Abstract
The leukocyte integrin LFA1 is indispensable for immune responses, orchestrating lymphocyte trafficking and adhesion. While LFA1 activation induces LFA1 clustering at the cell contact surface via outside-in signaling, the regulatory mechanisms remain unclear. Here, we uncovered a previously hidden function of the autophagosome component LC3 beyond its role in autophagy by bridging two seemingly unrelated pathways: LFA1 transport and autophagosome transport. LFA1 clusters co-trafficked with LC3, facilitating LFA1 accumulation at the contact surface. LC3b knockout decreased lymphocyte adhesiveness. LFA1 activation did not induce autophagy, whereas it increased mTOR and AMPK activity. LFA1-dependent AMPK activation enhances LFA1 and LC3 clustering and adhesion. Inhibiting Mst1 kinase-mediated LC3 phosphorylation promoted LC3-mediated LFA1 recruitment to the contact surface through direct interaction with RAPL, uncovering an unprecedented integrin recruitment route. These findings uncover a function of LC3 and expand our understanding of lymphocyte regulation via LFA1.
Subject terms: Integrins, Autophagy, Lymphocytes
LC3 is required for canonical and non-canonical autophagy. Here, the authors uncover LAPTIN, a function of LC3 triggered by integrin LFA1 outside-in signaling, which activates AMPK to drive the co-clustering of LFA1 and LC3, thereby increasing LFA1 transport and lymphocyte adhesion.
Introduction
The leukocyte integrin LFA1 (αLβ2) plays a fundamental role in lymphocyte trafficking and adhesion, which are essential for immune surveillance. LFA1 activation is controlled by bidirectional signaling, namely, inside-out and outside-in signaling1,2. Inside-out signaling, which is triggered by surface molecules other than integrins present in lymphocytes, such as the T-cell receptor (TCR) and chemokine receptors3, activates Rap1 to induce a conformational change in LFA1 from the inactive low-affinity/bent conformation (Low in Fig. 1a) to the active high-affinity/open conformation (High in Fig. 1a). LFA1 conformational changes driven by inside-out signaling lead to the binding of LFA1 to intercellular adhesion molecule 1 (ICAM1), which stabilizes LFA1 in its active state and induces outside-in signaling, resulting in the reorganization of cytoskeleton and cell spreading. The small GTPase Rap1 binds to guanosine triphosphate (GTP) and localizes to the membrane by exposing the geranyl-geranyl moiety at the C-terminus. GTP-bound active Rap1 plays a key role in integrin activation upon binding to effector molecules such as RAPL4,5 and RIAM6. The Rap1–RAPL complex activates the Ste20-like kinase Mst17,8, which phosphorylates NDR1 kinase; phosphorylated NDR1 recruits the LFA1 adaptor kindlin-3, which modulates LFA1 activity9. Rap1 also mediates the recruitment of the LFA1 adaptor talin1 to directly bind to LFA110,11. LFA1 activation is regulated by affinity, the duration of the LFA1–ICAM1 interaction dependent on the conformation of LFA1, and avidity, the LFA1–ICAM1 binding frequency regulated by LFA1 density at the cell contact surface2. We recently demonstrated that the conformational change in LFA1 to the active high-affinity form (High, Fig. 1a) is mediated by potent outside-in signaling, which activates Rap1, resulting in LFA1 accumulation at the contact surface (avidity increment) and recruitment of kindlin-3 and talin1 to the contact surface to form high-affinity LFA1 (affinity increment)11. We also demonstrated that the increase in LFA1 affinity under shear-flow is not induced by inside-out signaling alone, but rather involves the build-up of tension caused by simultaneous binding of ICAM1 and talin1 to LFA112. We thus elucidated the mechanism underlying the regulation of LFA1 affinity mediated by talin1 and kindlin-3; however, the detailed mechanism underlying LFA1 accumulation remains elusive.
Fig. 1. Identification of factors colocalizing with LFA1 intracellular clusters.
a LFA1 (αLβ2) intracellular cluster (ICC) formation concomitant with lymphocyte adhesion. Adhesion of lymphocytes (BAF/LFA1) to the ICAM1-displaying surface via LFA1 results in the formation of LFA1 ICCs inside the cell. LFA1 was visualized by fusing a SNAP tag to the C-terminus of β222. b Colocalization of β2-SNAP (shown in magenta) with factors involved in the regulation of vesicular trafficking (shown in green). Colocalized spots are indicated by arrows. Colocalization of LFA1 (β2) with TGN38 (TGN, n = 15), Rab7 (n = 9) or LC3 (n = 12) was quantified using Mander’s coefficient22. c Co-trafficking of β2-SNAP and CLIP-LC3 in adhered lymphocytes. Yellow arrows and cyan arrowheads indicate the ICCs containing both β2 and LC3 (see also Supplementary Movie 1). d Trajectories of co-trafficked ICCs indicated in (c). The area of the cell containing the visualized ICC is indicated by a dotted line. e Colocalization analysis of β2-SNAP with the ATG8 family proteins, LC3 (n = 14) and GABARAP (n = 14). f FRET assay between β2 and LC3. FRET between β2-mCherry and YPet-LC3 in adhered lymphocytes was measured (number of cells measured: n = 17). Lymphocytes expressing YPet-FL-mCherry24 (positive control, PC, n = 16), and β2-mCherry with YPet alone (n = 19) were used as positive and negative controls, respectively. The error bars represent the SEM (standard error of the mean). Statistical analyses were performed using unpaired nonparametric two-sided Student’s t test (e), one-way ANOVA with Dunnett correction (b) or Turkey correction (f) for multiple comparisons. Source data are provided as a Source Data file.
Macroautophagy (hereafter referred to as autophagy) is a crucial cell survival mechanism that rescues cells from nutrient depletion by degrading organelles to provide an energy source. It is induced by the inhibition of mechanistic target of rapamycin (mTOR) signaling caused by nutrient scarcity13. The subsequent signaling cascade promotes the conjugation of phosphatidylethanolamine (PE) to microtubule-associated protein 1 A/1B-light chain 3 (LC3), a key component that forms autophagosomes, which are double-membrane vesicle structures that engulf cytoplasmic components. Dynein-dependent transport of autophagosomes and fusion with lysosomes results in cargo degradation14. Wilkinson et al. reported that phosphorylation of LC3 at threonine 50 (T50) by Mst1 enhances autophagy by promoting dynein-dependent retrograde transport and fusion of autophagosomes with lysosomes15. However, the role of LC3 independent of autophagy regulation still remains unclear16.
There is growing evidence of non-canonical roles for LC3. LC3-associated phagocytosis (LAP), a process observed in macrophages, contributes to host defense17 and antigen presentation18–20, functioning independently of mTOR activity. Similarly, LC3-associated endocytosis (LANDO), which plays roles in neuroinflammation, receptor recycling, and neurodegeneration in central nervous system cells such as microglia, operates independently of both the mTOR pathway and the AMP-activated protein kinase (AMPK) pathway21. While both LAP and LANDO are involved in regulating protein degradation, the role of LC3 in the active, polarized transport of specific molecules or factors has not yet been reported. This highlights the need for further exploration of the non-canonical functions of LC3, particularly in processes beyond protein degradation.
In this study, we elucidated a mechanism regulating lymphocyte adhesion through the transport of LFA1 induced by outside-in signaling from high-affinity LFA1. We uncovered a previously unknown function of LC3 beyond its role in autophagy by connecting two seemingly unrelated pathways: the LFA1 transport pathway and the autophagosome transport pathway. We found that LFA1 clusters induced by outside-in signaling colocalized and co-trafficked with LC3 clusters, which are also formed in response to outside-in signaling. Deletion of LC3b and its homolog GABARAP decreased lymphocyte adhesiveness and reduced LFA1 clustering and accumulation at the cell contact surface, indicating that LC3 family proteins are involved in integrin-dependent lymphocyte adhesion. We used an autophagy flux probe and antibodies against components of canonical autophagy to demonstrate that outside-in signaling does not induce autophagy flux and is distinct from canonical autophagy signaling; outside-in signaling activates both mTOR and AMPK, whereas in the canonical autophagy pathway, nutrient starvation inhibits mTOR but activates AMPK. Intriguingly, AMPK activation promoted LFA1 and LC3 clustering and accumulation at the contact surface, thereby increasing lymphocyte adhesiveness. ATG16L1, which is crucial for LC3 lipidation in both canonical and non-canonical autophagy, also regulates LFA1 and LC3 clustering and adhesion. Analyses using truncation mutants of ATG16L1 functional domains suggest that LFA1 and LC3 clustering and adhesion depend on both canonical and non-canonical lipidation pathways regulated by ATG16L1. LC3 bound directly to RAPL, inhibiting its phosphorylation by Mst1. Deletion of the phosphorylation residue T50 increased LFA1 accumulation at the contact surface and significantly enhanced lymphocyte adhesiveness. The present study highlights unknown aspects of LC3 function and uncovers a previously unidentified route for LFA1 clustering and recruitment at the contact surface, providing important insight into the regulation of lymphocyte adhesiveness.
