Abstract
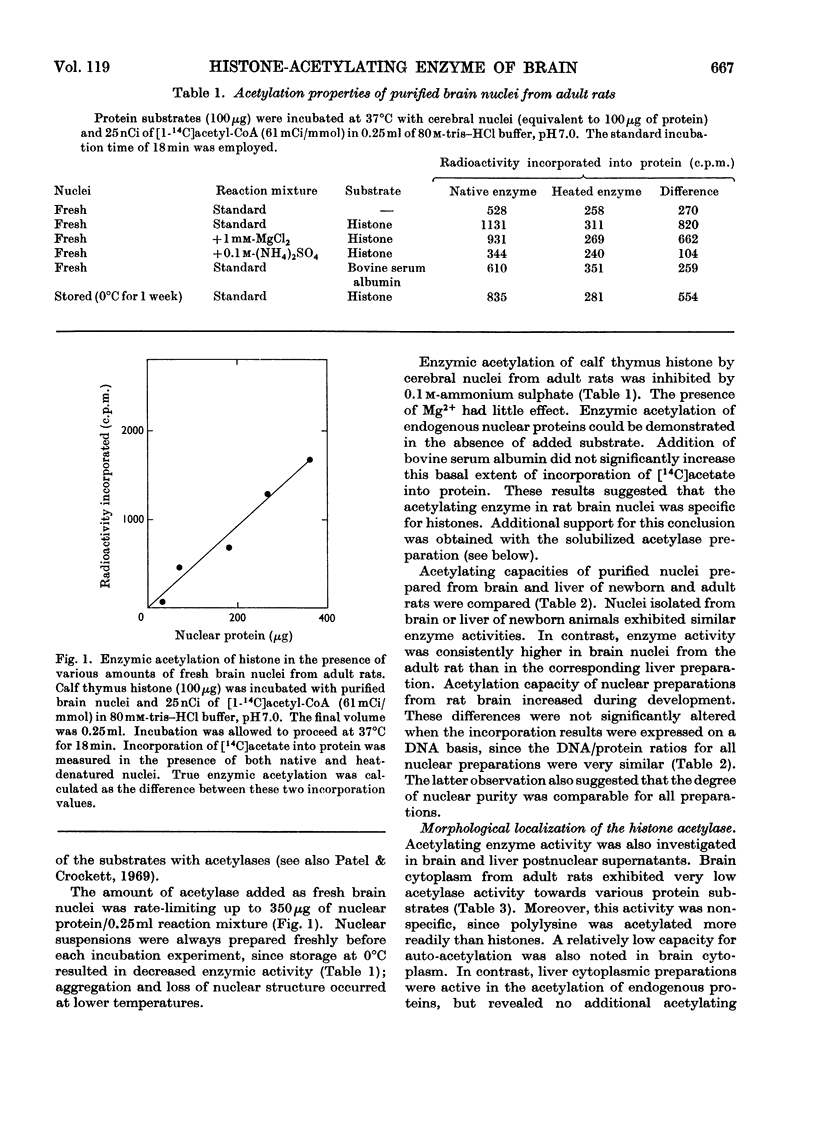
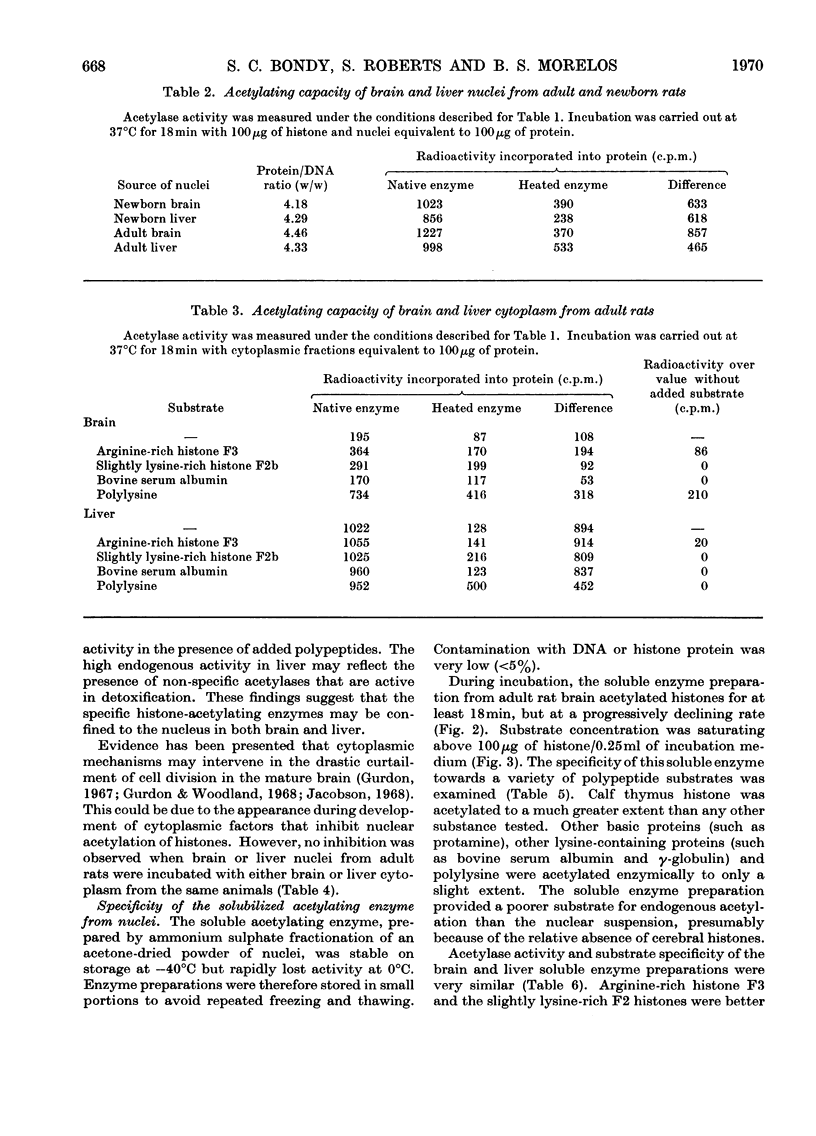
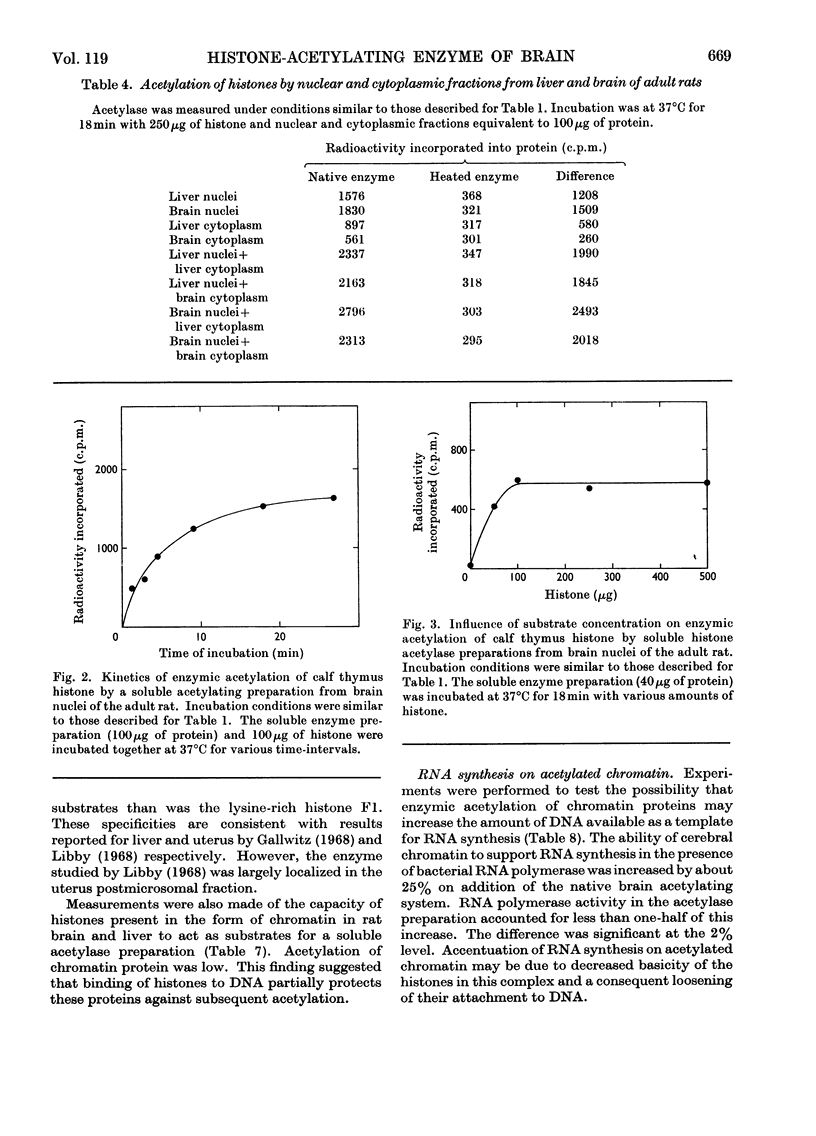
1. Acetylation of histones by an enzyme system derived from rat brain and liver (histone acetylase) was studied by using [1-14C]acetyl-CoA as the acetyl group donor. 2. The activity of this enzyme was largely confined to the nucleus. 3. Histone-acetylating activity of cerebral nuclei purified by centrifugation through 1.9m-sucrose was not altered by the presence of the cytoplasmic fraction. 4. Cerebral nuclei from adult rats exhibited greater histone-acetylating activity than did the corresponding preparation from newborn animals. 5. Nuclear acetylating activity was higher in brain than in liver of adult rats but not in newborn animals. 6. The partially purified enzyme from cerebral nuclei, prepared by ammonium sulphate fractionation of an acetone-dried powder, specifically catalysed histone acetylation. 7. Polylysine, protamine, serum albumin and γ-globulin were not enzymically acetylated by this preparation. 8. Soluble acetylating preparations from both brain and liver nuclei were more active towards arginine-rich F3 and slightly lysine-rich F2a and F2b histone fractions than towards the lysine-rich F1 fraction. 9. Enzymic acetylation of chromatin-bound proteins was much less extensive than that of free histones. 10. The high histone acetylase activity in mature brain may reflect the importance of this process in the genetic control of cerebral function.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN E. K., Jr, WALKER B. E. Incorporation of thymidine-H3 by cells in normal and injured mouse spinal cord. J Neuropathol Exp Neurol. 1962 Oct;21:597–609. doi: 10.1097/00005072-196210000-00007. [DOI] [PubMed] [Google Scholar]
