Abstract
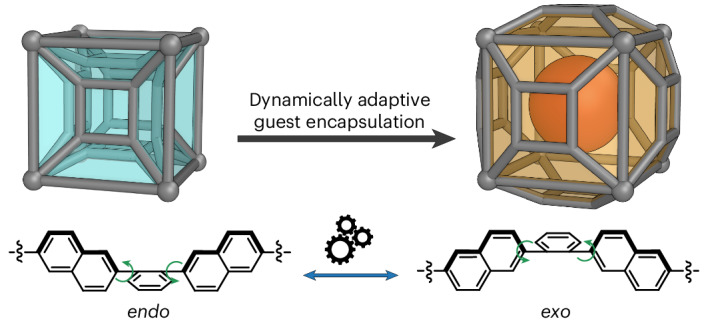
The creation of hosts capable of accommodating different guest molecules may enable these hosts to play useful roles in chemical purifications, among other applications. Metal–organic cages are excellent hosts for various guests, but they generally incorporate rigid structural units that hinder dynamic adaptation to specific guests. Here we report a conformationally adaptable pseudo-cubic cage that can dynamically increase its cavity volume to fit guests with differing sizes. This pseudo-cube incorporates a tetramine subcomponent with 2,6-naphthalene arms that cooperatively adopt a non-planar conformation, enabling the cage faces to switch between endo and exo states. A wide range of guest molecules were observed to bind within the cavity of this cage, spanning a range of sizes from 46% to 154% of the cavity volume of the empty cage. Experimental and computational evidence characterizes the flipping of cage faces from endo to exo, expanding the cavity upon binding of larger guests.
Subject terms: Molecular capsules, Self-assembly, Coordination chemistry
Synthetic nanocages that can adapt the size and shape of their cavity in response to a given guest have potential applications in various areas, including chemical purification. Now a flexible, pseudo-cubic metal–organic cage has been developed that is able to dynamically expand its cavity from 46% to 154% of its initial volume by flipping its cage faces.
Main
Biological molecules dynamically adapt their conformations to undertake their functions. For example, DNA supercoils enable the cell packaging and regulation of replication and transcription processes1,2. RNA adopts a loose chain configuration to transcribe information but folds to carry nucleic acids or to incorporate into ribosomes3, whereas proteins undergo conformational changes to bind ligands and catalyse or inhibit reactions4,5. The tremendous range of functions for these biomolecules thus depends on their ability to adapt their conformations.
Host–guest chemistry and molecular recognition likewise underpin a diverse array of functions in biology and chemistry6–8. Many artificial host molecules have been developed9–14, including polyhedral metal–organic cages, whose well-defined cavities serve as binding pockets11,12. Guest binding may be driven by solvophobic effects15,16, hydrogen bonding17,18, Coulombic interactions19, halogen bonding20 and arene stacking21,22. As described by Fischer’s lock-and-key principle23, size and shape complementarity between binding sites and guest molecules has a pivotal role in determining binding affinities24–26.
Enhancing the binding versatility of a supramolecular host can reduce the effort required to create a new receptor for a given guest molecule. More versatile hosts may be prepared by creating systems that involve variable numbers of assembling units27, incorporating structural adaptability through the introduction of mechanical bonds28,29, creating hosts with chemical switchability30–32 or imparting inherent adaptability to the structures33–35. In the field of metal–organic cages, the quest for cage designs that combine conformational flexibility with structural integrity remains an important challenge. In accordance with Koshland’s ‘induced fit’ theory, conformationally adaptable host molecules, with structural dynamics resembling those of proteins, may be able to reconfigure in the presence of different guests to optimize binding affinity36. However, the entropic demands associated with the assembly of metal–organic cages necessitate preorganization, highlighting the need to balance structural rigidity with adaptability37. Reported cases of metal–organic cages incorporating such adaptability are few, with strategies including the introduction of flexible ligands35,38, stereochemically adaptable vertices39,40 or geometrically flexible frameworks41–44. Here, we report the synthesis of a Zn8L6 pseudo-cubic cage with inherently non-planar faces, which can adopt endo or exo states. As each face can switch independently from exo to endo, the metal–organic cage possesses the capability to adjust the size and shape of its cavity in response to the specific molecules it binds, ensuring a versatile and optimized guest encapsulation. This work demonstrates that incorporating conformational flexibility into metal–organic cages is feasible while producing only a single discrete assembly and preventing the formation of coordination polymers, thus addressing an enduring challenge in the design of such adaptable systems.
Results and discussion
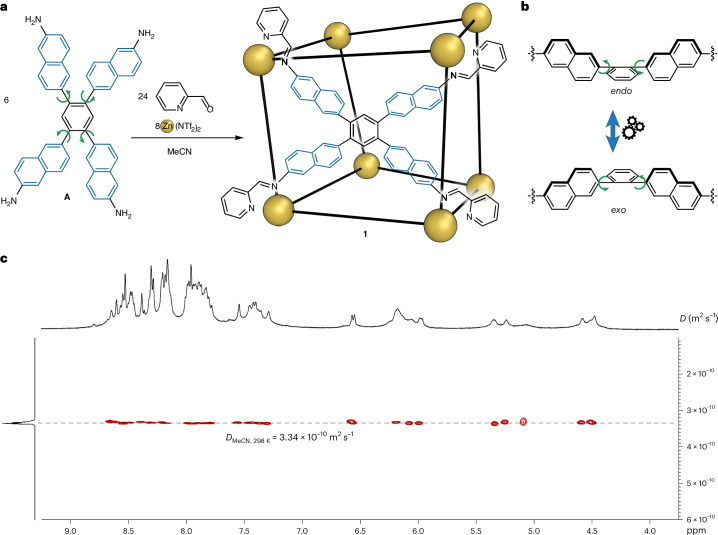
We sought to generate a conformationally adaptive cage by incorporating rotational subunits into the ligand arms. To implement this concept, the naphthalene-armed tetramine subcomponent A was designed and synthesized via a two-step route in 72% overall yield (Supplementary Scheme 1). Based on our previous work with rectangular tetramine subcomponents43, we hypothesized that subcomponent A would produce a pseudo-cubic cage with 2-formylpyridine and ZnII. The four 2,6-naphthyl groups panelling each face of the cage were designed to rotate so as to generate non-planar faces. This rotation could help in accommodating a guest, whereby each face may adopt an exo or endo configuration (Fig. 1a). The exo and endo ligand configurations are expected to have the same metal–metal distances, thus allowing each face to switch independently without introducing strain into the overall cage framework.
