Abstract
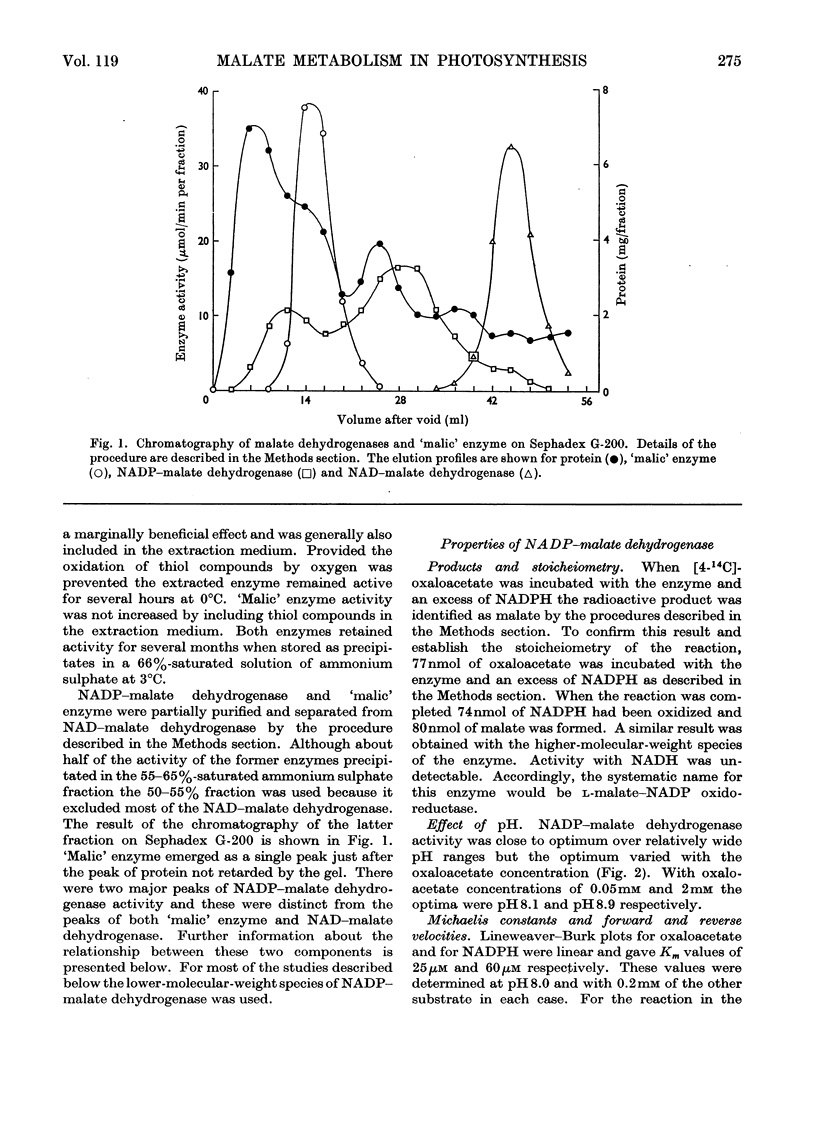
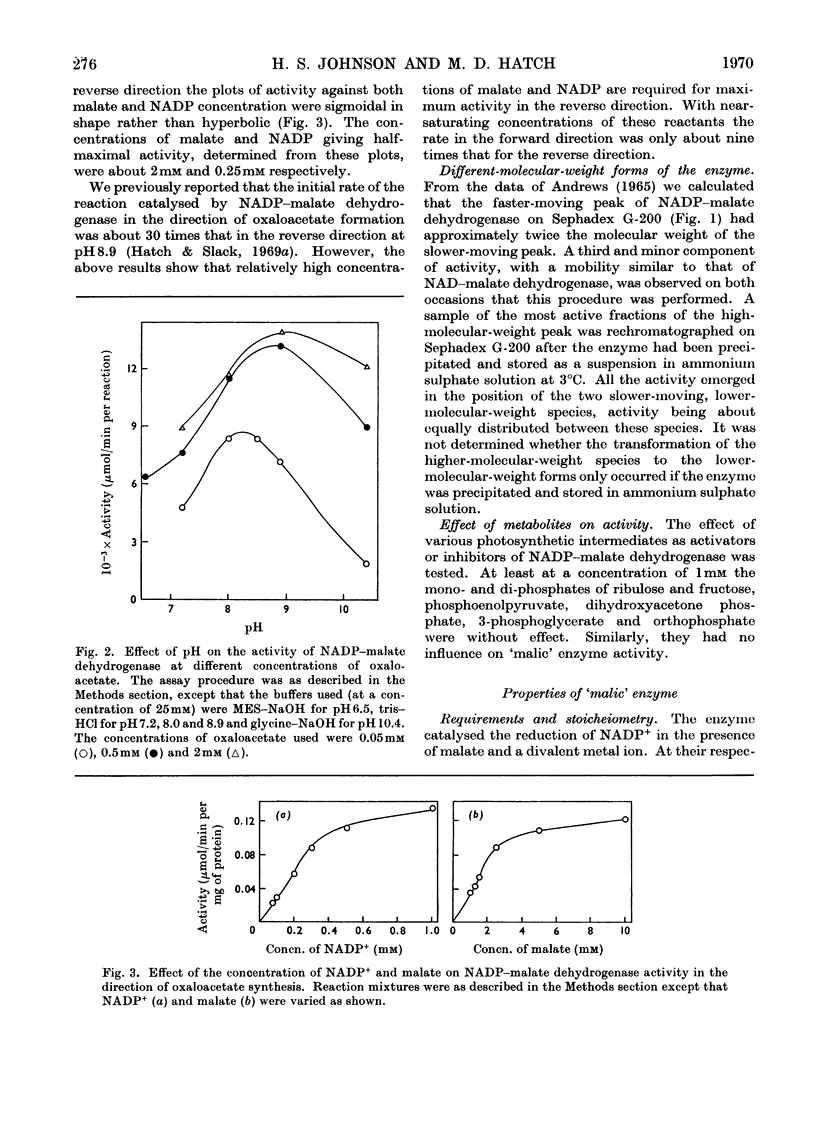
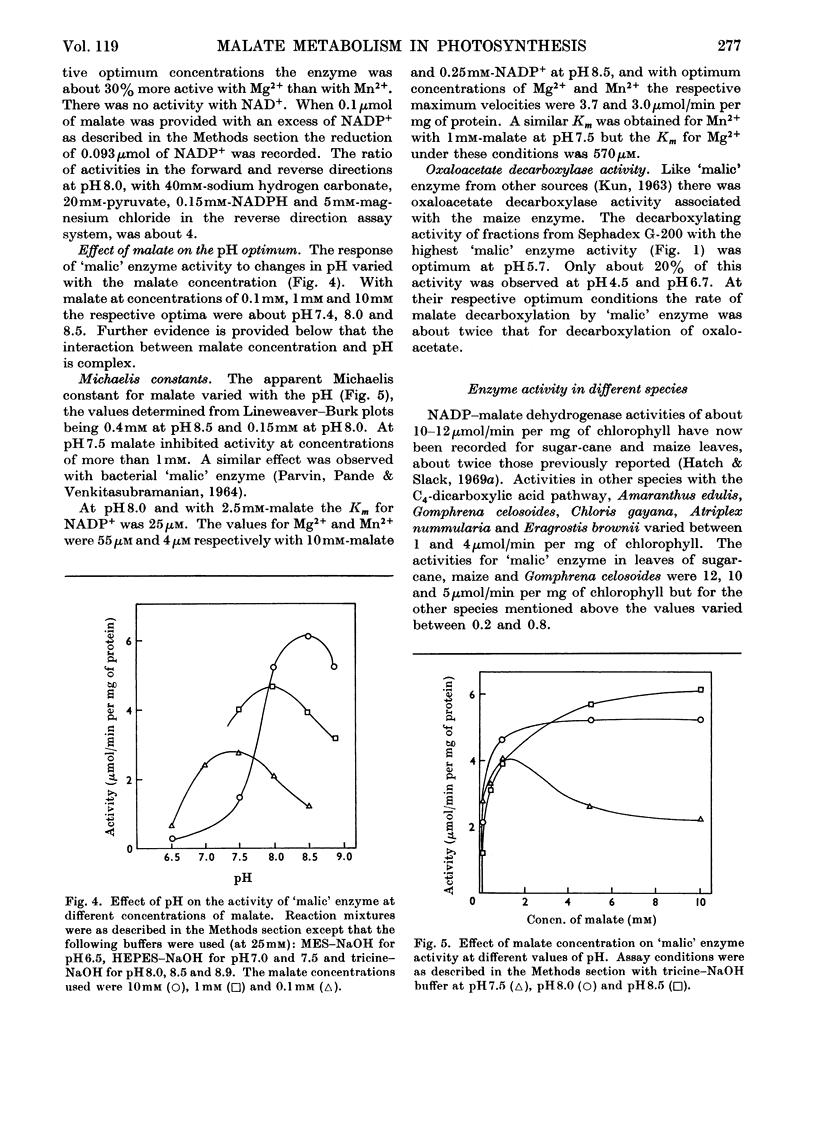
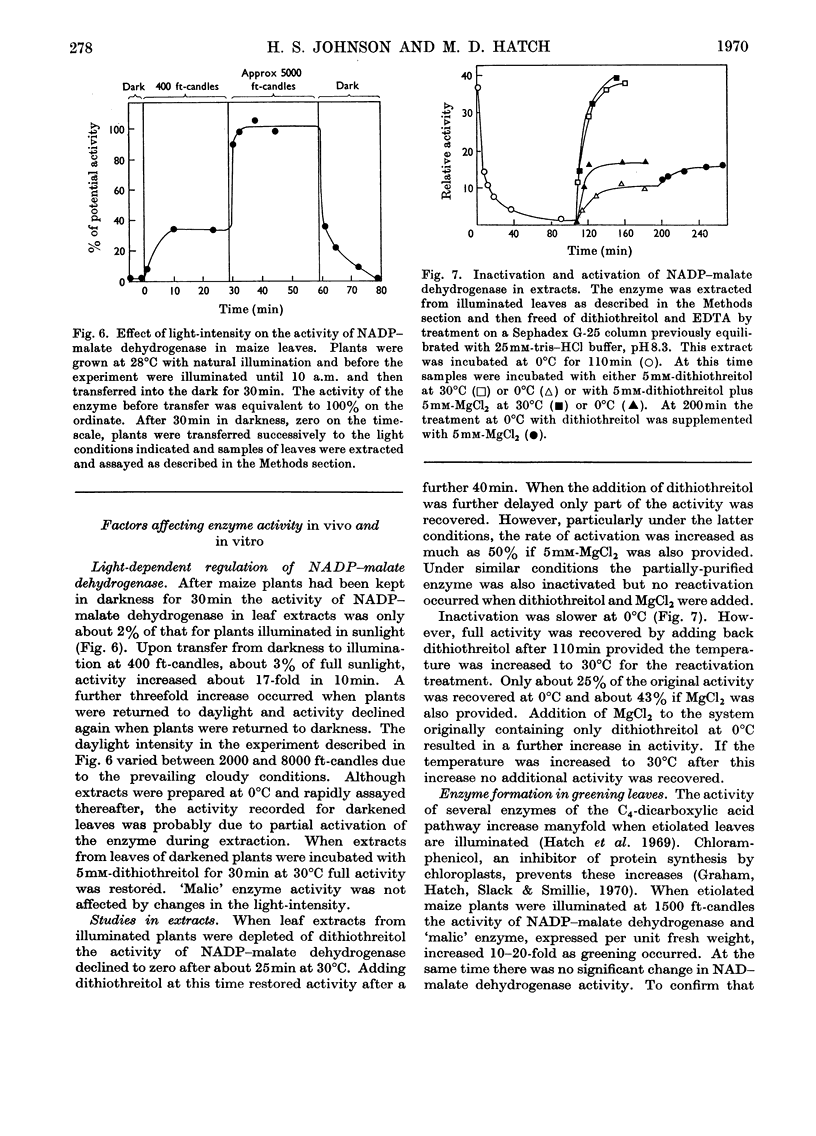
1. NADP–malate dehydrogenase and `malic' enzyme in maize leaf extracts were separated from NAD–malate dehydrogenase and their properties were examined. 2. The NADP–malate dehydrogenase was nicotinamide nucleotide-specific but otherwise catalysed a reaction comparable with that with the NAD-specific enzyme. By contrast with the latter enzyme, a thiol was absolutely essential for maintaining the activity of the NADP–malate dehydrogenase, and the initial velocity in the direction of malate formation, relative to the reverse direction, was faster. 3. For the `malic' enzyme reaction the Km for malate was dependent on pH and the pH optimum varied with the malate concentration. At their respective optimum concentrations the maximum velocity for this enzyme was higher with Mg2+ than with Mn2+. 