1 proteins lacking the N-terminal 100, 200, and 300 residues, respectively. These constructs were expressed in S. pombe strains from the thiamine-repressible nmt1 promoter (Maundrell, 1990) with a C-terminal HA6H tag encoding the HA epitope and hexahistidine residues (Shiozaki and Russell, 1997). Immunoblotting by anti-HA antibodies showed that, in the absence of thiamine, the truncated Wis1 proteins of predicted molecular weights were overproduced (Figure 2A).
Overexpression of wis1+ induces Spc1 activation even in the absence of stress stimuli (Shiozaki and Russell, 1995). Therefore, we first examined whether overexpression of the truncated Wis1 proteins could bring about Spc1 activation. The wild-type, ΔN100, ΔN200, and ΔN300 wis1 genes were overexpressed in a spc1:HA6H strain so that Spc1HA6H can be purified and probed by antibodies that recognize phosphorylated, active Spc1 (Shiozaki and Russell, 1997). Immunoblotting demonstrated strong activation of Spc1 in the strains overexpressing the wild-type, ΔN100, and ΔN200 Wis1 proteins (Figure 2A). On the other hand, the ΔN300 construct, which consists mostly of the kinase catalytic domain, failed to bring about detectable Spc1 activation. One possibility is that deletion of the entire N terminus prevents Wis1 from being phosphorylated and activated by upstream MAPKKKs. However, substitution of the MAPKKK phosphorylation sites in Wis1, Ser-469, and Thr-473, with aspartate, which mimics phosphorylation and activates Wis1 (Shiozaki et al., 1998; Nguyen and Shiozaki, 1999), did not potentiate ΔN300 in Spc1 activation (DDΔN300; Figure 2A).
Next, we examined whether these N-terminally truncated Wis1 proteins can respond to stress stimuli to activate Spc1. The wis1 null strain carrying the truncated wis1 constructs were grown in medium supplemented with low concentrations of thiamine so that the mutant Wis1 proteins were expressed from the nmt1 promoter at a low level comparable with that of endogenous Wis1 in wild-type cells (see MATERIALS AND METHODS). Under these conditions, the wis1 null strain expressing wild-type Wis1 from the plasmid construct showed Spc1 phosphorylation similar to that in the wis1+ strain before and after stress (Figure 2B, compare lanes 5–7 with lanes 1–3). We found that ΔN100 (our unpublished data) and ΔN200 complemented the wis1 null defect and Spc1 was activated after high osmolarity stress in the strains expressing these constructs (Figure 2B). In contrast, ΔN300 was not able to activate Spc1 upon osmostress, both in the presence (our unpublished data) and absence of thiamine (Figure 2B) to overproduce ΔN300. Almost identical results were obtained when these transformants were exposed to oxidative stress of H2O2 (our unpublished data).
These results indicate that the N-terminal 200 residues of Wis1 MAPKK are dispensable for activation of Spc1 MAPK in response to different forms of stress. On the other hand, the region adjacent to the kinase catalytic domain of Wis1, residue 201–300, is essential for Spc1 activation by Wis1.
Wis1ΔN300 Cannot Bind and Phosphorylate Spc1 MAPK
As described above, the mutant Wis1 proteins, ΔN300 and DDΔN300, cannot phosphorylate Spc1. To test whether these mutant Wis1 proteins are catalytically active as protein kinases, we examined the autophosphorylation activity of the purified ΔN300 and DDΔN300 proteins. ΔN300 and DDΔN300 constructs were expressed in E. coli as a fusion with GST. Purified GST-ΔN300 and GST-DDΔN300 proteins were incubated in the presence of Mg2+ and [γ-32P]ATP. Significant phosphorylation of both ΔN300 and DDΔN300 proteins was observed (Figure 3A). As expected, DDΔN300 showed an enhanced activity, ∼2.5-fold increase in 32P labeling, because of the activating mutations at the MAPKKK phosphorylation sites. These results suggest that the ΔN300 and DDΔN300 proteins are active as protein kinases.
Figure 3.
Residues 201–300 of Wis1 MAPKK are required for binding and phosphorylation of Spc1 MAPK. (A) Wis1ΔN300 is an active protein kinase. Bacterially produced GST-ΔN300 and GST-DDΔN300 proteins were tested for their autophosphorylation activity in the presence of [γ-32P]ATP. The samples were subjected to SDS-PAGE, followed by autoradiography. (B) Wis1ΔN300 cannot phosphorylate Spc1 in vitro. Activities of bacterially produced GST, GST-ΔN200, GST-ΔN300, or GST-DDΔN300 proteins were examined using GST-Spc1 as substrate. Phosphorylation of GST-Spc1 was detected by immunoblotting with anti-phospho-p38 antibodies. (C) Wis1ΔN300 cannot bind Spc1. Bacterially produced GST or different GST-Wis1 proteins were immobilized onto glutathione-beads and incubated with cell lysates from a spc1:myc strain (CA839). After extensive washes, proteins bound to the beads were analyzed with anti-GST and anti-myc antibodies.
We next tested whether these purified ΔN300 and DDΔN300 proteins can phosphorylate Spc1 in vitro. The GST-Spc1 fusion protein was added as substrate to the kinase assay reactions with ΔN300 and DDΔN300, which was followed by immunoblotting to detect Spc1 phosphorylation. As shown in Figure 3B, Spc1 phosphorylation was not detected even in the presence of excess amounts of the ΔN300 and DDΔN300, whereas the ΔN200 protein efficiently phosphorylated Spc1. Thus, the Wis1 kinase domain fragment ΔN300 cannot phosphorylate Spc1 MAPK, although the ΔN300 protein is active as a protein kinase. Together with the results from the aforementioned in vivo activity assays, these data demonstrated that residues 201–300 of Wis1 are essential for Wis1 MAPKK to phosphorylate its substrate, Spc1 MAPK.
Recent studies indicate that some of mammalian MAPKKs have an MAPK-binding site that promotes MAPK phosphorylation by MAPKKs (Xu et al., 1999; Enslen et al., 2000). We therefore examined the physical interaction of the truncated Wis1 proteins with Spc1 MAPK. GST-fusion proteins of the full-length and mutant Wis1 were bacterially produced and immobilized on glutathione-Sepharose beads, which were subsequently incubated with cell lysates from the wis1 null strain expressing myc epitope-tagged Spc1 (Spc1myc). After extensive washing, proteins bound to the beads were analyzed by immunoblotting (Figure 3C). Although Spc1myc was copurified with GST-Wis1 and GST-ΔN200, neither the GST-ΔN300 nor GST-DDΔN300 beads precipitated Spc1myc, indicating that residues 201–300 of Wis1 are required for stable association with Spc1. The defect of ΔN300 and DDΔN300 in interaction with Spc1 correlates well with their inability to phosphorylate Spc1. These results suggest that residues 201–300 of Wis1 contain an Spc1-binding site, which is important for Wis1 to bind and phosphorylate Spc1 MAPK.
