Abstract
Background:
The current study aims to identify the key pathways and potential therapeutic targets for pulmonary arterial hypertension (PAH) and to further evaluate the anti-PAH effects of isorhamnetin.
Methods:
The dataset of gene expression profiling for PAH (GSE113439) was downloaded from the gene expression omnibus (GEO) database. Isorhamnetin target genes were extracted from the comparative toxicogenomics database (CTD). Various bioinformatics methods were employed to identify the core pathways associated with PAH and potential intervention targets. Molecular docking was conducted between the interacting target and the candidate compound, isorhamnetin.
Results:
One thousand nine hundred sixty-two upregulated genes and 642 downregulated genes were identified. Molecular complex detection analyses revealed that the significant biological processes associated with upregulated genes included DNA damage response, mitotic cell cycle, and chromosome organization. In contrast, the significant biological processes related to downregulated genes encompassed cellular response to growth factor stimulus, response to growth factor, and blood vessel development. Immune infiltration analysis indicated that PAH is associated with significant changes in the distribution of immune cells and differential expression of immune checkpoints. Furthermore, 58 isorhamnetin targets were extracted from the CTD, and we identified 1 interacting gene, NFE2L2, among the differentially expressed genes (DEGs), DEGs related to ferroptosis, and isorhamnetin targets. Isorhamnetin demonstrated strong affinities with vascular endothelial growth factor (VEGF) receptors and transcription factors (ATM and ZNF24) associated with VEGFs, as well as the ferroptosis protein NFE2L2.
Conclusions:
Pulmonary arterial hypertension is characterized by a series of abnormalities in downstream molecular signaling pathways, including DNA damage, immune dysregulation, VEGF signaling deficiency, and the ferroptosis process. These may represent the core pathophysiological mechanisms of PAH. Ferroptosis-related genes, such as NFE2L2 and TF (ATM, ZNF24) associated with VEGFs, are potential therapeutic targets that contribute to the mechanisms mentioned above. Isorhamnetin is a promising candidate compound for the treatment of PAH.
Keywords: Pulmonary artery hypertension, bioinformatics, molecular docking, immune infiltration, isorhamnetin
Highlights
Based on network pharmacology, bioinformatics analysis, and molecular docking studies, we found that pulmonary artery hypertension (PAH) is associated with a series of abnormalities in downstream molecular signaling pathways, including DNA damage, immune dysregulation, vascular endothelial growth factor (VEGF) signal deficiency, and iron-induced apoptosis. Isorhamnetin emerges as a promising candidate compound for the treatment of PAH in these aspects.
Introduction
Pulmonary artery hypertension (PAH) is a severe, progressive disease that results in ongoing right ventricular remodeling and right heart failure. Pulmonary artery hypertension is characterized by increased pulmonary vascular resistance (PVR), arterial remodeling, in situ pulmonary arterial thrombosis (ISPAT), and stiffness of the pulmonary vascular walls. These pathological features are associated with multiple mechanisms, including endothelial damage, dysregulation of the immune system, and ionic metabolic abnormalities.1 The survival rate of PAH patients, confirmed for the first time, is approximately 60% over a 3-year period. Pulmonary artery hypertension is often referred to as the “cancer” of the cardiovascular system.2 Consequently, exploring the fundamental pathological pathways, identifying therapeutic targets, and screening potential chemical candidates for PAH are crucial for early diagnosis and treatment.
The mechanisms of PAH have been extensively studied in recent years; however, therapeutic targets and preventive methods have not yet been fully identified. Ferroptosis is a newly discovered form of cell death that differs from necrosis, apoptosis, and autophagy.3 It is primarily induced by iron-dependent lipid peroxidation, which leads to mitochondrial contraction, rupture of the mitochondrial membrane, cellular respiratory dysfunction, energy metabolism deficiency, and DNA damage.4,5 Previous studies have demonstrated that pulmonary vascular remodeling is influenced by factors such as oxidative stress, lipid peroxidation, and inflammation, all of which share molecular characteristics with the ferroptosis process.4,5 Additionally, it has been found that the activation of ferroptosis signaling coincides with the onset of idiopathic PAH.4 Ferroptosis is not only associated with heart and lung diseases, such as myocardial infarction and chronic obstructive pulmonary disease, but also plays a role in the pathogenesis and progression of PAH.6-8 However, the precise role of ferroptosis in the pathological mechanisms of PAH remains unclear.
In recent years, approximately 4000 plant-derived flavonoid compounds have been identified, many of which possess a variety of medicinal properties.9 Isorhamnoin is a specific class of flavonoid compounds primarily extracted from the fruits of “Hippophae rhamnoides” and the leaves of “Ginkgo biloba L.”Isorhamnoin exhibits anti-inflammatory and antioxidant effects and has the ability to protect vascular endothelial cells. Accumulating evidence suggests that isorhamnoin, as a supplemental agent, can be utilized to treat various diseases due to its pharmacological activities, including cardiovascular and cerebrovascular protection, anti-tumor effects, anti-inflammatory properties, antioxidant activity, organ protection, and obesity prevention.10-15 These studies indicate that isorhamnoin may be a promising candidate for combating PAH. Therefore, we conducted an integrated bioinformatics analysis and network pharmacology approach to investigate the core therapeutic targets of PAH, extending to ferroptosis signaling, and further assessed the anti-PAH effects.
Methods
Dataset
We conducted a gene expression profiling analysis of pulmonary arterial hypertension (GSE113439) using the gene expression omnibus (GEO) database (www.ncbi.nlm.nih.gov/geo/). This study employed microarray analysis to examine the gene expression profiles of patients with PAH compared to normal controls. The samples were collected from the tissues of 15 PAH cases, which included 6 cases of idiopathic PAH, 4 cases of PAH secondary to connective tissue disease, 4 cases of PAH secondary to congenital heart disease, and 1 case of chronic thromboembolic pulmonary hypertension. Additionally, 11 normal control samples were obtained from lung tissue adjacent to lung cancer resections.16
Differentially Expressed Genes
All microarray data were downloaded from the GEO database (http://www.ncbi.nih.gov/geo). The data are standardized, and the raw files were obtained in MINiML format. The limma package in the R software was employed to analyze the differentially expressed mRNAs (R software is developed by Synopsys in the United States). The adjusted P-value was assessed to correct for false positive results in the GEO datasets. A threshold of “Adjusted P < .05 and |Log2 Fold Change| > 1.5” was established to define the differential expression of mRNAs.17
Ferroptosis
Microarray data were downloaded from the GEO database (http://www.ncbi.nih.gov/geo). The raw data were obtained in MINiML format. Ferroptosis-related genes were identified from Liu et al’s18 systematic analysis of the aberrances and functional implications of ferroptosis in cancer. The analysis was conducted using statistical package for social sciences (SPSS) software (version 20.0). We used the Kolmogorov–Smirnov test to estimate the normal distribution of raw data of genes related to ferroptosis. Mann–Whitney U test and an independent sample t-test were used for non-normal distribution data and normal distribution data, respectively. The statistical results are presented using Grand Prism 8 software.