Fig. 1. Preparation and characterization of cage 1.
a, The synthesis of pseudo-cube 1 from tetramine subcomponent A. b, The two conformations of each face, as a result of the 2,6-naphthalyl rotational units. c, 1H NMR and 1H DOSY spectra of pseudo-cube 1, revealing a diffusion coefficient (D) of 3.34 × 10−10 m2 s−1 (400 MHz, 298 K, CD3CN).
Synthesis and characterization of pseudo-cube 1
The self-assembly of tetramine subcomponent A (6 equiv.), zinc(II) bis(trifluoromethanesulfonyl)imide (triflimide, NTf2−) (8 equiv.) and 2-formylpyridine (24 equiv.) generated ZnII8L6 cage 1, as confirmed by nuclear magnetic resonance (NMR) (Supplementary Figs. 1–16) and high-resolution electrospray ionization mass spectrometry (ESI-HRMS) experiments (Fig. 1b,c and Supplementary Fig. 17). After heating at 343 K overnight in CH3CN, all the 1H NMR signals of the product exhibited the same diffusion constant in the 1H diffusion-ordered spectroscopy (DOSY) NMR spectrum (Fig. 1c). Four sets of magnetically inequivalent ligand arm signals were observed (Supplementary Figs. 7–16), indicating the possible presence of a C3 symmetry axis, consistent with overall D3 or S6 point symmetry45. The ESI-HRMS spectrum of 1 indicated a Zn8L6(NTf2)16 composition (Supplementary Fig. 17). Single-crystal X-ray diffraction analysis confirmed the pseudo-cube structure of cage 1, with D3 point symmetry (Fig. 2, Supplementary Fig. 18 and Supplementary Table 1). Both enantiomers are present in the crystal structure. In one (Fig. 2), two antipodal metal vertices exhibited Λ handedness, with the others adopting Δ stereochemistry. Each face is enclosed by a tetratopic pyridyl-imine ligand incorporating tetramine A, with short and long axes of the tetratopic ligand mismatching in 6 out of the 12 edges of the pseudo-cube.
Fig. 2. Single-crystal structure of cage 1.

a, Oblique view of the crystal structure of 1, showing the Molovol44-calculated cavity in cyan and a cartoon representation of its all-endo conformation. b, Front view of the crystal structure, showing its all-endo conformation. C, grey; N, blue; fac-Δ-Zn, yellow; fac-Λ-Zn, purple. Hydrogens, counterions and solvent molecules have been omitted for clarity.
The crystal structure of cage 1 verifies the presence of the non-planar facial conformations. As seen in Fig. 2, steric hindrance between neighbouring naphthyl units led to a configuration in which the central phenyl moieties of each face protrude towards the cage interior. We label this face conformation endo, and the alternative arrangement, where the central phenyl is projected outwards from the centre, exo. Because steric hindrance between naphthyl groups precludes a face from adopting a planar conformation, each face of 1 may thus be assigned to either an endo or exo conformation.
Entropic factors may account for the all-endo initial facial conformation in the case of empty 1. The all-endo conformation would minimize the cavity volume when no guest is bound, thereby releasing the maximum number of solvent molecules into solution. The six endo faces of 1 were found to enclose a single central cavity (Fig. 2a) with a volume of 389 Å3, as calculated using MoloVol46 (Supplementary Fig. 19). We infer that the dynamic switching of these faces between the two conformations has a key role in allowing 1 to bind a wider range of guests than would otherwise be possible, as discussed below.
Guest binding studies of 1
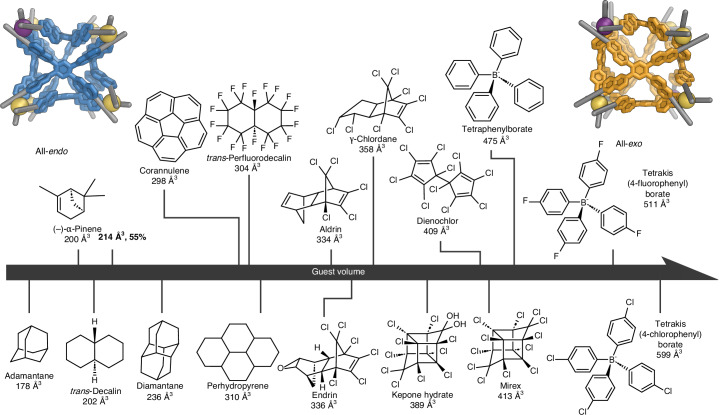
Host–guest studies revealed that 1 accommodated neutral and anionic guests of varying sizes (Fig. 3). The volumes of these guest molecules have been sphericity-corrected, spanning a range from 178 Å3 for adamantane to 599 Å3 for tetrakis(4-chlorophenyl)borate. This nearly fourfold volume difference between the smallest and largest guest encapsulated by 1 is remarkable compared with previous reports on flexible cages34,35,37,47. The addition of any of the guests shown in Fig. 3 to a solution of the cage resulted in either a shift of all 1H NMR signals or the emergence of at least one new set of signals for 1 (Supplementary Figs. 20–34). For all neutral guests, ESI-HRMS supported a 1:1 binding ratio, with distinct [H + G] peaks observed (Supplementary Figs. 35–47). No evidence for other binding ratios was observed. For the tetraarylborates, 1H NMR titrations suggested a 1:1 binding stoichiometry (Supplementary Figs. 48–53).
