Abstract
Many Drosophila species coexist by sharing their feeding and breeding sites, which may influence their oviposition choices in an interspecies social context. Whether and where to lay eggs is a crucial decision for female flies as it influences the success of their offspring, by minimizing the risk of predation, competition, or cannibalism. Significant gaps exist in our understanding of Drosophila oviposition dynamics in co-occurring species. Here we tested oviposition strategies of Drosophila melanogaster and its close relative Drosophila simulans under different conditions, to assess whether a single female would prefer to oviposit separately or together with another female, be it a conspecific or not. We find that ovipositing females, regardless whether they are conspecifics or not, prefer to oviposit at the same site. This might suggest that the flies regard the benefits of sharing oviposition sites as higher than the potential risks of competition or cannibalism. The willingness to share oviposition sites was lower when the nutritional value of the medium was increased by adding yeast, and was lost when flies were allowed to lay the eggs consecutively, instead of being tested together. The latter might be explained by our additional finding that females become attracted by the presence of other females on oviposition substrates and that this attraction is partly driven by visual cues. Ovipositing in groups might facilitate intra- and interspecific social feeding of same age offspring, as well as enrichment of microbes. However, this cooperation dynamic might change if another female’s offspring is already present, as it might be perceived as danger of competition or cannibalism.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10886-025-01576-4.
Keywords: Drosophila, Oviposition behavior, Niche utilization, Interspecies interaction, Coexistence, Aggregation
Introduction
Many Drosophila species in nature utilize the same feeding and breeding sites and are drawn to the same fermenting fruit baits, presenting a clear example of coexistence (Budnik & Brncic 1974). Drosophila melanogaster and Drosophila simulans are part of what is called the cosmopolitan guild, a group of species that share similar ecological niches (Atkinson & Shorrocks 1977). It has been suggested that the population dynamics of coexisting Drosophila species are likely influenced, at least in part, by interspecies competition (Budnik & Brncic 1974). While resource competition in animals can lead to exclusion, studies show that coexistence can be stable in certain environments or conditions, with one species not always completely eliminating the other, as shared resources could lead to cooperative behaviors (Ayala 1970; Budnik & Brncic 1974; Miller 1964). Competition between species is not limited to resource exploitation; it can also involve interference, where the presence of one species alters the efficiency or behavior of the other (Budnik & Brncic 1974).
The cooperation and competition dynamics are evident in insects, where density-dependent signals, such as pheromones or other chemosensory cues, can influence reproductive and oviposition behavior (Dumenil et al. 2016; Ferveur 2005; Tungadi et al. 2022). In Drosophila, females have been shown to adjust their egg-laying strategies based on the presence and density of other Drosophila eggs or larvae on a substrate (Bailly et al. 2023; Moreira-Soto et al. 2024). In Aedes aegypti mosquitoes it is known that females aggregate more often than expected in one oviposition site over others, when equal choices are offered (Costa-da-Silva et al. 2024). The ultimate decision to deposit eggs by a female insect demands a complex integration of sensory inputs, from finding a breeding site to assessing its quality (Costa-da-Silva et al. 2024). Reports in Drosophila show that oviposition engages multiple sensory modalities, including vision, olfaction, proprioception, and taste (Dweck et al. 2013; Liu et al. 2017). While sensory neurons on the ovipositor play a key role in the final decision to lay an egg (Chess & Ringo 1985; Takamura & Fuyama 1980), other appendages, such as the proboscis, wings, and legs, also contain taste receptors with sex-specific responses that may influence this decision-making process (Chyb 2004; Markow & O’Grady 2008; Meunier et al. 2000; Stocker 1994).
The selection of oviposition sites plays a critical role in the survival and fitness of an animal’s offspring (Richmond & Gerking 1978). In Drosophila flies, which have immobile egg and pupal stages, and larvae with limited mobility, the choice of oviposition site is paramount, as this means that the immature stages cannot move to better substrates (Markow 2015; Richmond & Gerking 1978). Therefore, poor site selection increases vulnerability to predation, and reduces chances for larval survival, directly influencing the reproductive success (Durisko et al. 2014; Refsnider & Janzen 2010). Also, laying eggs communally can lead to challenges such as resource competition, restricted growth, and even cannibalism when resources are exhausted (Bailly et al. 2023; Etienne et al. 2002; Narasimha et al. 2019; Wertheim et al. 2002). The significance of this choice is highlighted by numerous studies showing that females are highly selective about where they lay their eggs and can delay oviposition until they find an optimal substrate (Azanchi et al. 2013; Fanara et al. 2023; Joseph et al. 2009; Schwartz et al. 2012; Yang et al. 2008). Thus, natural selection is expected to exert strong pressures on behaviors related to oviposition, particularly under conditions of resource scarcity and high competition (Markow 2015).
In terms of joint egg laying, it has been suggested that females are more likely to be attracted to cues linked to favorable species, genotypes, and population densities (Beltramí et al. 2012). For example, in Drosophila suzukii, studies have shown that females are deterred from ovipositing when they detect the presence of D. melanogaster larvae. Additionally, when they detect allospecific egg cues, they avoid certain species while showing no preference for others (Kidera & Takahashi 2020). Similar results have been observed in D. melanogaster, where females were attracted to oviposit near conspecific eggs and some allospecifics but showed no preference for others (Moreira-Soto et al. 2024). Larvae of different species have been shown to interact, often with negative consequences for some species: D. simulans had a negative effect on Drosophila rufa and Drosophila immigrans, but its own fitness was unaffected (Takahashi et al. 2005).
In the present study, we investigated the oviposition preference of D. melanogaster and D. simulans under different conditions, given multiple oviposition sites, to assess whether they would prefer to oviposit separately or together with another female, and if the latter, whether it matters if the other female is a conspecific or not. We also tested aggregation at potential oviposition sites to determine whether flies follow conspecifics or heterospecifics using visual and/or olfactory cues when selecting where to lay their eggs. By examining these behaviors under various conditions, we expand the knowledge on the factors that affect coexistence in shared environments. As these species coexist in nature, they provide a valuable model for studying the complexities of niche utilization and species coexistence. The interplay between competition and cooperation in these sympatric species highlights the intricate behavioral and ecological mechanisms that allow for coexistence in shared environments, offering insights into how species balance resource use and reproductive strategies in natural systems.