Functional Enrichment and Protein-Protein Interaction Network
Gene Ontology (GO) is a widely utilized tool for annotating genes with their functions, particularly in the areas of molecular function (MF), biological processes (BP), and cellular components (CC). The Kyoto encyclopedia of genes and genomes (KEGG) enrichment analysis serves as a valuable practical resource for investigating gene functions and associated high-level genomic information. To gain a deeper understanding of the functions of differentially expressed genes (DEGs), functional enrichment analysis and a protein-protein interaction (PPI) network for the DEGs were established using Metascape (version: v3.5.20240101). Metascape is a web-based portal designed to provide comprehensive gene list annotation and analysis resources for experimental biologists. It is an effective and efficient tool for researchers to analyze and interpret OMICs-based studies in the era of big data. This online tool includes modules such as enrichment clustering, protein network analysis, multi-gene list meta-analysis, and transcription factor analysis. Additionally, the cytoscape plug-in for molecular complex detection (MCODE) was employed to explore key functional modules within the PPI network.19
Transcription Factor Analysis
Transcription regulatory relationships unraveled sentence-based text mining (TRRUST) (https://www.grnpedia.org/trrust/, version 2) is a manually curated database of transcriptional regulatory networks for humans and mice. This database includes 8444 and 6552 TF-target regulatory relationships of 800 human TFs and 828 mouse TFs, respectively. These relationships have been derived from 11 237 PubMed articles that detail small-scale experimental studies on transcriptional regulation. To facilitate efficient searches for regulatory relationships among over 20 million PubMed articles, the TRRUST database also provides information on the mode of regulation (activation or repression). Currently, 8972 (59.8%) of the regulatory relationships have a known mode of regulation.20 In the results of GO and KEGG analyses, down-regulated genes were found to be enriched in functional pathways related to cellular responses to growth factor stimuli, responses to growth factors, and blood vessel development. Consequently, in this database, we searched for the TFs associated with VEGF-A, VEGF-B, and VEGF-C, which are well-studied core components of arterial endothelial growth factor signaling. The analysis was conducted using SPSS software (version 20.0). We used the Kolmogorov–Smirnov test to estimate the normal distribution of raw data of transcription factors (TFs). Mann–Whitney U test and an independent sample t-test were used for non-normal distribution data and normal distribution data, respectively. The statistical results are presented using Grand Prism 8 software.
Immune Filtration Analysis
ImmuCellAI (Immune Cell Abundance Identifier) is a tool designed to estimate the abundance of 24 immune cell types from gene expression datasets, including RNA-Seq and microarray data. These 24 immune cells comprise 18 T-cell subtypes and 6 additional immune cells: B cells, NK cells, monocyte cells, macrophage cells, neutrophil cells, and dendritic cells (DCs).21,22
In our study, we analyzed the immune infiltration of PAH using a specialized tool. Additionally, we compared the expression levels of 10 immune checkpoint genes between PAH samples and control samples. Microarray data were downloaded from theGEO database (http://www.ncbi.nih.gov/geo) in MINiML format.23-25 We extracted the expression data of immune checkpoint genes and examined the expression values of these genes. The analysis was conducted using SPSS software (version 20.0). We used the Kolmogorov–Smirnov test to estimate the normal distribution of raw data of immune checkpoint genes. Mann–Whitney U test and an independent sample t-test were used for non-normal distribution data and normal distribution data, respectively. The statistical results are presented using Grand Prism 8 software.
Isorhamnetin Targets
Comparative toxicogenomics database (CTD) is a comprehensive, publicly accessible database designed to enhance our understanding of how environmental exposures affect human health. It offers meticulously curated information on chemical-gene/protein interactions, chemical-disease relationships, and gene-disease associations. This data is integrated with functional and pathway information to support the development of hypotheses regarding the mechanisms underlying diseases influenced by environmental factors. In this study, we utilized CTD to identify the targets of Isorhamnetin.26
Molecular Docking
NFE2L2 was identified as a common gene among DEGs, ferroptosis, and isorhamnetin, those interacting with isorhamnetin. Consequently, we conducted molecular docking to assess the binding affinities between isorhamnetin and NFE2L2. The crystal structures of the Nrf2/NFE2L2, VEGFR, ZNF24, and ATM proteins used for docking were obtained from the protein data bank (PDB), which is accessible at https://www.rcsb.org/.The PDB IDs for the 4 proteins are 7X5E 1FLT, 3LHR, and 7SIC, respectively.27-30
The 3-dimensional (3D) structure of the small molecule isorhamnetin was downloaded from the PubChem database (PubChem CID: 5281654), and energy minimization was performed using the MMFF94 force field. AutoDock Vina 1.2.3 software, developed by the Scripps Research Institute, was utilized for molecular docking. Prior to docking, PyMol 2.5.5 was employed to remove water molecules, salt ions, and small molecules.31 The coordinates for docking are listed in Table 1. Additionally, we used ADFRsuite 1.03 to convert all processed small molecules and receptor proteins into the PDBQT format for docking with AutoDock Vina 1.2.3 docking.32 During the docking process, the global search granularity was set to 32, while all other parameters were maintained at their default settings. The docking conformation with the highest output score was considered the binding conformation, and the docking results were visualized using PyMOL 2.5.5.
Table 1.
Ferroptosis Genes Expression Between PAH Group and Control Group
| Genes | PAH group (n = 15) | Control group (n = 11) | Kolmogorov–Smirnov test | Independent Samples test | Mann–Whitney U test | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | Std. Error | n | Mean | Std. Error | P | T value | P (2-tailed) | P | |
| ACSL4 | 15 | 9.776 | 0.105 | 11 | 8.706 | 0.175 | .173 | 5.55 | < .001 | |
| ALOX15 | 15 | 8.090 | 0.301 | 11 | 8.116 | 0.236 | .172 | −0.063 | .950 | |
| ATP5MC3 | 15 | 7.065 | 0.044 | 11 | 7.106 | 0.035 | .200 | −0.675 | .506 | |
| CARS | 15 | 8.223 | 0.079 | 11 | 7.873 | 0.091 | .200 | 2.902 | .008 | |
| CDKN1A | 15 | 9.746 | 0.164 | 11 | 10.073 | 0.188 | .200 | −1.306 | .204 | |
| CISD1 | 15 | 8.704 | 0.098 | 11 | 8.828 | 0.086 | .200 | −0.907 | .374 | |
| DPP4 | 15 | 9.892 | 0.125 | 11 | 8.389 | 0.150 | .200 | 7.743 | < .001 | |
| FANCD2 | 15 | 6.679 | 0.124 | 11 | 5.991 | 0.057 | .154 | 4.466 | < .001 | |
| FDFT1 | 15 | 11.221 | 0.066 | 11 | 11.137 | 0.105 | .078 | 0.714 | .482 | |
| GLS2 | 15 | 6.845 | 0.038 | 11 | 6.887 | 0.043 | .200 | −0.722 | .477 | |
| GPX4 | 15 | 11.433 | 0.058 | 11 | 11.763 | 0.060 | .200 | −3.887 | .001 | |
| HSPA5 | 15 | 11.880 | 0.090 | 11 | 10.913 | 0.105 | .200 | 7.004 | < .001 | |
| HSPB1 | 15 | 12.653 | 0.045 | 11 | 12.865 | 0.046 | .200 | −3.218 | .004 | |
| LPCAT3 | 15 | 11.326 | 0.069 | 11 | 11.727 | 0.070 | .200 | −4.021 | < .001 | |
| MT1G | 15 | 12.026 | 0.163 | 11 | 11.845 | 0.169 | .200 | 0.754 | .458 | |
| NCOA4 | 15 | 10.606 | 0.049 | 11 | 9.885 | 0.114 | .065 | 5.805 | < .001 | |
| NFE2L2 | 15 | 10.909 | 0.057 | 11 | 10.141 | 0.097 | .200 | 7.229 | < .001 | |
| RPL8 | 15 | 13.035 | 0.021 | 11 | 13.087 | 0.031 | .200 | −1.423 | .168 | |
| SAT1 | 15 | 12.417 | 0.062 | 11 | 12.199 | 0.085 | .200 | 2.134 | .043 | |
| SLC1A5 | 15 | 9.700 | 0.101 | 11 | 10.007 | 0.097 | .200 | −2.128 | .044 | |
| SLC7A11 | 8.460 | 0.306 | 11 | 6.105 | 0.126 | .135 | 7.125 | < .001 | ||
| TFRC | 15 | 10.808 | 0.127 | 11 | 9.890 | 0.158 | .200 | 4.586 | < .001 | |
| CS | 15 | 10.785 | 0.058 | 11 | 10.630 | 0.021 | .006 | 2.197 | < .001 | .038 |
| EMC2 | 15 | 9.310 | 0.058 | 11 | 8.706 | 0.095 | .043 | 5.713 | < .001 | < .001 |
Pulmonary arterial hypertension (PAH) group (n = 15): GSM3106326, GSM3106327, GSM3106328, GSM3106329, GSM3106330, GSM3106331, GSM3106332, GSM3106333, GSM3106334, GSM3106335, GSM3106336, GSM3106337, GSM3106338, GSM3106339, GSM3106340; Control group (n = 11): GSM3106341, GSM3106342, GSM3106343, GSM3106344, GSM3106345, GSM3106346, GSM3106347, GSM3106348, GSM3106349, GSM3106350, GSM3106351. PAH, pulmonary arterial hypertension.