Fig. 3. The range of guest molecules used in this study, all of which exhibited 1:1 binding with 1.
The sphericity-corrected volumes of guests ranged from 178 to 599 Å3 (summarized in Supplementary Table 2), with larger guests flipping more faces of 1 from endo to exo during dynamically adaptive guest encapsulation. The all-endo structure here used for illustration is the single-crystal X-ray diffraction structure of 1. The all-exo structure is a structure of B(p-Cl-C6H4)−⊂1 optimized with GFN2-xTB53, which gave six exo faces in the minimization. C: grey, blue (for endo faces) or orange (for exo faces); fac-Δ-Zn: yellow; fac-Λ-Zn: purple. The host hydrogen atoms in both conformations and the guest in the all-exo structure are omitted, and the vertices including the imine-pyridyl ligand moieties have been simplified, for clarity.
In accordance with Rebek’s rule48, optimal binding with 1 would correspond to a guest molecule with a volume of approximately 214 Å3, which corresponds to 55% of the cavity volume of 1 in the crystal. Many of the guests shown in Fig. 3 exceed this volume, with the largest guest having a volume of 599 Å3, corresponding to 154% of the initial cavity volume of 1.
Conformational transformation of 1 upon guest binding
During investigations of guest binding within 1, in most cases, only one set of guest peaks was observed, implying fast guest exchange on the NMR timescale (Supplementary Fig. 20). However, we noted splitting of the cage signals into at least two sets, consistent with different environments in slow exchange on the NMR timescale. One host set corresponds to the original signals of the empty cage, whereas the other emerges upon guest addition and gradually intensifies over time. Since only a single set of shifted 1H NMR signals was observed for the guest molecule, we infer that the cage undergoes a structural reconfiguration, slow on the NMR timescale, allowing it to optimise its conformation to accommodate the guest. Directly studying the conformations of the cage using ¹H NMR in these cases proved challenging, so we turned to alternative techniques to elucidate these structural changes.
As our attempts to obtain X-ray-quality crystals of the host–guest complexes of 1 were not successful, we turned to 1H-1H nuclear Overhauser effect spectroscopy (NOESY) measurements to probe the conformations of the guest⊂1 complexes in solution. As shown in Fig. 4a,b, selectively irradiating at frequencies corresponding to guest signals revealed which host protons were in closest proximity to the guest. When a face is in an endo conformation, the f, g and h protons point to the interior of the cavity and will thus exhibit nuclear Overhauser effects (NOEs) with the guest, as observed during smaller guest binding. By contrast, exo faces have the j, k and i protons pointing inwards. These protons are thus adjacent to the guest when larger guests are bound. When the tetrakis(4-chlorophenyl)borate anion was bound, its phenylene protons showed NOEs with protons j and k (Fig. 4c,d). Minor NOE signals were also observed with protons g and i. No NOEs were observed between the guest and any imino-pyridine protons. We do not anticipate that the large tetrakis(4-chlorophenyl)borate anion could bind to the exterior of the cage without being in proximity to these protons. Hence, all these observations support the hypothesis that the cage face conformations switch to exo to accommodate this large anion within the cavity.
Fig. 4. One-dimensional (1D) NOESY spectra of host–guest complexes of 1, showing the host conformational changes upon guest binding.
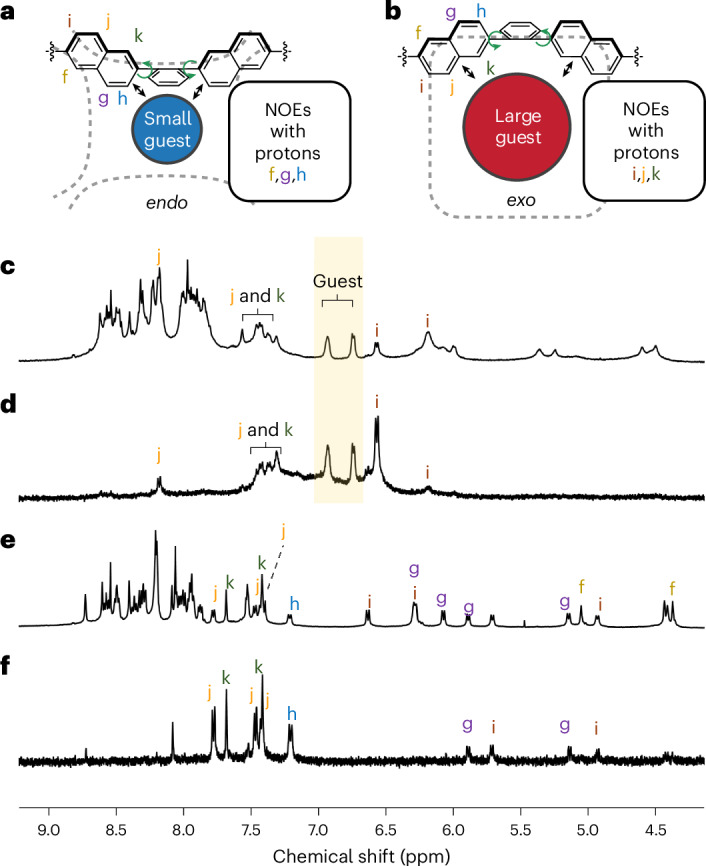
a,b, Illustration of different naphthalene protons to show NOEs between guest molecules and host panels in endo (a) versus exo (b) configurations: When a small guest is bound in proximity to an endo panel, protons f, g and h will exhibit NOEs with the guest. Larger guests will show NOEs with protons i, j and k with an exo host panel. c,d, 1H NMR spectrum (c) and 1D selective gradient NOESY spectrum (irradiated as highlighted at 7.03–6.69 ppm) (d) of B(p-Cl-C6H4) −⊂1. e,f, 1H NMR spectrum (e) and 1D selective gradient NOESY spectrum (irradiated at 2.08–2.00 ppm) (f) of diamantane⊂1.