Results
Oviposition Site Preference of D. melanogaster and D. simulans in 4 Choice Assays
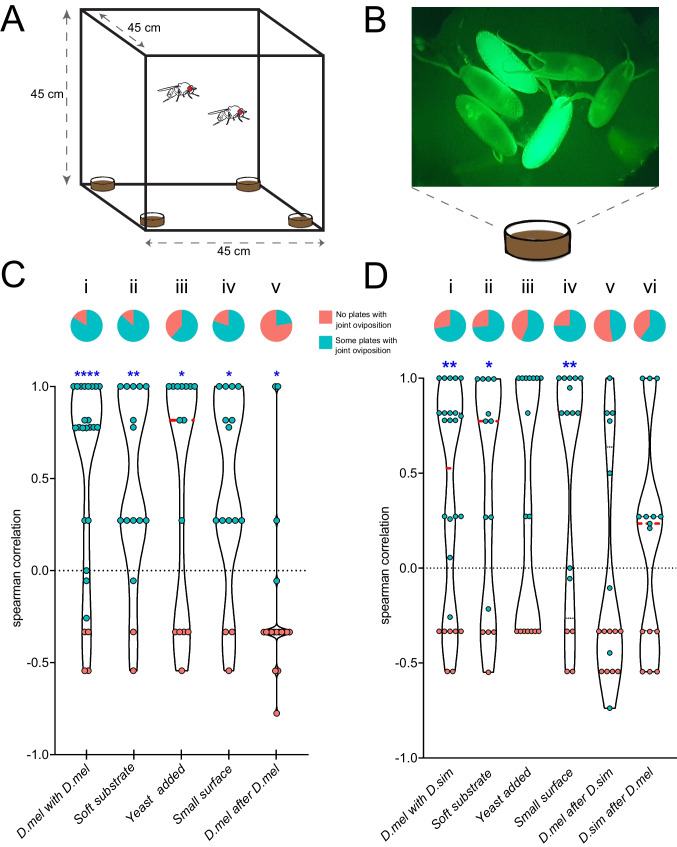
We examined the oviposition preference for 4 equal oviposition substrates (Fig. 1A), to determine if D. melanogaster females would prefer to oviposit together with females of D. simulans or conspecifics. In order to later distinguish D. melanogaster from D. simulans eggs, we used a D. melanogaster fly line (RRID:BDSC_4534) expressing GFP ubiquitously under control of Act5C (Fig. 1B). The cuticular chemicals of this fly line are indeed similar to that of wild type D. melanogaster, as the wild type samples group close to the mutant fly line in the UMAP (Fig. S1). Further, we found that only 4.6% of the chemical compounds have difference in abundance between this fly line and the wild type flies (Tukey’s test for multiple comparisons of means (P < 0.05)). We found no differences in the abundances of the male specific compounds: (Z)−11-Octadecen-1-yl acetate (cVA), (Z)−7-Tricosene and (Z)−7-Pentacosene, as well as female-specific compounds: (Z,Z)−7,11-Pentacosadiene, (Z,Z)−7,11-Heptacosadiene, and (Z,Z)−7,11-Nonacosadiene, reported for D. melanogaster (Khallaf et al. 2021). Given this, the results from this fly line in oviposition experiments should be transferable to wild type flies.
Fig. 1.
(A) Visual representation of the 4-choice oviposition setup. (B) Example image of fluorescent eggs from D. melanogaster (RRID:BDSC_4534) and non-fluorescent eggs from D. melanogaster. (C) Oviposition assays in D. melanogaster (RRID:BDSC_4534 and wild type for i-iv; 2 wild type flies for v) tested under different conditions (i-v). (D) Oviposition assays of D. simulans with D. melanogaster (RRID:BDSC_4534 for i-iv; wild type for v-vi) tested under different conditions (i-vi). Top graph, proportion of assays that had no plates with joint oviposition (red) or at least one plate with joint oviposition (green; see Table S1); bottom graph, distribution of spearman correlation values for all individual assays; dashed red lines indicate the median and dashed black lines indicate the quartiles. High values depict more joint, low values depict more separate oviposition. Blue stars indicate statistical difference from zero (n = 15–26, Wilcoxon signed-rank test, *P < 0.05; **P < 0,01; ***P < 0.001, ****P < 0.0001). For raw data of (C) and (D) see Supplemental Data 1
When first testing the oviposition choice of two synchronously tested females in a four-choice assay (Fig. 1A) we found a significant correlation of the number of eggs on a given oviposition site of the 2 flies tested, regardless, whether they were conspecific (Fig. 1Ci) or allospecific (Fig. 1Di). Obviously, the flies preferentially oviposit on the same sites, even if they have several oviposition sites available. Drosophila larvae are known to cooperate while digging themselves into a substrate (Durisko et al. 2014). We therefore asked whether the females’ preference to oviposit together is driven by this, and is reduced when cooperative digging is not needed. Thus, we repeated the assay using 4 plates with softer agar. Despite the potentially reduced need for larval cooperation the female flies still oviposited together, be it with conspecifics (Fig. 1Cii) or allospecifics (Fig. 1Dii). It is possible that substrate hardness, and consequently the need for larval cooperation to soften the substrate, do not primarily govern the females' preference for joint oviposition sites; however, further tests are needed to confirm this.
Drosophila larvae are known to mainly consume microbes growing on the oviposition substrate and it was shown that flies during oviposition transfer microbes to the substrate (Bakula 1969; Stamps et al. 2012). One could, hence, speculate that female flies by laying eggs together increase the chance of their offspring for a nutritionally rich substrate. In reverse, a nutritionally rich substrate could reduce the motivation for cooperative oviposition. Indeed, when we added yeast to the substrate generally more flies decided to lay eggs, with 88.7% of the assays resulting in egg laying, compared to an average of 58.9% in all assays (Table S2), suggesting that yeast improved the substrate quality. Furthermore, at least the willingness of D. melanogaster to share the niche with D. simulans disappeared (Fig. 1Diii), while the cooperative oviposition of conspecific D. melanogaster females remained unchanged (Fig. 1Ciii).
We next tested the flies’ preferences in a situation of increased competition, by reducing the surface they had to oviposit on. Under these conditions only 36.9% of the experiments resulted in egg laying, suggesting that this reduction indeed is sensed by the females (Fig. 1Civ). However, in the cases where the flies did oviposit, we observed that despite the potentially increased competition the preference to oviposit together remained, both for conspecific and allospecific flies (Fig. 1Civ and Div).