Estimating Toxify Profiles of Isorhamnetin
ADMETlab 3.0 (available at: https://admetlab3.scbdd.com/server/evaluation) is the second updated version of the web server that offers a comprehensive and efficient platform for evaluating ADMET-related parameters, physicochemical properties, and medicinal chemistry characteristics involved in the drug discovery process.33 In our study, we assessed the toxicity profiles of isorhamnetin using ADMETlab (version 3.0). The imported SMILES representation of isorhamnetin is as follows: COC1=C(C=CC(=C1)C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O or O1C2=C([H])C(=C([H])C(=C2C(C(=C1C1C([H])=C([H])C(=C(C=1[H])OC([H])([H])[H])O[H])O[H])=O)O[H])O[H].
Statement
We do not use artificial intelligence (AI) assistive technologies (such as large language models (LLMs), chatbots, or image creators).
The study is secondary and has no ethical implications.
Results
Identification of DEGs in PAH
Figure 1 illustrates the workflow chart. The microarray dataset GSE113439 was obtained from the GEO database. An adjusted P-value threshold of less than .05 and a |log2FC| of 1.5 or greater were applied. Consequently, 22 841 genes were included in the analysis. We identified a total of 2604 DEGs, comprising 1962 upregulated genes and 642 downregulated genes. The results were visualized using volcano plots (Figure 2A and B).
Figure 1.
Work flowchart.
Figure 2.
Analysis of GSE113439. (A) Volcano plot of DEGs between the PAH samples and control samples. (B) Heatmap of DEGs between the PAH samples and control samples. (C, D) Gene ontology and KEGG analysis of upregulated genes, the data were presented as Log2(fold change). (E, F) Gene ontology and KEGG analysis of downregulated genes, the data were presented as—Log10 (P value).
Enrichment ofDEGs and establishment ofPPI network
To investigate the biological functions of these DEGs,GO andKEGG pathway enrichment analyses were conducted on 1962 upregulated genes and 642 downregulated genes associated with PAH, utilizing the Metascape online tool. The results revealed that the top functional GO terms for upregulated DEGs included the mitotic cell cycle and DNA damage response, while the downregulated DEGs were primarily associated with vasculature development and cellular responses to growth factor stimuli (Figure 2C, E). The leading KEGG pathways identified were herpes simplex virus 1 infection for upregulated genes and pathways in cancer for downregulated genes (Figure 2D, F). Additionally, the MCODE plug-in was employed to explore significant gene clustering modules. The findings indicated that DNA damage response, mitotic cell cycle, and chromosome organization were enriched in upregulated genes, whereas cellular responses to growth factor stimuli, responses to growth factors, and blood vessel development were enriched in downregulated genes. These results, illustrated in Figure 3, suggest that cell proliferation and DNA damage play a significant role in the molecular pathology of PAH, alongside a reduction in vascular VEGF signaling.
Figure 3.
Protein-protein interaction network of enriched terms with MCODE plug-in estimation. (A) Protein-protein interaction network of up-regulated genes, colored by cluster ID, where nodes that share the same cluster ID are typically close to each other. (B) Protein-protein interaction network of up-regulated genes, colored by P-value, where terms containing more genes tend to have a more significant P-value. (C) Protein-protein interaction network of down-regulated genes, where nodes that share the same cluster ID are typically close to each other. (D) Protein-protein interaction network of down-regulated genes, colored by P-value, where terms containing more genes tend to have a more significant P-value. (E) MCODE estimation (Top 10).
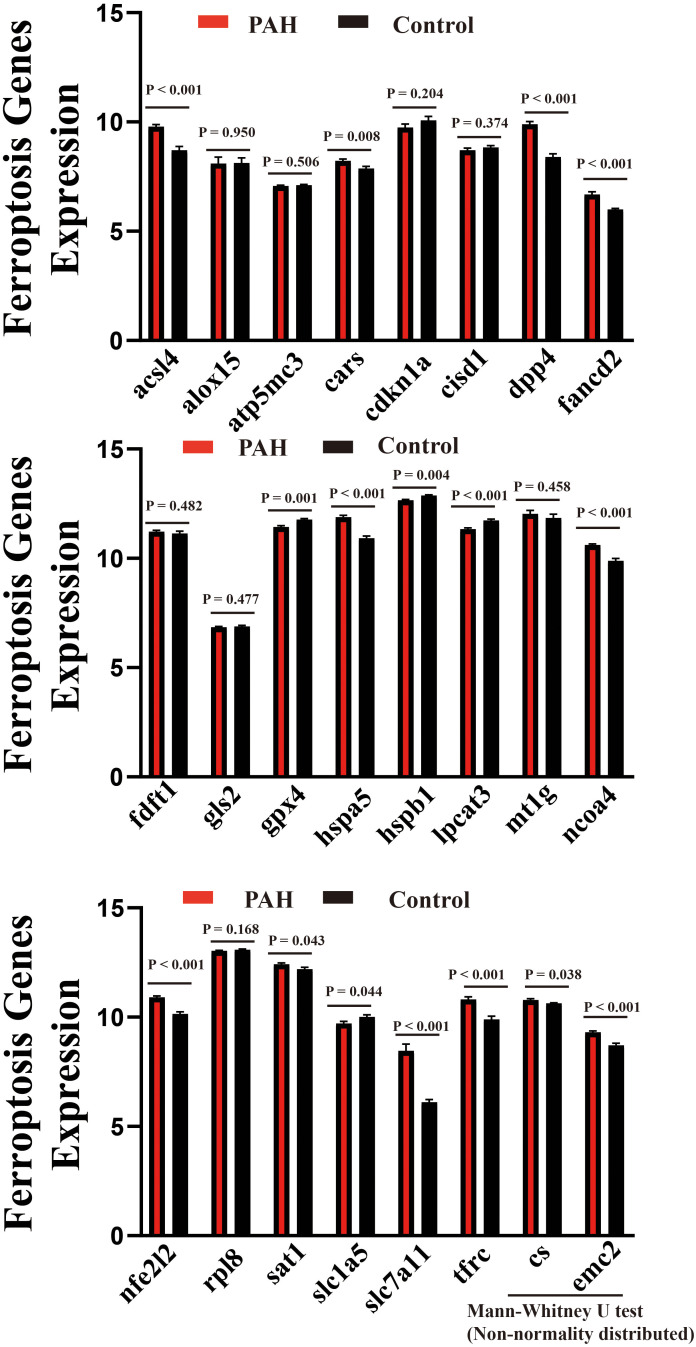
Differentially Expressed Genes of Ferroptosis-Related Genes
Twenty-four ferroptosis-related genes were identified and analyzed. The results indicate that genes associated with ferroptosis, such as ACSL4, CARS, CS, DPP4, EMC2, FANCD2, HSPA5, NCOA4, NFE2L2, SLC7A11, SAT1, and TFRC, were significantly upregulated in 15 samples of PAH. In contrast, GPX4, HSPB1, SLC1a5 and LPCAT3 were significantly downregulated. These findings suggest that the ferroptosis process may play a role in the pathology of PAH. The results are illustrated in Figure 4 and Table 1.