In the case of diamantane (Fig. 4e,f), the observed NOEs suggest spatial proximity between the guest molecule and both sides of the host naphthalenes, indicating that 1 may adopt different conformations featuring both endo and exo faces to accommodate diamantane. Given the number of signals present, we infer that the multiple conformations are probably in fast exchange due to the structural flexibility of the cage faces. The integral ratios between outward-facing and inward-facing naphthalene signals (Supplementary Fig. 54) suggest that more exo than endo faces may be present in these conformations, provided that the distances between the naphthalene protons and the guest remain comparable.
Differing NOE correlations between the three ligand arms that meet at each vertex also support the assignment of exo ligand conformations following the binding of large guests within 1 Supplementary Fig. 55). The f protons from different ligand arms showed cross peaks in the NOESY spectrum of empty 1 (Supplementary Fig. 56), whereas, following the binding of larger guests, such as diamantane (Supplementary Figs. 57–59) and trans-perfluorodecalin (Supplementary Figs. 60–62), fewer inter-f cross peaks were found, in favour of more f–i cross peaks, consistent with more naphthalenes adopting the conformation required in exo ligand faces (Fig. 4b).
Expansion of 1 upon guest binding
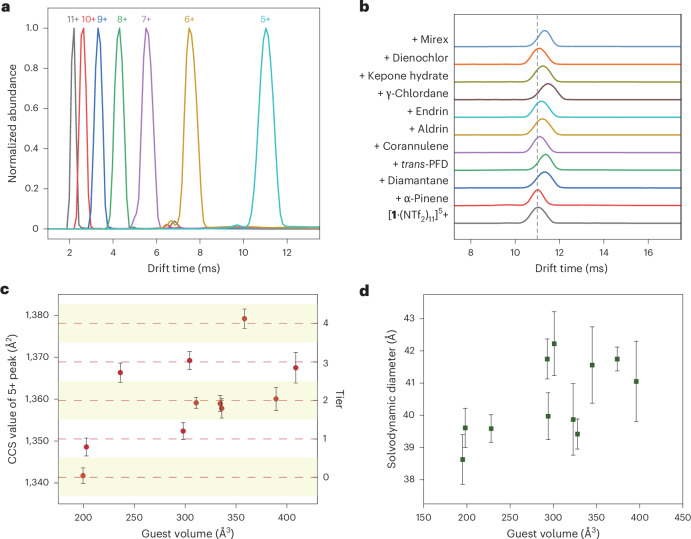
Increasing numbers of exo faces should result in an increase in the measured size of 1. A series of ion mobility mass spectrometry (IMS) (Supplementary Figs. 63–85) and 1H DOSY experiments (Supplementary Figs. 87–97) were therefore carried out to gauge the increasing size of 1 upon guest binding. As shown in Fig. 5a, cage 1 generates a characteristic series of ions that travel with different drift time. As the collisional cross-section (CCS) value is proportional to the drift time, slower ions, with lower charges, provide the most sensitive means of probing CCS. We thus chose the guest adducts of the [1·(NTf2)11]5+ ion, which was the smallest-charged ion that was consistently observed, for analysis of CCS values. Figure 5b shows mobilograms of the 5+ ions of 1 and its host–guest complexes, showing that guest binding results in variable increases in drift time.
Fig. 5. Mass spectrometry and NMR reveal how cage 1 increases its size in discrete increments upon binding progressively larger guests.
a, Normalized mobilograms of ions corresponding to cage 1 with varying numbers of counterions. b,c, Stacked, normalized mobilograms (b) and CCS values (c) of the [1·(NTf2)11]5+ ion from cage 1 and its host–guest complexes, showing how larger guests lead to larger CCS values that cluster into tiers, which we infer to correspond to increasing numbers of exo cage faces. Guest volumes are corrected for sphericity. The data are presented as mean values ± standard deviation from the CCS values of ~1,000–3,000 distinct ion peaks, with details provided in Supplementary Table 3 and Supplementary Information section 5.4. d, The solvodynamic diameter of cage 1 and its various host–guest complexes from 1H DOSY data. The data are presented as mean values ± standard deviation from the solvodynamic diameters, calculated from ~20–40 individual NMR signals with the Stokes–Einstein equation. For details, please see Supplementary Table 4 and Supplementary Information section 5.5.
CCS values obtained using the Mason–Schamp equation49–51 are summarized in Fig. 5c and Supplementary Table 3, where they are plotted against the guest volumes. Examination of the CCS values suggested that they cluster into five tiers. We thus fitted the CCS values to the linear expression
| 1 |
where and ΔCCS stand for the smallest observed CCS value of guest⊂1 and the tier increment, respectively. Each tier of data points (highlighted with horizontal stripes in Fig. 5c) was assigned a tier number n from 0 to 4. Linear least-squares fitting of the CCS values using equation (1) provided a tier increment ∆CCS of 9.2 Å2 and a smallest CCS guest⊂1 value of 1,341 Å2 (Supplementary Fig. 86), with an R2 of 0.997. The quality of fit obtained suggests that the CCS values may describe a quantized system, where a discrete number of cage faces become exo during adaptation to the size and shape of each specific guest.