As Drosophila larvae have been reported to feed on Drosophila eggs (Narasimha et al. 2019), we finally asked whether a fly would avoid places where older eggs (that might present future danger for cannibalism) are already present. We therefore let the two tested flies lay their eggs successively. For this, the first fly was allowed to oviposit alone, and only after it was removed the second fly was tested. When testing allospecific combinations both D. melanogaster and D. simulans females lost their preference for joint oviposition, when the eggs/larvae of the first fly were already present (Fig. 1Dv+vi). Interestingly, when D. melanogaster females were tested after conspecific females had already oviposited, the flies even significantly avoided the already occupied oviposition plates (Fig. 1Cv).
Oviposition site aggregation of mated females of D. melanogaster and D. simulans
To investigate whether females are influenced by the presence or absence of other females when selecting an oviposition site we used a similar setup as before. However, instead of oviposition plates, we introduced four traps (i.e. small containers filled with fly food that could be entered but not exited easily), using a pipette tip as the entry point (Fig. 2A). This setup allows us to test whether flies follow each other into potential oviposition sites, in contrast to the oviposition assay, where they might visit food plates at different times, guided by other cues.
Fig. 2.
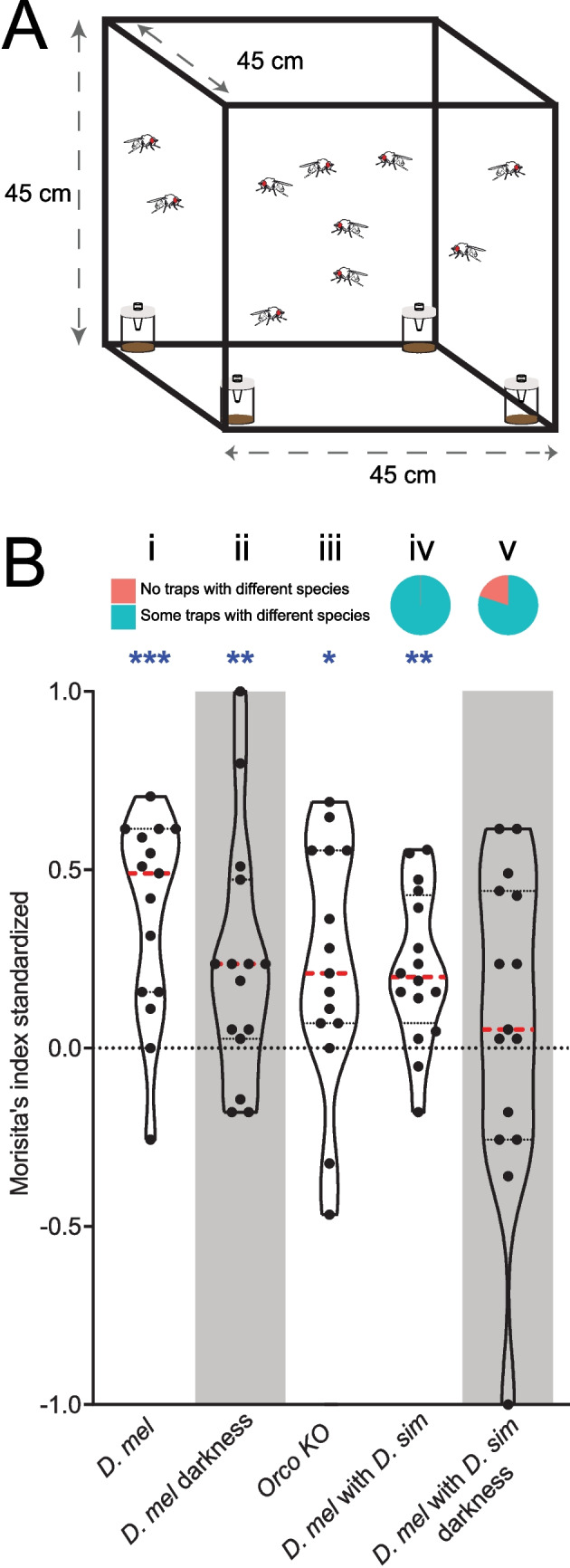
(A) Visual representation of the 4-choice trap assay. (B) Trap choices under different conditions (i-v). Top, proportion of assays in which traps only had one of the 2 species (red) or with at least one trap with both species (green); bottom, distribution of the Morisita’s index of aggregation for the different assays. Dashed red lines indicate the median and dashed black lines indicate the quartiles. High values depict more aggregation in traps. Gray areas indicate the assays performed in darkness. Blue stars indicate statistical difference from zero (n = 15–16, Wilcoxon signed-rank test, *P < 0.05; **P < 0,01; ***P < 0.001, ****P < 0.0001). For raw data of (B) see Supplemental Data 1
Instead of testing two individual flies, we also increased the number of tested flies to 10 (i.e. either 10 D. melanogaster females, or 5 D. melanogaster and 5 D. simulans females) per assay. Again, D. melanogaster females did not randomly end up in the different traps, but rather aggregated in some traps (resulting in a positive Morista’s index in Fig. 2B), regardless whether they were tested with conspecifics (Fig. 2Bi) or with D. simulans females (Fig. 2Biv). The conspecific aggregation remained in the absence of visual cues (i.e. when tested in darkness; Fig. 2Bii) and when partly anosmic flies (i.e. flies with a non-functional olfactory co-receptor Orco; Fig. 2Biii) were tested. Obviously, several sensory modalities are involved in this conspecific aggregation. Interestingly, however, D. melanogaster’s aggregation with D. simulans disappeared in darkness, suggesting that D. melanogaster females are not attracted by the different smell (Fig. S1) of D. simulans females and their tendency to join D. simulans females is mainly driven by visual cues (Fig. 2v).