Figure 4.
Identification of ferroptosis related genes with Wilcox test between PAH samples and control samples.
Transcription Factors
The results of the KEGG analysis and MCODE estimation indicated that the cellular response to growth factor stimulus, response to growth factor, and blood vessel development were the top pathways affected in the down-regulated genes associated with PAH. It is well established that VEGF signaling plays a crucial role in blood vessel activities. These findings suggest a potential reduction in VEGF signaling. Consequently, we investigated theTFs regulating the expression of VEGF-A, VEGF-B, and VEGF-C by consulting the TRRUSS database. We identified 58 TFs for VEGF-A, 7 TFs for VEGF-B, and 2 TFs for VEGF-C, as presented in Figure 5A. Among the TFs for VEGF-A, 6 common TFs (RB1, HDAC2, HIF1A, ZNF24, ATM, MEF2C) were found in the up-regulatedDEGs, while 1 TF (ID3) was identified in the down-regulated DEGs. In contrast, only 1 common TF (HIF1A) was identified for VEGF-B among the DEGs. No common TFs were found between the DEGs and TFs for VEGF-C. These results are displayed in Figure 5B. The differentially expressed TFs showed statistical significance between the PAH samples and control samples, as illustrated in Figure 5C and Table 2.
Figure 5.
Transcriptional factor analysis of VEGFs. (A) TFs for VEGFs regulation. (B) Intersection of TFs and DEGs. (C) Transcriptional factor analysis with Wilcox test between PAH samples and control samples.
Table 2.
Transcriptional Factor Expression between PAH Group and Control Group
| Genes | PAH Group (n = 15) | Control Group (n = 11) | Kolmogorov–Smirnov test | Independent Samples test | Mann–Whitney U test | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | Std. Error | n | Mean | Std. Error | P | T value | P (2-tailed) | P | |
| HDAC2 | 15 | 9.425 | 0.059 | 11 | 8.724 | 0.059 | .130 | 8.147 | < .001 | |
| HIF1A | 15 | 11.628 | 0.111 | 11 | 10.477 | 0.155 | .200 | 6.205 | < .001 | |
| ID3 | 15 | 9.079 | 0.136 | 11 | 9.736 | 0.179 | .200 | −2.974 | .007 | |
| MEF2C | 15 | 10.426 | 0.062 | 11 | 9.776 | 0.109 | .200 | 5.516 | < .001 | |
| ATM | 15 | 9.222 | 0.077 | 11 | 8.459 | 0.105 | .040 | 5.986 | < .001 | < .001 |
| RB1 | 15 | 10.213 | 0.034 | 11 | 9.446 | 0.074 | .009 | 9.466 | < .001 | < .001 |
| ZNF24 | 15 | 9.128 | 0.039 | 11 | 8.300 | 0.146 | < .001 | 5.493 | < .001 | < .001 |
Pulmonary arterial hypertension (PAH) group (n = 15): GSM3106326, GSM3106327, GSM3106328, GSM3106329, GSM3106330, GSM3106331, GSM3106332, GSM3106333, GSM3106334, GSM3106335, GSM3106336, GSM3106337, GSM3106338, GSM3106339, GSM3106340; Control group (n = 11): GSM3106341, GSM3106342, GSM3106343, GSM3106344, GSM3106345, GSM3106346, GSM3106347, GSM3106348, GSM3106349, GSM3106350, GSM3106351. PAH, pulmonary arterial hypertension.
Immune Characteristics in Pulmonary Arterial Hypertension
The raw microarray data of PAH were input into the immune cell abundance identifier. The results indicated that the distribution and infiltration of immune cells in PAH samples were altered compared to control samples. The affected immune cells included dendritic cells (DC), macrophages, natural killer (NK) cells, neutrophils, CD8+ T cells, inflammatory Helper T 17 cells, T follicular helper (Tfh) cells, cytotoxic T cells, central memory T cells, and effector memory T cells. The infiltration score slightly increased in PAH samples (0.866) compared to control samples (0.837) (P = .02). These results are illustrated in Figure 6 and Table 3. Additionally, 8 immune checkpoint genes were significantly expressed, with 4 highly expressed genes (CD274, HAVCR2, PDCD1LG2) and 4 lowly expressed genes (IGSF8, LAG3, SIGLEC15, TIGIT) compared to the control. These findings are presented in Figure 7 and Table 4. Overall, these results suggest that the pathology of PAH is associated with immune responses and inflammation.
Figure 6.
Immune filtration analysis. (A) Immune cell abundance in sample (B) Immune cell abundance in groups.
Table 3.
Immune Filtration Analysis between PAH Group and Control Group
| Immune Cells | PAH Group (n = 15) |
Control Group (n = 11) |
P |
|---|---|---|---|
| Dendritic cell | 0.05 | 0.132 | < .001 |
| Bcell | 0.042 | 0.035 | .720 |
| Monocyte | 0.068 | 0.064 | .980 |
| Macrophage | 0.175 | 0.082 | .011 |
| NK | 0.028 | 0.055 | < .001 |
| Neutrophil | 0.286 | 0.167 | < .001 |
| CD4_T | 0.131 | 0.127 | .940 |
| CD8_T | 0.051 | 0.128 | < .001 |
| Natural killer T cell | 0.039 | 0.040 | .900 |
| Gamma_delta | 0.130 | 0.176 | .069 |
| CD4_naive | 0.001 | 0.001 | 1.000 |
| Tr1 | 0.006 | 0.005 | .700 |
| Natural regulatory T cell | 0.002 | 0.000 | .066 |
| Induced regulatory T cell | 0.005 | 0.003 | .120 |
| Th1 | 0.003 | 0.002 | .140 |
| Th2 | 0.005 | 0.007 | .140 |
| Th17 | 0.002 | 0.008 | < .001 |
| Tfh | 0.004 | 0.008 | .022 |
| CD8_naive | 0.001 | 0.002 | .150 |
| Cytotoxic | 0.001 | 0.002 | .016 |
| Exhausted | 0.001 | 0.001 | .830 |
| Mucosal-associated invariant T cell | 0.002 | 0.002 | .330 |
| Central_memory | 0.006 | 0.002 | .004 |
| Effector_memory | 0.000 | 0.004 | .001 |
| Infiltration score | 0.866 | 0.837 | .020 |
Pulmonary arterial hypertension (PAH) group (n = 15): GSM3106326, GSM3106327, GSM3106328, GSM3106329, GSM3106330, GSM3106331, GSM3106332, GSM3106333, GSM3106334, GSM3106335, GSM3106336, GSM3106337, GSM3106338, GSM3106339, GSM3106340; Control group (n = 11): GSM3106341, GSM3106342, GSM3106343, GSM3106344, GSM3106345, GSM3106346, GSM3106347, GSM3106348, GSM3106349, GSM3106350, GSM3106351. NK, natural killer; PAH, pulmonary arterial hypertension; Tfh, T follicular helper
Figure 7.
