Abstract
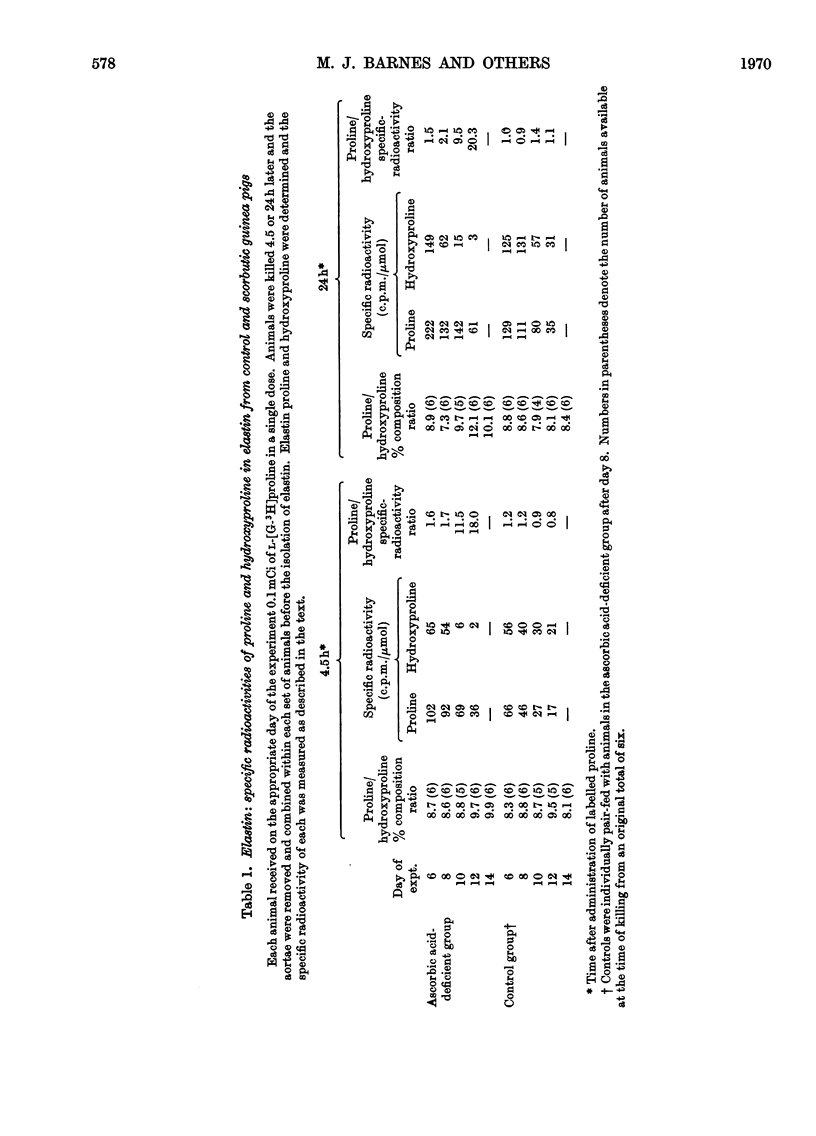
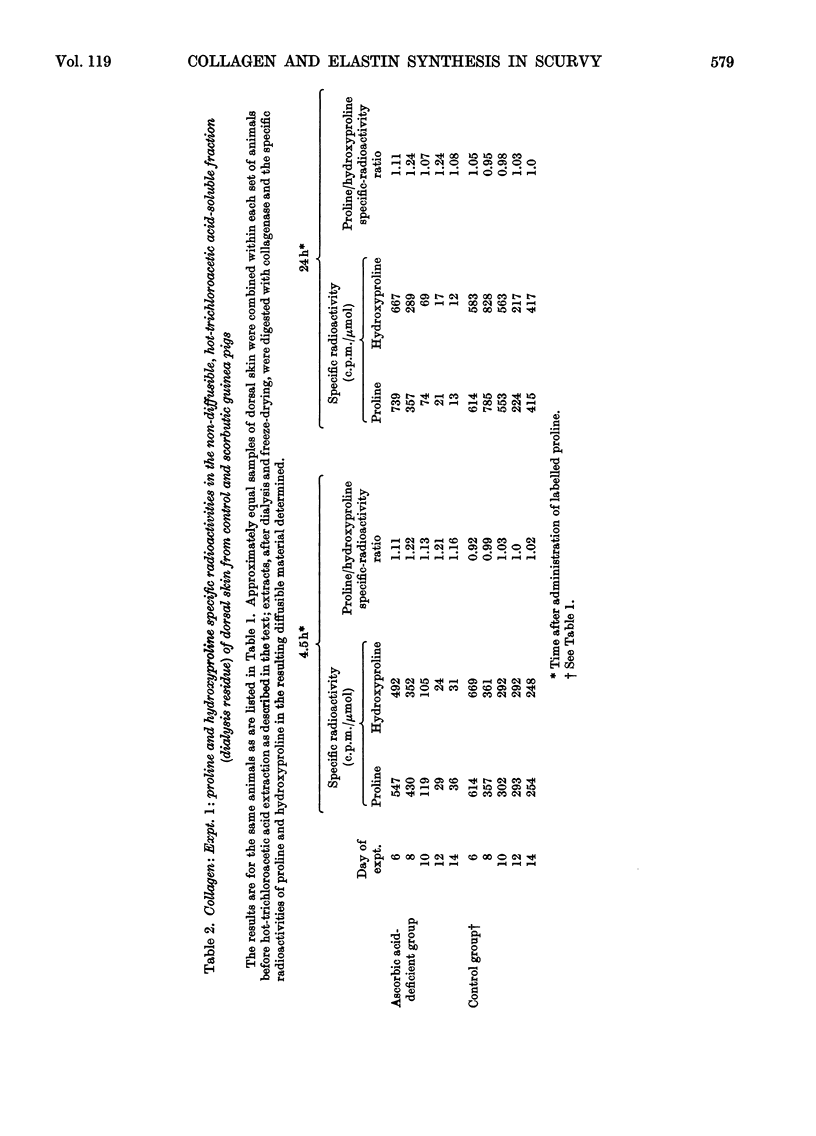
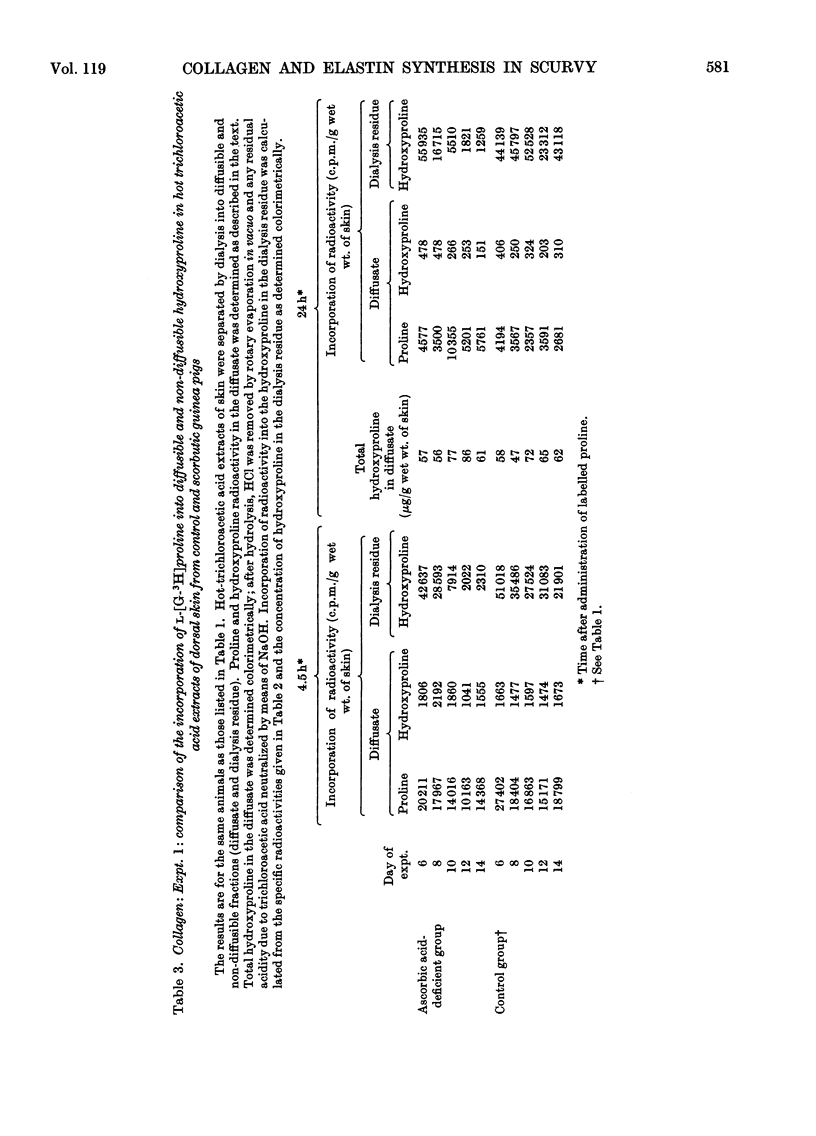
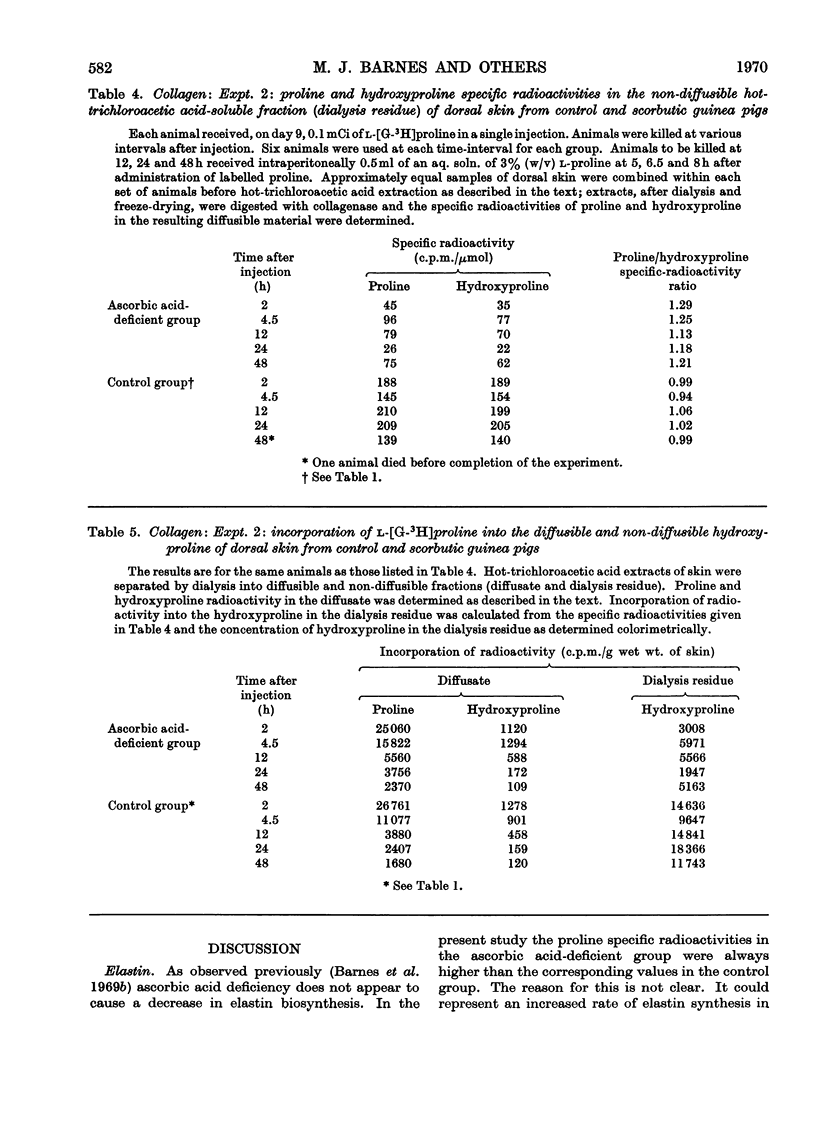
1. After the administration of l-[G-3H]proline to guinea pigs deprived of ascorbic acid for increasing periods of time, the specific radioactivities of proline and hydroxyproline in skin collagen and aortic elastin were determined at various time-intervals after administration of the labelled compound with a view to studying the formation and degradation of collagen and elastin both deficient in hydroxyproline. 2. As judged from the incorporation of radioactivity into elastin proline, elastin synthesis was not decreased in the ascorbic acid-deficient animals. There was however, a rapid decline in the specific radioactivity of elastin hydroxyproline. The proline/hydroxyproline specific-radioactivity ratio was approx. 1.5:1 after 6 days and 20:1 after 12 days of ascorbic acid deprivation, in contrast with the ratio of 1:1 in controls. The results suggested that the effect of ascorbic acid deficiency on elastin biosynthesis could be regarded as simply an elimination of hydroxylation of elastin proline with the formation and retention of a polymer increasingly deficient in hydroxyproline. 3. Collagen proline and hydroxyproline specific radioactivities were derived from material that was soluble in hot trichloroacetic acid, non-diffusible and collagenase-degradable. In contrast with elastin, there was a rapid decline in the specific radioactivity of proline as well as hydroxyproline in collagen from the ascorbic acid-deficient animals. However, the