Abstract
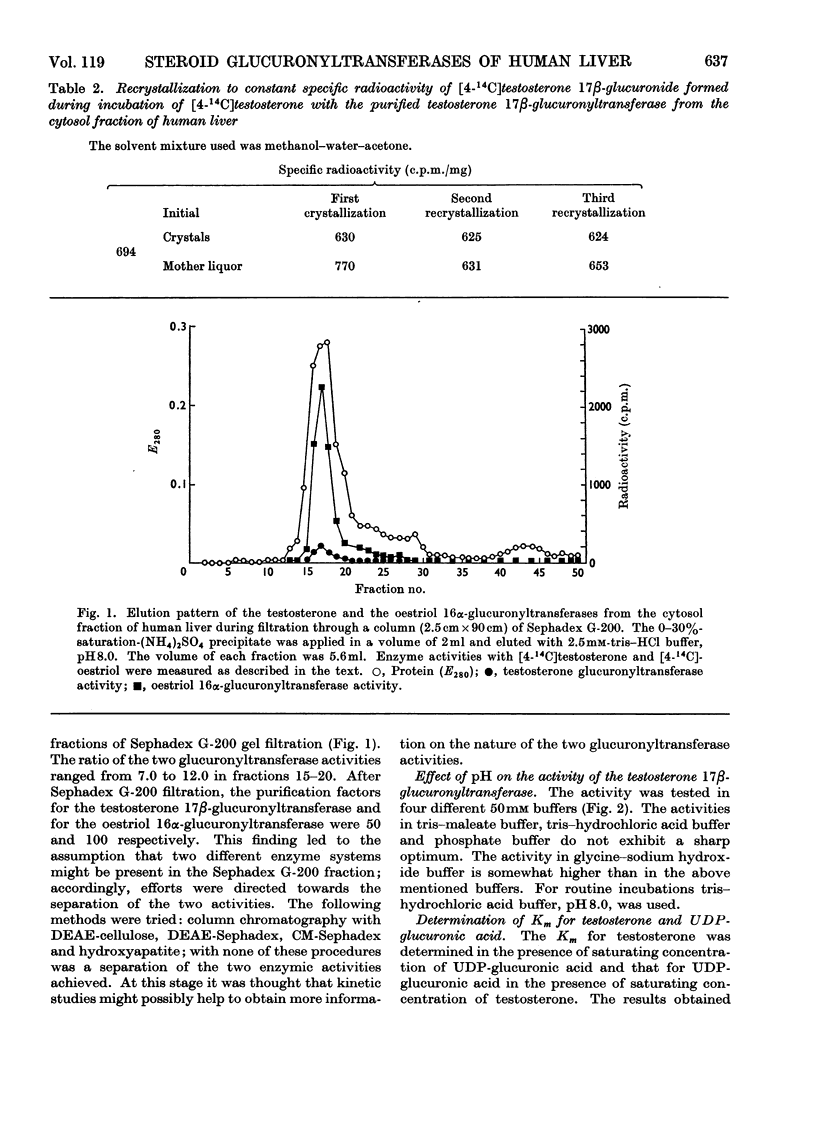
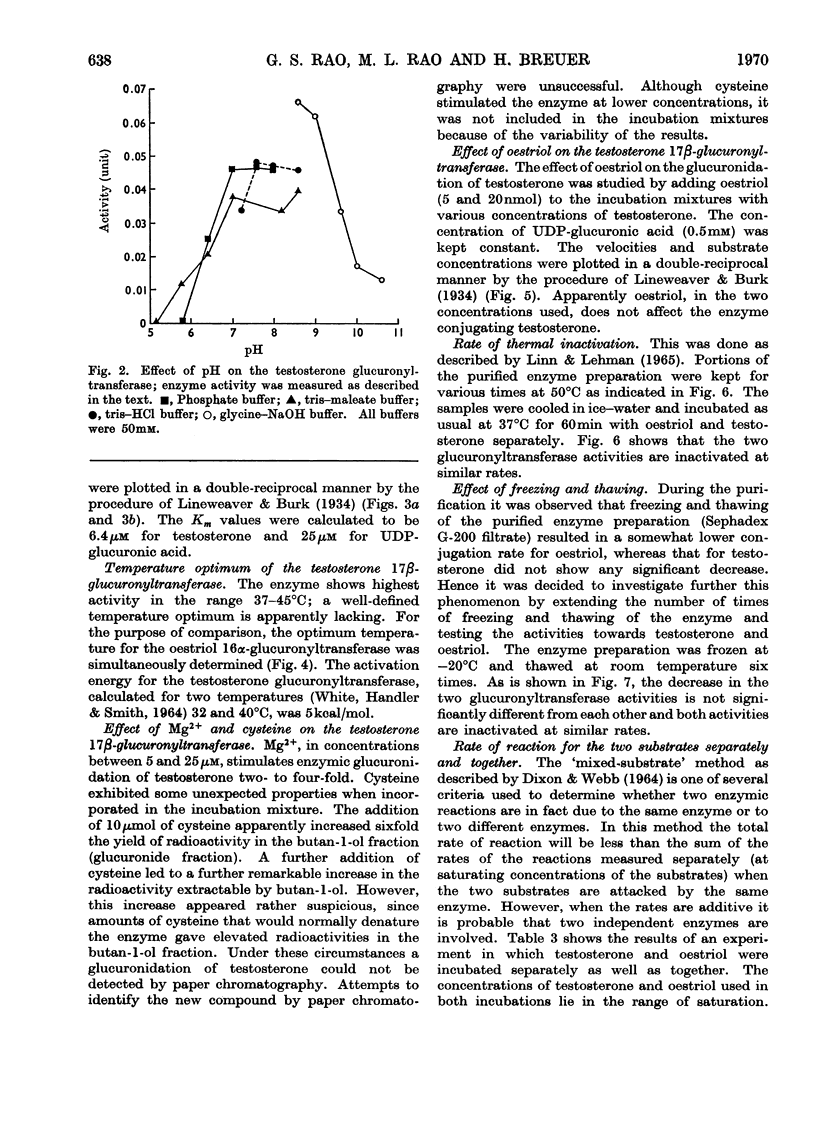
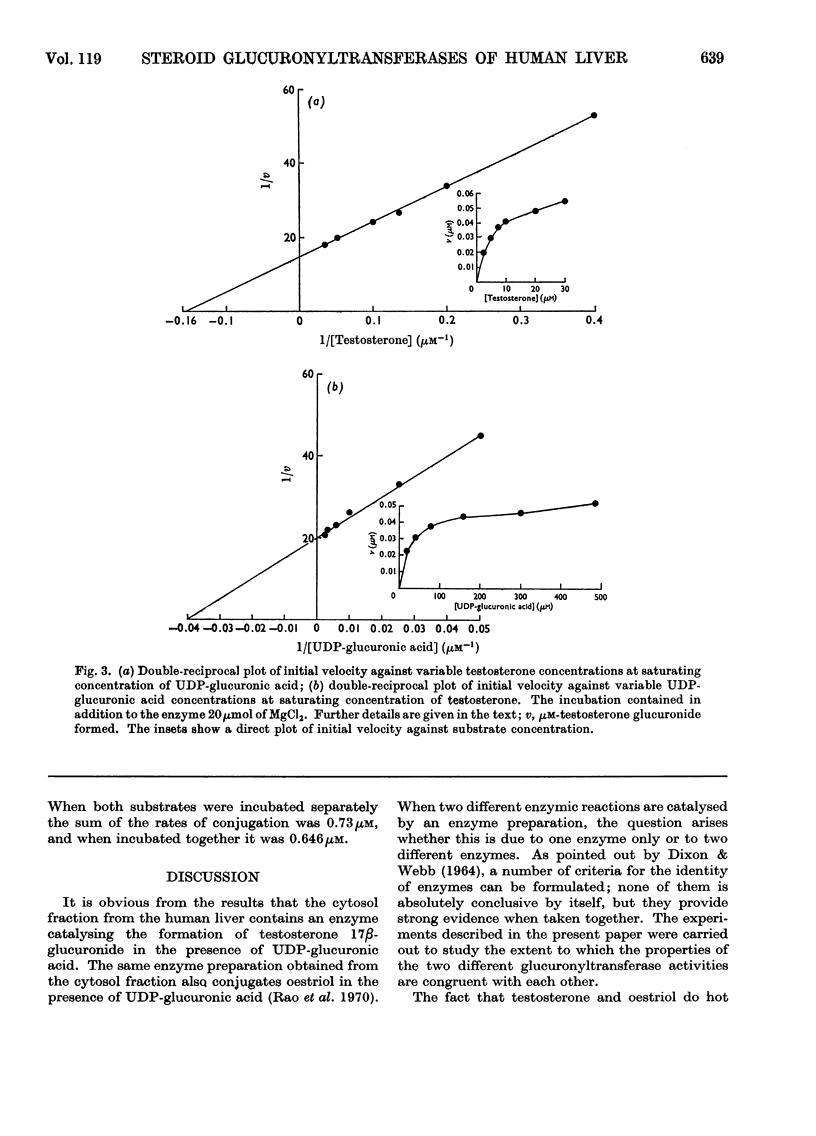
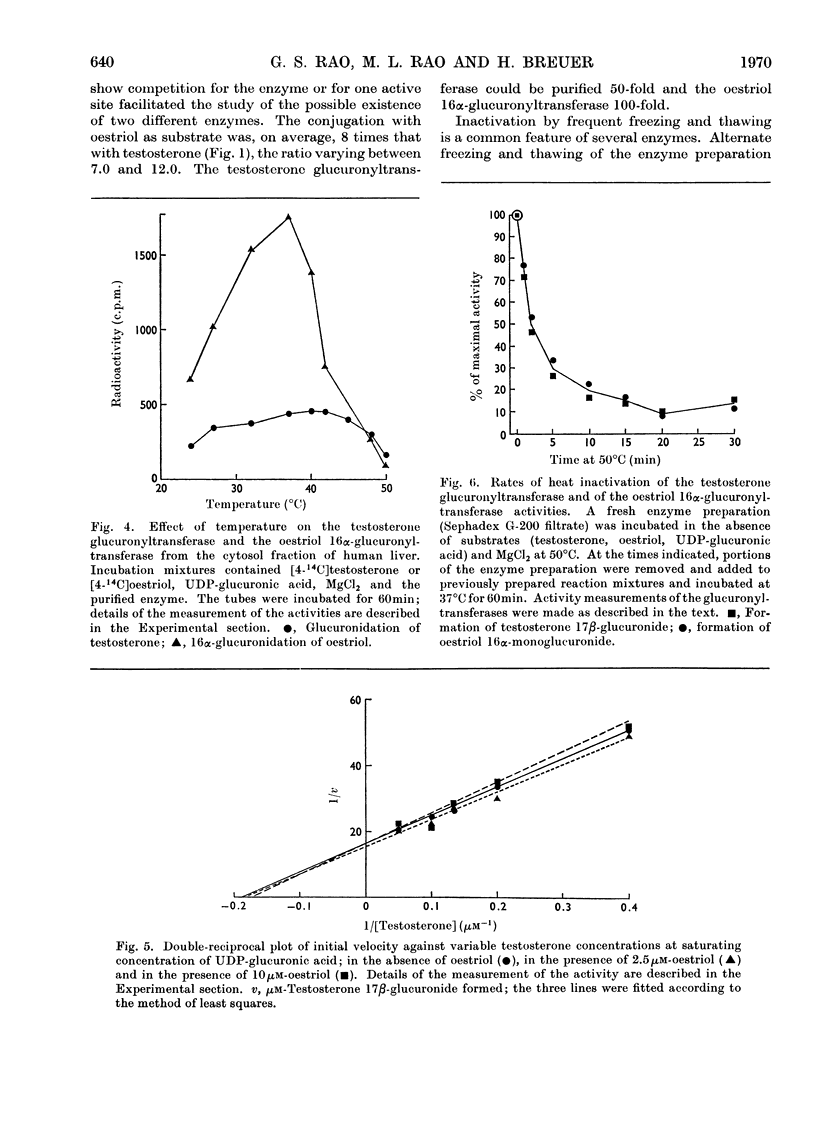
An enzyme that conjugates the 17β-hydroxyl group of testosterone was found in the cytosol fraction of human liver. The same enzyme preparation also conjugates the 16α-hydroxyl group of oestriol. The enzymic activity could not be sedimented by centrifuging the cytosol fraction at 158000gav. for 120min. The testosterone-conjugating as well as the oestriol-conjugating activities were found in the precipitate obtained after 30% saturation of the cytosol fraction with ammonium sulphate. Filtration of the precipitate through Sephadex G-200 enriched the testosterone-conjugating enzyme 50-fold and the oestriol-conjugating enzyme 100-fold. No separation of the two activities was achieved. With labelled testosterone the product