Comparison of expression levels of immune checkpoint genes between PAH samples (n = 15) and control samples (n = 11).
Table 4.
Immune Checkpoint Expression between PAH Group and Control Group
| Genes | PAH Group (n = 15) | Control Group (n = 11) | Kolmogorov–Smirnov test | Independent Samples test | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | Std. Error | n | Mean | Std. Error | P | T value | P (2-tailed) | |
| CD274 | 15 | 8.462 | 0.203 | 11 | 7.790 | 0.167 | .20 | 2.425 | .023 |
| CTLA4 | 15 | 7.191 | 0.157 | 11 | 6.786 | 0.110 | .19 | 1.959 | .062 |
| HAVCR2 | 15 | 8.849 | 0.128 | 11 | 8.194 | 0.137 | .20 | 3.437 | .002 |
| IGSF8 | 15 | 8.406 | 0.076 | 11 | 8.873 | 0.050 | .06 | −4.566 | .000 |
| ITPRIPL1 | 15 | 6.756 | 0.037 | 11 | 6.742 | 0.031 | .20 | 0.265 | .793 |
| LAG3 | 15 | 7.071 | 0.042 | 11 | 7.241 | 0.033 | .20 | −2.999 | .006 |
| PDCD1 | 15 | 7.976 | 0.042 | 11 | 8.089 | 0.037 | .20 | −1.926 | .066 |
| PDCD1LG2 | 15 | 8.969 | 0.111 | 11 | 8.582 | 0.123 | .20 | 2.306 | .030 |
| SIGLEC15 | 15 | 6.882 | 0.041 | 11 | 7.057 | 0.069 | .20 | −2.3 | .030 |
| TIGIT | 15 | 6.448 | 0.088 | 11 | 6.732 | 0.083 | .08 | −2.28 | .032 |
Pulmonary arterial hypertension (PAH) group (n = 15): GSM3106326, GSM3106327, GSM3106328, GSM3106329, GSM3106330, GSM3106331, GSM3106332, GSM3106333, GSM3106334, GSM3106335, GSM3106336, GSM3106337, GSM3106338, GSM3106339, GSM3106340; Control group (n = 11): GSM3106341, GSM3106342, GSM3106343, GSM3106344, GSM3106345, GSM3106346, GSM3106347, GSM3106348, GSM3106349, GSM3106350, GSM3106351. PAH, pulmonary arterial hypertension.
Identification of Isorhamnetin
We extracted 58 isorhamnetin-interacting genes from the CTD. These genes are highly enriched in various biological processes, including responses to hormones, response to xenobiotic stimuli, response to oxidative stress, inorganic substances, peptides, and chemical stress. Additionally, they are involved in the regulation of inflammatory responses, responses to molecules of bacterial origin, toxic substances, and lipopolysaccharides (Figure 8A, D, E). The most enriched pathways include the AGE-RAGE signaling pathway in diabetic complications, pathways in cancer, lipid metabolism and atherosclerosis, fluid shear stress and atherosclerosis, Chagas disease, toxoplasmosis, chemical carcinogenesis involving reactive oxygen species, diabetic cardiomyopathy, Th17 cell differentiation, and hepatitis B (Figure 8B, C, E). Furthermore, 2604 DEGs, 24 ferroptosis-related genes, and 58 isorhamnetin targets identified through various algorithms were intersected using Venn diagram analysis. One interacting gene, NFE2L2, was identified among the DEGs, DEGs related to ferroptosis, and the targets of isorhamnetin. We consider that NFE2L2 contributes to the pathological mechanism of PAH related to ferroptosis. Additionally, NFE2L2 may serve as a potential therapeutic target for isorhamnetin in the treatment of PAH. The results are illustrated in Figure 9A and B.
Figure 8.
Functional enrichments of isorhamnetin targets. (A, B) Gene oncology and KEGG pathway analyses. (C, D) Protein-protein interaction functional network analysis, color by cluster and P-value respectively. (E) Molecular Complex Detection estimation. Blue color denotes GO analysis and orange color denotes KEGG analysis.
Figure 9.
Molecular docking of isorhamnetin and identified common genes. (A) Identification of intersecting genes of isorhamnetin targets, DEGs, and ferroptosis-related genes. (B) Interaction of isorhamnetin targets. (C-F) Binding capacity of isorhamnetin and predicted hub regulatory targets, including NFE2L2 (PDB ID: 7X5E), VEGFR (PDB ID: 1FLT), ZNF24 (PDB ID: 3LHR), and ATM (PDB ID: 7SIC).
Binding Capacity of Isorhamnetin to NFE2L2, VEGFR, ATM, and ZNF24
Molecular docking simulation technology is a convenient and effective method for exploring the interactions between small molecules and target proteins. Therefore, we utilized Vina 1.2.3 software to estimate the binding affinity of isorhamnetin to NFE2L2, VEGFR, ZNF24, and ATM. The results of the molecular docking indicate that chemical-protein interactions with a docking energy of less than 5 kcal/mol suggest a strong binding affinity.34
Isorhamnetin forms hydrogen-bonding interactions with NFE2L2 at specific amino acid sites, including PHE-490, ARG-499, ASN-482, and MET-485. The establishment of these hydrogen bonds enhances the binding affinity between proteins and small molecules. Additionally, Isorhamnetin exhibits hydrophobic interactions with NFE2L2 at the amino acid site MET-485, which may contribute to strong van der Waals forces between the molecules. The calculated affinity score of −6.869 kcal/mol between isorhamnetin and NFE2L2 indicates a favorable binding capacity of Isorhamnetin to NFE2L2 (Figure 9C and Table 5). Isorhamnetin forms hydrogen-bonding interactions with VEGFR at specific amino acid sites, including CYS-68, GLY-59, and GLN-37. Furthermore, isorhamnetin also forms hydrophobic interactions with VEGFR at the amino acid sites THR-31 and ARG-56, with a binding energy of −6.508 kcal/mol (Figure 9D and Table 2). For ZNF24, isorhamnetin forms hydrophobic interactions at amino acid sites, including LEU-84, PHE-57, LEU-65, LEU-87, and LEU-61 (−6.882 kcal/mol) (Figure 9E and Table 5). Isorhamnetin forms hydrogen-bonding interactions with ATM at several amino acid sites, including CYS-2770, THR-2773, and LYS-2717. Isorhamnetin also forms hydrophobic interactions with ATM at the amino acid sites LEU-2877, TRP-2769, LEU-2767, PRO-2699, and LEU-2715, with a binding energy of −7.878 kcal/mol (Figure 9 F and Table 2).