Results
LFA1 clusters colocalize with Rab7 and LC3, autophagosome components
Previously, we showed formation of an intracellular cluster (ICC) of LFA1 in adhered lymphocytes nearby the cell contact area (Supplementary Fig. 1a)22. To identify factors involved in the regulation of LFA1 ICC formation and transport, LFA1 was co-stained with antibodies against vesicular transport regulators and visualized by super-resolution microscopy using lymphocytes expressing SNAP tag–fused integrin β2 to detect ICCs in adhered lymphocytes (Fig. 1a, Supplementary Fig. 1b)22. LFA1 did not clearly colocalize with the trans-Golgi marker TGN38, the lysosome marker LAMP1, the recycling endosome marker Rab11, a component of the ESCRT-III/multivesicular body, VPS4, and formerly known regulator Rab2723. However, LFA1 colocalized with Rab7 and LC3 (Fig. 1b, Supplementary Fig. 1c–e). LFA1 ICCs localized to the Rab7- and LC3-positive sub-cellular region (Supplementary Fig. 1f), and three-dimensional data revealed colocalization with LC3 (Supplementary Fig. 2). Simultaneous monitoring of LFA1 and LC3 with a spinning disk microscope revealed that LFA1 co-trafficked with LC3 (Fig. 1c, d, Supplementary Movie 1). The staining of lymphocytes for the LC3 homolog γ-aminobutyric acid receptor-associated protein (GABARAP) showed the colocalization of GABARAP with clusters harboring LC3/LFA1, suggesting that LFA1 and ATG8 family proteins (i.e., LC3 and GABARAP) reside in similar cluster-like compartments (Fig. 1e).
To further investigate the relationship between LFA1 and LC3, we established a Förster resonance energy transfer (FRET) assay system (Supplementary Fig. 3). We expressed a yellow fluorescent protein, YPet, fused to LC3b (YPet-LC3b) and a red fluorescent protein, mCherry, fused to β2 (β2-mCherry) in lymphocytes. As a positive control, lymphocytes expressing tandemly fused YPet-FL-mCherry, which undergoes intramolecular FRET24, were used, while cells expressing YPet alone and β2-mCherry served as a negative control. We observed that FRET from YPet to mCherry in cells expressing YPet-LC3 and β2-mCherry was significantly stronger than that in cells expression YPet and β2-mCherry, suggesting that LFA1 and LC3 are in close proximity, allowing FRET to occur (Fig. 1f).
ATG8 family proteins are important for LFA1- and ICAM1-dependent lymphocyte adhesiveness
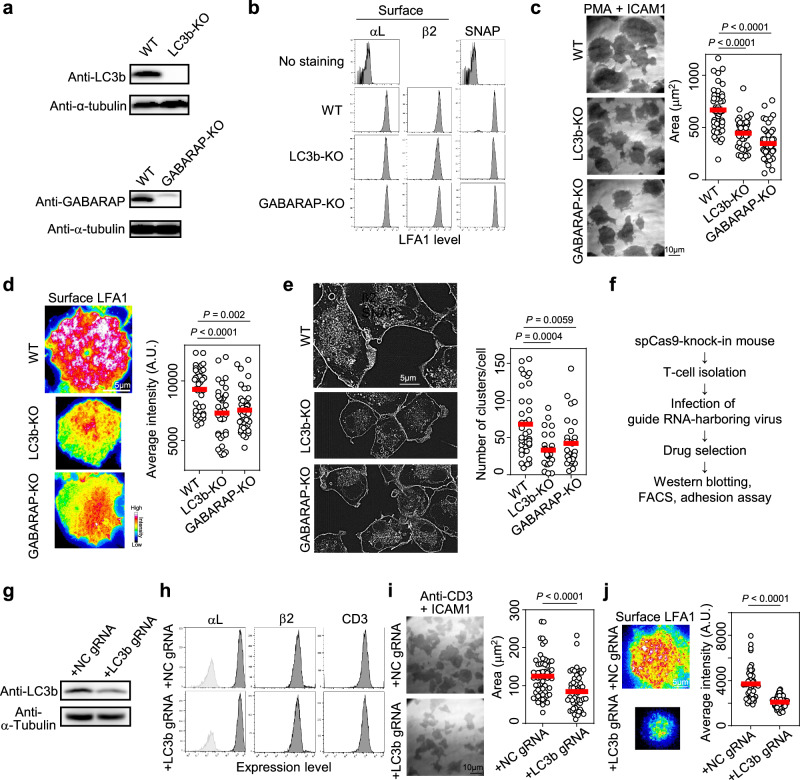
To determine the importance of ATG8 family proteins [LC3b and GABARAP, the dominant paralogs in lymphocytes according to GENE SKYLINE (ImmGen)] for cell adhesion, we established a lymphocyte cell line deficient in the expression of both proteins (Fig. 2a). Deletion of Map1lc3b and Gabarap did not affect the surface expression of LFA1 (Fig. 2b); however, Map1lc3b and Gabarap knockout (KO) cells, which are termed LC3b-KO and GABARAP-KO cells from this point onward, presented significantly lower adhesiveness than did wild-type (WT) cells (Fig. 2c). The phonotype of the Rab7a-deficient cells (Rab7a-KO cells) was similar to that of the LC3b/GABARAP-KO cells (Supplementary Fig. 1g–i). ATG8 family proteins induced LFA1 accumulation at the cell contact surface (Fig. 2d), suggesting that these proteins regulate LFA1-dependent lymphocyte adhesion by modulating LFA1 levels at the contact surface. Knockout of ATG8 family proteins decreased the number of LFA1 ICCs formed in response to cell adhesion (Supplementary Fig. 4a, b, Fig. 2e), suggesting that ATG8 proteins play a crucial role in LFA1 clustering.
Fig. 2. Importance of ATG8 family proteins for LFA1-dependent lymphocyte adhesion.
a Western blotting analysis of LC3b and GABARAP in BAF/LFA1. LC3b-KO: BAF/LFA1 with deletion of LC3b; GABARAP-KO: BAF/LFA1 with deletion of GABARAP. Two independent experiments were performed. b FACS analysis of LFA1 expression in BAF/LFA1 cells. Surface expression of LFA1 was monitored with monoclonal antibodies (Mean Fluorescence Intensity, MFI: αL [No staining, WT, LC3b-KO, GABARAP-KO] = [83.3, 22400, 22300, 26500], β2 [WT, LC3b-KO, GABARAP-KO] = [23300, 16000, 23900]). Total expression of LFA1 was monitored using the SNAP tag protein fused to β2 (MFI: SNAP [No staining, WT, LC3b-KO, GABARAP-KO] = [77.1, 33700, 30300, 39900]). c Adhesion assay. Areas of cell adhesion were monitored by interference reflection microscopy. WT: n = 45; LC3b-KO: n = 45; GABARAP-KO: n = 48. d Accumulation of LFA1 at the contact surface. Surface LFA1 was stained with a dye-conjugated non-blocking monoclonal antibody and adhered to ICAM1-coated dishes in the presence of PMA. The fluorescence intensity of LFA1 at the contact surface was measured by TIRFM. WT: n = 36; LC3b-KO: n = 35; GABARAP-KO: n = 42. e LFA1 clustering inside the cell. Intracellular clusters were visualized using a SNAP tag fused to β2 by SRRF microscopy. The size of clusters was measured using Image J (clusters were defined as particles >0.05 µm2). WT: n = 36; LC3b-KO: n = 27; GABARAP-KO: n = 31. f Working flow describing the preparation of T-cell blasts with deletion of the gene of interest by the CRISPR/Cas9 system. g Western blotting analysis of LC3b in primary T-cell blasts. NC: negative control. Two independent experiments were performed. h FACS analysis of LFA1 surface expression in T-cell blasts. i Adhesion assay using T-cell blasts. Cells were stimulated with an anti-CD3 monoclonal antibody and applied onto ICAM1-coated dishes. NC: n = 60; LC3b: n = 54. j Accumulation of LFA1 at the contact surface of adhered T-cell blasts. NC: n = 51; LC3b: n = 63. Statistical analyses were performed using unpaired nonparametric two-sided Student’s t test (i, j), or one-way ANOVA with Dunnett correction (c–e) for multiple comparisons. Source data are provided as a Source Data file.
To examine the importance of LC3 in cell adhesion in primary lymphocytes, we established T-cell blasts with decreased LC3 expression using T cells from human codon-optimized Streptococcus pyogenes Cas9 (hSpCas9) knock-in (Cas9-KI) mice25. T cells isolated from Cas9-KI mice were cultured in the presence of guide RNA–harboring retrovirus, and the infected cells were selected with puromycin (Fig. 2f) as described previously26,27. The downregulation of LC3 in T cells was verified by western blotting (Fig. 2g), whereas the surface expression levels of LFA1 and CD3 were identical (Fig. 2h). We found that TCR- and ICAM1-dependent cell adhesion and accumulation of LFA1 at the T-cell contact surface were also dependent on LC3b (Fig. 2i, j). Collectively, these results indicate that ATG8 family proteins play important roles as positive regulators of lymphocyte adhesiveness.
Establishment of SNAP KI mice reveals the importance of LC3 for LFA1 clustering in primary T cells
To further investigate the importance of LC3 for LFA1 clustering in primary T cells, we generated SNAP-KI mice by inserting the gene at the C-terminus of the LFA1 β2 subunit (Supplementary Fig. 5a, b) because visualization of mouse LFA1 in primary T cells was difficult with antibodies. Expression of SNAP-fused β2 in T cells isolated from SNAP KI mice was verified (Supplementary Fig. 5c). Colocalization of β2 and LC3 was observed in primary T cells and B cells that adhered to ICAM1 (Supplementary Fig. 5d). Next, we crossed SNAP-KI mice with Cas9-KI mice to generate SNAP-KI;Cas9-KI mice, which were used to establish T-cell blasts with decreased LC3 expression as described above (Fig. 2f). Downregulation of LC3 in T-cell blasts had no effect on the expression of β2-SNAP, surface LFA, or CD3, which is consistent with the results shown in Fig. 2h (Supplementary Fig. 5e). However, decreased LC3 expression in T-cell blasts decreased LFA1 ICC formation (Supplementary Fig. 5f). These results support the notion that LC3 promotes LFA1 clustering during lymphocyte adhesion as shown in Fig. 2e.