The corresponding 1H DOSY results are shown in Fig. 5d. The solvodynamic diameters, calculated via the Stokes–Einstein equation, also exhibited a positive correlation with increasing guest size, but the values are distributed more evenly than the CCS values.
The different conditions probed by the two methods may account for the different results obtained. As ions desolvate during IMS measurements, van der Waals interactions between proximate naphthyl groups would be maximized during the formation of a structure with the minimum number of exo faces to accommodate a given guest. These internal solvation effects52 are thus anticipated to favour a single conformation with a discrete number of endo faces and a correspondingly well-defined CCS. In contrast, in the liquid phase of the DOSY measurements, acetonitrile solvation may stabilize host–guest complex microstates with varying exo–endo face ratios simultaneously. As these microstates equilibrate in fast exchange on the NMR timescale (Supplementary Fig. 20–34), DOSY would be expected to report a diffusion rate intermediate between them.
Possible conformations and cavity states of 1
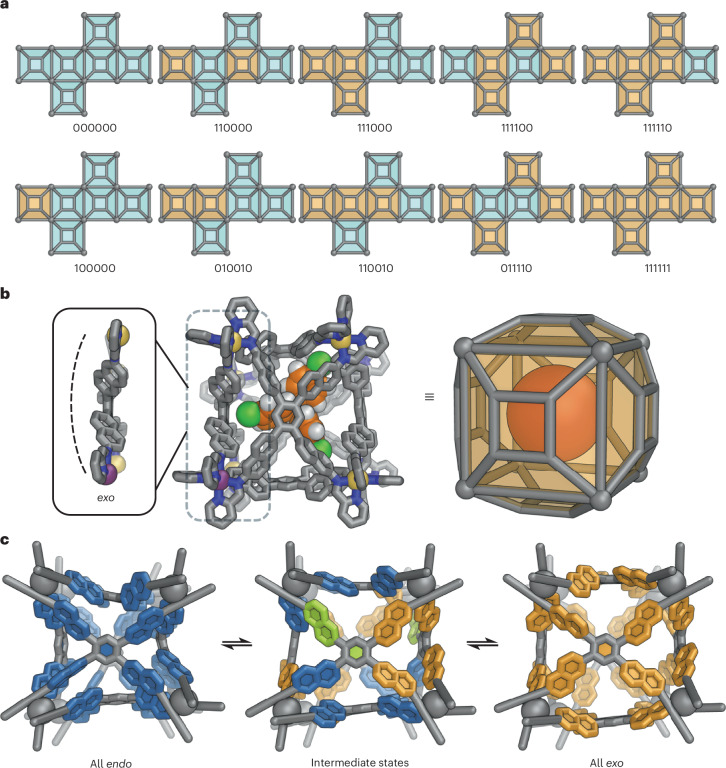
On the basis of molecular modelling, we hypothesized that each face of 1 has local minima corresponding to endo and exo configurations, with all of the conformations shown in Fig. 6a potentially accessible. We also inferred that the energetic difference between these conformations would be considerably less than the energy of guest binding. This view of the guest-binding behaviour of 1 was consistent with the CCS values obtained (Fig. 5c), in which five of the cavity volume states may represent the five tiers found in the plot. However, 1H NMR spectra of the host–guest complexes do not show clear-cut evidence of cage desymmetrization, suggesting that the conversion between different cage conformations occurs in fast exchange. Figure 6b and Supplementary Fig. 98 shows an GFN2-xTB53-optimized structure of B(p-Cl-C6H4)−⊂1, which suggests that 1 expands its cavity by adopting an all-exo conformation to accommodate this large anionic guest.
Fig. 6. Illustration of possible conformations for cage 1.
a, Enumeration of the ten possible states that cage 1 can adopt, with differing face conformations. A blue colour and ‘0’ denote endo faces, while orange and ‘1’ represent exo faces. b, The GFN2-xTB53 model of B(p-Cl-C6H4)−⊂1, minimised to give six exo faces (111111 in a above, ‘all-exo’ in c below). C, grey or orange (guest); N, blue; fac-Δ-Zn, yellow orange; fac-Λ-Zn, purple; Cl, green; H, white. Hydrogen atoms of the cage are omitted for clarity. c, An illustration of the pathway for conversion between the all-endo to the all-exo conformation of 1, calculated with the GFN-FF54 potential and computational tools from the energy landscape framework (Supplementary Video 1). The metal vertices are simplified for clarity. Blue, lime and orange denotes endo, intermediate and exo states of the structure, respectively.
To further understand the conversion process between the bistable states of the faces of 1, we conducted a simulation of the transition of 1 from fully endo to fully exo. The crystal structure of empty 1 and a modelled structure of B(p-Cl-C6H4)−⊂1 were used to define the starting and end points, and were optimized with the GFN-FF method54 before the simulation. A 40-step path, with 40 transition states between the two states, was found and is visualized in Fig. 6c and Supplementary Video 1. This pathway suggests that the naphthyls can rotate individually and, in some steps, concertedly during the transition. These rotations expand the cavity of the cage in response to the presence of guest molecules and help optimize the host to provide the best fit in each case. The energy landscape for this pathway is summarized in a disconnectivity graph55,56 and an energy profile based on all the configurations identified along the pathway (Supplementary Figs. 99 and 100 and Supplementary Table 5).