proline/hydroxyproline specific-radioactivity ratio in all samples from scorbutic animals was consistently slightly above 1:1. The results suggest the appearance in place of collagen, but in rapidly diminishing amounts, of a partially hydroxylated collagen in which the degree of hydroxylation may be decreased only by approx. 10%. 4. Incorporation of radioactivity into the diffusible hydroxyproline in skin remained relatively high despite the rapid decline in the incorporation of radioactivity into collagen. This observation is interpreted as indicative of an increasing degree of degradation of partially hydroxylated collagen to diffusible peptides. An alternative explanation might be that partially hydroxylated peptides are released to an increasing extent from ribosomes before they attain a length at least sufficient to render them non-diffusible. In either case it implies the accumulation in scurvy of low-molecular-weight peptides enriched in proline and deficient in hydroxyproline and could explain the failure to accumulate a high-molecular-weight collagen deficient in hydroxyproline. 5. It is thought, however, that, in addition, an inhibition of ribosomal amino acid incorporation leading to decreased synthesis of partially hydroxylated collagen may also occur, perhaps secondarily to impaired hydroxylation.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey A. J., Fowler L. J., Peach C. M. Identification of two interchain crosslinks of bone and dentine collagen. Biochem Biophys Res Commun. 1969 Jun 6;35(5):663–671. doi: 10.1016/0006-291x(69)90456-2. [DOI] [PubMed] [Google Scholar]