Signaling cascade required for LFA1+LC3+ cluster formation
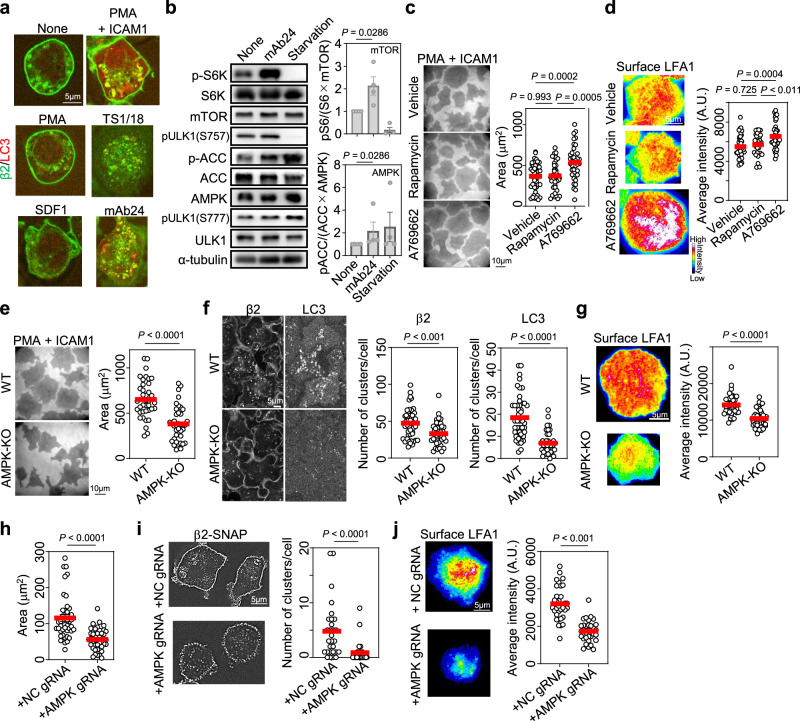
To determine whether inside-out or outside-in signaling is the integrin activation signal important for LC3 and LFA1 clustering, we examined the effect of bidirectional signaling stimuli on LC3 and LFA1 co-clustering. Inside-out signaling stimulators such as PMA and SDF1 did not clearly induce co-clustering, especially clustering of LC3 (Fig. 3a). Outside-in signaling induced by the pan-β2 monoclonal antibody TS1/18 did not clearly induce co-clustering, whereas outside-in signaling induced by ICAM1 in the presence of PMA, and mAb24, a monoclonal antibody that induces high-affinity LFA1 formation2,11, strongly induced co-clustering. This finding suggests that high-affinity–dependent outside-in signaling is important for the formation of LFA1+LC3+ ICCs (Fig. 3a).
Fig. 3. Outside-in signaling–induced LC3 clustering is driven by AMPK activation.
a Role of outside-in signaling in LC3 and LFA1 co-clustering. Merged images of β2-SNAP (green) and CLIP-LC3 (red) in BAF/LFA1 cells are shown. Inside-out stimulation was mediated by PMA and SDF1. Outside-in stimulation was mediated by the following monoclonal antibodies coated on the glass bottom dish: TS1/18, which induces low-affinity dependent outside-in signaling22, and mAb24, which induces high-affinity LFA1-dependent outside-in signaling. Both inside-out and outside-in signaling pathways are activated by ICAM1 + PMA, which induces the binding of lymphocyte LFA1 to ICAM1 in a PMA stimulation-dependent manner. ICAM1-dependent adhesion of BAF/LFA1 was already verified11. Three independent experiments were performed. Scale bar: 5 µm. b Western blotting analysis of the phosphorylation of mTOR and AMPK substrates. S6K: p70 S6 kinase, a substrate of mTOR; ACC: acetyl-CoA carboxylase, a substrate of AMPK; ULK1: Unc-51 like autophagy activating kinase 1. mTOR: n = 4; AMPK: n = 4. c Effects of rapamycin and A769662 on lymphocyte adhesion. Vehicle: n = 42; rapamycin: n = 37; A769662: n = 37. d Effects of rapamycin and A769662 on LFA1 levels at the contact surface. Vehicle: n = 42; rapamycin: n = 32; A769662: n = 37. e Effect of AMPK deletion on lymphocyte adhesion. WT: n = 38; AMPK-KO: n = 39. f Effect of AMPK deletion on LFA1 and LC3 clustering inside BAF/LFA1 cells adhered to ICAM1-coated dishes. WT: n = 48; AMPK-KO: n = 39. g Effect of AMPK deletion on LFA1 levels at the contact surface of BAF/LFA1 cells. WT: n = 36; AMPK-KO: n = 38. h Effect of AMPK deletion on T-cell blast adhesion. NC: n = 41; AMPK: n = 42. i Effect of AMPK deletion on LFA1 clustering in T-cell blasts. NC: n = 29; AMPK: n = 34. j Effect of AMPK deletion on LFA levels at the contact surface of T-cell blasts. NC: n = 34; AMPK: n = 32. The error bars represent the SEM. Statistical analyses were performed using unpaired (b, e–j) nonparametric two-sided Student’s t test, or one-way ANOVA with Turkey correction (c, d) for multiple comparisons. Source data are provided as a Source Data file.
Next, we compared the effects of outside-in signaling and starvation on the signaling cascade by measuring mTOR activity after exposure to each stimulus. Nutrient starvation inhibits mTOR activity28. We found that outside-in signaling promoted the phosphorylation of p70 S6 kinase (S6K), a substrate of mTOR, whereas starvation decreased the phosphorylation of S6K (Fig. 3b). The autophagy machinery is activated by the dephosphorylation of Unc-51 like autophagy activating kinase 1 (ULK1), a key inducer of the complex required for autophagosome formation, at Ser-757 (S757)29. This was reproduced in lymphocytes under starvation condition; however, outside-in signaling did not decrease S757 phosphorylation (Fig. 3b, Supplementary Fig. 6a). Phosphorylation of ULK1 at Ser-777 (S777) by AMPK is another marker of autophagosome formation13. Starvation promoted the activity of AMPK against acetyl-CoA carboxylase (ACC), a representative substrate for AMPK, and the phosphorylation of ULK1 at Ser777. Outside-in signaling also increased AMPK activity against ACC, but did not obviously increase ULK1 phosphorylation at S777 (Fig. 3b, Supplementary Fig. 6a). These results collectively suggest that outside-in stimulation does not simply hijack the canonical autophagy machinery but rather activates both mTOR and AMPK without affecting ULK1 phosphorylation.
The importance of these signaling cascades for lymphocyte function was further analyzed by modulating signaling pathways using the mTOR inhibitor rapamycin and the AMPK agonist A76966230. Inhibition of mTOR activity by rapamycin and activation of AMPK by A769662 were confirmed in lymphocytes (Supplementary Fig. 6b); however, drug treatment did not affect the surface expression of LFA1 before adhesion (Supplementary Fig. 6c). Although rapamycin had no effect on lymphocyte adhesiveness, A769662 increased lymphocyte adhesiveness by upregulating LFA1 at the cell contact surface (Fig. 3c, d). In Prkaa1 knock-out (AMPK-KO) lymphocytes (Supplementary Fig. 6d, e), which presented the same LFA1 expression levels as the WT, AMPK positively regulated lymphocyte adhesiveness (Fig. 3e), LFA1 clustering (Fig. 3f), and LFA1 accumulation at the contact surface (Fig. 3g). These findings suggest that outside-in signaling relies mainly on AMPK signaling to control LFA1-dependent adhesion. Consistently, LC3 clustering induced by outside-in signaling was also regulated by AMPK (Fig. 3f). To further determine the role of AMPK in lymphocyte adhesion, we established primary AMPK-KO T-cell blasts (Supplementary Fig. 6f, g) using SNAP-KI;Cas9-KI mice as described above (Supplementary Fig. 5a, Fig. 2f). Decreased AMPK expression in T-cell blasts reduced cell adhesiveness (Fig. 3h), LFA1 ICC formation (Fig. 3i), and LFA1 levels at the cell contact surface (Fig. 3j). These results support that AMPK controls LFA1 and LC3 clustering mediated by outside-in signaling.
Integrin outside-in signaling does not induce autophagosome degradation
In the canonical autophagy pathway, starvation inhibits mTOR activity and induces LC3 lipidation, leading to the occurrence of autophagy flux for the degradation of engulfed substrates31. Concomitant with autophagosome formation, autophagy adaptors such as p62 recruit ubiquitinated substrates32. Unlike the canonical autophagy pathway, outside-in signaling does not stimulate p62 clustering or the degradation of LC3-II, the lipidated form of LC3 (Supplementary Fig. 7a, b), whereas lipidation of LC3 per se is important for LFA1-dependent lymphocyte adhesion (Supplementary Fig. 7c, d). To further investigate the role of outside-in signaling in autophagy induction, we used an autophagy flux probe (Supplementary Fig. 8a)33 to examine the response to outside-in signaling stimulation (Supplementary Fig. 8a–c). Although outside-in signaling did not affect the GFP/RFP ratio, exposure to starvation conditions significantly decreased the GFP/RFP ratio, suggesting that integrin-dependent LC3 clustering does not induce autophagy flux (Supplementary Fig. 8d, e). Collectively, these results suggest that LC3-mediated lymphocyte adhesion is not correlated with the occurrence of canonical autophagy flux.