Our computational results suggested that the endo and exo face configurations of 1 are nearly isoenergetic. Although the results from the methods we used, especially force field (FF) methods, may exhibit some degree of uncertainty, they still provide valuable insights into the energetic differences between the various conformations of 1. The fully exo conformation of 1 is lower in energy than the fully endo conformation of 1 when calculated with the GFN-FF method, but higher in energy using density functional theory (DFT) at the r2SCAN-3c level57. When an implicit acetonitrile solvation model was applied, the fully exo structure became energetically comparable to the endo starting point. With our experimental observations in mind, namely that certain guests drive the cage towards exo conformations, such subtle differences in the energies underscore the importance of suitable guest molecules in triggering the conformational change of 1. The comparison of energy landscapes calculated with DFT and GFN-FF methods also indicates the important role of aromatic stacking interactions in the energy differences among the conformations. Whereas DFT calculations attempt to capture these interactions, FF methods are more likely to neglect them. Therefore, the difference in the energy landscapes calculated with different methods indicates that endo conformations are more favourable when the cavity is empty, while the presence of a guest would disrupt the stacking interactions, thereby destabilizing the endo conformations and pushing the cage towards exo structures.
GFN-FF produces a highest-energy barrier of 34.9 kJ mol−1 for the all-endo to all-exo conversion pathway, versus 58.9 kJ mol−1 using r2SCAN-3c. Estimates from variable-temperature NMR results (Supplementary Figs. 101–104 and Supplementary Tables 6 and 7) suggested an energy barrier of 4–9 kJ mol−1 for the structural conversion, lower than the calculated barriers. As the calculations were performed using the empty cage structure, the observed difference suggests that guests reduce these barriers through non-covalent interactions and facilitate the structural transformation of 1 by stabilizing higher-energy intermediate structures, accounting for the fast NMR exchange between conformations of 1 at room temperature. We also note that entropic effects and intermolecular interactions from guest binding have not been considered in the calculations. The mechanistic characteristics of the interconversion process are the main focus of interest in the simulations. By complementing our experimental observations, these computational results provide a more comprehensive understanding of the structural dynamics of 1.
Conclusion
The introduction of 2,6-naphthalene rotating struts into tetramine subcomponent A enables the resulting pseudo-cube 1 to bind guest molecules with a wide range of sizes and shapes. From an initial all-endo conformation, the ability of the cage panels to adopt exo conformations allows 1 to bind guest molecules ranging from 178 to 599 Å3. Our results thus allow us to infer that 1 can adopt ten volume-quantized states through face reconfigurations, leading to seven accessible cavity volumes. This approach of integrating conformationally switchable faces into coordination cages may be generalizable across different classes of metal–organic cage, enabling augmentation of their versatility as molecular receptors.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41557-024-01708-5.
Supplementary information
Supplementary Figs. 1–104, Tables 1–7 and Schemes 1 and 2.
Atom coordinates for the optimized structure shown in Fig. 6b.
Atom coordinates for the optimized pathway shown in Fig. 6c.
Single-crystal XRD data of empty cage 1 (CCDC 2367409).
A multimedia representation of the optimized pathway shown in Fig. 6c.
Source data
Source data for Fig. 5c (IMS) and Fig. 5d (1H DOSY).
Acknowledgements
This study was supported by the UK Engineering and Physical Sciences Research Council (EPSRC) (EP/T031603/1-JRN and EP/P027067/1-JRN). We thank the Diamond Light Source (UK) for the synchrotron beamtime on I19 (CY29890). H.X. acknowledges the Cambridge Trust and China Scholarship Council for PhD funding. A.W.H. is the recipient of an Astex Pharmaceuticals Sustaining Innovation Postdoctoral Award. P.C.P.T. acknowledges the Engineering and Physical Sciences Research Council via project EP/S024220/1 EPSRC Centre for Doctoral Training in Automated Chemical Synthesis Enabled by Digital Molecular Technologies. P.P. acknowledges the Alexander von Humboldt Foundation for a Feodor Lynen Research Fellowship. GFN2-xTB calculations were performed using resources provided by the Cambridge Service for Data Driven Discovery (CSD3) operated by the University of Cambridge Research Computing Service (www.csd3.cam.ac.uk), provided by Dell EMC and Intel using Tier-2 funding from the Engineering and Physical Sciences Research Council (capital grant EP/T022159/1) and DiRAC funding from the Science and Technology Facilities Council (www.dirac.ac.uk).
Author contributions
H.X. and J.R.N. conceived the study and wrote the manuscript. H.X. performed the synthetic work. H.X. collected the X-ray data and refined the structure with assistance from T.K.R. H.X., A.W.H. and L.S. conducted the ion-mobility mass spectrometry studies. P.C.P.T. performed the GFN2-xTB calculation of the structure in Fig. 6b. D.J.W. and P.P. performed the pathway calculations using software written and maintained by D.J.W. and coworkers. H.X. and J.D.T. fitted the NMR titration data to the binding models. H.X. led the project overall. All the authors contributed to the manuscript preparation.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Data availability
The authors declare that all data supporting the findings of this study are included within the Article and its Supplementary Information and are also available from the authors upon request. Crystallographic data for the structures reported in this paper have been deposited at the Cambridge Crystallographic Data Centre under deposition number 2367409 (empty cage 1). Copies of these data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41557-024-01708-5.
References
- 1.Sinden, R. R. in DNA Structure and Function 95–133 (Academic Press, 1994).