Table 5.
Molecular Docking of the Interaction of Isorhamnetin and Target Genes
| Targets | PDB ID | Chemical | Coordinate | Grid Size | Docking Score (kcal/mol) | Hydrophobic Interactions | Hydrogen Bonds |
|---|---|---|---|---|---|---|---|
| Nrf2/NFE2L2 | 7X5E | Isorhamnetin | x = −59.12, y = −18.90, z = 4.03 |
x = 60, y = 60, z = 60 |
−6.869 | MET-485 | PHE-490, MET-485, ASN-482, ARG-499 |
| VEGFR | 1FLT | Isorhamnetin | x = −1.41, y = 7.71, z = 14.51 |
x = 20, y = 20, z = 20 |
−6.508 | THR-31, ARG-56 | CYS-68, GLY-59, GLN-37 |
| ZNF24 | 3LHR | Isorhamnetin | x = 4.485, y = 32.47, z = 12.05 |
x = 45, y = 45, z = 45 |
−6.882 | LEU-84, PHE-57, LEU-65, LEU-87, LEU-61 | |
| ATM | 7SIC | Isorhamnetin | x = 120.83, y = 166.20, z = 93.39 |
x = 20, y = 20, z = 20 |
−7.878 | LEU-2877, TRP-2769, LEU-2767, PRO-2699, LEU-2715 | CYS-2770, THR-2773, LYS-2717 |
PDB, protein data bank.
Toxicity Prediction of Isorhamnetin
We conducted ADMET predictions using ADMETlab 3.0 and found no acute toxicity associated with the oral administration of isorhamnetin. While there is no validated evidence of carcinogenicity, skin sensitization was identified (see Table 6). These results suggest that the oral administration of isorhamnetin is safe.
Table 6.
Toxicophore Rules
| Property | Value | Comment |
|---|---|---|
| Acute toxicity rule | 0 | 20 substructures; acute toxicity during oral administration |
| Genotoxic carcinogenicity rule | 0 | 117 substructures;carcinogenicity or mutagenicity |
| Nongenotoxic carcinogenicity rule | 0 | 23 substructures; carcinogenicity through nongenotoxic mechanisms |
| Skin sensitization rule | 8 alerts | 155 substructures; skin irritation |
| Aquatic toxicity rule | 0 | 99 substructures; toxicity to liquid (water) |
| Nonbiodegradable rule | 1 alerts | 19 substructures; non-biodegradable |
| SureChEMBL rule | 0 | 164 substructures; MedChem unfriendly status |
| Toxicophores rule | 1 alerts | 154 toxic substructures from FAF-Drug 4 |
Discussion
Pulmonary arterial hypertension, a well-studied form of pulmonary hypertension, is classified as pre-capillary pulmonary hypertension. Advances in genetics and molecular medicine have led to the identification of genetic variations and susceptibilities associated with the onset of PAH. Factors such as genetic mutagenesis, DNA damage and repair, and the activation of cell death pathways contribute to the pathological processes underlying PAH. The most extensively studied mutated gene is BMPR2. In Western populations, 70% to 80% of patients with hereditary PAH and 10% to 20% of patients with idiopathic PAH (IPAH) have been found to carry mutations in the BMPR2 gene. These mutations are associated with early onset, severe clinical phenotypes, and poor outcomes. Although the exact causes and mechanisms of PAH remain unclear, it is generally accepted that multiple factors, pathways, and processes are involved. Currently, molecularly targeted therapies such as ambrisentan and tadalafil are employed to improve clinical conditions or exercise tolerance. However, there are still no effective drugs available to delay disease progression. Therapeutic interventions for PAH primarily focus on managing pathophysiological processes and controlling complications associated with the condition. Therefore, identifying key regulatory genes and functional pathways is essential.
In this study, we utilized GSE113439 to analyze the DEGs associated with PAH. We identified highly expressed genes in PAH samples that were significantly enriched in biological pathways related to DNA damage response, the mitotic cell cycle, and chromosome organization. Additionally, we observed notable changes in the expression levels of genes associated with ferroptosis. These findings suggest an activation of cell death pathways, cell protection mechanisms, and cell cycle progression in the development of PAH. Conversely, downregulated genes were significantly enriched in pathways associated with cellular responses to growth factor stimuli, responses to growth factors, and blood vessel development. It is well known that pulmonary artery endothelial cell (PAEC) dysfunction is the most common inducer of the occurrence and progression of PAH, and that VEGF signaling contributes to these functional processes.35 Chakraborty et al35 discovered that the loss of microvessels and the reduction ofVEGF-induced tip cell formation were crucial mechanisms in PAH.36 We interpreted the enrichment results of downregulated genes as indicating a decrease in vascular VEGF signaling. Consequently, we conducted a search forTFs that regulate VEGF-A, B, and C, identifying 7 common TFs with validated functions that overlapped with DEGs. Among these common TFs, ATM and ZNF24 are involved in repressing VEGF-A transcription. These 2 genes could potentially serve as targets for modulating reduced VEGF signaling.
Recent studies have confirmed that patients with PAH exhibit iron metabolic abnormalities, which are correlated with a decline in athletic performance, decreased survival rates, and the worsening of clinical symptoms.5,37 Ferroptosis is precisely regulated at multiple levels, including the epigenetic, transcriptional, and post-transcriptional levels. Zhang and colleagues identified 8 ferroptosis-related genes in lung tissue from patients with PAH, which included 4 driver genes (IDH1, DPP4, HIF1A, ACSL4) and 3 suppressor genes (SLC7A11, HIF1A, PLIN2). Their results indicated that both promoting and inhibiting factors coexisted in the progression of ferroptosis.5 In our study, we identified 24 ferroptosis-related genes. Eleven genes (ACSL4, CARS, CS, DPP4, EMC2, FANCD2, HSPA5, NCOA4, NFE2L2, SLC7A11, and TFRC) were significantly upregulated in 15 PAH samples compared to the control samples, while GPX4, HSPB1, LPCAT3, and RPL8 were significantly downregulated. The results of the independent comparison of ferroptosis-related genes between the PAH samples and control samples differed from those obtained through the DEGs screening. The significance of the comparison results between the 2 sample groups was assessed using the Wilcoxon test, whereas in the DEGs screening process, we established a threshold of |Log2 (Fold Change)| > 1.5. Importantly, NFE2L2 has been identified as the central regulatory target of isorhamnetin, ferroptosis, and DEGs associated with PAH. The complex alterations in cytokine activation, inflammation, cellular immunity, and autoantibody responses suggest that PAH is, in part, an autoimmune and inflammatory disease.13,38 In the current study, we performed an immune infiltration analysis and assessed the expression levels of immune checkpoints. We observed a significant increase in the immune infiltration score in PAH samples compared to control samples. Furthermore, there were notable changes in the expression levels of immune checkpoints. The dysregulation of these immune checkpoints indicates abnormal immune responses and contributes to the pathophysiology of PAH.