MAPK-docking Motifs in Residues 201–300 of Wis1 MAPKK
MAPK-docking sites have been identified at the N termini of different MAPKKs from yeast to human, and a consensus sequence has been proposed (Bardwell and Thorner, 1996; Bardwell et al., 2001). This consensus sequence consists of two basic amino acid residues followed by a spacer (1–6 residues) and an L/I-X-L/I sequence (Figure 4A). Because residues 201–300 of Wis1 are required for binding to Spc1 MAPK, we carefully examined this region and found sequences closely related to the MAPK-docking site consensus at residues 234–243 and 260–265 (Figure 4A). To examine the significance of these sequences, two mutant wis1 genes, wis1-2RE and wis1-4RE, were constructed. In wis1-2RE, Arg-234 and Arg-235 are replaced by glutamate to disrupt the first motif, whereas wis1-4RE has additional glutamate substitutions at Arg-260 and Arg-261 to disrupt both motifs; equivalent mutations in the MAPK-docking site of the budding yeast Ste7 MAPKK result in significantly reduced affinity to MAPKs (Bardwell et al., 2001). Together with wild-type wis1+, these mutant genes were expressed as GST-fusion in E. coli and were used in the Spc1myc coprecipitation assay. As shown in Figure 4B, significantly reduced amounts of Spc1 were copurified with Wis1-2RE and Wis1-4RE, comparing to that with wild-type Wis1. Wis1-4RE showed a lower affinity to Spc1 than Wis1-2RE (Figure 4B), whereas mutations only to the second motif (Arg-260, 261→Glu) did not affect binding to Spc1 (our unpublished data). It is likely that both sequence motifs, residues 234–243 and 260–265, contribute to Spc1 binding.
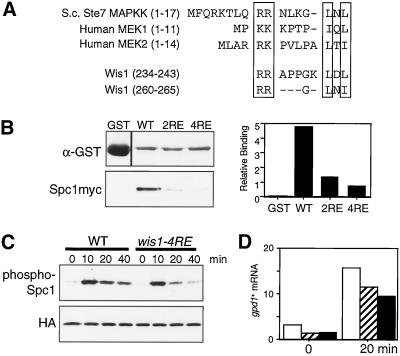
Figure 4.
MAPK docking site motifs in Wis1 MAPKK. (A) Residues 201–300 of Wis1 contain two sequences that resemble the MAPK-docking sites in Saccharomyces cerevisiae Ste7 and human MEK1/MEK2 MAPKKs. The conserved two basic amino acid residues and L/I-X-L/I motifs are boxed. Either two or all four of the conserved arginine residues were substituted with glutamate to create the wis1-2RE or wis1-4RE alleles, respectively. (B) Glutathione-beads conjugated with bacterially produced GST, GST-Wis1, GST-Wis1-2RE, or GST-Wis1-4RE were mixed with S. pombe cell lysates from a spc1:myc strain (CA839). After extensive washes, proteins bound to the beads were analyzed by immunoblotting with anti-GST and anti-myc antibodies and quantified using the Storm System. (C) Defect in Spc1 activation in MAPK docking site mutant cells. wis1:GFP spc1:HA6H (CA1174) and wis1-4RE:GFP spc1:HA6H (CA1314) cells were grown to early-log phase at 30°C in YES medium and aliquots were harvested at the indicated time points after adding 0.3 M KCl to the cultures. Spc1HA6H was purified by Ni2+-nitrilotriacetic acid chromatography, followed by immunoblotting with anti-phospho-p38 MAPK and anti-HA antibodies. (D) The levels of the gpd1+ mRNA were determined by Northern blotting in the wis1:GFP spc1:HA6H (CA1174, open bars), wis1-4RE:GFP spc1:HA6H (CA1314, hatched bars), and wis1NLS:GFP spc1:HA6H (CA1225, filled bars) strains before and after a 20-min osmostress treatment by 0.3 M KCl. The quantified data are normalized using the leu1+ probe as control. Numbers are in arbitrary units.
We further examined whether these MAPK-docking site motifs in Wis1 are important for stress signaling to Spc1 in vivo. The chromosomal wis1+ locus was replaced with the wis1-2RE or wis1-4RE mutant alleles by homologous recombination so that the mutant genes were expressed from the endogenous wis1 promoter. Stress-induced activation of Spc1 in the constructed wis1 mutant strains were monitored by immunoblotting against phosphorylated Spc1. Unexpectedly, little difference was observed between wild-type and the MAPK-docking site mutants in Spc1 activation by strong osmostress with 0.6 M KCl (our unpublished data). It is possible that the residual affinity of Wis1-2RE and Wis1-4RE to Spc1 (Figure 4B) may still be sufficient to bring about activation of Spc1 under such conditions. However, when wis1-4RE cells were exposed to milder osmostress by 0.3 M KCl, significantly reduced Spc1 phosphorylation was observed, particularly at later time points after osmostress; at 20- and 40-min time points, Spc1 phosphorylation in wis1-4RE cells was only 50–60% of that in the control strain (Figure 4C). Activation of Spc1 leads to expression of gpd1+ (Degols et al., 1996), a glycerol-3-phosphate dehydrogenase gene (Pidoux et al., 1990), which is important to protect cells from high osmolarity. In the wis1-4RE cells the induced expression of gpd1+ in response to 0.3 M KCl was reduced by ∼30%, comparing to that in wild-type cells (Figure 4D), which indicated that the osmostress response in wis1-4RE cells was indeed compromised. On the other hand, we detected no apparent defect in Spc1 activation with the wis1-2RE strain under the same stress condition (our unpublished data). These results suggest that the MAPK docking site motifs at residues 234–243 and 260–265 of Wis1 contribute to Spc1 activation in vivo.
Cytoplasmic Localization of Wis1 Is Dependent on the NES in Its N Terminus
In addition to stress-signaling to Spc1 MAPK, we also tested the N-terminally truncated Wis1 proteins for their subcellular localization, using plasmid constructs that express wild-type and mutant Wis1 fused at the C terminus with the GFP. Consistent with previous immunolocalization studies (Gaits et al., 1998), wild-type Wis1-GFP showed solely cytoplasmic localization and little GFP signal was seen in the nuclear region (Figure 5A). Notably, deletion of the N-terminal 100 residues or more abolished the nuclear exclusion of Wis1, and ΔN100-GFP was detected in both the nucleus and cytoplasm, with a higher level of the protein in the nucleus. These results indicate that the N-terminal region of Wis1 contains a sequence required for the specific cytoplasmic localization of Wis1 MAPKK. Further deletion analyses showed that truncation of the residues 1–18 was sufficient to abrogate the exclusion of Wis1 from the nucleus (Figure 5A).
