Abstract
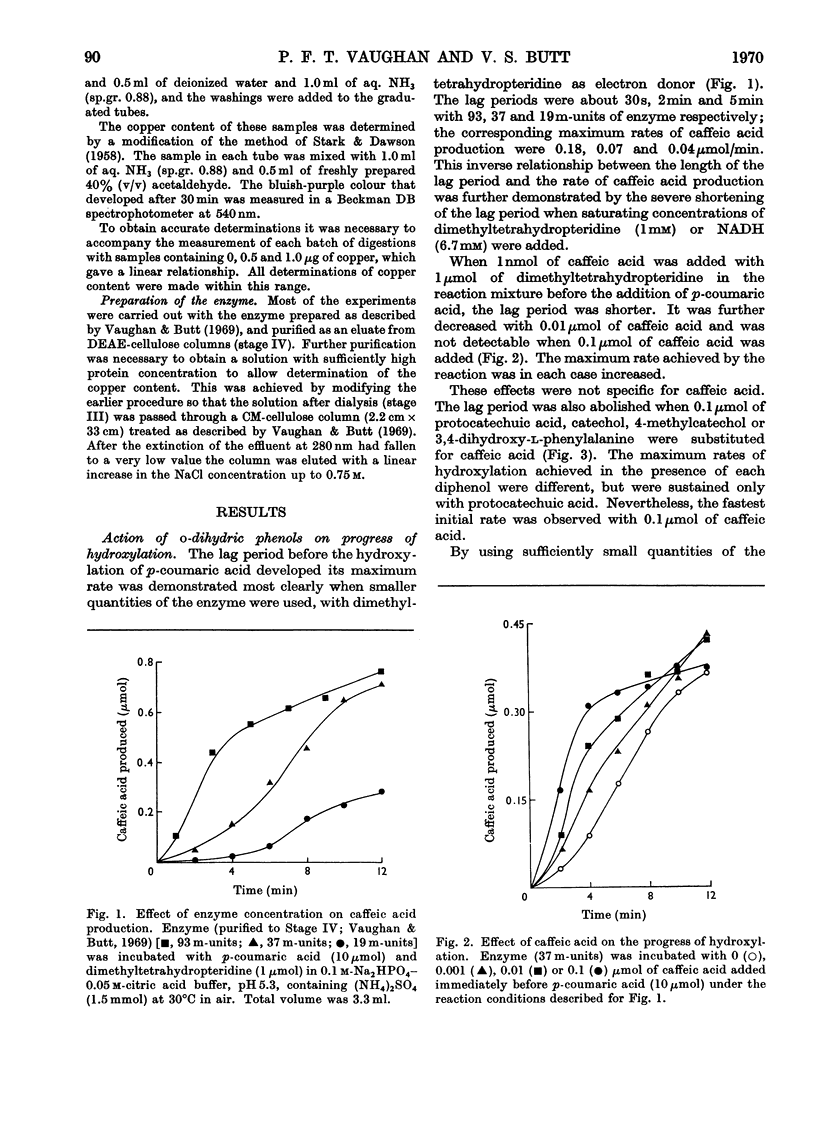
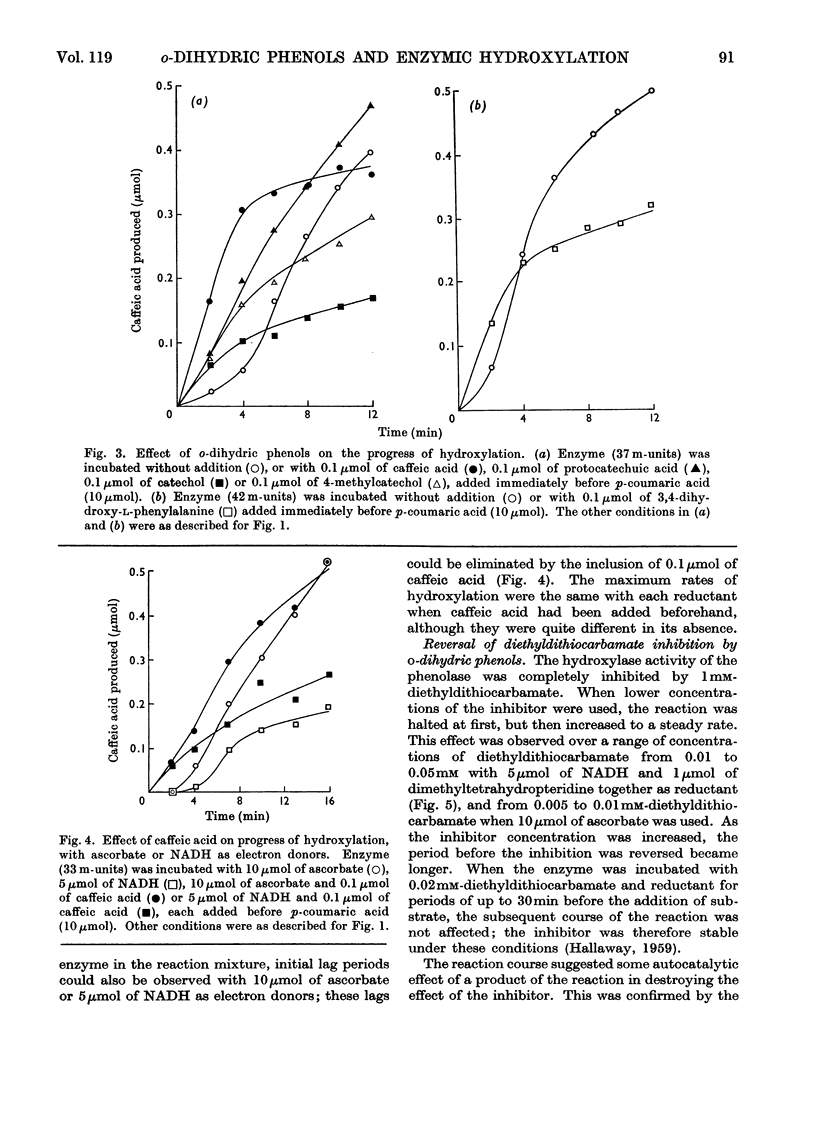
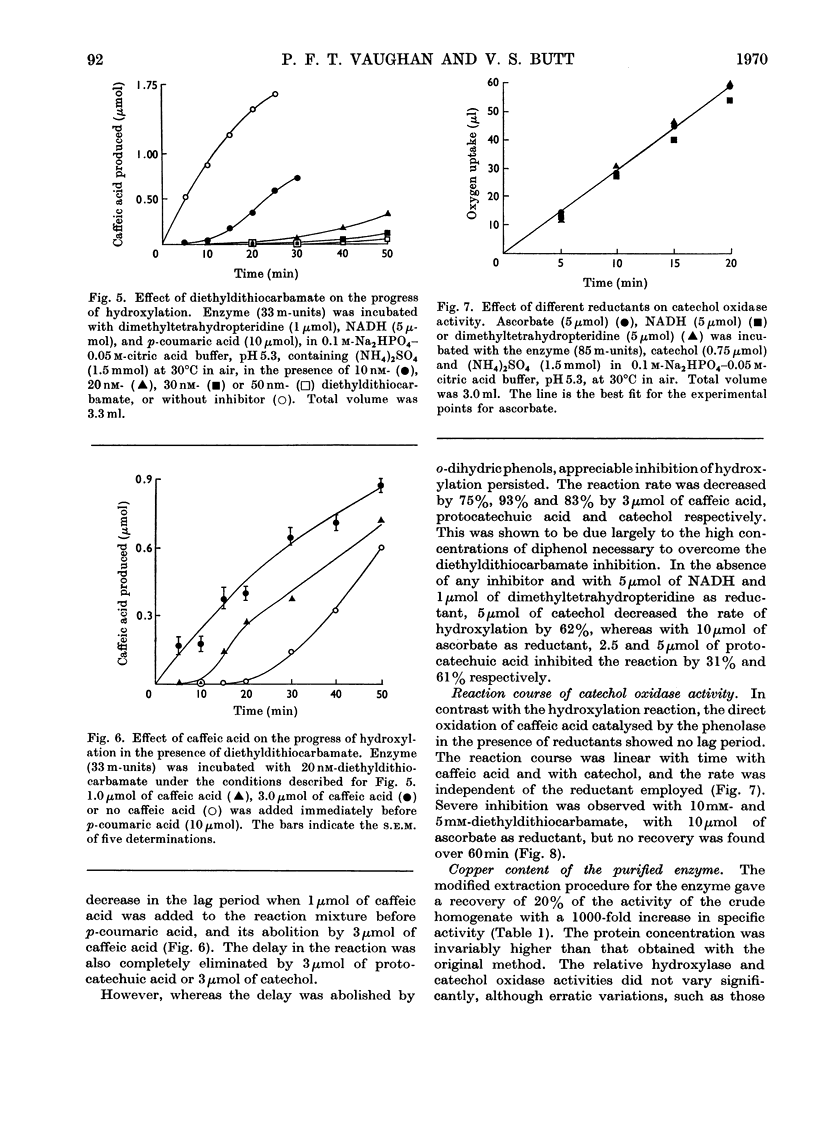
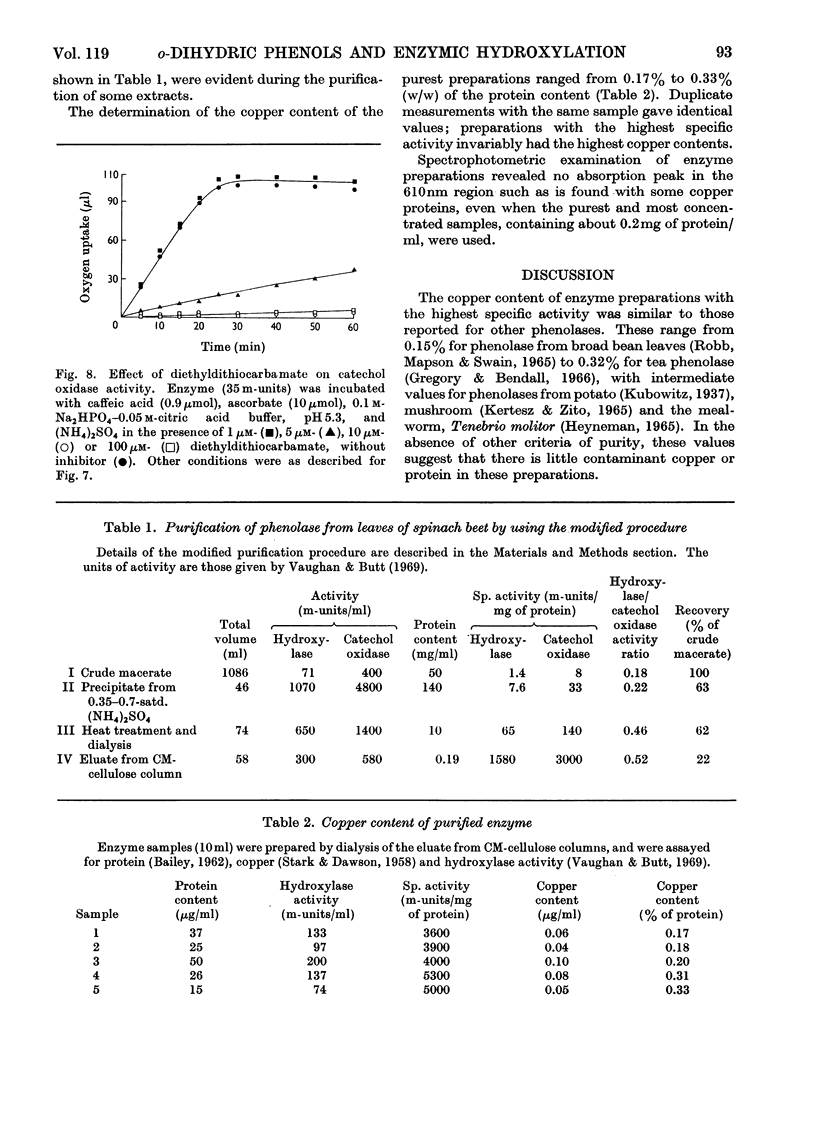
1. Under defined conditions, the hydroxylation of p-coumaric acid catalysed by a phenolase from leaves of spinach beet (Beta vulgaris L.) was observed to develop its maximum rate only after a lag period. 2. By decreasing the reaction rate with lower enzyme concentrations or by increasing it with higher concentrations of reductants, the length of the lag period was inversely related to the maximum rate subsequently developed. 3. Low concentrations of caffeic acid or other o-dihydric phenols abolished this lag period. With caffeic acid, the rate of hydroxylation was independent of the reductant employed. 