- Bailey A. J., Peach C. M. Isolation and structural identification of a labile intermolecular crosslink in collagen. Biochem Biophys Res Commun. 1968 Dec 9;33(5):812–819. doi: 10.1016/0006-291x(68)90233-7. [DOI] [PubMed] [Google Scholar]
- Barnes M. J., Constable B. J., Kodicek E. Excretion of hydroxyproline and other amino acids in scorbutic guinea-pigs. Biochim Biophys Acta. 1969 Jul 30;184(2):358–365. doi: 10.1016/0304-4165(69)90038-5. [DOI] [PubMed] [Google Scholar]
- Barnes M. J., Constable B. J., Kodicek E. Studies in vivo on the biosynthesis of collagen and elastin in ascorbic acid-deficient guinea pigs. Biochem J. 1969 Jun;113(2):387–397. doi: 10.1042/bj1130387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley J. P., Hanson A. N. The hydroxyproline of elastin. Biochim Biophys Acta. 1969 Mar;175(2):339–344. doi: 10.1016/0005-2795(69)90011-7. [DOI] [PubMed] [Google Scholar]
- Bornstein P. Comparative sequence studies of rat skin and tendon collagen. I. Evidence for incomplete hydroxylation of individual prolyl residues in the normal proteins. Biochemistry. 1967 Oct;6(10):3082–3093. doi: 10.1021/bi00862a015. [DOI] [PubMed] [Google Scholar]
- Butler W. T. Partial hydroxylation of certain lysines in collagen. Science. 1968 Aug 23;161(3843):796–798. doi: 10.1126/science.161.3843.796. [DOI] [PubMed] [Google Scholar]
- Chou W. S., Savage J. E., O'Dell B. L. Role of copper in biosynthesis of intramolecular cross-links in chick tendon collagen. J Biol Chem. 1969 Nov 10;244(21):5785–5789. [PubMed] [Google Scholar]