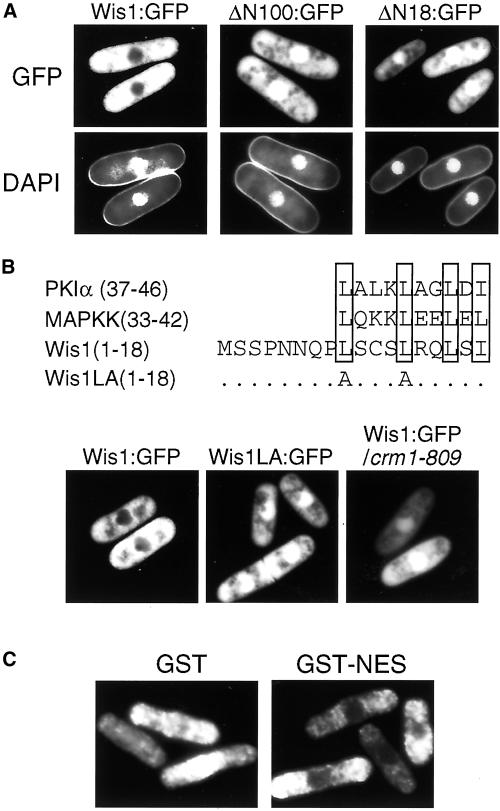
Figure 5.
Cytoplasmic localization of Wis1 MAPKK is dependent on a NES sequence at its N terminus. (A) Δwis1 cells (CA894) were transformed with pREP41-wis1:GFP, pREP41-wis1ΔN100:GFP, or pREP41-wis1ΔN18:GFP to express Wis1-GFP, ΔN100-GFP, or ΔN18:GFP, respectively, from the thiamine-repressible nmt41 promoter. The transformants were grown at 30°C for 16 h in EMM2 without thiamine and visualized directly with fluorescence microscopy after 4,6-diamidino-2-phenylindole staining. (B) Nuclear exclusion of Wis1 requires its NES sequence and the exportin Crm1. A leucine-rich region in the N terminus of Wis1 shows similarity to NES sequences found in PKIα and Xenopus MAPKK. In the wis1LA allele, the first two leucine residues were substituted with alanine. Δwis1 cells (CA894) were transformed with pREP41-wis1:GFP or pREP41-wis1LA:GFP plasmids, whereas crm1-809 mutant cells (CA1068) were transformed with pREP41-wis1:GFP plasmid. The transformants were observed as in A. (C) NES sequence found in Wis1 (residues 7–20) was fused to GST to construct the pREP1-GST:NES plasmid. Together with pREP1-GST, this plasmid was used to transform the wild-type S. pombe strain (PR109). The transformants were grown as in A and subjected to immunofluorescence microscopy by anti-GST antibodies.
Consistent with these observations, we identified in residues 1–18 a sequence very similar to the NES sequences found in the inhibitor protein of cAMP-dependent protein kinase, PKIα (Wen et al., 1995), as well as in the N terminus of Xenopus MAPKK (Fukuda et al., 1996) (Figure 5B). Residues 9–18 of Wis1 and the NES sequences in PKIα and Xenopus MAPKK share conserved four leucine/isoleucine residues that are crucial for NES activity. To determine whether the putative NES in Wis1 is responsible for its localization excluded from the nucleus, we substituted Leu-9 and Leu-13 with alanine to construct the Wis1LA mutant (Figure 5B). Like the ΔN mutant Wis1 proteins, Wis1LA expressed with a GFP-tag was not excluded from the nucleus, which suggests that Leu-9 and Leu-13 are essential for Wis1 nuclear export, as reported with the equivalent mutations in the NES of PKIα and Xenopus MAPKK. Furthermore, we also found that the nuclear exclusion of Wis1 is dependent on Crm1 exportin in fission yeast. Crm1 functions as a nuclear receptor for NES in proteins to be exported, and fission yeast crm1 mutants are defective in nuclear export mediated by NES (Fornerod et al., 1997; Fukuda et al., 1997b; Ossareh-Nazari et al., 1997; Stade et al., 1997). As shown in Figure 5B, wild-type Wis1-GFP localized both in the nucleus and the cytoplasm in the crm1-809 mutant strain, cellular localization very similar to that of the NES-defective Wis1LA in crm1+ cells.
We also tested whether the NES sequence found in Wis1 is functional when fused to unrelated proteins. As expected, the GST protein carrying the Wis1 NES at the C terminus (GST-NES) was excluded from the nucleus, whereas unfused GST distributed both in the nucleus and the cytoplasm when expressed in S. pombe (Figure 5C).
Taken together, these experiments demonstrate that the specific cytoplasmic localization of Wis1 MAPKK is mediated by its NES sequence at the N terminus.
Wis1 MAPKK Enters the Nucleus in Response to Stress Stimuli
The discovery of NES in Wis1 MAPKK strongly suggests that Wis1 MAPKK shuttles between the cytoplasm and the nucleus, rather than localizing constitutively in the cytoplasm. For careful examination of Wis1 localization in living cells, we constructed a fission yeast strain in which chromosomal wis1+ was replaced with the wis1:GFP fusion gene. In this strain, Wis1-GFP was expressed from the endogenous wis1 promoter to avoid artifacts by overexpression. This wis1:GFP strain is indistinguishable from untagged wis1+ strains in stress sensitivities and stress-induced Spc1 activation, indicating that the GFP-tag does not affect the Wis1 function (our unpublished data). Consistent with earlier observations on the wis1:GFP plasmid (Figure 5), Wis1-GFP was localized exclusively in the cytoplasm under normal growth conditions (Figure 6A). However, when cells were exposed to osmostress, GFP signals were detected both in the nucleus and the cytoplasm within 5 min, indicating that a fraction of Wis1-GFP entered the nucleus. Nuclear exclusion of Wis1 resumed around 20 min after osmostress, and by 40 min, the Wis1-GFP staining was localized only in the cytoplasm. It was also observed that homogeneous GFP staining before the stress became punctate after osmostress, which was particularly evident at later time points (Figure 6A). Unfused GFP did not show punctate staining even after osmostress (our unpublished data).