Discussion
Drosophilid flies are known to have species-specific chemical cues, and these cues provide information for intra- and inter-specific communication (Antony et al. 1985; Bartelt et al. 1985; Ferveur 2005; Khallaf et al. 2021; Laturney & Billeter 2016; Moreira-Soto et al. 2024; Tungadi et al. 2023; Yew et al. 2009). Here, we investigate the niche overlap of the two closely related drosophilids D. melanogaster and D. simulans that are known to inhabit the same habitats in nature (Atkinson & Shorrocks 1977). Like other species, these two species exhibit species specific chemical profiles both as adults and on their eggs (Fig. S1; (Moreira-Soto et al. 2024)). We investigate the female flies’ preference to cluster their eggs with those of other females and the dependency of this behavior on whether the other females are conspecifics or instead belong to the closely related species. Our results indicate that ovipositing females, whether from the same species or not, tend to cluster their eggs on the same oviposition sites. However, the preference attenuated when the nutritional value of the medium increased (added yeast), and this preference was lost when females were allowed to lay eggs sequentially rather than simultaneously, thus encountering previously laid eggs or larvae (Fig. 1C,D).
These results suggest that the advantages of cooperation with same age larvae outweigh the risks of competition both within and between both species. This could potentially lead to larvae forming social foraging groups and by that enhancing their ability to burrow into difficult to penetrate substrate (Durisko et al. 2014). The group foraging strategy offers several benefits: first, it enables larvae to penetrate the fruit more quickly, where temperature and humidity are more stable than on the surface, and where they may be better shielded from parasitoids; second, burrowing by larvae can help to break down and soften the food, making it easier to consume; and finally, the larval digging may also mix the food substrate, potentially preventing the growth of competing molds and encouraging the development of beneficial yeast species (Bakula 1969; Durisko et al. 2014; Rohlfs 2005a, 2005b; Stamps et al. 2012).
Joint oviposition potentially presents several other advantages. It is known that ovipositing females inoculate the food with beneficial yeast (Stamps et al. 2012). In addition, the flies’ association with conspecifics or other species could help them find suitable food more quickly than they could on their own (Sarin & Dukas 2009). Studies on social behavior in larvae have shown that there is aggregation of larvae even when the food is very soft and easy to penetrate, suggesting that larvae may benefit from copying the choices of others, which could give them information to find a good substrate (Durisko & Dukas 2013). Also, Orengo and Prevosti (1994) showed that larval survival of Drosophila subobscura and Drosophila pseudoobscura was improved by the presence of allospecific larvae (i.e. D. pseudoobscura or D. subobscura), indicating a positive interaction.
On the other hand, reports show that aggregation comes with costs, when larvae compete for food, which might limit their growth and adult body size, and could eventually lead to cannibalism when resources are depleted (Allee 1927; Courchamp et al. 1999; Etienne et al. 2002; Narasimha et al. 2019; Vijendravarma et al. 2013; Wertheim et al. 2002). In accordance with this, competition between D. melanogaster and D. simulans when grown together for several generations, resulted in D. melanogaster displacing D. simulans (Hedrick 1972; Hedrick & King 1996). While in our experiments, despite of these potential risks, the flies decided to cluster their eggs, this effect disappeared when we let the flies oviposit successively (Fig. 1Bv and Cv). In a recent study we showed that the presence of eggs attracts ovipositing females (Moreira-Soto et al. 2024). However, the lack of attraction reported here for successively ovipositing females may be due to the 24 h delay between the tests of the two flies, which may have resulted in the first larvae already hatching before the second fly was being tested. It has been reported that first instar larvae can predate on conspecifics (Ahmad et al. 2015; Vijendravarma et al. 2013), meaning that young larvae might represent a risk for the offspring of the ovipositing female. Furthermore, interspecific interactions can have asymmetric effects, where one species is competitively superior, negatively impacting the other, while remaining unaffected by the presence of the other species (Takahashi et al. 2005).
Do flies actually follow each other to potential oviposition sites? When we tested groups of gravid females in a trap assay where they could decide to enter traps together or not, again we found joint choices regardless, whether flies were conspecifics or not (Fig. 2i and iv). While joint choices remained even in the absence of visual or the reduction of olfactory cues when we tested the aggregation within D. melanogaster (Fig. 2iii+iv), these flies lost their attraction to D. simulans, when tested in darkness (Fig. 2v). It is already established that oviposition in Drosophila melanogaster is affected by social cues (Sarin & Dukas 2009), with naïve flies, after seeing other flies, increasing their own oogenesis (Bailly et al. 2023) and obviously even mimicking the flies’ oviposition choices (Battesti et al. 2012). On the other hand, D. melanogaster mated females are known to use aggregation pheromones that strongly attract other flies (Bartelt et al. 1985). Therefore, the social context driving oviposition choice probably is detected by multiple sensory modalities. Our data suggest that the joint oviposition is partly driven by the flies following each other, which again seems to be governed by several sensory modalities.
One example of an aggregation pheromone in Drosophila melanogaster is 11-cis-vaccenyl acetate (cVA), a chemical produced exclusively by males and transferred to females during copulation (Bartelt et al. 1985). This pheromone can attract flies over distance. At closer distance, the flies could rely on visual cues but also on chemicals sensed by sensory neurons on the ovipositor (Chess & Ringo 1985), the proboscis, wings, and legs to help them decide whether to lay an egg (Chyb 2004; Meunier et al. 2000). Additional tests would be needed to determine if the order in which cues are detected could have an effect on the oviposition outcome.
Previous studies of larval aggregation have shown that D. melanogaster exhibited a greater degree of aggregation than D. simulans (Durisko et al. 2014). They proposed that one of the benefits of group borrowing is that it facilitates larval penetration of food to hide from parasitoid wasps. Interestingly, D. melanogaster and D. simulans have different defense strategies against parasitoids with D. melanogaster exhibiting specific avoidance behavior against parasitoid wasps (Ebrahim et al. 2015), while D. simulans exhibits greater physiological immune responses (Lefevre et al. 2012). It could be that the greater larval sociality observed among D. melanogaster larvae serves to increase their burrowing ability as a strategy to reduce parasitism. In ovipositing adult females, this could also be one of the factors that explains why we saw significant preference to oviposit together with conspecifics in all treatments tested synchronously (Fig. 1C), and significant aggregation of conspecific females in oviposition sites, even in darkness (Fig. 2).
In conclusion, our findings provide new insights into the oviposition behavior of D. melanogaster and D. simulans, revealing a preference for social oviposition, even in an interspecific context. This indicates that the benefits of social oviposition, in many cases, outweigh the risks of competition. This aligns with the principles of the Allee effect, where smaller, isolated populations face greater challenges in fitness and survival due to difficulties in cooperation and resource acquisition (Courchamp et al. 1999). The results also suggest that chemical cues and social interactions both could play a role in shaping these oviposition choices. Future studies could explore the ecological consequences of such social strategies in natural populations, as well as the mechanisms underlying these behaviors. Understanding these dynamics broadens our understanding of cooperation, competition, and resource use in species that share overlapping ecological niches.