Clustering of LFA1 and LC3 and clustering-dependent adhesion are mediated by both non-canonical and canonical autophagy lipidation pathways
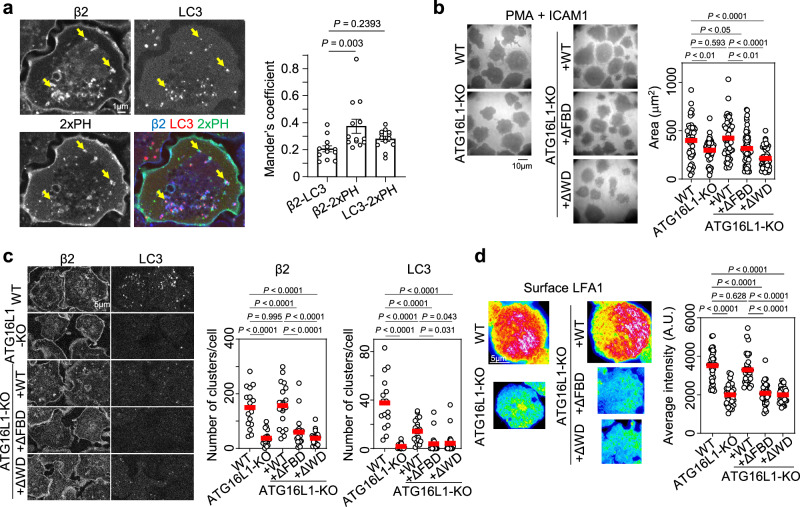
The independence of the canonical autophagy pathway on the newly identified outside-in–dependent LFA1 regulation by LC3 prompted us to examine the relevance of the non-canonical autophagy pathway. In the non-canonical autophagy, single-membrane vesicle, which is positive for LC3, is formed34. Durgan et al. reported that the double-membrane vesicle is exclusively modified by PE, whereas the single-membrane vesicle is modified by both PE and phosphatidyl serine (PS)35. To investigate the features of lipid modification in LFA1+LC3+ICC, we used a PS-specific probe, a tandem fusion of the evectin-2 pleckstrin-homology (PH) domain fused with GFP (2×PH-GFP)36–38. We found that 2×PH-GFP colocalized with both LFA1 and LC3 slightly more frequently than LFA1 colocalized with LC3 (Fig. 4a), suggesting that LFA1+LC3+ICCs feature lipid modifications induced by non-canonical autophagy.
Fig. 4. The relevance of the non-canonical autophagy in outside-in signaling-dependent LFA1–LC3 co-clustering and lymphocyte adhesion.
a Colocalization analysis of LFA1 + LC3+ cluster with a tandem fusion of the evectin-2 pleckstrin-homology (PH) domain (2×PH) fused with GFP. The colocalization indices of LFA1 (β2) with LC3 or 2×PH, and that of LC3 with 2×PH were calculated (n = 13). b Effects of ATG16L1 deletion and expression of ATG16L1 mutants on lymphocyte adhesion. WT: n = 47; ATG16L1-KO: n = 57; ATG16L1-KO expressing GFP-fused ATG16L1 WT (+WT, n = 49), ΔFBD (+ΔFBD, n = 57), or ΔWD (+ΔWD, n = 51) protein. c Effects of ATG16L1 deletion and expression of ATG16L1 mutants on LFA1 and LC3 clustering inside BAF/LFA1 cells adhered to ICAM1-coated dishes. WT: n = 16; ATG16L1-KO: n = 18; +WT: n = 18; +ΔFBD: n = 22; +ΔWD: n = 23. d Effects of ATG16L1 deletion and expression of ATG16L1 mutants on LFA1 levels at the contact surface of BAF/LFA1 cells. WT: n = 37; ATG16L1-KO: n = 33; +WT: n = 32; +ΔFBD: n = 36; +ΔWD: n = 34. The error bars represent the SEM. Statistical analyses were performed using one-way ANOVA with Dunnett correction (a), or Turkey correction (b–d) for multiple comparisons. Source data are provided as a Source Data file.
To further prove the relevance of non-canonical autophagy with LFA1+LC3+ICC, we examined the effect of ATG16L1 mutation on the LFA1 outside-in signaling–dependent process. ATG16L1, a component of the ATG5-ATG12-ATG16L1 complex, is essential for the lipidation of LC3 and its recruitment to membranes39. Fletcher et al. demonstrated that the FIP200-binding domain (FBD) in the central region of ATG16L1 is required for canonical autophagy, whereas the WD40 domain (WD) in the C-terminal region is required for non-canonical autophagy (Supplementary Fig. 9a)40. We generated lymphocytes lacking Atg16l1, and exclusively expressing either the FBD deletion mutant (ΔFBD) or the WD deletion mutant (ΔWD) of ATG16L1 to examine the dependence of these domains on LFA1+LC3+ICC-dependent lymphocyte adhesion (Supplementary Fig. 9b–d). We found that ATG16L1 knockout reduced cell adhesiveness, which was restored by the expression of ATG16L1-WT (Fig. 4b). Interestingly, the expression of either ATG16L1-ΔFBD or ATG16L1-ΔWD failed to restore cell adhesiveness (Fig. 4b). Consistently, LFA1 and LC3 intracellular clustering (Fig. 4c) and LFA1 levels at the contact surface (Fig. 4d) were rescued by WT but not by ΔFBD or ΔWD expression, suggesting that LFA1+LC3+ICC formation relies on both non-canonical and canonical autophagy lipidation pathways in the context of ATG16L1 function.
RAPL binding to LC3 inhibits Mst1 dependent LC3 phosphorylation
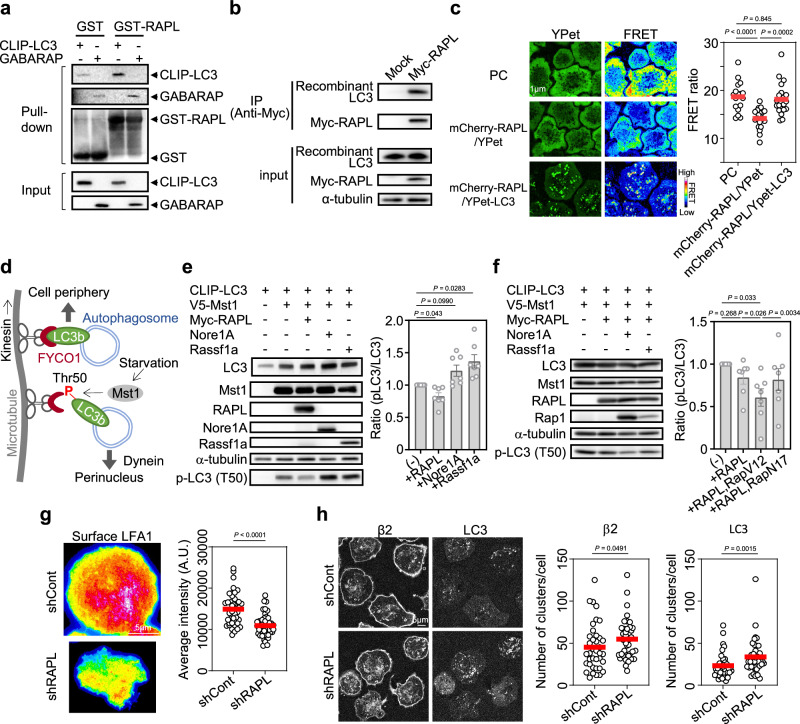
We next explored the mechanisms underlying the regulation of LFA1 transport and LFA1-dependent cell adhesion by LC3 through a series of biochemical experiments. We previously isolated RAPL, a protein encoded by the Rassf5 gene, as a downstream effector of active Rap1 and a direct interacting protein of LFA1 at the cytoplasmic tail of αL4. RAPL plays important roles in lymphocyte adhesiveness and polarity by directly binding to LFA14,5; however, the mechanism by which RAPL is involved in integrin regulation remains unclear. A search for RAPL effector molecules by yeast two-hybrid screening identified GABARAP as a candidate interactor of RAPL. Pull-down assays using GST-fused RAPL showed that RAPL directly binds to both LC3 and GABARAP (Fig. 5a), consistent with a recent interactome analysis of ATG8 family proteins41. To further verify this interaction, we performed an immunoprecipitation assay of Myc-tagged RAPL with recombinant LC3 and GABARAP and found that even in the absence of other factors, both proteins can bind to RAPL (Fig. 5b, Supplementary Fig. 10a). The interaction of LC3 with RAPL in cells was also confirmed via FRET between mCherry-tagged RAPL and YPet-tagged LC3 (Fig. 5c, Supplementary Fig. 10b, c). Next, we investigated the effect of RAPL on LC3 phosphorylation at T50 by Mst1 on the basis of previous findings showing that under starvation conditions Mst1 directly phosphorylates LC3 at T50 and promotes autophagy by inhibiting kinesin-dependent autophagosome recruitment to the cell periphery42 (Fig. 5d). Although the RAPL homolog proteins Nore1A, an alternative isoform of the Rassf5 gene, and Rassf1a, a close homolog of the Rassf5 gene43, promoted the phosphorylation of LC3 by Mst1, RAPL significantly inhibited the activity of Mst1 toward LC3 in 293 T cell lysates (Fig. 5e). RAPL does not attenuate the interaction between Mst1 and LC3, but rather potentiates the interaction, suggesting that inhibitory conformational change of Mst1 is induced by RAPL binding (Supplementary Fig. 10d). The presence of active Rap1 increased the inhibitory effect of RAPL on Mst1-mediated LC3 phosphorylation (Fig. 5f). These results suggest that an active Rap1–RAPL axis is crucial for the inhibition of Mst1 kinase activity toward LC3. The effect of RAPL on LC3-mediated LFA1 regulation was examined by establishing a RAPL-knockdown lymphocyte cell line (Supplementary Fig. 10e–g), which showed that RAPL is important for the accumulation of LFA1 at the contact surface (Fig. 5g). RAPL knockdown promoted LFA1 and LC3 clustering instead of suppressing it (Fig. 5h). Collectively, these results indicate that RAPL regulates LFA1 accumulation at the contact surface, but does not play a critical role in co-clustering of LFA1 and LC3, suggesting that RAPL functions in the step after LFA1 clustering.