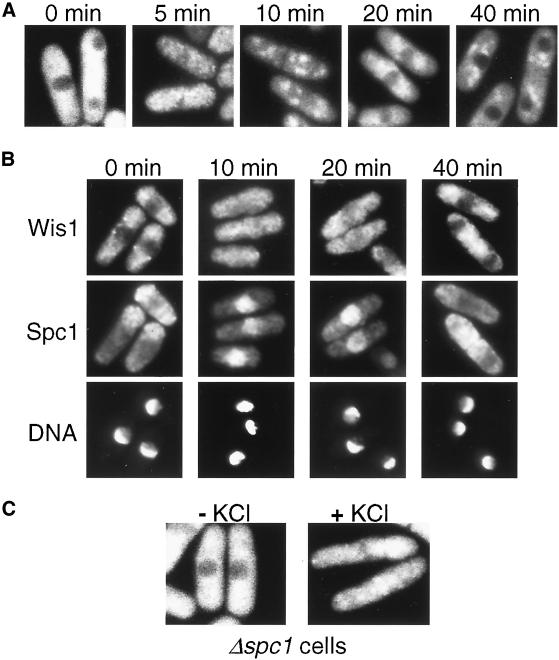
Figure 6.
Wis1 MAPKK translocates into the nucleus in response to stress. (A) Strain CA1155, in which chromosomal wis1+ was replaced with the wis1:GFP fusion gene, was grown to early-log phase at 30°C in YES medium and exposed to 0.6 M KCl for the indicated times. Cells were visualized directly by fluorescence microscopy. (B) Transient accumulation of Wis1 and Spc1 in the nucleus in response to osmostress. The wis1:GFP spc1:myc strain (CA1282) was cultured and exposed to 0.6 M KCl as in A. Cells were fixed and subjected to immunofluorescence microscopy with anti-myc antibodies to determine Spc1 localization. Localization of Wis1-GFP (top) and cell nuclei stained by DAPI (bottom) are also shown. (C) Localization of Wis1-GFP in the Δspc1 wis1:GFP strain (CA1286) was examined as in A before and after 5-min osmostress by 0.6 M KCl.
The spc1:myc strains, in which the chromosomal copy of the spc1+ gene was tagged with the sequence encoding the myc epitope, were successfully used to demonstrate stress-induced nuclear accumulation of Spc1 MAPK (Gaits et al., 1998; Gaits and Russell, 1999). Therefore, the spc1:myc allele was introduced to the wis1:GFP strain by a genetic cross to simultaneously monitor cellular localization of Wis1-GFP and Spc1myc. At different time points after osmostress, cells were fixed and processed for immunofluorescence microscopy (Figure 6B). Within 10 min after osmostress, Spc1myc accumulated in the nucleus and the nuclear exclusion of Wis1-GFP disappeared. A slight decrease in nuclear Wis1-GFP was observed at 20 min, when nuclear accumulation of Spc1myc was still evident. By 40 min, Wis1-GFP was exported from the nucleus and the Spc1myc protein was distributed largely to the cytoplasm. These observations strongly suggest that not only Spc1 MAPK but also at least a fraction of Wis1 MAPKK enters the nucleus in response to stress.
Because Wis1 physically interacts with Spc1 through its MAPK docking sites (Figure 4), it is possible that nuclear transport of Spc1 upon stress stimuli may also convey Wis1 into the nucleus. However, we observed that Wis1-GFP translocated into the nucleus after osmostress even in the spc1 null mutant (Figure 6C). Thus, the stress-induced translocation of Wis1 MAPKK into the nucleus is not dependent on Spc1 MAPK.
NES in Wis1 MAPKK Is Not Essential for Regulation of Spc1 MAPK Localization
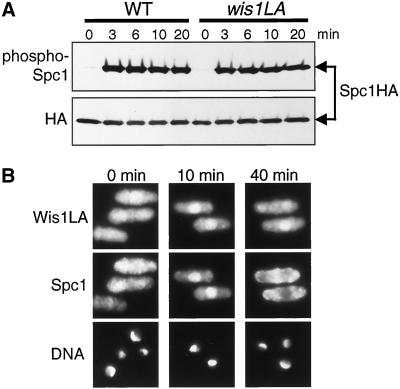
Studies in mammalian cells suggest that the NES of MAPKK plays critical roles in ensuring cytoplasmic localization of ERK MAPKs in quiescent cells (Fukuda et al., 1997b) and in relocalizing nuclear ERKs to the cytoplasm after stimulation (Adachi et al., 2000) (see INTRODUCTION). Therefore, we decided to study whether the NES in Wis1 MAPKK is also important for the cellular localization of Spc1 MAPK before and after stress stimuli, by substituting the chromosomal wis1+ locus with the NES-defective wis1 mutant gene wis1LA:GFP (Figure 5B). In this strain, Wis1LA-GFP was expressed from the endogenous wis1 promoter. We first compared this wis1LA:GFP strain with the control wis1:GFP strain for stress-induced Spc1 activation (Figure 7A). Strong phosphorylation of Spc1 was observed in both strains upon osmostress, although careful quantification showed 10–20% reduced Spc1 phosphorylation in the wis1LA strain. Thus, the NES in Wis1 does not have a major role in stress-induced activation of Spc1 MAPK.
Figure 7.
NES of Wis1 MAPKK is not essential for Spc1 MAPK activation and localization. (A) wis1:GFP spc1:HA6H (CA1174) and wis1LA:GFP spc1:HA6H (CA1175) strains were grown to early-log phase at 30°C in YES medium and exposed to 0.6 M KCl for the indicated times. Spc1HA6H was purified by Ni2+-nitrilotriacetic acid chromatography, followed by immunoblotting with anti-phospho-p38 and anti-HA antibodies. (B) wis1LA:GFP spc1:myc strain (CA1170) was cultured and stressed as in A. Cells were fixed and subjected to immunofluorescence microscopy with anti-myc antibodies. Like in wild-type cells, Spc1 transiently accumulates in the nucleus upon stress in the wis1LA mutant strain.
Fluorescent microscopy confirmed that Wis1LA-GFP was not excluded from the nucleus because of the mutation in the NES (Figure 7B). After 10-min osmostress, nuclear signal of Wis1LA-GFP increased, which was notable even after 40 min, indicating stress-induced translocation of Wis1LA-GFP to the nucleus. Importantly, no apparent difference in Spc1 localization was observed between the wis1:GFP (Figure 6B) and wis1LA:GFP (Figure 7B) strains along the time course after osmostress; nuclear accumulation upon stress and subsequent export of Spc1 MAPK seemed to be normal in the wis1LA:GFP strain. These results suggest that the NES in Wis1 MAPKK is not essential for the regulation of Spc1 MAPK localization, which contrasts with the NES function proposed for vertebrate MAPKKs.
Nuclear Targeting of Spc1 MAPK Requires Cytoplasmic Wis1 MAPKK
Although the NES of Wis1 is not essential for phosphorylation and localization of Spc1 MAPK, it is still possible that the cytoplasmic localization of Wis1 has significance in stress signaling by the Spc1 cascade. To further pursue such a possibility, the NES of Wis1 was replaced with a NLS sequence from the simian virus 40 large-T antigen PKKKRKV (Kalderon et al., 1984), so that Wis1 was targeted to the nucleus and the amount of cytoplasmic Wis1 was reduced. This mutant construct, wis1NLS, was integrated to the chromosome with a C-terminal GFP-tag sequence to replace the wis1+ locus, so that Wis1NLS-GFP was expressed from the endogenous wis1 promoter. As expected, the majority of Wis1NLS-GFP was found in the nucleus (Figure 8B), which contrasts well with Wis1-GFP (Figure 6) and Wis1LA-GFP (Figure 7B).