Methods
Fly stocks
The study utilized wild-type flies that were acquired from the National Drosophila Species Stock Centre (NDSSC; http://blogs.cornell.edu/drosophila/) and the Kyoto stock center (Kyoto DGGR; https://kyotofly.kit.jp/cgi-bin/stocks/index.cgi): D. melanogaster (Hansson’s lab) and D. simulans flies (Stock no. 14021‐0251.01). We also used mutant flies acquired from the Bloomington Drosophila Stock Center (BDSC; https://bdsc.indiana.edu/): D. melanogaster that expresses GFP ubiquitously under control of Act5C (RRID:BDSC_4534), and D. melanogaster with an Orco gene mutation (RRID:BDSC_23130). All flies were raised under specific conditions: a temperature of 25 °C, a 12-h light and 12-h dark cycle, and 70% relative humidity. The flies were reared at standard density in vials with standard cornmeal diet, with males and females together. Per liter of diet, it consists of 118 g of beet syrup, 11 g of brewer's yeast, 95 g of yellow cornmeal, 4.1 g of agar (Carl Roth ®), 2.4 ml of Propionic acid (> 99% pure, 13.4 M; Carl Roth ®) and 3.3 ml of 30% Nipagin (Sigma-Aldrich ®). The care and treatment of all flies adhered to applicable ethical regulations.
Chemical Analyses
Thermal Desorption-Gas Chromatography-Mass Spectrometry (TD-GC–MS)
In order to compare the chemical profiles of the mutant D. melanogaster mated females (RRID:BDSC_4534) with the wild type flies, newly hatched females were left with males to mate, which does not exclude multiple matings. Individual 10-day old flies were decapitated to avoid them from escaping. They were placed in standard microvials in thermal desorption tubes and transferred into a GERSTEL thermal desorption unit (www.gerstel.de) using a GERSTEL MPS 2 XL multipurpose sampler. We analyzed at least 6 replicates. All the cuticular chemical profiles from wild type flies (16 species) were generated in a previous study (Moreira-Soto et al. 2024) following the same procedure.
Regarding the GC–MS system, an Agilent GC 7890 A coupled with an MS 5975 C inert XL MSD unit (www.agilent.com) was utilized, featuring an HP5-MS UI column (19091S-433UI; Agilent Technologies). Volatiles were initially desorbed at 250 °C for 8 min, then trapped at − 50 °C by means of liquid nitrogen cooling. To introduce the components into the GC column, the vaporizer injector was progressively heated to 270 °C at a rate of 12 °C per second and maintained at that temperature for 5 min. The GC oven temperature was held at 50 °C for 3 min, then increased at a rate of 15 °C per minute to 250 °C, where it was held for 3 min, before being further increased to 280 °C at a rate of 20 °C per minute, and maintained for 20 min. For the MS, the transfer line, source, and quadrupole were kept at 270 °C, 230 °C, and 150 °C, respectively.
The raw GC/MS data were converted to AIA format using MSD ChemStation (Agilent Technologies). These converted files were then imported into R (version 4.1.0), where the XCMS package was employed for peak detection and retention time alignment (Smith et al. 2006). For peak detection in XCMS, the centWave algorithm was applied with the following settings: ∆m/z tolerance of 30 ppm, minimum peak width of 3 s, maximum peak width of 50 s, and a signal-to-noise threshold of 20. Retention time correction was achieved using the obiwarp function, and peaks were grouped with parameters of an m/z width of 0.1, a base width of 5, and a minimum fraction of 0.1. All chromatographic peaks occurring before 540 s and after 1980 s were excluded. This analysis was done to compare the chemical profiles of mutant D. melanogaster mated females, along the chemical profiles of 16 Drosophila species, including D. simulans and D. melanogaster, taken from a previous study (Moreira-Soto et al. 2024).
The XCMS data (intensities of compounds, i.e., features with distinct m/z (mass-to-charge ratios)) was normalized by the sum of all features per sample. From this, samples were compared using a Uniform Manifold Approximation and Projection (UMAP) in R (4.1.0) with umap package (McInnes et al. 2020), using the default values for the parameters (n_neighbors = 15, min_dist = 0.1). We tested for statistical difference in the abundance from all compounds found, with Tukey’s test for multiple comparisons of means (P < 0.05), using GraphPad Prism v. 9 (https://www.graphpad.com).
Behavioral Experiments
Oviposition Assays
All behavioral experiments were conducted throughout multiple months, with the different test situations being mixed, to avoid that they were run with different cohorts of flies. Tests were carried out to determine whether a single D. melanogaster fly would oviposit together or separately with a single conspecific or, in another set of experiments, with a D. simulans fly when multiple oviposition sites were available. In order to distinguish eggs from two different D. melanogaster females or from one D. melanogaster and one D. simulans female, we used a D. melanogaster fly line (RRID:BDSC_4534) expressing GFP ubiquitously under control of Act5C. This mutant fly line was used for all assays where 2 flies were tested together (Fig. 1 Ci-iv and Di-iv). We tested these mutant D. melanogaster flies against D. simulans, or wild type D. melanogaster as control, using 7–10 days old female flies. These flies were separated by sex upon hatching and maintained in groups in vials with fly food. The day before the assays, males and females were placed together (in group) in vials containing 5% sucrose and yeast powder for 24 h before the assays began.
In cubic cages of 45 cm per side, we set up the oviposition assays for 24 h, where flies had 4 equal small petri dishes (diameter, 3.5 cm) containing fly food, in several different conditions. Afterwards, the eggs on each petri dish were counted (assays that did not yield in any oviposition were excluded from further analyses). Using individual flies, we also tested several different conditions that potentially would give the flies less reasons to cooperate: we either used half the amount of agar in the fly food (see recipe above) to soften the substrate or enriched the oviposition substrate with 20 µl of a 2% yeast solution. On the other hand, we tested increasing competition by making the oviposition surface smaller, using Eppendorf tubes (diameter 1 cm) filled with fly food as oviposition sites. For the treatments, the number of replicates was between 15 and 26 (see Supplementary Data 1). All behavioral experiments were performed under normal white light at 25 °C and 70% humidity.