Fig. 5. RAPL inhibits the phosphorylation of LC3 at Thr50 by Mst1.
a Direct binding between RAPL and ATG8 family proteins. Pull-down assays were performed using purified GST-fused RAPL and 293T lysates expressing CLIP-LC3 or flag-GABARAP. Three independent experiments were performed. b Interaction of RAPL with purified LC3. Immunoprecipitation of myc-tagged RAPL in 293T cell lysate was followed by the addition of recombinant LC3b. Myc-RAPL and LC3 were detected with anti-Myc and anti-LC3 antibodies, respectively. Two independent experiments were performed. c FRET assay between RAPL and LC3. FRET between mCherry-RAPL and YPet-LC3 in adhered lymphocytes was measured (number of cells measured: n = 20). Lymphocytes expressing YPet-FL-mCherry24 (positive control, PC, n = 17), or mCherry-RAPL with YPet alone (n = 20) were used as positive and negative controls, respectively. d Role of LC3 phosphorylation in the canonical autophagy pathway. In the presence of nutrient starvation, Mst1 phosphorylates LC3b at Thr50 to inhibit binding of LC3b to FYCO1, an adaptor protein for kinesin, thereby promoting dynein-dependent transport of autophagosomes to the perinuclear region42. e Specific inhibition of Mst1-mediated LC3 phosphorylation at Thr50 by RAPL in 293T cells. Indicated tagged proteins were overexpressed in 293T cells and detected by western blotting with lysates and tag-specific antibodies. For Nore1A, α-tubulin and phosphorylated LC3 (p-LC3), antigen specific antibodies were used. N = 7. f Increased inhibition of LC3 phosphorylation caused by Rap1 activation in 293T cells. N = 7. g Effect of RAPL knockdown on LFA1 accumulation at the contact surface. BAF/LFA1 cells infected with virus harboring control shRNA (shCont): n = 40 or shRNA against RAPL (shRAPL): n = 44. h Effect of RAPL knockdown on LFA1(β2) and LC3 clustering. shCont: n = 38; shRAPL: n = 39. The error bars represent the SEM. Statistical analyses were performed using unpaired (g, h) nonparametric two-sided Student’s t tests, or one-way ANOVA with Dunnett correction (e), or Turkey correction (c, f) for multiple comparisons. The error bars represent the SEM. Source data are provided as a Source Data file.
LC3 dephosphorylation induces LFA1 accumulation and lymphocyte adhesiveness
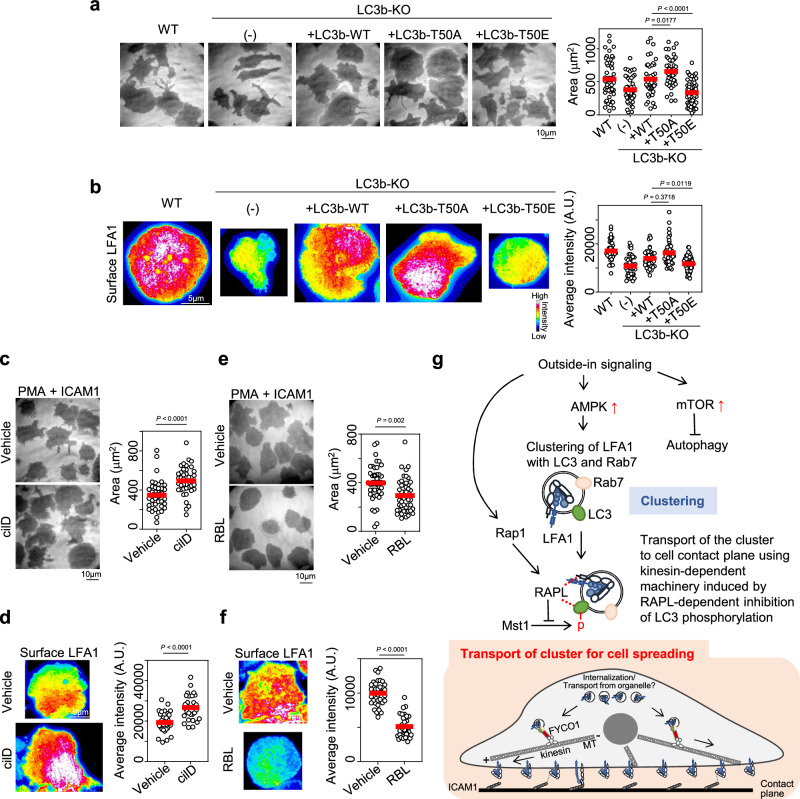
The function of RAPL and the effect of LC3 phosphorylation by Mst1 on LFA1 were examined by generating LC3b mutants mimicking phosphorylated LC3 (T50E) and dephosphorylated LC3 (T50A). These mutants were expressed in LC3b-KO lymphocytes (Supplementary Fig. 11a, b). Compared with WT LC3, dephosphorylated LC3 increased cell adhesiveness, whereas phosphorylated LC3 decreased adhesiveness (Fig. 6a). Consistent with these results, contact surface LFA1 levels were higher in T50A and lower in T50E than in the WT (Fig. 6b), suggesting that inhibition of LC3 phosphorylation is important for LFA1-dependent lymphocyte adhesion. Because LC3 phosphorylation promotes the dynein-dependent retrograde transport of LC3+ autophagosomes to fuse with lysosomes near the nucleus (Fig. 5d), we tested the effects of the dynein inhibitor ciliobrevin D on lymphocyte adhesiveness and LFA1 accumulation. Inhibition of dynein promoted lymphocyte adhesiveness and LFA1 accumulation (Fig. 6c, d), indicating that suppressing dynein-dependent transport is important for LFA1-dependent cell adhesion. In contrast, the kinesin-1 inhibitor Rose Bengal Lactone44 inhibited lymphocyte adhesiveness and LFA1 accumulation (Fig. 6e, f). We also examined the relevance of the kinesin adaptor FYCO1, which directly binds to LC3 and kinesin-1 (Fig. 5d)42,45. FYCO1 reduction, although partial, inhibited cell adhesion and LFA1 accumulation (Supplementary Fig. 11c–f). Taken together, these results suggest that the suppression of LC3 phosphorylation by the Rap1–RAPL axis promotes kinesin- and FYCO1-dependent transport of LFA1 to the cell contact surface.
Fig. 6. Dephosphorylation of LC3 promotes LFA1-mediated lymphocyte adhesion.
a Effect of LC3b phosphorylation at T50 on lymphocyte adhesion to ICAM1-coated dishes under PMA stimulation. LC3b with mutation of T50 to Ala (LC3b-T50A, dephosphorylated form, n = 49) or to Glu (LC3b-T50E, constitutively phosphorylated form, n = 60) or without mutation (LC3b-WT, n = 45) was introduced into LC3b-knockout BAF/LFA1 cells (LC3b-KO). WT: parental cells before LC3b deletion, n = 48; (-): LC3b-KO without CLIP-LC3b expression, n = 47. b Effect of LC3b phosphorylation on LFA1 levels at the contact surface of the cells shown in (a). WT: n = 40; (-): n = 44; LC3b-WT: n = 40; LC3b-T50A: n = 45; LC3b-T50E: n = 68. c Effect of ciliobrevin D (cilD), a dynein inhibitor, on lymphocyte adhesiveness. Vehicle: n = 38; cilD: n = 40. d Effect of cilD on the contact surface level of LFA1. Vehicle: n = 30; cilD: n = 31. e Effects of Rose Bengal Lactone (RBL), a kinesin inhibitor, on lymphocyte adhesiveness. Vehicle: n = 43; RBL: n = 48. f Effect of RBL on LFA1 levels at the contact surface. Vehicle: n = 36; RBL: n = 36. g A model of LC3-mediated LFA1 transport to enhance lymphocyte adhesion. Outside-in signaling activates AMPK and induces the formation of intracellular clusters to facilitate lymphocyte adhesion. Activated LFA1 forms intracellular clusters, which are partly derived from surface LFA1, with Rab7 and LC3b. Direct interaction of LC3b with RAPL inhibits LC3b phosphorylation at T50 promoted by Rap1 activation via outside-in signaling, leading to FYCO1- and kinesin-dependent transport of LFA1+LC3+ clusters to the cell contact surface and promoting the accumulation of LFA1 at the surface to increase lymphocyte adhesion. Statistical analyses were performed using unpaired nonparametric two-sided Student’s t tests (c–f), or one-way ANOVA with Dunnett correction (a, b) for multiple comparisons. Source data are provided as a Source Data file.