4. The NADP–malate dehydrogenase in green leaves was rapidly inactivated in the dark and was reactivated when plants were illuminated. Reactivation of the enzyme extracted from darkened leaves was achieved simply by adding a thiol compound. 5. The activity of both enzymes was low in etiolated leaves of maize plants grown in the dark but increased 10–20-fold, together with chlorophyll, when leaves were illuminated. 6. The activity of these enzymes in different species with the C4-dicarboxylic acid pathway was compared and their possible role in photosynthesis was considered.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R., Johnson H. S. Further studies on a new pathway of photosynthetic carbon dioxide fixation in sugar-cane and its occurrence in other plant species. Biochem J. 1967 Feb;102(2):417–422. doi: 10.1042/bj1020417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R. NADP-specific malate dehydrogenase and glycerate kinase in leaves and evidence for their location in chloroplasts. Biochem Biophys Res Commun. 1969 Mar 10;34(5):589–593. doi: 10.1016/0006-291x(69)90778-5. [DOI] [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R. Photosynthesis by sugar-cane leaves. A new carboxylation reaction and the pathway of sugar formation. Biochem J. 1966 Oct;101(1):103–111. doi: 10.1042/bj1010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R. Studies on the mechanism of activation and inactivation of pyruvate, phosphate dikinase. A possible regulatory role for the enzyme in the C4 dicarboxylic acid pathway of photosynthesis. Biochem J. 1969 May;112(5):549–558. doi: 10.1042/bj1120549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARVIN R., PANDE S. V., VENKITASUBRAMANIAN T. A. PURIFICATION AND PROPERTIES OF MALATE DEHYDROGENASE (DECARBOXYLATING) FROM MYCOBACTERIUM 607. Biochim Biophys Acta. 1964 Nov 22;92:260–277. doi: 10.1016/0926-6569(64)90184-1. [DOI] [PubMed] [Google Scholar]
- RAVAL D. N., WOLFE R. G. Malic dehydrogenase. IV. pH dependence of the kinetic parametrs. Biochemistry. 1962 Nov;1:1118–1123. doi: 10.1021/bi00912a024. [DOI] [PubMed] [Google Scholar]
- Slack C. R., Hatch M. D. Comparative studies on the activity of carboxylases and other enzymes in relation to the new pathway of photosynthetic carbon dioxide fixation in tropical grasses. Biochem J. 1967 Jun;103(3):660–665. doi: 10.1042/bj1030660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C. R., Hatch M. D., Goodchild D. J. Distribution of enzymes in mesophyll and parenchyma-sheath chloroplasts of maize leaves in relation to the C4-dicarboxylic acid pathway of photosynthesis. Biochem J. 1969 Sep;114(3):489–498. doi: 10.1042/bj1140489. [DOI] [PMC free article] [PubMed] [Google Scholar]