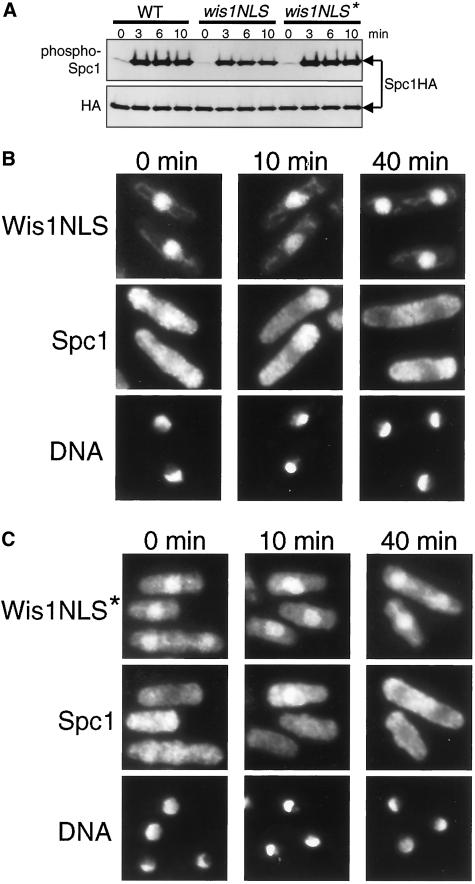
Figure 8.
Cytoplasmic localization of Wis1 MAPKK is essential for nuclear translocation of Spc1 MAPK in response to stress. (A) NES of wis1:GFP was substituted with the wild-type and mutant NLS from the simian virus 40 large-T antigen to construct wis1NLS:GFP and wis1NLS*:GFP alleles, respectively. The wis1:GFP spc1:HA6H (CA1174), wis1NLS:GFP spc1:HA6H (CA1225), and wis1NLS*:GFP spc1:HA6H (CA1207) strains were grown to early-log phase at 30°C in YES medium and exposed to 0.6 M KCl for the indicated times. Spc1HA6H was purified by Ni2+-nitrilotriacetic acid chromatography, followed by immunoblotting with anti-phospho-p38 and anti-HA antibodies. Spc1 phosphorylation in the wis1NLS strain was partially compromised, whereas the wis1NLS* strain showed normal Spc1 activation. (B and C) wis1NLS:GFP spc1:myc (CA1212) (B) and wis1NLS*: GFP spc1:myc (CA1296) (C) strains were exposed to 0.6 M KCl for the indicated times. Cells were fixed and subjected to immunofluorescence microscopy with anti-myc antibodies. The wis1NLS strain was completely defective in nuclear accumulation of Spc1 after osmostress, which was only partially restored by the NLS* mutation.
Immunoblotting to detect phosphorylated Spc1 showed that osmostress-induced activation of Spc1 was partially compromised in the wis1NLS:GFP strain; the levels of phosphorylated Spc1 were ∼30% reduced from those observed in the control wis1:GFP strain (Figure 8A). More surprisingly, immunofluorescence microscopy demonstrated that the wis1NLS strain was completely defective in stress-induced nuclear localization of Spc1 MAPK (Figure 8B). The majority of Spc1 was observed in the cytoplasm even after osmostress and never accumulated in the nucleus. Osmostress-induced expression of gpd1+ in the wis1NLS:GFP strain was also reduced by ∼45% in comparison with the control wis1:GFP cells, indicating compromised stress responses in the mutant cells.
To confirm whether nuclear accumulation of Wis1 is responsible for the observed wis1NLS defect in the nuclear targeting of Spc1, a point mutation, PKKKRKV→PKAKRKV (Kalderon et al., 1984), was introduced to the NLS of wis1NLS:GFP. As expected, this mutant protein, Wis1NLS*-GFP, was detectable in the cytoplasm, although the concentration of Wis1NLS*-GFP was still high in the nucleus (Figure 8C). Importantly, osmostress-induced Spc1 phosphorylation in the wis1:GFP and wis1NLS*:GFP strains was comparable (Figure 8A), indicating that the increased level of cytoplasmic Wis1NLS* can bring about normal Spc1 activation. However, nuclear targeting of Spc1 upon stress was not completely restored by the NLS* mutation (Figure 8C); nuclear accumulation of Spc1 was visible in only ∼40% of the wis1:NLS* cells, whereas ∼70% of the control wis1:GFP cells showed apparent nuclear staining of Spc1 after 10 min of osmostress. Moreover, we often observed that Spc1 staining in the nucleus of wis1:NLS* was weaker than that observed in wild-type cells.
These results suggest that cytoplasmic localization of Wis1 plays an important role in stress-induced nuclear targeting of Spc1 MAPK, and nuclear accumulation of Spc1 upon stress is compromised when the level of cytoplasmic Wis1 is reduced in the wis1NLS and wis1NLS* mutants.
DISCUSSION
In the fission yeast stress MAPK cascade, Wis1 is the only MAPKK that phosphorylates and activates Spc1 MAPK in response to diverse forms of stress (Millar et al., 1995; Shiozaki and Russell, 1995; Degols et al., 1996). Our detailed analyses of the N-terminal, noncatalytic domain of Wis1 have identified two functionally important regions; residues 201–300 for binding Spc1 MAPK and an NES sequence at residues 9–18. Although vertebrate MEK MAPKKs have an MAPK-docking site and an NES sequence, Wis1 is the first MAPKK in lower eukaryotes in which both of these sequence elements have been identified. This prompted us to evaluate the importance of these sequences in MAPKK by introducing specific mutations in the chromosomal wis1+ gene. Unlike transient transfection experiments in mammalian cells, such fission yeast strains express only mutant forms of MAPKKs at physiological levels, which provide an ideal model system to evaluate the significance of an MAPK-docking site and NES in MAPKK.