The results of this study led us to propose an unconventional transport model of LFA1 ICCs regulated by LC3-mediated trafficking (Fig. 6g). In this model, Rab7+LC3+LFA1+ clusters, which are partly derived from internalized LFA1 (Supplementary Fig. 11g), are formed in response to outside-in signaling following AMPK activation. mTOR activation by outside-in signaling blocks the commitment of the pathway to autophagy induction. Concomitant with outside-in signaling enhancement, Rap1 activation induces the dephosphorylation of LC3 by RAPL. Dephosphorylated LC3 promotes ICC transport in the anterograde direction (contact plane), which is mediated by kinesin and FYCO1.
Discussion
Here, we uncovered a pathway of LFA1 transport mediated by the autophagy component LC3. LC3 co-clustered with LFA1 and co-trafficked with LFA1 in response to outside-in signaling induced by high-affinity LFA1 formation. Deletion of LC3b or GABARAP decreased LFA1 accumulation at the contact surface and lymphocyte adhesiveness, suggesting that LC3 controls lymphocyte adhesion by modulating LFA1 transport.
We found that LFA1-dependent outside-in signaling activates both mTOR and AMPK and further demonstrated that AMPK activation induces LFA1 clustering, whereas Rap1 activation enhances the inhibition of Mst1-mediated LC3 phosphorylation by RAPL to promote anterograde transport of LC3-containing LFA1 ICCs to the contact surface, thereby increasing lymphocyte adhesiveness.
In previous work, we showed that outside-in signaling is a key inducer of the positive feedback loop mediating the transport and accumulation of LFA1 and its activators Rap1, talin1, and kindlin-311; however, the mechanism by which the transport of these signalosome components is regulated to promote LFA1 activation remains unknown. The present findings shed light on the missing link between LFA1 activation and the signalosome transport machinery, revealing that the latter is partly controlled by the LC3-mediated autophagosome transport pathway.
The noncanonical autophagy pathway mediated by LC3 is important for organelle regulation21. LAP, the most studied phenomenon among noncanonical autophagy pathways, occurs in macrophages and is involved in host defense against bacterial infection17 and antigen presentation18–20. Toll-like receptors (TLRs) initiate LAP by forming LC3-associated phagosomes, which are formed independently of mTOR and AMPK46. Another well-known noncanonical autophagy pathway, LANDO, which is associated with neuroinflammation, receptor recycling, and neurodegeneration of cells in the central nervous system such as microglia, also functions independently of mTOR and AMPK21. In the present study, we showed that integrin outside-in signaling induced the activation of both mTOR and AMPK, among which AMPK plays a critical role in the polarized transport/accumulation of LFA1 at the cell contact plane. Furthermore, we showed that LFA1 regulation in lymphocytes depends on both FBD and WD in ATG16L1, whereas either canonical autophagy or non-canonical autophagy exclusively depend on FBD or WD, respectively40. Therefore, the pathways identified in this study are qualitatively different from LAP and LANDO in terms of mTOR dependency and the requirement of ATG16L1 functional domains, and we thus defined them as LC3-Associated Protein Transport induced by IntegriN (LAPTIN). The discovery of LAPTIN broadens our understanding of global regulation of membrane trafficking.
Rap1-mediated integrin activation is a key process for leukocyte adhesion1,2. We and others have shown that Rap1 plays three main roles in the regulation of integrin activation: (1) direct binding and recruitment of talin1 to the cell contact surface10,11, (2) activation of the downstream kinases Mst1 and NDR1 for the recruitment of kindlin-3 to the cell contact surface7,9, and (3) recruitment of LFA1 via RAPL through the formation of the Rap1–RAPL–LFA1 complex4. Regarding (3), the precise mechanism underlying the transport of LFA1 by RAPL and Rap1 remains to be determined. In this study, we showed that the Rap1–RAPL complex inhibits LC3 phosphorylation via direct interaction of RAPL with LC3, which promotes anterograde transport of LFA1/LC3 ICCs to the cell contact surface. This evidence improves our understanding of RAPL-mediated LFA1 regulation by elucidating an intracellular transport pathway for LFA1. The inhibition of Mst1-dependent phosphorylation of LC3 by RAPL is unexpected, as we previously showed that RAPL enhances Mst1-dependent phosphorylation of NDR19. Bitra et al. reported that the Rassf family, to which RAPL belongs, has a dual regulatory effect on Mst1: Rassf proteins promote Mst1-dependent phosphorylation of histone H2B but inhibit phosphorylation of forkhead box O (FoxO)47, suggesting that the regulatory effect of Rassf proteins on Mst1 is substrate specific. In the case of LC3, RAPL functions as an inhibitory regulator of Mst1.
We demonstrated the importance of both LC3B and GABARAP in regulating LFA1 ICC formation and adhesion. Furthermore, our findings suggest that the conjugation of LC3B with FYCO1, facilitates LFA1 transport, with RAPL-mediated inhibition of LC3 phosphorylation at Thr50 playing a critical regulatory role in this process. However, because Thr50 is not conserved in GABARAP48, the mechanism by which GABARAP regulates LFA1 transport remains unclear. The crystal structure of the FYCO1-LC3A complex49 indicates that FYCO1 binds to hydrophobic pockets (HP1 and HP2), which are common interaction sites for multiple LC3 substrates50. This binding involves the conserved Lys49 and Lys51 residues that flank Thr50 in LC3A/B. In GABARAP, these residues are similarly conserved as Lys46 and Lys48, respectively, but Thr50 in LC3B is replaced by Lys47 in GABARAP48. Structural analyses of the binding properties of LC3B and GABARAP with their shared binding partner, PLEKHM1, revealed that neither Thr50 in LC3B nor Lys47 in GABARAP is critical for the PLEKHM1 interaction at HP1 of ATG8 family proteins, including LC3B and GABARAP. Moreover, conserved Lys49 and Lys51 residues in LC3B and identical residues in GABARAP are important for PLEKHM1 binding48, suggesting that FYCO1-ATG8 family protein interactions are also primarily stabilized by conserved lysine residues rather than the presence of a threonine at position 50 in LC3. Indeed, GABARAP can associate with FYCO1, although this interaction is weaker than that between LC3 and FYCO151, indicating a possible role for GABARAP-dependent LFA1 transport through the GABARAP–FYCO1 interaction independent of the phosphorylation of the threonine flanked by lysines. On the other hand, the comparatively lower affinity of GABARAP for FYCO1 also raises the possibility that an alternative mechanism may mediate GABARAP-dependent LFA1 transport. Future studies are needed to explore whether distinct regulatory pathways may operate in parallel or complementarily to facilitate LFA1 trafficking and lymphocyte adhesion.
This study highlights the importance of LC3 in the regulation of LFA1 transport and lymphocyte adhesion, although the precise mechanism underlying LC3-mediated LFA1 regulation needs to be addressed. There are several possible pathways to explain the role of the LC3-mediated transport machinery in regulating T-cell adhesion and LFA1 accumulation besides the kinesin-mediated regulation reported here: (a) Membrane trafficking and recycling: The noncanonical autophagy machinery, such as LANDO, participates in the recycling of proteins, including amyloid β, TLR4, and CD3652. Although the dependency of LAPTIN on mTOR and AMPK differs from that of LANDO, recycling in LAPTIN may ensure the availability and proper localization of LFA1 to T-cell contact surfaces, thereby facilitating lymphocyte adhesion. (b) Cytoskeletal remodeling: in the canonical autophagy machinery, LC3 enhances actin nucleation via direct interaction with junction mediating and regulatory protein (JMY), an actin regulator harboring an Arp2/3-activating sequence53. This pathway could modulate the actin cytoskeleton in response to integrin outside-in signaling, leading to LFA1 clustering and polarized transport during lymphocyte adhesion. (c) Alternative signaling modulation: the LC3-related autophagy machinery affects various signaling pathways, which could also be involved in T-cell adhesion and migration. For example, Rho activity is regulated by LC3 via interaction with the Rho-GEF domain of AKAP-Lbc54. It is conceivable that LC3 interacts with other small GTPases involved in integrin activation. Itoh et al. reported that OATL1, a Rab33b GAP that directly interacts with ATG8 homologs, regulates autophagosome maturation55. Similar interactions between LC3 and other ATG8 homologs could potentially regulate LFA1 outside-in–dependent LC3 clustering at adhesion sites.
Building upon previous findings and experimental results, this study provides insight into the uncharacterized roles of LC3 and reveals a previously unknown route mediating LFA1 recruitment at the contact surface. The present findings improve our understanding of the dynamic regulation of T-cell adhesion and highlight the involvement of the LC3-mediated transport machinery in autophagy-unrelated processes. The colocalization and co-trafficking of LFA1 with LC3 regulated by AMPK and the Rap1–RAPL–Mst1 axis suggest a close interplay between autophagy and adhesion signaling pathways, adding a new dimension to our understanding of T-cell biology and immune responses, which may lead to the development of new drugs and therapeutic approaches.