To test the oviposition of D. simulans with D. melanogaster, or 2 flies of the latter species, in a sequential manner, we set up the same 4 choice oviposition assay using petri dishes as described before, but one fly was left 24 h to oviposit, and was then removed before adding the other fly. In this case, eggs were counted after the first fly had the opportunity to oviposit, and then counted again after the second fly oviposited. This approach also allowed us to use only wild type flies for the sequential assays, as the eggs were counted separately, making fluorescent labeling unnecessary.
All the experiments were conducted in blocks, with each treatment tested across multiple months. To assess the preference for oviposition, we only used the data of the positive assays, i.e. assays in which both flies laid eggs (see Table S2). From these positive assays we calculated the Spearman’s Rank Correlation Coefficient using the function cor in the stats R package 4.1.0, where a value close to 1 means that they lay eggs on the same place, and values close to −1 where the eggs were laid separately. For example, if one fly lays 7 eggs on plate A and the other fly lays 15 eggs on the same plate A, we reach a Spearman correlation of 1. If one fly lays 18 eggs on plate A, and the other fly lays 25 eggs on plate A, and 3 eggs on plate B, the Spearman correlation drops to 0.82. On the other hand, if they lay eggs separately, where one fly lays 20 eggs on plate A, and the other lays 7 eggs on plate B and 2 eggs on plate C, we obtain a Spearman correlation of −0.54. To reach a Spearman correlation of −1, both flies need to lay the same number of eggs, distributed equally in 2 plates for each fly; for example, one fly lays 10 eggs in plate A and 10 in plate B, and the other fly lays 10 in plate C and 10 in plate D. To statistically test if the correlations were significantly different from zero, Wilcoxon signed-rank tests were conducted using GraphPad Prism v. 9 (https://www.graphpad.com).
Aggregation Assays
In order to test, whether females are influenced by the presence or absence of other females when they target an oviposition site, we used a similar setup as before, but instead of oviposition plates now installed 4 traps. We tested 5 D. melanogaster flies against 5 conspecifics or 5 D. simulans, using 7–10 days old female flies mated the day before the assays, and left in vials with 5% saccharose and yeast powder until the assays started. In cubic cages of 45 cm per side, we set up the trap assays for 24 h, where flies had 4 equal vials with fly food (diameter, 3.5 cm) provided with a pipette tip as a one-way entrance, i.e. where they could get in but not come out (Fig. 2A). Experiments were performed under normal white light at 25 °C and 70% humidity. To test the role of vision on this behavior we repeated the tests with no light. In order to test the role of olfaction in the behavior, we used D. melanogaster flies with a mutation rendering their olfactory coreceptor Orco non-functional (RRID:BDSC_23130).
Flies on each of the traps were counted, and separated morphologically by species in the case of mixed assays, using descriptions from McEvey (2019). In order to assess the aggregation, we calculated Morisita’s standardized aggregation index using the vegan R package (Oksanen et al. 2022). Values closer to 1 indicate a significant aggregation, while values closer to 0 indicate a random distribution, and values closer to −1 indicate a uniform distribution of flies in the traps. For example, if 9 flies are found in 1 trap, and the other 3 traps are empty, we reach a standardized Morisita’s index of 1. If we find 5 flies on trap A, 2 flies on traps B and C, and 1 fly on trap D, the standardized Morisita’s index calculated is 0.05. If we find 1 fly in each of the 4 traps, we reach a standardized Morisita’s index of −1. To statistically test if the indexes were significantly different from zero, Wilcoxon signed-rank tests were conducted using GraphPad Prism v. 9 (https://www.graphpad.com).
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Ibrahim Alali, Silke Trautheim, Roland Spieß and Kerstin Weniger for technical support. We are grateful to Dr. Andres Moreira-Soto for suggestions on statistical analyses. Wild-type flies were obtained from the National Drosophila Species Stock Center, Cornell University and KYOTO Stock Center.
Author Contribution
R.M. and M.K. designed the experiments. R.M. conducted experiments, wrote the main manuscript and prepared figures. M.K. and B.H. edited the text and figures.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported through funding by the Max Planck Society. Further funding was provided by the German Academic Exchange Service (DAAD, No. 57440921 to RDM-S).
Data Availability
All the raw data and other relevant data supporting the findings of this study are provided as supplementary files.
Declarations
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmad M, Chaudhary SU, Afzal AJ, Tariq M (2015) Starvation-induced dietary behaviour in Drosophila melanogaster larvae and adults. Sci Rep 5:14285. 10.1038/srep14285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allee WC (1927) Animal aggregations. Q Rev Biol 2(3):367–398. 10.1086/394281 [Google Scholar]
- Antony C, Davis TL, Carlson DA, Pechine JM, Jallon JM (1985) Compared behavioral responses of male Drosophilamelanogaster (Canton S) to natural and synthetic aphrodisiacs. J Chem Ecol 11(12):1617–1629. 10.1007/bf01012116 [DOI] [PubMed] [Google Scholar]
- Atkinson W, Shorrocks B (1977) Breeding site specificity in the domestic species of Drosophila. Oecologia 29:223–232 [DOI] [PubMed] [Google Scholar]
- Ayala FJ (1970) Competition, coexistence, and evolution. In: Hecht MK, Steere WE (eds) Essays in Evolution and Genetics in Honor of Th. Dobzhansky (pp. 121–158). Appleton-Century-Crofts