We have demonstrated that residues 201–300 immediately N terminal to the kinase domain are indispensable for the Wis1 activity to phosphorylate Spc1 both in vivo and in vitro. This region in Wis1 is also essential for binding to Spc1, implying that stable Wis1-Spc1 interaction is important for phosphorylation of Spc1 by Wis1. Residues 234–243 (RRAPPGKLDL) and 260–265 (RRGLNI) resemble the proposed consensus sequence for a MAPK-docking site in MAPKKs (Bardwell and Thorner, 1996; Bardwell et al., 2001). Unlike in Wis1, such MAPK-docking site sequences are at the very N termini in other MAPKKs, which usually have a very short N-terminal noncatalytic sequence. Thus, the arrangement of MAPK-docking sites immediately N terminal to the catalytic domain seems to be conserved among different MAPKKs, including Wis1. Mutations of the MAPK docking site motifs in the Wis1-2RE and -4RE proteins result in significantly reduced affinity to Spc1, indicating that these motifs in Wis1 indeed contribute to interaction with Spc1. Moreover, strains expressing the Wis1-4RE mutant shows reduced activation of Spc1, particularly when cells are exposed to mild stress, suggesting that the two MAPK-docking motifs found in Wis1 promote stress signaling to Spc1 MAPK in vivo.
On the other hand, we found that the N-terminal 200 residues of Wis1 are dispensable for Spc1 activation in response to different forms of stress. This region contains a proline-rich sequence similar to the SH3 domain-binding site in budding yeast Pbs2 MAPKK. Although we cannot rule out the possibility that this proline-rich sequence in Wis1 also interacts with an SH3-domain protein, such interaction may not be directly involved in stress signaling to Wis1.
We also identified in the Wis1 N terminus an NES sequence that is important for maintaining Wis1 in the cytoplasm. First, residues 9–18 of Wis1 show a significant similarity to known NES sequences, and mutations in this region abrogate Wis1 localization excluded from the nucleus. Second, specific cytoplasmic localization of Wis1 is disturbed in the crm1-809 strain, an exportin mutant defective in NES-dependent protein export from the nucleus (Fukuda et al., 1997a). Last, GST fused to this NES sequence from Wis1 is exported from the nucleus. Although Gaits and Russell (1999) previously reported that Wis1 localization is not regulated by Crm1, the data collected in this study unequivocally demonstrate that cytoplasmic localization of Wis1 is dependent on its NES and the exportin Crm1.
Studies in mammalian cells suggest that the NES of MAPKK plays critical roles in the regulation of ERK MAPK localization through two different mechanisms. First, the NES of MAPKK ensures the cytoplasmic localization of MAPKK, which serves as a cytoplasmic anchor for MAPK in unstimulated cells (Fukuda et al., 1997b). Second, MAPK translocated to the nucleus upon stimuli is relocalized to the cytoplasm by forming a complex with MAPKK diffusing into the nucleus. The MAPK–MAPKK complex in the nucleus is exported to the cytoplasm because of the NES in MAPKK (Adachi et al., 2000). In contrast to these models in mammalian cells, we found that the fission yeast mutant expressing the NES-defective Wis1 MAPKK (Wis1LA) exhibits normal regulation of Spc1 MAPK localization. Thus, Wis1 NES is not essential for cytoplasmic anchoring of Spc1 nor for relocalization of nuclear Spc1 to the cytoplasm during stress adaptation. Although both ERK and Spc1 MAPKs are transiently localized in the nucleus in response to activating stimuli, the mechanisms that operate nuclear import and export of these MAPKs might be quite different.
However, analyses of wis1NLS and wis1NLS* mutants strongly suggest that also in S. pombe cytoplasmic localization of MAPKK is important for MAPK regulation. In wis1NLS cells, where most of Wis1 is localized in the nucleus, Spc1 phosphorylation upon stress is partially compromised, suggesting that full activation of the Spc1 cascade requires Wis1 to be in the cytoplasm. Wis1 may need to be in the cytoplasm to be activated by upstream MAPKKKs, which seem to be localized mainly in the cytoplasm (our unpublished data). More importantly, the wis1NLS mutant is completely defective in nuclear accumulation of Spc1 upon stress. We also found that the wis1NLS* mutant, which has a partially inactive NLS attached to Wis1, is also imperfect in stress-induced nuclear localization of Spc1 in spite of normal Spc1 phosphorylation during stress. In the wis1NLS and wis1NLS* strains, the levels of cytoplasmic Wis1 are lower than that in wild-type cells, which is likely to be responsible for the observed defect in the nuclear targeting of Spc1. Thus, in addition to phosphorylating Spc1, cytoplasmic Wis1 may play a critical role in transporting Spc1 into the nucleus upon stress stimuli. How does cytoplasmic Wis1 MAPKK mediate translocation of Spc1 MAPK into the nucleus? Another important discovery in this study is that not only Spc1 MAPK but also Wis1 is transported into the nucleus in response to osmostress. The Wis1 protein (∼70 kDa) seems to be too large to enter the nucleus by diffusion. In addition, we have observed that stress-induced translocation of Wis1 into the nucleus is not dependent on Spc1. Therefore, it is likely that Wis1 translocation into the nucleus after stress is regulated by an active transport mechanism independent of Spc1, although Wis1 contains no apparent NLS sequence. One possible explanation for the dependency of Spc1 nuclear localization on cytoplasmic Wis1 is that nuclear transport of Spc1 is coupled with Wis1 translocation from the cytoplasm to the nucleus. Consistently, both Wis1 and Spc1 enter the nucleus about the same time, within 5–10 min after osmostress. On the other hand, Wis1 is exported from the nucleus more rapidly than nuclear Spc1, suggesting that nuclear export of Spc1 is independent of that of Wis1.
In summary, we have demonstrated that, like vertebrate MEKs, the fission yeast Wis1 MAPKK has a MAPK-docking site and a NES sequence in its N-terminal, noncatalytic domain. Although the NES in vertebrate MAPKK is believed to be important to promote cytoplasmic localization of MAPK, our mutagenesis analyses in vivo strongly suggest that cytoplasmic localization of Wis1 MAPKK, which is maintained by NES, is critical for nuclear targeting of Spc1 MAPK. Furthermore, we demonstrated that, like Spc1 MAPK, Wis1 MAPKK is translocated into the nucleus in response to stress stimuli. Also in mammalian cells, nuclear translocation of MEK1 upon stimulation was observed at least when the NES in MEK1 was mutationally inactivated. (Jaaro et al., 1997; Tolwinski et al., 1999). Thus, translocation of MAPKKs from the cytoplasm to the nucleus could be an integral part of the signaling mechanism conserved among different MAPK cascades.
ACKNOWLEDGMENTS
We thank Hisashi Tatebe, Jesús Aguirre, and Ted Powers for advice in microscopy, for critical discussions, and Mitsuhiro Yanagida for a crm1 strain. We are also grateful to Doris Lui and Hiroki Tanaka for technical assistance. A.N.N. and A.D.I. were supported by the National Institutes of Health Molecular and Cellular Biology Training Program at University of California, Davis (T32 GM-07377). A.N.N. and A. D. I. were also supported by the George Lee Fellowship and the National Institutes of Health-IMSD Graduate Student Award, respectively. This research was supported by grants awarded to K.S from National Institutes of Health (GM-59788) and from the University of California Institute for Mexico and the United States/Consejo Nacional de Ciencia y Tecnología de México.