Methods
Reagents and antibodies
RPMI-1640 cell culture medium (R8758) and anti-V5 tag antibody (011-23591) were purchased from Fuji Film-Wako. IMDM (12440-061) was from gibco. Phorbol 12-myristate 13-acetate (PMA, P1585), anti-α-tubulin antibody (T9026), anti-flag M2 antibody (F1804), aprotinin (A6279), cOmplete ULTRA tablets (5-892-791-001), and Rose Bengal Lactone (328960) were from Sigma-Aldrich. Lympholyte-M (CL5031) was from Cedarlane. SNAP-Cell 647-SiR (S9102S), SNAP-Cell 505-Star (S9103S), pSNAPf vector (N9183S), CLIP-Cell Starter Kit (E9200), CLIP-Cell TMR-Star (S9219S), and anti-SNAP-tag antibody (P9310S) were from New England Biolabs. Anti-phospho LC3 antibody (AP4013), and anti-CD18 antibody (BS6922) were from Bioworld Technology. Anti-LC3 antibodies [8E10(M186-3), 4E12(M152-3)], anti-p62 antibody (PM045), and anti-GABARAP antibody (PM037) were from MBL. PE anti-human CD11a antibody (350605), anti-human β2 antibody (mAb24, 363402), anti-mouse LFA-1 antibody (H155-78, 141002), and MojoSort Mouse CD3 T Cell Isolation Kit (480031) were from BioLegend. Ciliobrevin D (250401) was from Calbiochem. KOD-Plus-Mutagenesis Kit (SMK-101) was from TOYOBO. Anti-VPS4 (sc-32922) and anti-Rab27 (sc-74586) antibodies were from Santa Cruz Biotechnology. Anti-Rab11 (610656) and anti-TGN38 (610898) were from BD Biosciences. Anti-LAMP1 antibody (ab320851) and recombinant human LC3B protein (ab103506) were from Abcam. Recombinant human GABARAP protein (ATGP0465) was from NKMAXBio. Anti-Rab7 antibody (9367S), anti-ULK1 antibody (8054T), anti-phospho-ULK1 (Ser757) antibody (14202T), anti-mTOR antibody (2983T), anti-p70 S6 kinase antibody (2708T), anti-phospho-p70 S6 kinase (Thr389) antibody (9234T), anti-acetyl-CoA carboxylase antibody (3662S), anti-phospho-acetyl-CoA carboxylase (Ser79) antibody (3661S), AMPKα antibody (2603S), anti-rabbit IgG HRP-linked antibody (7074S), and anti-mouse IgG HRP–linked antibody (7076S) were from Cell Signaling Technology. Anti-phospho-ULK1 (Ser777) antibody (ABC213) was from Millipore. A-769662 (HY-50662) was from MedChemExpress. Lane marker reducing sample buffer (5×)(39000), Alexa Fluor 488-, 555-, or 633- conjugated anti-rabbit and anti-mouse antibodies (A-11034, A-21429, A-21071, A-11029, A-21424, A-21052), and Lipofectamine 2000 (11668019) were from Thermo Scientific. Anti-RAPL antibody, and anti-Nore1A antibody were generated in house from rats4. Anti-myc antibody (9E10), anti-human αL antibody (TS2/4), and anti-human β2 antibody (TS1/18) were purified from hybridomas purchased from ATCC. CoraLite Plus 488 anti-human CD11a (TS2/4) (CL488-65194), Myc-Trap Magnetic agarose (ytma-10), and DYKDDDDK Fab-Trap agarose (ffa-10) were from Proteintech.
Plasmid construction, virus preparation, and cell establishment/purification
The mouse LC3b gene was cloned from an in-house cDNA library of the mouse spleen. The CLIP-tag sequence was amplified from the pCLIPf vector and fused to the 5′-terminus of LC3b. The CLIP-LC3b chimera was subcloned and inserted into the lentiviral vector CSII-EF-MCS. Point mutants of LC3b were generated using a KOD-Plus-Mutagenesis Kit. The construction of the pX458 plasmid harboring guide RNA-encoding oligonucleotides was performed as described previously11, except for the following sequences of the target genes: Rab7 (AGACTGGAACCGTTCTTGAC, GTCATCATCCTGGGGGACTC), LC3b (GGAGCTTTGGTGAGCCCCGC, TGAGCTGCAAGCGCCGTCTG), GABARAP (TCAGAGCGGCGCTTCTCGAA, CTGGTACAGCTGACCCATCG), ATG5 (GATGAAAGGCCGCTCCGTCG, AAGAGTCAGCTATTTGACGT), AMPK (ACGCTTGGTGTCGGCACCTT, GAGTTAAATGGTGGTCGTCC), FYCO1 (ATGATGGCATTCGATTTGTC, AAGCTTCGACGAGATGCGGC). The production of lentiviruses for gene expression and knockdown of RAPL, and determination of the infectious titer were performed as described previously9. We used a previously generated BAF/LFA1 lymphocyte cell line derived from the mouse pro-B Ba/F3 cell line (RIKEN, RCB476), in which we deleted mouse β1, β2, β3, and β7, and introduced human αL and β2 to effectively monitor LFA1-related phenotypes in lymphocytes11. This cell line was modified by fusing the SNAP-tag at the C-terminus of human β2 to monitor the localization and transport of LFA1 using photostable SNAP-tag fluorescent ligands. BAF/LFA1 cells with a SNAP-tag (BAF/LFA1-SNAP) were used as parental cells for deletion and introduction of genes of interest as described above. For FRET analysis BAF/LFA1 cells expressing β2-mCherry and either YPet alone or YPet-LC3 were established. The expression levels of YPet and mCherry were adjusted using flow cytometry sorting. Similarly, BAF/LFA1 cells expressing mCherry-RAPL and either YPet alone or YPet-LC3 were establised in the same manner. For a positive control of FRET, a feredoxin-like (FL) linker peptide flanked by YPet and mCherry, which constituitively facilitate FRET in the absence of tension24, was introduced into BAF/LFA1 cells. Primary B cells and T cells were purified from the spleens of 12–16 week old C57BL/6 mice using a MojoSort Mouse Pan B Cell Isolation Kit and Mouse CD3 T cell Isolation Kit, respectively.
Confocal imaging analyses
BAF/LFA1 cells expressing SNAP tag-fused human β2 were stained with SNAP Cell SiR-647 (1/1000 dilution) for 1 h at 37 °C and washed with dye-free medium for 1 h. Stained cells were adhered to ICAM1-coated 35-mm glass bottom dishes in the presence of 100 ng/mL PMA at 37 °C for 30 min, and fixed with 2% paraformaldehyde. To visualize intracellular factors, the cells were permeabilized with 0.2% Triton-X in PBS, washed with 1% BSA, and stained with antibodies against the cellular factors of interest for 30 min at room temperature. The cells were further stained with Alexa Fluor–conjugated secondary antibodies. Super-resolution imaging was performed using a Dragonfly spinning disk microscope with the Super-Resolution Radial Fluctuation (SRRF) stream setting, which allows visualization of localization at 50–150 nm spatial resolution through time-dependent processing of the obtained images56. For simultaneous live imaging, BAF/LFA1 cells expressing β2-SNAP and CLIP-LC3 were stained with SNAP cell 505 and CLIP cell TMR as described above. The movement of the stained proteins was visualized using simultaneous monitoring mode in Fusion software (Oxford instruments) with a dual camera equipped with Dragonfly. ICCs positive for β2 and LC3 were analyzed using the Fiji manual tracking mode.
Cell adhesion assay
Glass bottom dishes with a diameter of 35 mm (Matsunami, D11130H) were coated with anti-human IgG antibody (15 µg/mL, Rockland, 609-4103), washed with PBS containing 0.1% BSA, and further coated with human ICAM1-Fc (1–3 µg/mL). BAF/LFA1 cells were washed with IL3-free medium and placed on ICAM1-coated 35-mm dishes in the presence of 100 ng/mL PMA. After application and incubation at 37 °C for 15–45 min, differential interference contrast (DIC) microscopy and interference reflection microscopy (IRM) images were captured by MetaMorph (Molecular Devices) using IX81 microscope (Olympus). The areas of IRM were measured with Fiji and statistically analyzed with Prism (GraphPad Software). Statistical significance was computed using the Student’s t-test or one-way ANOVA.