- Cooper G. W., Prockop D. J. Intracellular accumulation of protocollagen and extrusion of collagen by embryonic cartilage cells. J Cell Biol. 1968 Sep;38(3):523–537. doi: 10.1083/jcb.38.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh K., Nimni M. E. A defect in the intramolecular and intermolecular cross-linking of collagen caused by penicillamine. II. Functional groups involved in the interaction process. J Biol Chem. 1969 Apr 10;244(7):1787–1795. [PubMed] [Google Scholar]
- Efron M. L., Bixby E. M., Hockaday T. D., Smith L. H., Jr, Meshorer E. Hydroxyprolinemia. 3. The origin of free hydroxyproline in hydroxyprolinemia. Collagen turnover. Evidence for biosynthetic pathway in man. Biochim Biophys Acta. 1968 Sep 3;165(2):238–250. doi: 10.1016/0304-4165(68)90052-4. [DOI] [PubMed] [Google Scholar]
- GOULD B. S., MANNER G., GOLDMAN H. M., STOLMAN J. M. Some aspects of collagen formation. Ann N Y Acad Sci. 1960 Mar 29;85:385–398. doi: 10.1111/j.1749-6632.1960.tb49969.x. [DOI] [PubMed] [Google Scholar]
- GROSS J. Studies on the formation of collagen. IV. Effect of vitamin C deficiency on the neutral salt-extractible collagen of skin. J Exp Med. 1959 Jun 1;109(6):557–569. doi: 10.1084/jem.109.6.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb A. A., Kaplan A., Udenfriend S. Further evidence for the accumulation of a hydroxyproline-deficient, collagenase-degradable protein during collagen biosynthesis in vitro. J Biol Chem. 1966 Apr 10;241(7):1551–1555. [PubMed] [Google Scholar]
- HAUSMANN E., NEUMAN W. F. Conversion of proline to hydroxyproline and its incorporation into collagen. J Biol Chem. 1961 Jan;236:149–152. [PubMed] [Google Scholar]