- Azanchi R, Kaun KR, Heberlein U (2013) Competing dopamine neurons drive oviposition choice for ethanol in Drosophila. Proc Natl Acad Sci U S A 110(52):21153–21158. 10.1073/pnas.1320208110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly TPM, Kohlmeier P, Etienne RS, Wertheim B, Billeter JC (2023) Social modulation of oogenesis and egg laying in Drosophila melanogaster. Curr Biol 33(14):2865-2877 e2864. 10.1016/j.cub.2023.05.074 [DOI] [PubMed] [Google Scholar]
- Bakula M (1969) The persistence of a microbial flora during postembryogenesis of Drosophila melanogaster. J Invertebr Pathol 14(3):365–374. 10.1016/0022-2011(69)90163-3 [DOI] [PubMed] [Google Scholar]
- Bartelt RJ, Schaner AM, Jackson LL (1985) cis-Vaccenyl acetate as an aggregation pheromone in Drosophilamelanogaster. J Chem Ecol 11(12):1747–1756. 10.1007/bf01012124 [DOI] [PubMed] [Google Scholar]
- Battesti M, Moreno C, Joly D, Mery F (2012) Spread of social information and dynamics of social transmission within Drosophila groups. Curr Biol 22(4):309–313. 10.1016/j.cub.2011.12.050 [DOI] [PubMed] [Google Scholar]
- Beltramí M, Medina-Muñoz MC, Del Pino F, Ferveur J-F, Godoy-Herrera R (2012) Chemical cues influence pupation behavior of Drosophilasimulans and Drosophilabuzzatii in nature and in the laboratory. PLoS ONE 7(6):e39393. 10.1371/journal.pone.0039393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnik M, Brncic D (1974) Preadult competition between Drosophilapavani and Drosophilamelanogaster, Drosophilasimulans, and Drosophilawillistoni. Ecology 55(3):657–661. 10.2307/1935157 [Google Scholar]
- Chess KF, Ringo JM (1985) Oviposition site selection by Drosophila melanogaster and Drosophila simulans. Evolution 39:869–877. 10.1111/j.1558-5646.1985.tb00428.x [DOI] [PubMed] [Google Scholar]
- Chyb S (2004) Drosophila gustatory receptors: from gene identification to functional expression. J Insect Physiol 50(6):469–477. 10.1016/j.jinsphys.2004.03.012 [DOI] [PubMed] [Google Scholar]
- Costa-da-Silva AL, Cabal S, Lopez K, Boloix J, Rodriguez BG, Marrero KM, Bellantuono AJ, DeGennaro M (2024) Female Aedesaegypti mosquitoes use communal cues to manage population density at breeding sites. Commun Biol 7(1):143. 10.1038/s42003-024-05830-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchamp F, Clutton-Brock T, Grenfell B (1999) Inverse density dependence and the Allee effect. Trends Ecol Evol 14(10):405–410. 10.1016/S0169-5347(99)01683-3 [DOI] [PubMed] [Google Scholar]
- Dumenil C, Woud D, Pinto F, Alkema JT, Jansen I, Van Der Geest AM, Roessingh S, Billeter JC (2016) Pheromonal cues deposited by mated females convey social information about egg-laying sites in Drosophilamelanogaster. J Chem Ecol 42(3):259–269. 10.1007/s10886-016-0681-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durisko Z, Dukas R (2013) Attraction to and learning from social cues in fruitfly larvae. Proc Biol Sci 280(1767):20131398. 10.1098/rspb.2013.1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durisko Z, Kemp R, Mubasher R, Dukas R (2014) Dynamics of social behavior in fruit fly larvae. PLoS ONE 9(4):e95495. 10.1371/journal.pone.0095495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dweck HK, Ebrahim SA, Kromann S, Bown D, Hillbur Y, Sachse S, Hansson BS, Stensmyr MC (2013) Olfactory preference for egg laying on citrus substrates in Drosophila. Curr Biol 23(24):2472–2480. 10.1016/j.cub.2013.10.047 [DOI] [PubMed] [Google Scholar]
- Ebrahim SA, Dweck HK, Stokl J, Hofferberth JE, Trona F, Weniger K, Rybak J, Seki Y, Stensmyr MC, Sachse S, Hansson BS, Knaden M (2015) Drosophila avoids parasitoids by sensing their semiochemicals via a dedicated olfactory circuit. PLoS Biol 13(12):e1002318. 10.1371/journal.pbio.1002318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne RS, Wertheim B, Hemerik L, Schneider P, Powell J (2002) The interaction between dispersal, the Allee effect and scramble competition affects population dynamics. Ecol Model 148:152–168. 10.1016/S0304-3800(01)00417-3 [Google Scholar]
- Fanara JJ, Beti MIL, Gandini L, Hasson E (2023) Oviposition behaviour in Drosophilamelanogaster: Genetic and behavioural decoupling between oviposition acceptance and preference for natural fruits. J Evol Biol 36(1):251–263. 10.1111/jeb.14109 [DOI] [PubMed] [Google Scholar]
- Ferveur J-F (2005) Cuticular hydrocarbons: their evolution and roles in Drosophila pheromonal communication. Behav Genet 35(3):279–295. 10.1007/s10519-005-3220-5 [DOI] [PubMed] [Google Scholar]
- Hedrick PW (1972) Factors responsible for a change in interspecific competitive ability in Drosophila. Evolution 26(4):513–522. 10.1111/j.1558-5646.1972.tb01957.x [DOI] [PubMed] [Google Scholar]
- Hedrick PW, King E (1996) Genetics and the environment in interspecific competition: a study using the sibling species Drosophila melanogaster and Drosophila simulans. Oecologia 108:72–78. 10.1007/BF00333216 [DOI] [PubMed] [Google Scholar]
- Joseph RM, Devineni AV, King IFG, Heberlein U (2009) Oviposition preference for and positional avoidance of acetic acid provide a model for competing behavioral drives in Drosophila. Proc Natl Acad Sci U S A 106(27):11352. 10.1073/pnas.0901419106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khallaf MA, Cui R, Weißflog J, Erdogmus M, Svatoš A, Dweck HK, Valenzano DR, Hansson BS, Knaden M (2021) Large-scale characterization of sex pheromone communication systems in Drosophila. Nat Commun 12(1):4165. 10.1038/s41467-021-24395-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidera H, Takahashi KH (2020) Chemical cues from competitors change the oviposition preference of Drosophilasuzukii. Entomol Exp Appl 168(4):304–310. 10.1111/eea.12889 [Google Scholar]
- Laturney M, Billeter JC (2016) Drosophilamelanogaster females restore their attractiveness after mating by removing male anti-aphrodisiac pheromones. Nat Commun 7:12322. 10.1038/ncomms12322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre T, de Roode JC, Kacsoh BZ, Schlenke TA (2012) Defence strategies against a parasitoid wasp in Drosophila: fight or flight? Biol Lett 8(2):230–233. 10.1098/rsbl.2011.0725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Zhang K, Li Y, Su W, Hu K, Jin S (2017) Enterococci mediate the oviposition preference of Drosophilamelanogaster through sucrose catabolism. Sci Rep 7(1):13420. 10.1038/s41598-017-13705-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markow TA (2015) The secret lives of Drosophila flies. Elife 4:e06793. 10.7554/eLife.06793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markow TA, O’Grady P (2008) Reproductive ecology of Drosophila. Funct Ecol 22(5):747–759. 10.1111/j.1365-2435.2008.01457.x [Google Scholar]