- 2.Corless, S. & Gilbert, N. Effects of DNA supercoiling on chromatin architecture. Biophys. Rev.8, 245–258 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tinoco, I. & Bustamante, C. How RNA folds. J. Mol. Biol.293, 271–281 (1999). [DOI] [PubMed] [Google Scholar]
- 4.Billas, I. M. L. et al. Structural adaptability in the ligand-binding pocket of the ecdysone hormone receptor. Nature426, 91–96 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Haliloglu, T. & Bahar, I. Adaptability of protein structures to enable functional interactions and evolutionary implications. Curr. Opin. Struct. Biol.35, 17–23 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehn, J.-M. Supramolecular chemistry: receptors, catalysts, and carriers. Science227, 849–856 (1985). [DOI] [PubMed] [Google Scholar]
- 7.Houk, K. N., Leach, A. G., Kim, S. P. & Zhang, X. Binding affinities of host–guest, protein–ligand, and protein–transition-state complexes. Angew. Chem. Int. Ed.42, 4872–4897 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Schreiber, G., Haran, G. & Zhou, H. X. Fundamental aspects of protein–protein association kinetics. Chem. Rev.109, 839–860 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tozawa, T. et al. Porous organic cages. Nat. Mater.8, 973–978 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Zhou, H. C., Long, J. R. & Yaghi, O. M. Introduction to metal–organic frameworks. Chem. Rev.112, 673–674 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Yoshizawa, M., Klosterman, J. K. & Fujita, M. Functional molecular flasks: new properties and reactions within discrete, self-assembled hosts. Angew. Chem. Int. Ed.48, 3418–3438 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Chakrabarty, R., Mukherjee, P. S. & Stang, P. J. Supramolecular coordination: self-assembly of finite two- and three-dimensional ensembles. Chem. Rev.111, 6810–6918 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crini, G. Review: a history of cyclodextrins. Chem. Rev.114, 10940–10975 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Ogoshi, T., Yamagishi, T. A. & Nakamoto, Y. Pillar-shaped macrocyclic hosts pillar[n]arenes: new key players for supramolecular chemistry. Chem. Rev.116, 7937–8002 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Whitehead, M., Turega, S., Stephenson, A., Hunter, C. A. & Ward, M. D. Quantification of solvent effects on molecular recognition in polyhedral coordination cage hosts. Chem. Sci.4, 2744–2751 (2013). [Google Scholar]
- 16.Schneider, H.-J. Binding mechanisms in supramolecular complexes. Angew. Chem. Int. Ed.48, 3924–3977 (2009). [DOI] [PubMed] [Google Scholar]
- 17.Liu, Y., Zhao, W., Chen, C. H. & Flood, A. H. Chloride capture using a C–H hydrogen-bonding cage. Science365, 159–161 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Bravin, C., Badetti, E., Scaramuzzo, F. A., Licini, G. & Zonta, C. Triggering assembly and disassembly of a supramolecular cage. J. Am. Chem. Soc.139, 6456–6460 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Custelcean, R. Anion encapsulation and dynamics in self-assembled coordination cages. Chem. Soc. Rev.43, 1813–1824 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Langton, M. J., Robinson, S. W., Marques, I., Félix, V. & Beer, P. D. Halogen bonding in water results in enhanced anion recognition in acyclic and rotaxane hosts. Nat. Chem.6, 1039–1043 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Liu, W. et al. XCage: a tricyclic octacationic receptor for perylene diimide with picomolar affinity in water. J. Am. Chem. Soc.142, 3165–3173 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Dannhäuser, J. et al. σ-Donor and π-acceptor stacking interactions in a trans-2-linked C60–cobalt(II) tetraphenylporphyrin diad. Angew. Chem. Int. Ed.45, 3368–3372 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Fischer, E. Einfluss der Configuration auf die Wirkung der Enzyme. Chem. Ber.27, 2985–2993 (1894). [Google Scholar]
- 24.Lehn, J. M. Supramolecular chemistry—scope and perspectives molecules, supermolecules, and molecular devices (Nobel Lecture). Angew. Chem. Int. Ed.27, 89–112 (1988). [Google Scholar]
- 25.McTernan, C. T., Davies, J. A. & Nitschke, J. R. Beyond platonic: how to build metal–organic polyhedra capable of binding low-symmetry, information-rich molecular cargoes. Chem. Rev.122, 10393–10437 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wittenberg, J. B. & Isaacs, L. Complementarity and preorganization. In Supramolecular Chemistry (eds Gale, P. A. & Steed, J. W.) (Wiley, 2012).
- 27.Katagiri, Y., Tsuchida, Y., Matsuo, Y. & Yoshizawa, M. An adamantane capsule and its efficient uptake of spherical guests up to 3 nm in water. J. Am. Chem. Soc.143, 21492–21496 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Frey, J., Tock, C., Collin, J. P., Heitz, V. & Sauvage, J. P. A [3]rotaxane with two porphyrinic plates acting as an adaptable receptor. J. Am. Chem. Soc.130, 4592–4593 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Yu, H.-M. et al. Self-assembly of cluster-mediated 3D catenanes with size-specific recognition behavior. J. Am. Chem. Soc.145, 25103–25108 (2023). [DOI] [PubMed] [Google Scholar]
- 30.Lee, H. et al. Light-powered dissipative assembly of diazocine coordination cages. J. Am. Chem. Soc.144, 3099–3105 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dekhtiarenko, M. et al. Playing with the cavity size of exTTF-based self-assembled cages. Org. Chem. Front.10, 1803–1810 (2023). [Google Scholar]
- 32.Sivalingam, V., Parbin, M., Krishnaswamy, S. & Chand, D. K. Cage-to-cage transformations in self-assembled coordination cages using ‘acid/base’ or ‘guest binding-induced strain’ as stimuli. Angew. Chem. Int. Ed. e202403711 (2024). [DOI] [PubMed]