4. Hydroxylation was inhibited by diethyldithiocarbamate, but with low inhibitor concentrations hydroxylation recovered after a lag period. This lag could again be abolished by the addition of high concentrations of caffeic acid or other o-dihydric phenols. 5. Catechol oxidase activity showed no lag period, and did not recover from diethyldithiocarbamate inhibition. 6. The purified enzyme contained 0.17–0.33% copper; preparations with the highest specific activity were found to have the highest copper content. 7. The results are interpreted to suggest that the oxidation of o-dihydric phenols converts the enzymic copper into a species catalytically active in hydroxylation. This may represent the primary function for the catechol oxidase activity of the phenolase complex. The electron donors are concerned mainly, but not entirely, in the reduction of o-quinones produced in this reaction.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gregory R. P., Bendall D. S. The purification and some properties of the polyphenol oxidase from tea (Camellia sinensis L.). Biochem J. 1966 Dec;101(3):569–581. doi: 10.1042/bj1010569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALLAWAY M. The stability of sodium diethyldithiocarbamate in biochemical experiments. Biochim Biophys Acta. 1959 Dec;36:538–540. doi: 10.1016/0006-3002(59)90199-4. [DOI] [PubMed] [Google Scholar]
- Heyneman R. A. Final purification of a latent phenolase with mono- and diphenoloxidase activity from Tenebrio molitor. Biochem Biophys Res Commun. 1965 Oct 26;21(2):162–169. doi: 10.1016/0006-291x(65)90103-8. [DOI] [PubMed] [Google Scholar]
- MASON H. S. Mechanisms of oxygen metabolism. Adv Enzymol Relat Subj Biochem. 1957;19:79–233. doi: 10.1002/9780470122648.ch2. [DOI] [PubMed] [Google Scholar]
- Pierpoint W. S. The enzymic oxidation of chlorogenic acid and some reactions of the quinone produced. Biochem J. 1966 Feb;98(2):567–580. doi: 10.1042/bj0980567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz S. H., Warner M. C. 3,4-dihydroxy-L-phenylalanine as the tyrosinase cofactor. Occurrence in melanoma and binding constant. J Biol Chem. 1967 Nov 25;242(22):5308–5314. [PubMed] [Google Scholar]
- Vaughan P. F., Butt V. S. The hydroxylation of p-coumaric acid by an enzyme from leaves of spinach beet (Beta vulgaris L.). Biochem J. 1969 Jun;113(1):109–115. doi: 10.1042/bj1130109. [DOI] [PMC free article] [PubMed] [Google Scholar]