- McEvey S (2019) Drosophila melanogaster and D. simulans (Diptera: Drosophilidae)—morphological differences. In. figshare. 10.6084/m9.figshare.7981505.v1
- McInnes L, Healy J, Melville J (2020) UMAP: Uniform Manifold Approximation and Projection for Dimension Reduction. arXiv pre-print server. https://arxiv.org/abs/1802.03426
- Meunier N, Ferveur J-F, Marion-Poll F (2000) Sex-specific non-pheromonal taste receptors in Drosophila. Curr Biol 10(24):1583–1586. 10.1016/s0960-9822(00)00860-5 [DOI] [PubMed] [Google Scholar]
- Miller RS (1964) Larval competition in Drosophila melanogaster and D. simulans. Ecology 45(1):132–148. 10.2307/1937114 [Google Scholar]
- Moreira-Soto RD, Khallaf MA, Hansson BS, Knaden M (2024) How conspecific and allospecific eggs and larvae drive oviposition preference in Drosophila. Chem Senses 49:bjae012. 10.1093/chemse/bjae012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimha S, Nagornov KO, Menin L, Mucciolo A, Rohwedder A, Humbel BM, Stevens M, Thum AS, Tsybin YO, Vijendravarma RK (2019) Drosophilamelanogaster cloak their eggs with pheromones, which prevents cannibalism. PLoS Biol 17(1):e2006012. 10.1371/journal.pbio.2006012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen J, Simpson GL, Blanchet FG, Kindt R, Legendre P, Minchin PR, O'Hara RB, Solymos P, Stevens MHH, Szoecs E, Wagner H, Barbour M, Bedward M, Bolker B, Borcard D, Carvalho G, Chirico M, Caceres MD, Durand S, … Weedon J (2022) vegan: Community Ecology Package. In (Version 2.6–2) https://CRAN.R-project.org/package=vegan.
- Orengo DJ, Prevosti A (1994) Preadult competition between Drosophila subobscura and Drosophila pseudoobscura. J Zool Syst Evol Res 32(1):44–50. 10.1111/j.1439-0469.1994.tb00469.x [Google Scholar]
- Refsnider JM, Janzen FJ (2010) Putting eggs in one basket: ecological and evolutionary hypotheses for variation in oviposition-site choice. Annu Rev Ecol Evol Syst 41(1):39–57. 10.1146/annurev-ecolsys-102209-144712 [Google Scholar]
- Richmond RC, Gerking JL (1978) Oviposition site preference in Drosophila. Behav Genet 9(3):233–241. 10.1007/BF01071304 [DOI] [PubMed] [Google Scholar]
- Rohlfs M (2005a) Clash of kingdoms or why Drosophila larvae positively respond to fungal competitors. Front Zool 2(1):2. 10.1186/1742-9994-2-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlfs M (2005b) Density-dependent insect-mold interactions: effects on fungal growth and spore production. Mycologia 97(5):996–1001. 10.3852/mycologia.97.5.996 [DOI] [PubMed] [Google Scholar]
- Sarin S, Dukas R (2009) Social learning about egg-laying substrates in fruitflies. Proc Biol Sci 276(1677):4323–4328. 10.1098/rspb.2009.1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz NU, Zhong L, Bellemer A, Tracey WD (2012) Egg laying decisions in Drosophila are consistent with foraging costs of larval progeny. PLoS ONE 7(5):e37910. 10.1371/journal.pone.0037910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, Want EJ, O’Maille G, Abagyan R, Siuzdak G (2006) XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal Chem 78(3):779–787. 10.1021/ac051437y [DOI] [PubMed] [Google Scholar]
- Stamps JA, Yang LH, Morales VM, Boundy-Mills KL (2012) Drosophila regulate yeast density and increase yeast community similarity in a natural substrate. PLoS ONE 7(7):e42238. 10.1371/journal.pone.0042238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker RF (1994) The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res 275:3–26. 10.1007/BF00305372 [DOI] [PubMed] [Google Scholar]
- Takahashi KH, Kimura MT, Benton T (2005) Intraspecific and interspecific larval interaction in Drosophila assessed by integrated fitness measure. Oikos, 111(3), 574–581. https://www.jstor.org/stable/3548649
- Takamura T, Fuyama Y (1980) Behaviour genetics of choice of oviposition sites in Drosophila melanogaster. I. Genetic variability and analysis of behavior. Behav Genet 10:105–120. 10.1007/BF01067322 [DOI] [PubMed] [Google Scholar]
- Tungadi TD, Shaw B, Powell G, Hall DR, Bray DP, Harte SJ, Farman DI, Wijnen H, Fountain MT (2022) Live Drosophila melanogaster larvae deter oviposition by Drosophila suzukii. Insects 13(8):688. 10.3390/insects13080688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tungadi TD, Powell G, Shaw B, Fountain MT (2023) Factors influencing oviposition behaviour of the invasive pest, Drosophilasuzukii, derived from interactions with other Drosophila species: potential applications for control. Pest Manag Sci 79(11):4132–4139. 10.1002/ps.7693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijendravarma RK, Narasimha S, Kawecki TJ (2013) Predatory cannibalism in Drosophila melanogaster larvae. Nat Commun 4:1789. 10.1038/ncomms2744 [DOI] [PubMed] [Google Scholar]
- Wertheim B, Marchais J, Vet LEM, Dicke M (2002) Allee effect in larval resource exploitation in Drosophila: an interaction among density of adults, larvae, and micro-organisms. Ecol Entomol 27(5):608–617. 10.1046/j.1365-2311.2002.00449.x [Google Scholar]
- Yang CH, Belawat P, Hafen E, Jan LY, Jan YN (2008) Drosophila egg-laying site selection as a system to study simple decision-making processes. Science 319(5870):1679–1683. 10.1126/science.1151842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yew JY, Dreisewerd K, Luftmann H, Müthing J, Pohlentz G, Kravitz EA (2009) A new male sex pheromone and novel cuticular cues for chemical communication in Drosophila. Curr Biol 19(15):1245–1254. 10.1016/j.cub.2009.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the raw data and other relevant data supporting the findings of this study are provided as supplementary files.