- HURYCH J., SHVAPIL M. INFLUENCE OF CHELATING AGENTS ON THE BIOSYNTHESIS OF COLLAGEN. Biochim Biophys Acta. 1965 Feb 15;97:361–363. doi: 10.1016/0304-4165(65)90108-x. [DOI] [PubMed] [Google Scholar]
- Hagopian A., Bosmann H. B., Eylar E. H. Glycoprotein biosynthesis: the localization of polypeptidyl: N-acetylgalactosaminyl, collagen: glucosyl, and glycoprotein:galactosyl transferases in HeLa cell membrane fractions. Arch Biochem Biophys. 1968 Nov;128(2):387–396. doi: 10.1016/0003-9861(68)90045-3. [DOI] [PubMed] [Google Scholar]
- Hausmann E. Cofactor requirements for the enzymatic hydroxylation of lysine in a polypeptide precursor of collagen. Biochim Biophys Acta. 1967 Apr 11;133(3):591–593. doi: 10.1016/0005-2795(67)90566-1. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Prockop D. J. Enzymatic hydroxylation of proline and lysine in protocollagen. Proc Natl Acad Sci U S A. 1967 Mar;57(3):782–789. doi: 10.1073/pnas.57.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVENE C. I., GROSS J. Alterations in state of molecular aggregation of collagen induced in chick embryos by beta-aminopropionitrile (lathyrus factor). J Exp Med. 1959 Nov 1;110:771–790. doi: 10.1084/jem.110.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITOMA C., SMITH T. E. Studies on the role of ascorbic acid in collagen synthesis. J Biol Chem. 1960 Feb;235:426–428. [PubMed] [Google Scholar]
- PETERKOFSKY B., UDENFRIEND S. ENZYMATIC HYDROXYLATION OF PROLINE IN MICROSOMAL POLYPEPTIDE LEADING TO FORMATION OF COLLAGEN. Proc Natl Acad Sci U S A. 1965 Feb;53:335–342. doi: 10.1073/pnas.53.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads R. E., Udenfriend S. Substrate specificity of collagen proline hydroxylase: hydroxylation of a specific proline residue in bradykinin. Arch Biochem Biophys. 1969 Aug;133(1):108–111. doi: 10.1016/0003-9861(69)90493-7. [DOI] [PubMed] [Google Scholar]