- 33.Zhan, Y.-Y. et al. Induced-fit expansion and contraction of a self-assembled nanocube finely responding to neutral and anionic guests. Nat. Commun.9, 4530 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang, J., Ju, Y.-Y., Low, K.-H., Tan, Y.-Z. & Liu, J. A molecular transformer: a π-conjugated macrocycle as an adaptable host. Angew. Chem. Int. Ed.60, 11814–11818 (2021). [DOI] [PubMed] [Google Scholar]
- 35.Jiang, W.-L., Huang, B., Zhao, X.-L., Shi, X. & Yang, H.-B. Strong halide anion binding within the cavity of a conformation-adaptive phenazine-based Pd2L4 cage. Chem9, 2655–2668 (2023). [Google Scholar]
- 36.Koshland, D. E. Application of a theory of enzyme specificity to protein synthesis. Proc. Natl Acad. Sci.44, 98–104 (1958). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martín Díaz, A. E. & Lewis, J. E. M. Structural flexibility in metal–organic cages. Front. Chem.9, 706462 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samanta, D., Mukherjee, S., Patil, Y. P. & Mukherjee, P. S. Self-assembled Pd6 open cage with triimidazole walls and the use of its confined nanospace for catalytic Knoevenagel- and Diels–Alder reactions in aqueous medium. Chem. Eur. J.18, 12322–12329 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Rizzuto, F. J. & Nitschke, J. R. Stereochemical plasticity modulates cooperative binding in a CoII12L6 cuboctahedron. Nat. Chem.9, 903–908 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Huang, Y.-H. et al. Dynamic stereochemistry of M8Pd6 supramolecular cages based on metal-center lability for differential chiral induction, resolution, and recognition. Angew. Chem. Int. Ed.63, e202315053 (2024). [DOI] [PubMed] [Google Scholar]
- 41.Mirtschin, S., Slabon-Turski, A., Scopelliti, R., Velders, A. H. & Severin, K. A coordination cage with an adaptable cavity size. J. Am. Chem. Soc.132, 14004–14005 (2010). [DOI] [PubMed] [Google Scholar]
- 42.García-Simón, C. et al. Complete dynamic reconstruction of C60, C70, and (C59N)2 encapsulation into an adaptable supramolecular nanocapsule. J. Am. Chem. Soc.142, 16051–16063 (2020). [DOI] [PubMed] [Google Scholar]
- 43.Huang, Y.-H. et al. Dynamic metallosupramolecular cages containing 12 adaptable pockets for high-order guest binding beyond biomimicry. J. Am. Chem. Soc.145, 23361–23371 (2023). [DOI] [PubMed] [Google Scholar]
- 44.Bloch, W. M. et al. Maximized axial helicity in a Pd2L4 cage: inverse guest size-dependent compression and mesocate isomerism. Chem. Sci.14, 1524–1531 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davies, J. A. et al. Tetramine aspect ratio and flexibility determine framework symmetry for Zn8L6 self-assembled structures. Angew. Chem. Int. Ed.62, e202217987 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maglic, J. B. & Lavendomme, R. MoloVol: an easy-to-use program for analyzing cavities, volumes and surface areas of chemical structures. J. Appl. Crystallogr.55, 1033–1044 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berry, S. N., Qin, L., Lewis, W. & Jolliffe, K. A. Conformationally adaptable macrocyclic receptors for ditopic anions: analysis of chelate cooperativity in aqueous containing media. Chem. Sci.11, 7015–7022 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mecozzi, S. & Rebek, J. Jr. The 55% solution: a formula for molecular recognition in the liquid state. Chem. Eur. J.4, 1016–1022 (1998).
- 49.Ruotolo, B. T., Benesch, J. L. P., Sandercock, A. M., Hyung, S.-J. & Robinson, C. V. Ion mobility–mass spectrometry analysis of large protein complexes. Nat. Protoc.3, 1139–1152 (2008). [DOI] [PubMed] [Google Scholar]
- 50.Bush, M. F. et al. Collision cross sections of proteins and their complexes: a calibration framework and database for gas-phase structural biology. Anal. Chem.82, 9557–9565 (2010). [DOI] [PubMed] [Google Scholar]
- 51.Campuzano, I. et al. Structural characterization of drug-like compounds by ion mobility mass spectrometry: comparison of theoretical and experimentally derived nitrogen collision cross sections. Anal. Chem.84, 1026–1033 (2012). [DOI] [PubMed] [Google Scholar]
- 52.Ottosson, C.-H. & Cremer, D. Intramolecularly stabilized phenylsilyl and anthrylsilyl cations. Organometallics15, 5309–5320 (1996). [Google Scholar]
- 53.Bannwarth, C. et al. Extended tight-binding quantum chemistry methods. Wiley Interdiscip. Rev. Comput. Mol. Sci.11, e1493 (2021). [Google Scholar]
- 54.Spicher, S. & Grimme, S. Robust atomistic modeling of materials, organometallic, and biochemical systems. Angew. Chem. Int. Ed.59, 15665–15673 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Becker, O. M. & Karplus, M. The topology of multidimensional potential energy surfaces: theory and application to peptide structure and kinetics. J. Chem. Phys.106, 1495–1517 (1997). [Google Scholar]
- 56.Wales, D. J., Miller, M. A. & Walsh, T. R. Archetypal energy landscapes. Nature394, 758–760 (1998). [Google Scholar]
- 57.Grimme, S., Hansen, A., Ehlert, S. & Mewes, J.-M. r2SCAN-3c: a ‘Swiss army knife’ composite electronic-structure method. J. Chem. Phys.154, 064103 (2021). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figs. 1–104, Tables 1–7 and Schemes 1 and 2.
Atom coordinates for the optimized structure shown in Fig. 6b.
Atom coordinates for the optimized pathway shown in Fig. 6c.
Single-crystal XRD data of empty cage 1 (CCDC 2367409).
A multimedia representation of the optimized pathway shown in Fig. 6c.
Source data for Fig. 5c (IMS) and Fig. 5d (1H DOSY).
Data Availability Statement
The authors declare that all data supporting the findings of this study are included within the Article and its Supplementary Information and are also available from the authors upon request. Crystallographic data for the structures reported in this paper have been deposited at the Cambridge Crystallographic Data Centre under deposition number 2367409 (empty cage 1). Copies of these data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif. Source data are provided with this paper.