- Adams D. H. The relationship between cellular nucleic acids in the developing rat cerebral cortex. Biochem J. 1966 Feb;98(2):636–640. doi: 10.1042/bj0980636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy S. C., Roberts S. Developmental and regional variations in ribonucleic acid synthesis on cerebral chromatin. Biochem J. 1969 Nov;115(2):341–349. doi: 10.1042/bj1150341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy S. C., Roberts S. Hybridizable ribonucleic acid of rat brain. Biochem J. 1968 Oct;109(4):533–541. doi: 10.1042/bj1090533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy S. C. The ribonucleic acid metabolism of the brain. J Neurochem. 1966 Oct;13(10):955–959. doi: 10.1111/j.1471-4159.1966.tb10291.x. [DOI] [PubMed] [Google Scholar]
- Byvoet P. Differences in turnover between histones and their acetyl N-terminal groups. Biochim Biophys Acta. 1968 Jun 26;160(2):217–223. doi: 10.1016/0005-2795(68)90089-5. [DOI] [PubMed] [Google Scholar]
- Byvoet P. Metabolic integrity of deoxyribonucleohistones. J Mol Biol. 1966 Jun;17(2):311–318. doi: 10.1016/s0022-2836(66)80143-2. [DOI] [PubMed] [Google Scholar]
- CHOU T. C., LIPMANN F. Separation of acetyl transfer enzymes in pigeon liver extract. J Biol Chem. 1952 May;196(1):89–103. [PubMed] [Google Scholar]
- Caspary E. A., Sewell F. M. Histone acetylation in scrapie-affected mouse brain. Experientia. 1968 Aug 15;24(8):793–794. doi: 10.1007/BF02144871. [DOI] [PubMed] [Google Scholar]
- Chalkley G. R., Maurer H. R. Turnover of template-bound histone. Proc Natl Acad Sci U S A. 1965 Aug;54(2):498–505. doi: 10.1073/pnas.54.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLange R. J., Fambrough D. M., Smith E. L., Bonner J. Calf and pea histone IV. II. The complete amino acid sequence of calf thymus histone IV; presence of epsilon-N-acetyllysine. J Biol Chem. 1969 Jan 25;244(2):319–334. [PubMed] [Google Scholar]
- Dick C., Johns E. W. A quantitative comparison of histones from immature and mature erythroid cells of the duck. Biochim Biophys Acta. 1969 Mar;175(2):414–418. doi: 10.1016/0005-2795(69)90020-8. [DOI] [PubMed] [Google Scholar]