Isorhamnetin (3-methylquercetin) is a natural compound that belongs to the class of 3-O-methylated metabolites of quercetin. Isorhamnetin demonstrates both preventive and therapeutic effects on cardiovascular and cerebrovascular diseases, including anti-atherosclerotic properties, protection of endothelial cells, anti-myocardial ischemia effects, anti-hypotension actions, anti-hypoglycemic effects, and anti-thrombotic properties, among others.13 The mechanisms underlying these beneficial effects are associated with its antioxidant, anti-inflammatory, and anti-mitochondrial-dependent effects on cell apoptosis.10-15 REN et al14 found that isorhamnetin significantly improved outcomes in acute lung injury, asthma, and non-small cell lung cancer. Isorhamnetin can suppress the production of reactive oxygen species (ROS), mitochondrial arachidonic acid (AA) and iron-induced dysfunction, as well as glutathione (GSH) reduction. In our study, we identified 58 target genes that interact with isorhamnetin. These target genes intersected with DEGs associated with PAH, ferroptosis-related genes, and the transcription factor NFE2L2. NFE2L2 was initially recognized as a crucial regulator of redox homeostasis in cells. Subsequent research has revealed that NFE2L2 is also responsible for maintaining protein homeostasis, regulating the pentose phosphate pathway, and facilitating amino acid and carbohydrate metabolism.39-42
Previous studies have indicated that NFE2L2 plays a crucial role in enhancing the body’s defense against ferroptosis. NFE2L2 regulates iron balance and the ferroptosis process through HERC2, VAMP8, and NCOA4. Elevated levels of NFE2L2 amplify its effects.43 Consequently, we conducted molecular docking studies to investigate the binding capabilities of isorhamnetin to NFE2L2 and other transcription factors (VEGFR, ATM, and ZNF24) associated with VEGF signaling. Our findings revealed that isorhamnetin exhibited strong affinities for these crucial regulators of PAH. These results suggest that isorhamnetin could be a promising natural compound in the fight against PAH.
Conclusion
Based on the results of the current analysis, we found that PAH is characterized by a series of abnormalities in downstream molecular signaling pathways, including DNA damage, immune dysregulation, VEGF signaling deficiency, and the ferroptosis process. These factors may be the core pathophysiological mechanisms of PAH. Ferroptosis-related genes, such as NFE2L2 and TF (including ATM and ZNF24) associated with VEGF signaling, serve as potential candidate therapeutic targets that contribute to the mechanisms mentioned above. Isorhamnetin is identified as a candidate compound for the treatment of PAH.
Funding Statement
The authors declared that this study has received no financial support.
Footnotes
Ethics Committee Approval: Not applicable. All original materials can be obtained from the PubMed database (GSE113439) and corresponding study (Mura et al16, Respirology 2019;24(11):1104-1110).
Peer-review: Internally peer reviewed.
Acknowledgments: We thank the providers of the protein structures in the PDB database (Sengoku et al28, Wiesmann et al27, Volkman et al., and Warren et al30), and to the providers of the microarray of PAH in the GEO database (Mura et al16).
Author Contributions: Concept – Y.L., C.S.; Design – Y.L.; Supervision – Y.L.; Resources – W.X., C.S., Y.L.; Materials – W.X., C.S., Y.L.; Data Collection and/or Processing – W.X., C.S., Y.L.; Analysis and/or Interpretation – W.X., C.S., Y.L.; Literature Search – W.X., C.S., Y.L.; Writing – W.X., C.S., Y.L.; Critical Review – C.S., Y.L.
Declaration of Interests: The authors have no conflict of interest to declare.
References
- 1. Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2023;61(1):1 144. ( 10.1183/13993003.00879-2022) [DOI] [PubMed] [Google Scholar]
- 2. Omura J, Habbout K, Shimauchi T, et al. Identification of long noncoding RNA H19 as a new biomarker and therapeutic target in right ventricular failure in pulmonary arterial hypertension. Circulation. 2020;142(15):1464 1484. ( 10.1161/CIRCULATIONAHA.120.047626) [DOI] [PubMed] [Google Scholar]
- 3. Sumneang N, Siri-Angkul N, Kumfu S, Chattipakorn SC, Chattipakorn N. The effects of iron overload on mitochondrial function, mitochondrial dynamics, and ferroptosis in cardiomyocytes. Arch Biochem Biophys. 2020;680:108241. ( 10.1016/j.abb.2019.108241) [DOI] [PubMed] [Google Scholar]
- 4. Zou HX, Qiu BQ, Lai SQ, et al. Iron metabolism and idiopathic pulmonary arterial hypertension: new insights from bioinformatic analysis. BioMed Res Int. 2021;2021:5669412. ( 10.1155/2021/5669412) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang F, Liu H. Identification of ferroptosis-associated genes exhibiting altered expression in pulmonary arterial hypertension. Math Biosci Eng. 2021;18(6):7619 7630. ( 10.3934/mbe.2021377) [DOI] [PubMed] [Google Scholar]
- 6. Miyamoto HD, Ikeda M, Ide T, et al. Iron Overload via heme Degradation in the endoplasmic reticulum Triggers Ferroptosis in myocardial ischemia-reperfusion Injury. JACC Basic Transl Sci. 2022;7(8):800 819. ( 10.1016/j.jacbts.2022.03.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Günes Günsel G, Conlon TM, Jeridi A, et al. The arginine methyltransferase PRMT7 promotes extravasation of monocytes resulting in tissue injury in COPD. Nat Commun. 2022;13(1):1303. ( 10.1038/s41467-022-28809-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xie SS, Deng Y, Guo SL, et al. Endothelial cell ferroptosis mediates monocrotaline-induced pulmonary hypertension in rats by modulating NLRP3 inflammasome activation. Sci Rep. 2022;12(1):3056. ( 10.1038/s41598-022-06848-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cristina Marcarini J, Ferreira Tsuboy MS, Cabral Luiz R, Regina Ribeiro L, Beatriz Hoffmann-Campo C, Ségio Mantovani M. Investigation of cytotoxic, apoptosis-inducing, genotoxic and protective effects of the flavonoid rutin in HTC hepatic cells. Exp Toxicol Pathol. 2011;63(5):459 465. ( 10.1016/j.etp.2010.03.005) [DOI] [PubMed] [Google Scholar]
- 10. Dong GZ, Lee JH, Ki SH, et al. AMPK activation by isorhamnetin protects hepatocytes against oxidative stress and mitochondrial dysfunction. Eur J Pharmacol. 2014;740:634 640. ( 10.1016/j.ejphar.2014.06.017) [DOI] [PubMed] [Google Scholar]
- 11. Chi G, Zhong W, Liu Y, et al. Isorhamnetin protects mice from lipopolysaccharide-induced acute lung injury via the inhibition of inflammatory responses. Inflamm Res. 2016;65(1):33 41. ( 10.1007/s00011-015-0887-9) [DOI] [PubMed] [Google Scholar]
- 12. Sun M, Deng R, Wang Y, et al. Sphingosine kinase 1/sphingosine 1-phosphate/sphingosine 1-phosphate receptor 1 pathway: A novel target of geniposide to inhibit angiogenesis. Life Sci. 2020;256:117988. ( 10.1016/j.lfs.2020.117988) [DOI] [PubMed] [Google Scholar]
- 13. Gong G, Guan YY, Zhang ZL, et al. Isorhamnetin: a review of pharmacological effects. Biomed Pharmacother. 2020;128:110301. ( 10.1016/j.biopha.2020.110301) [DOI] [PubMed] [Google Scholar]