of the reaction, testosterone 17β-glucuronide, was identified by paper chromatography and by crystallization to constant specific radioactivity. Testosterone 17β-glucuronyltransferase was active between pH7.0 and 8.6 in tris–HCl and tris–maleate buffers. The apparent Km values for testosterone and UDP-glucuronic acid were 6.4 and 25μm respectively. The enzyme was active between 37 and 45°C; the activation energy was calculated to be 5kcal/mol. Oestriol did not influence the glucuronidation of testosterone. Controlled heating as well as alternate freezing and thawing of the purified enzyme preparation led to an inactivation of both testosterone-conjugating and oestriol-conjugating activities at similar rates. Testosterone and oestriol, when incubated together, gave a reaction rate that was approximately equal to the sum of the rates when the two substrates were incubated separately. The present findings suggest that testosterone and oestriol are conjugated by two separate enzymes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- LINN S., LEHMAN I. R. AN ENDONUCLEASE FROM NEUROSPORA CRASSA SPECIFIC FOR POLYNUCLEOTIDES LACKING AN ORDERED STRUCTURE. I. PURIFICATION AND PROPERTIES OF THE ENZYME. J Biol Chem. 1965 Mar;240:1287–1293. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Rao G. S., Rao M. L., Breuer H. Partial purification and kinetics of oestriol 16 alpha-glucuronyltransferase from the cytosol fraction of human liver. Biochem J. 1970 Jul;118(4):625–634. doi: 10.1042/bj1180625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. G., Hall P. F. The side-chain cleavage of cholesterol and cholesterol sulfate by enzymes from bovine adrenocortical mitochondria. Biochemistry. 1969 Jul;8(7):2987–2997. doi: 10.1021/bi00835a046. [DOI] [PubMed] [Google Scholar]