- FRENSTER J. H., ALLFREY V. G., MIRSKY A. E. REPRESSED AND ACTIVE CHROMATIN ISOLATED FROM INTERPHASE LYMPHOCYTES. Proc Natl Acad Sci U S A. 1963 Dec;50:1026–1032. doi: 10.1073/pnas.50.6.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallwitz D. Acetylation of histones by a kinase from rat liver nucle. Biochem Biophys Res Commun. 1968 Jul 26;32(2):117–121. doi: 10.1016/0006-291x(68)90355-0. [DOI] [PubMed] [Google Scholar]
- Gallwitz D., Sekeris C. E. The acetylation of histones of rat liver nuclei in vitro by acetyl-CoA. Hoppe Seylers Z Physiol Chem. 1969 Feb;350(2):150–154. doi: 10.1515/bchm2.1969.350.1.150. [DOI] [PubMed] [Google Scholar]
- Gershey E. L., Vidali G., Allfrey V. G. Chemical studies of histone acetylation. The occurrence of epsilon-N-acetyllysine in the f2a1 histone. J Biol Chem. 1968 Oct 10;243(19):5018–5022. [PubMed] [Google Scholar]
- Gurdon J. B., Woodland H. R. The cytoplasmic control of nuclear activity in animal development. Biol Rev Camb Philos Soc. 1968 May;43(2):233–267. doi: 10.1111/j.1469-185x.1968.tb00960.x. [DOI] [PubMed] [Google Scholar]
- HINDLEY J. The relative ability of reconstituted nucleohistones to allow DNA-dependent RNA synthesis. Biochem Biophys Res Commun. 1963 Jul 26;12:175–179. doi: 10.1016/0006-291x(63)90184-0. [DOI] [PubMed] [Google Scholar]
- Hydén H., McEwen B. A glial protein specific for the nervous system. Proc Natl Acad Sci U S A. 1966 Feb;55(2):354–358. doi: 10.1073/pnas.55.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNS E. W., PHILLIPS D. M., SIMSON P., BUTLER J. A. Improved fractionations of arginine-rich histones from calf thymus. Biochem J. 1960 Dec;77:631–636. doi: 10.1042/bj0770631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob M., Stevenin J., Jund R., Judes C., Mandel P. Rapidly-labelled ribonucleic acids in brain. J Neurochem. 1966 Aug;13(8):619–628. doi: 10.1111/j.1471-4159.1966.tb09870.x. [DOI] [PubMed] [Google Scholar]