- Ross R. The fibroblast and wound repair. Biol Rev Camb Philos Soc. 1968 Feb;43(1):51–96. doi: 10.1111/j.1469-185x.1968.tb01109.x. [DOI] [PubMed] [Google Scholar]
- Rucker R. B., Parker H. E., Rogler J. C. Effect of copper deficiency on chick bone collagen and selected bone enzymes. J Nutr. 1969 May;98(1):57–63. doi: 10.1093/jn/98.1.57. [DOI] [PubMed] [Google Scholar]
- STONE N., MEISTER A. Function of ascorbic acid in the conversion of proline to collagen hydroxyproline. Nature. 1962 May 12;194:555–557. doi: 10.1038/194555a0. [DOI] [PubMed] [Google Scholar]
- Sandberg L. B., Weissman N., Smith D. W. The purification and partial characterization of a soluble elastin-like protein from copper-deficient porcine aorta. Biochemistry. 1969 Jul;8(7):2940–2945. doi: 10.1021/bi00835a037. [DOI] [PubMed] [Google Scholar]
- Udenfriend S. Formation of hydroxyproline in collagen. Science. 1966 Jun 3;152(3727):1335–1340. doi: 10.1126/science.152.3727.1335. [DOI] [PubMed] [Google Scholar]
- VAN ROBERTSON W. B., HIWETT J., HERMAN C. The relation of ascorbic acid to the conversion of proline to hydroxyproline in the synthesis of collagen in the carrageenan granuloma. J Biol Chem. 1959 Jan;234(1):105–108. [PubMed] [Google Scholar]
- WEISSMAN N., SHIELDS G. S., CARNES W. H. CARDIOVASCULAR STUDIES ON COPPER-DEFICIENT SWINE. IV. CONTENT AND SOLUBILITY OF THE AORTIC ELASTIN, COLLAGEN, AND HEXOSAMINE. J Biol Chem. 1963 Sep;238:3115–3118. [PubMed] [Google Scholar]