Footnotes
DOI: 10.1091/mbc.02–03–0043.
REFERENCES
- Adachi M, Fukuda M, Nishida E. Two co-existing mechanisms for nuclear import of MAP kinase: passive diffusion of a monomer and active transport of a dimer. EMBO J. 1999;18:5347–5358. doi: 10.1093/emboj/18.19.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi M, Fukuda M, Nishida E. Nuclear export of MAP kinase (ERK) involves a MAP kinase kinase (MEK)-dependent active transport mechanism. J Cell Biol. 2000;148:849–856. doi: 10.1083/jcb.148.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfa C, Fantes P, Hyams J, McLeod M, Warbrick E. Experiments with Fission Yeast. A Laboratory Course Manual. Cold Spring Harbor, NY: Cold Spring Harbor Press; 1993. [Google Scholar]
- Bardwell AJ, Flatauer LJ, Matsukuma K, Thorner J, Bardwell L. A conserved docking site in MEKS mediates high-affinity binding to MAP kinases and cooperates with a scaffold protein to enhance signal transmission. J Biol Chem. 2001;276:10374–10386. doi: 10.1074/jbc.M010271200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell L, Thorner J. A conserved motif at the amino termini of MEKs might mediate high-affinity interaction with the cognate MAPKs. Trends Biochem Sci. 1996;21:373–374. [PubMed] [Google Scholar]
- Basi G, Schmid E, Maundrell K. TATA box mutations in the Schizosaccharomyces pombe nmt1 promoter affect transcription efficiency but not the transcription start point or thiamine repressibility. Gene. 1993;123:131–136. doi: 10.1016/0378-1119(93)90552-e. [DOI] [PubMed] [Google Scholar]
- Brewster JL, de Valoir T, Dwyer ND, Winter E, Gustin MC. An osmosensing signal transduction pathway in yeast. Science. 1993;259:1760–1763. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- Brunet A, Roux D, Lenormand P, Dowd S, Keyse S, Pouysségur J. Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor-induced gene expression and cell cycle entry. EMBO J. 1999;18:664–674. doi: 10.1093/emboj/18.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RH, Sarnecki C, Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol Cell Biol. 1992;12:915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb MH, Goldsmith EJ. How MAP kinases are regulated. J Biol Chem. 1995;270:14843–14846. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- Degols G, Shiozaki K, Russell P. Activation and regulation of the Spc1 stress-activated protein kinase in Schizosaccharomyces pombe. Mol Cell Biol. 1996;16:2870–2877. doi: 10.1128/mcb.16.6.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enslen H, Brancho DM, Davis RJ. Molecular determinants that mediate selective activation of p38 MAP kinase isoforms. EMBO J. 2000;19:1301–1311. doi: 10.1093/emboj/19.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornerod M, Ohno M, Yoshida M, Mattaj IW. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M, Nishida E. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997a;390:308–311. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Gotoh I, Gotoh Y, Nishida E. Cytoplasmic localization of mitogen-activated protein kinase kinase directed by its NH2-terminal, leucine-rich short amino acid sequence, which acts as a nuclear export signal. J Biol Chem. 1996;271:20024–20028. doi: 10.1074/jbc.271.33.20024. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Gotoh Y, Nishida E. Interaction of MAP kinase with MAP kinase kinase: its possible role in the control of nucleocytoplasmic transport of MAP kinase. EMBO J. 1997b;16:1901–1908. doi: 10.1093/emboj/16.8.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaits F, Degols G, Shiozaki K, Russell P. Phosphorylation and association with the transcription factor Atf1 regulate localization of Spc1/Sty1 stress-activated kinase in fission yeast. Genes Dev. 1998;12:1464–1473. doi: 10.1101/gad.12.10.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaits F, Russell P. Active nucleocytoplasmic shuttling required for function and regulation of stress-activated kinase Spc1/Sty1 in fission yeast. Mol Biol Cell. 1999;10:1395–1407. doi: 10.1091/mbc.10.5.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez FA, Seth A, Raden DL, Bowman DS, Fay FS, Davis RJ. Serum-induced translocation of mitogen-activated protein kinase to the cell surface ruffling membrane and the nucleus. J Cell Biol. 1993;122:1089–1101. doi: 10.1083/jcb.122.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan K, Dixon JE. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Hagan IM, Hyams JS. The use of cell division cycle mutants to investigate the control of microtubule distribution in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1988;89:343–357. doi: 10.1242/jcs.89.3.343. [DOI] [PubMed] [Google Scholar]
- Heim R, Cubitt AB, Tsien RY. Improved green fluorescence. Nature. 1995;373:663–664. doi: 10.1038/373663b0. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. MAP kinase pathways in yeast: for mating and more. Cell. 1995;80:187–197. doi: 10.1016/0092-8674(95)90402-6. [DOI] [PubMed] [Google Scholar]
- Hill CS, Treisman R. Transcriptional regulation by extracellular signals: mechanisms and specificity. Cell. 1995;80:199–211. doi: 10.1016/0092-8674(95)90403-4. [DOI] [PubMed] [Google Scholar]
- Jaaro H, Rubinfeld H, Hanoch T, Seger R. Nuclear translocation of mitogen-activated protein kinase kinase (MEK1) in response to mitogenic stimulation. Proc Natl Acad Sci USA. 1997;94:3742–3747. doi: 10.1073/pnas.94.8.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon D, Roberts BL, Richardson WD, Smith AE. A short amino acid sequence able to specify nuclear location. Cell. 1984;39:499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Karin M, Hunter T. Transcriptional control by protein phosphorylation: signal transmission from the cell surface to the nucleus. Curr Biol. 1995;5:747–757. doi: 10.1016/s0960-9822(95)00151-5. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- Lenormand P, Sardet C, Pages G, L'Allemain G, Brunet A, Pouyssegur J. Growth factors induce nuclear translocation of MAP kinases (p42mapk and p44mapk) but not of their activator MAP kinase kinase (p45mapkk) in fibroblasts. J Cell Biol. 1993;122:1079–1088. doi: 10.1083/jcb.122.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Takekawa M, Saito H. Activation of yeast PBS2 MAPKK by MAPKKKs or by binding of an SH3-containing osmosensor. Science. 1995;269:554–558. doi: 10.1126/science.7624781. [DOI] [PubMed] [Google Scholar]
- Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Maundrell K. nmt1 of fission yeast. J Biol Chem. 1990;265:10857–10864. [PubMed] [Google Scholar]
- Maundrell K. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene. 1993;123:127–130. doi: 10.1016/0378-1119(93)90551-d. [DOI] [PubMed] [Google Scholar]
- Millar JBA, Buck V, Wilkinson MG. Pyp1 and Pyp2 PTPases dephosphorylate an osmosensing MAP kinase controlling cell size at division in fission yeast. Genes Dev. 1995;9:2117–2130. doi: 10.1101/gad.9.17.2117. [DOI] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Moriguchi T, Gotoh Y, Nishida E. Activation of two isoforms of mitogen-activated protein kinase kinase in response to epidermal growth factor and nerve growth factor. Eur J Biochem. 1995;234:32–38. doi: 10.1111/j.1432-1033.1995.032_c.x. [DOI] [PubMed] [Google Scholar]
- Nguyen AN, Shiozaki K. Heat shock-induced activation of stress MAP kinase is regulated by threonine- and tyrosine-specific phosphatases. Genes Dev. 1999;13:1653–1663. doi: 10.1101/gad.13.13.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossareh-Nazari B, Bachelerie F, Dargemont C. Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science. 1997;278:141–144. doi: 10.1126/science.278.5335.141. [DOI] [PubMed] [Google Scholar]
- Pidoux AL, Fawell EH, Armstrong J. Glyerol-3-phosphate dehydrogenase homologue from Schizosaccharomyces pombe. Nucleic Acids Res. 1990;18:7145. doi: 10.1093/nar/18.23.7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posas F, Saito H. Osmotic activation of the HOG MAPK pathway via Ste11p MAPKKK: scaffold role of Pbs2p MAPKK. Science. 1997;276:1702–1705. doi: 10.1126/science.276.5319.1702. [DOI] [PubMed] [Google Scholar]
- Raitt DC, Posas F, Saito H. Yeast Cdc42 GTPase and Ste20 PAK-like kinase regulate Sho1-dependent activation of the Hog1 MAPK pathway. EMBO J. 2000;19:4623–4631. doi: 10.1093/emboj/19.17.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samejima I, Mackie S, Fantes PA. Multiple modes of activation of the stress-responsive MAP kinase pathway in fission yeast. EMBO J. 1997;16:6162–6170. doi: 10.1093/emboj/16.20.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samejima I, Mackie S, Warbrick E, Weisman R, Fantes PA. The fission yeast mitotic regulator win1+ encodes a MAP kinase kinase kinase that phosphorylates and activates Wis1 MAP kinase kinase in response to high osmolarity. Mol Biol Cell. 1998;9:2325–2335. doi: 10.1091/mbc.9.8.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer HJ, Weber MJ. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol. 1999;19:2435–2444. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh J-C, Wilkinson MG, Buck V, Morgan BA, Makino K, Millar JBA. The Mcs4 response regulator coordinately controls the stress-activated Wak1-Wis1-Sty1 MAP kinase pathway and fission yeast cell cycle. Genes Dev. 1997;11:1008–1022. doi: 10.1101/gad.11.8.1008. [DOI] [PubMed] [Google Scholar]
- Shieh JC, Martin H, Millar JBA. Evidence for a novel MAPKKK-independent pathway controlling the stress activated Sty1/Spc1 MAP kinase in fission yeast. J Cell Sci. 1998;111:2799–2807. doi: 10.1242/jcs.111.18.2799. [DOI] [PubMed] [Google Scholar]
- Shiozaki K, Akhavan-Niaki H, McGowan CH, Russell P. Protein phosphatase 2C encoded by ptc1+ is important in the heat shock response of fission yeast. Mol Cell Biol. 1994;14:3743–3751. doi: 10.1128/mcb.14.6.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozaki K, Russell P. Cell-cycle control linked to the extracellular environment by MAP kinase pathway in fission yeast. Nature. 1995;378:739–743. doi: 10.1038/378739a0. [DOI] [PubMed] [Google Scholar]
- Shiozaki K, Russell P. Conjugation, meiosis and the osmotic stress response are regulated by Spc1 kinase through Atf1 transcription factor in fission yeast. Genes Dev. 1996;10:2276–2288. doi: 10.1101/gad.10.18.2276. [DOI] [PubMed] [Google Scholar]
- Shiozaki K, Russell P. Stress-activated protein kinase pathway in cell cycle control of fission yeast. Methods Enzymol. 1997;283:506–520. doi: 10.1016/s0076-6879(97)83040-6. [DOI] [PubMed] [Google Scholar]
- Shiozaki K, Shiozaki M, Russell P. Mcs4 mitotic catastrophe suppressor regulates the fission yeast cell cycle through the Wik1-Wis1-Spc1 kinase cascade. Mol Biol Cell. 1997;8:409–419. doi: 10.1091/mbc.8.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozaki K, Shiozaki M, Russell P. Heat stress activates fission yeast Spc1/Sty1 MAPK by a MEKK-independent mechanism. Mol Biol Cell. 1998;9:1339–1349. doi: 10.1091/mbc.9.6.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stade K, Ford CS, Guthrie C, Weis K. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell. 1997;90:1041–1050. doi: 10.1016/s0092-8674(00)80370-0. [DOI] [PubMed] [Google Scholar]
- Tolwinski NS, Shapiro PS, Goueli S, Ahn NG. Nuclear localization of mitogen-activated protein kinase kinase 1 (MKK1) is promoted by serum stimulation and G2-M progression. Requirement for phosphorylation at the activation lip and signaling downstream of MKK. J Biol Chem. 1999;274:6168–6174. doi: 10.1074/jbc.274.10.6168. [DOI] [PubMed] [Google Scholar]
- Warbrick E, Fantes PA. The wis1 protein is a dosage-dependent regulator of mitosis in Schizosaccharomyces pombe. EMBO J. 1991;10:4291–4299. doi: 10.1002/j.1460-2075.1991.tb05007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen W, Meinkoth JL, Tsien RY, Taylor SS. Identification of a signal for rapid export of proteins from the nucleus. Cell. 1995;82:463–473. doi: 10.1016/0092-8674(95)90435-2. [DOI] [PubMed] [Google Scholar]
- Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- Wilkinson MG, Samuels M, Takeda T, Toone WM, Shieh J-C, Toda T, Millar JBA, Jones N. The Atf1 transcription factor is a target for the Sty1 stress-activated MAP kinase pathway in fission yeast. Genes Dev. 1996;10:2289–2301. doi: 10.1101/gad.10.18.2289. [DOI] [PubMed] [Google Scholar]
- Xu B, Wilsbacher JL, Collisson T, Cobb MH. The N-terminal ERK-binding site of MEK1 is required for efficient feedback phosphorylation by ERK2 in vitro and ERK activation in vivo. J Biol Chem. 1999;274:34029–34035. doi: 10.1074/jbc.274.48.34029. [DOI] [PubMed] [Google Scholar]
- Zheng CF, Guan KL. Cytoplasmic localization of the mitogen-activated protein kinase activator MEK. J Biol Chem. 1994;269:19947–19952. [PubMed] [Google Scholar]