- Jacobson C. O. Reactivation of DNA synthesis in mammalian neuron nuclei after fusion with cells of an undifferentiated fibroblast line. Exp Cell Res. 1968 Oct;53(1):316–318. doi: 10.1016/0014-4827(68)90385-6. [DOI] [PubMed] [Google Scholar]
- Kimberlin R. H., Anger H. S. DNA synthesis in the glial cells of scrapie-affected mouse brain. J Neurochem. 1969 Apr;16(4):543–548. doi: 10.1111/j.1471-4159.1969.tb06853.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langan T. A. Histone phosphorylation: stimulation by adenosine 3',5'-monophosphate. Science. 1968 Nov 1;162(3853):579–580. doi: 10.1126/science.162.3853.579. [DOI] [PubMed] [Google Scholar]
- Martenson R. E., Deibler G. E., Kies M. W. Extraction of rat myelin basic protein free of other basic proteins of whole central nervous system tissue. An analysis of its electrophoretic heterogeneity. J Biol Chem. 1969 Aug 25;244(16):4268–4272. [PubMed] [Google Scholar]
- Mould D. L. Inherited characteristics affecting neurotropic behaviour of scrapie. Biochem J. 1969 Feb;111(3):17P–17P. doi: 10.1042/bj1110017pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A. B., Cohen M. M. Histone acetylation: cytological evidence in human lymphocytes. Exp Cell Res. 1969 Feb;54(2):257–260. doi: 10.1016/0014-4827(69)90245-6. [DOI] [PubMed] [Google Scholar]
- NEIDLE A., WAELSCH H. HISTONES: SPECIES AND TISSUE SPECIFICITY. Science. 1964 Sep 4;145(3636):1059–1061. doi: 10.1126/science.145.3636.1059. [DOI] [PubMed] [Google Scholar]
- Piha R. S., Cuénod M., Waelsch H. Metabolism of histones of brain and liver. J Biol Chem. 1966 May 25;241(10):2397–2404. [PubMed] [Google Scholar]
- Pogo B. G., Allfrey V. G., Mirsky A. E. RNA synthesis and histone acetylation during the course of gene activation in lymphocytes. Proc Natl Acad Sci U S A. 1966 Apr;55(4):805–812. doi: 10.1073/pnas.55.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo B. G., Pogo A. O., Allfrey V. G., Mirsky A. E. Changing patterns of histone acetylation and RNA synthesis in regeneration of the liver. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1337–1344. doi: 10.1073/pnas.59.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadgopal A., Bonner J. The relationship between histone and DNA synthesis in HeLa cells. Biochim Biophys Acta. 1969 Aug 20;186(2):349–357. doi: 10.1016/0005-2787(69)90013-6. [DOI] [PubMed] [Google Scholar]
- Sluyser M. Binding of hydrocortisone to rat liver histones. J Mol Biol. 1966 Aug;19(2):591–595. doi: 10.1016/s0022-2836(66)80029-3. [DOI] [PubMed] [Google Scholar]
- Spelsberg T. C., Tankersley S., Hnilica L. S. The interaction of RNA polymerase with histones. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1218–1225. doi: 10.1073/pnas.62.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevely W. S., Stocken L. A. Histone phosphorylation and cell division. Biochem J. 1968 Sep;109(3):24P–25P. doi: 10.1042/bj1090024pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunaga K., Koide S. S. Identification of a prednisolone derivative interacting with calf thymus histones. J Pharm Sci. 1968 Dec;57(12):2116–2119. doi: 10.1002/jps.2600571219. [DOI] [PubMed] [Google Scholar]
- Teng C. S., Hamilton T. H. Role of chromatin in estrogen action in the uterus. II. Hormone-induced synthesis of nonhistone acidic proteins which restore histone-inhibited DNA-dependent RNA synthesis. Proc Natl Acad Sci U S A. 1969 Jun;63(2):465–472. doi: 10.1073/pnas.63.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi L. G., Kornguth S. E. Characterization and immunochemical localization of a basic protein from pig brain. II. Peptide maps and tissue-specific nuclear localization. J Biol Chem. 1968 May 25;243(10):2507–2513. [PubMed] [Google Scholar]
- ZOMZELY C. E., ROBERTS S., RAPAPORT D. REGULATION OF CEREBRAL METABOLISM OF AMINO ACIDS-3. CHARACTERISTICS OF AMINO ACID INCORPORATION INTO PROTEIN OF MICROSOMAL AND RIBOSOMAL PREPARATIONS OF RAT CEREBRAL CORTEX. J Neurochem. 1964 Aug;11:567–582. doi: 10.1111/j.1471-4159.1964.tb11454.x. [DOI] [PubMed] [Google Scholar]