- 14. Ren X, Han L, Li Y, et al. Isorhamnetin attenuates TNF-α-induced inflammation, proliferation, and migration in human bronchial epithelial cells via MAPK and NF-κB pathways. Anat Rec (Hoboken). 2021;304(4):901 913. ( 10.1002/ar.24506) [DOI] [PubMed] [Google Scholar]
- 15. Li S, Cheng CS, Zhang C, et al. Edible and herbal plants for the prevention and management of COVID-19. Front Pharmacol. 2021;12:656103. ( 10.3389/fphar.2021.656103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mura M, Cecchini MJ, Joseph M, Granton JT. Osteopontin lung gene expression is a marker of disease severity in pulmonary arterial hypertension. Respirology. 2019;24(11):1104 1110. ( 10.1111/resp.13557) [DOI] [PubMed] [Google Scholar]
- 17. Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics. 2012;16(5):284 287. ( 10.1089/omi.2011.0118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu Z, Zhao Q, Zuo ZX, et al. Systematic analysis of the aberrances and functional implications of ferroptosis in cancer. iScience. 2020;23(7):101302. ( 10.1016/j.isci.2020.101302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou Y, Zhou B, Pache L, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10(1):1523. ( 10.1038/s41467-019-09234-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Han H, Cho JW, Lee S, et al. TRRUST v2: an expanded reference database of human and mouse transcriptional regulatory interactions. Nucleic Acids Res. 2018;46(D1):D380 D386. ( 10.1093/nar/gkx1013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miao YR, Zhang Q, Lei Q, et al. ImmuCellAI: A unique method for comprehensive T-cell subsets abundance prediction and its application in cancer immunotherapy. Adv Sci (Weinh). 2020;7(7):1902880. ( 10.1002/advs.201902880) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miao YR, Xia M, Luo M, Luo T, Yang M, Guo AY. ImmuCellAI-mouse: a tool for comprehensive prediction of mouse immune cell abundance and immune microenvironment depiction. Bioinformatics. 2022;38(3):785 791. ( 10.1093/bioinformatics/btab711) [DOI] [PubMed] [Google Scholar]
- 23. Zeng D, Li M, Zhou R, et al. Tumor microenvironment characterization in gastric cancer identifies prognostic and immunotherapeutically relevant gene signatures. Cancer Immunol Res. 2019;7(5):737 750. ( 10.1158/2326-6066.CIR-18-0436) [DOI] [PubMed] [Google Scholar]
- 24. Wang J, Sun J, Liu LN, et al. Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat Med. 2019;25(4):656 666. ( 10.1038/s41591-019-0374-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deng S, Zhang Y, Wang H, et al. ITPRIPL1 binds CD3ε to impede T cell activation and enable tumor immune evasion. Cell. April 25 2024;187(9):2305 2323.e33. ( 10.1016/j.cell.2024.03.019) [DOI] [PubMed] [Google Scholar]
- 26. Davis AP, Wiegers TC, Johnson RJ, Sciaky D, Wiegers J, Mattingly CJ. Comparative Toxicogenomics Database (CTD): update 2023. Nucleic Acids Res. 2023;51(D1):D1257 D1262. ( 10.1093/nar/gkac833) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wiesmann C, Fuh G, Christinger HW, Eigenbrot C, Wells JA, de Vos AM. Crystal structure at 1.7 A resolution of VEGF in complex with domain 2 of the Flt-1 receptor. Cell. 1997;91(5):695 704. ( 10.1016/s0092-8674(00)80456-0) [DOI] [PubMed] [Google Scholar]
- 28. Sengoku T, Shiina M, Suzuki K, et al. Structural basis of transcription regulation by CNC family transcription factor, Nrf2. Nucleic Acids Res. 2022;50(21):12543 12557. ( 10.1093/nar/gkac1102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eberhardt J, Santos-Martins D, Tillack AF, Forli S, AutoDock V. AutoDock Vina 1.2.0: New docking methods, expanded force field, and python bindings. J Chem Inf Model. 2021;61(8):3891 3898. ( 10.1021/acs.jcim.1c00203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Warren C, Pavletich NP. Structure of the human ATM kinase and mechanism of Nbs1 binding. eLife. 2022;11. ( 10.7554/eLife.74218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ravindranath PA, Forli S, Goodsell DS, Olson AJ, Sanner MF. AutoDockFR: advances in protein-ligand docking with explicitly specified Binding Site flexibility. PLOS Comput Biol. 2015;11(12):e1004586. ( 10.1371/journal.pcbi.1004586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fu L, Shi S, Yi J, et al. ADMETlab 3.0: an updated comprehensive online ADMET prediction platform enhanced with broader coverage, improved performance, API functionality and decision support. Nucleic Acids Res. 2024;52(W1):W422 W431. ( 10.1093/nar/gkae236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li B, Rui J, Ding X, Yang X. Exploring the multicomponent synergy mechanism of Banxia Xiexin Decoction on irritable bowel syndrome by a systems pharmacology strategy. J Ethnopharmacol. 2019;233:158 168. ( 10.1016/j.jep.2018.12.033) [DOI] [PubMed] [Google Scholar]
- 34. Ma C, Wang X, Zhang L, et al. Super enhancer-associated circular RNA-CircKrt4 regulates hypoxic pulmonary artery endothelial cell dysfunction in mice. Arterioscler Thromb Vasc Biol. 2023;43(7):1179 1198. ( 10.1161/ATVBAHA.122.318842) [DOI] [PubMed] [Google Scholar]
- 35. Chakraborty A, Nathan A, Orcholski M, et al. Wnt7a deficit is associated with dysfunctional angiogenesis in pulmonary arterial hypertension. Eur Respir J. 2023;61(6):1 47. ( 10.1183/13993003.01625-2022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lan M, Wu S, Fernandes TM. Iron deficiency and pulmonary arterial hypertension. Nutr Clin Pract. 2022;37(5):1059 1073. ( 10.1002/ncp.10884) [DOI] [PubMed] [Google Scholar]
- 37. Thenappan T, Ormiston ML, Ryan JJ, Archer SL. Pulmonary arterial hypertension: pathogenesis and clinical management. BMJ. 2018;360:j5492. ( 10.1136/bmj.j5492) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38(4):769 789. ( 10.1080/03602530600971974) [DOI] [PubMed] [Google Scholar]
- 39. Mitsuishi Y, Taguchi K, Kawatani Y, et al. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell. 2012;22(1):66 79. ( 10.1016/j.ccr.2012.05.016) [DOI] [PubMed] [Google Scholar]
- 40. Pajares M, Jiménez-Moreno N, García-Yagüe ÁJ, et al. Transcription factor NFE2L2/NRF2 is a regulator of macroautophagy genes. Autophagy. 2016;12(10):1902 1916. ( 10.1080/15548627.2016.1208889) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu P, Dodson M, Li H, et al. Non-canonical NRF2 activation promotes a pro-diabetic shift in hepatic glucose metabolism. Mol Metab. 2021;51:101243. ( 10.1016/j.molmet.2021.101243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Anandhan A, Dodson M, Shakya A, et al. NRF2 controls iron homeostasis and ferroptosis through HERC2 and VAMP8. Sci Adv. 2023;9(5):eade9585. ( 10.1126/sciadv.ade9585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang JH, Shin BY, Han JY, et al. Isorhamnetin protects against oxidative stress by activating Nrf2 and inducing the expression of its target genes. Toxicol Appl Pharmacol. 2014;274(2):293 301. ( 10.1016/j.taap.2013.10.026) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a