Western blotting and biochemical assays
293 T cells were transfected to obtain protein-overexpressing cell extracts as described previously with some modifications9. 293T cells were transfected with pcDNA3, pcDNA4, or CSII-EF-MCS encoding flag-tagged GABARAP, CLIP- or flag-tagged LC3, Myc-tagged RAPL, V5-tagged Mst1, Nore1A, or Myc-tagged Rassf1a using polyethylenimine. Cells (0.5–2 × 106) were lysed with lysis buffer [1% NP-40, 25 mM Tris-HCl (pH 7.5), 2% glycerol, 150 mM NaCl, 1 mM EDTA, 1 mM NaVO4, 10 mM NaF, 1% PMSF, 1/100 aprotinin, and cOmplete Ultra protease inhibitor cocktail]. The cell lysates were treated with sample buffer at 99 °C for 5 min and subjected to SDS-PAGE and immunoblotting. Pull-down assay was performed as described previously11 using GST or GST-fused RAPL proteins expressed in Escherichia coli BL21 (DE3) lysate and CLIP-tagged LC3 or flag-tagged GABARAP expressed in 293 T cell lysate. The GST-fused proteins, CLIP-LC3, and flag-GABARAP were detected by anti-GST, anti-CLIP, and anti-GABARAP antibodies, respectively in western blotting. For the immunoprecipitation assay between RAPL and ATG8 family proteins, lysates from mock-transfected or Myc-RAPL-transfected 293T cells were mixed with Myc-Trap magnetic agarose beads, rotated at 4 °C for 1 h, and washed with wash buffer [0.05% NP-40, 25 mM Tris-HCl (pH 7.5), 300 mM NaCl, 0.5 mM EDTA]. Subsequently, 0.3 µg of recombinant LC3b or GABARAP was mixed with the washed beads in lysis buffer, rotated at 4 °C for 1 h, and washed by wash buffer. Myc-RAPL, LC3, and GABARAP were detected by anti-Myc, anti-LC3, and anti-GABARAP antibodies, respectively in western blotting.
Flow cytometry
Surface and intracellular integrin levels and CD3 levels were measured using flow cytometry as described previously11. Briefly, the cells were stained with SNAP ligand at 37 °C for 30 min, or with TS2/4 or TS1/18 on ice for 30 min. The latter cells were further stained with anti-mouse eFluor 660 IgG. The stained cells were analyzed by flow cytometry on a FACSCalibur or Attune 4 Nxt instrument. The obtained data were analyzed with FlowJo software (BD Science)
FRET analysis
Ratiometric FRET analyses of live BAF/LFA1 cells were performed using a Dragonfly dual-camera mode equipped with a 100× oil-immersion objective. The cells were excited at 488 nm, and the emission intensities of the donor (using a 521/38 nm filter) and acceptor (using a 594/43 nm filter) were recorded simultaneously. Background signals were subtracted from each image using Fiji software. Ratiometric FRET values were calculated by dividing the mean intensity of the acceptor signal by that of the donor signal, as described previously57.
Preparation of retrovirus for guide RNA expression
Retroviruses harboring guide RNA were prepared following a protocol described previously26,27. The GFP gene in MRIG, a retroviral vector for gRNA expression, was replaced with a puromycin resistance gene, and the constructed plasmid was named MRIG-puro. Negative control (NC, non-targeting sequence; GCGAGGTATTCGGCTCCGCG), LC3b, and AMPK guide RNA sequences were introduced into MRIG-puro. 293T cells (3.5 × 105 cells) were cultured in 12-well plates. At 24 h after cultivation, the 293T cells were transfected with MRIG-puro harboring guide RNA and pCL-eco, the retroviral packaging vector58, using Lipofectamine 2000. The culture supernatant was centrifuged at 1000 ×g for 10 min 24 h after transfection, and the supernatant was used for retroviral transduction of guide RNA into T cells.
Preparation of mouse T-cell blasts with deletion of the gene of interest
All animal experiments were performed in accordance with protocols approved by the Animal Care and Use Committee of Kansai Medical University (approval no. 24-058). The mice used in this study were maintained under specific pathogen–free conditions (dark/light cycle: 12-h light/12-h dark, temperature: 22 °C, humidity: 50%) in the animal facility at Kansai Medical University. Mice expressing Streptococcus pyogenes CRISPR-associated protein 9 (spCas9) (C57BL/6J-congenic H11Cas9, RRID: IMSR_JAX: 028239, Cas9-KI)25 at 8–16 weeks of age were used for T-cell blast preparation. Both male and female mice were used. T-cell blasts with deletion of the gene of interest were prepared following the protocol described previously26,27. Briefly, primary mouse CD3+ T cells were purified from splenocytes by negative selection using the MojoSort Mouse CD3 T Cell Isolation Kit after red blood cells were removed by incubation for 1 min in ACK buffer. Isolated T cells (5 × 106 cells) were cultured on 8 µg/mL anti-CD3 and 8 µg/mL anti-CD28–coated 12-well plates in complete medium [IMDM supplemented with 4% fetal calf serum (FCS), penicillin/streptomycin, and 55 µM 2-mercaptoethanol]. At 24 h after stimulation, the medium was gently replaced with the culture supernatant of retroviruses harboring guide RNA supplemented with 8 µg/mL polybrene and 100 U/mL IL-2. The plate was centrifuged at 780 ×g for 1.5 h at 25–30 °C to enhance the efficiency of infection. After centrifugation, the medium was replaced with a complete medium supplemented with 100 U/mL IL-2. T cells were cultured for 12 h and infected with retrovirus followed by centrifugation as described above. The medium was then replaced with a complete medium containing 100 U/mL IL-2, and the T cells were cultured for 4 h, and then mixed with puromycin (final concentration: 4 µg/mL). Two days after cultivation, dead T cells were removed using Lympholyte-M. T cells were further cultured for 1–3 days in a complete medium supplemented with 100 U/mL IL-2 and 4 µg/mL puromycin and used for assays.
β2-SNAP knock-in (SNAP-KI) mice were generated using the CRISPR–Cas9 system59. SNAP-KI mice were crossed with Cas9-KI mice to generate SNAP-KI;Cas9-KI mice. T cells from SNAP-KI;Cas9-KI mice were isolated and cultured to obtain T-cell blasts with gene deletion as described above.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
The authors thank T. Taguchi and Y. Uchida for kindly providing the 2×PH-GFP plasmid, P. Schwartzberg for kindly providing the MRIG plasmid, R. Hamaguchi for excellent technical assistance, and Y. Ueda and Y. Kamioka for helpful discussions. pMRX-IP-GFP-LC3-RFP-LC3ΔG was a gift from Noboru Mizushima (Addgene plasmid #84572; RRID: Addgene_84572). The FL tension sensor module was a gift from Carsten Grashoff (Addgene plasmid # 101170; http://n2t.net/addgene:101170; RRID: Addgene_101170). This study was supported by KAKENHI (20K07331) (to N.K.), (22H02623) (to T.K.), the Takeda Science Foundation (to N.K.), Japan Science and Technology Agency Core Research for Evolutional Science and Technology (JPMJCR1863) (to Y.M.-K.), the Research Grant D1 from Kansai Medical University (to N.K.), and the Strategic Project for Proofreading and Submission Support of International Academic Papers by Kansai Medical University (to N.K.).
Author contributions
N.K. and T.K. conceived the experiments. N.K. designed the experiments, and performed the experiments and analyses. Y.M.-K. supported the observation by lattice light-sheet microscopy. K.T. and N.K. created the genetically modified mice. N.K., G.P., and T.K. wrote the manuscript.
Peer review
Peer review information
Nature Communications thanks Minsoo Kim and Jose Nieto-Torres for their contribution to the peer review of this work. A peer review file is available.
Data availability
The data are available within the Supplementary Information and Source Data file. Both the Supplementary Information and the Source Data files are provided with this paper. Source data are provided with this paper.
Competing interests
The authors declare no competing interests
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-025-56631-1.
References
- 1.Kinashi, T. & Katagiri, K. Regulation of immune cell adhesion and migration by regulator of adhesion and cell polarization enriched in lymphoid tissues. Immunology116, 164–171 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kondo, N., Ueda, Y. & Kinashi, T. LFA1 Activation: insights from a single-molecule approach. Cells11, 1751 (2022). [DOI] [PMC free article] [PubMed]
- 3.Dustin, M. L. & Springer, T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature341, 619–624 (1989). [DOI] [PubMed] [Google Scholar]
- 4.Katagiri, K., Maeda, A., Shimonaka, M. & Kinashi, T. RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nat. Immunol.4, 741–748 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Katagiri, K. et al. Crucial functions of the Rap1 effector molecule RAPL in lymphocyte and dendritic cell trafficking. Nat. Immunol.5, 1045–1051 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Lafuente, E. M. et al. RIAM, an Ena/VASP and Profilin ligand, interacts with Rap1-GTP and mediates Rap1-induced adhesion. Dev. Cell7, 585–595 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Katagiri, K., Imamura, M. & Kinashi, T. Spatiotemporal regulation of the kinase Mst1 by binding protein RAPL is critical for lymphocyte polarity and adhesion. Nat. Immunol.7, 919–928 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Katagiri, K. et al. Mst1 controls lymphocyte trafficking and interstitial motility within lymph nodes. EMBO J.28, 1319–1331 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kondo, N. et al. NDR1-dependent regulation of kindlin-3 controls high-affinity LFA-1 binding and immune synapse organization. Mol. Cell. Biol.37, e00424-16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu, L. et al. Structure of Rap1b bound to talin reveals a pathway for triggering integrin activation. Nat. Commun.8, 1744 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kondo, N., Ueda, Y. & Kinashi, T. Kindlin-3 disrupts an intersubunit association in the integrin LFA1 to trigger positive feedback activation by Rap1 and talin1. Sci. Signal14, eabf2184 (2021). [DOI] [PubMed]
- 12.Kamioka, Y. et al. Distinct bidirectional regulation of LFA1 and alpha4beta7 by Rap1 and integrin adaptors in T cells under shear flow. Cell Rep.42, 112580 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim, J., Kundu, M., Viollet, B. & Guan, K. L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol.13, 132–141 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimura, S., Noda, T. & Yoshimori, T. Dynein-dependent movement of autophagosomes mediates efficient encounters with lysosomes. Cell Struct. Funct.33, 109–122 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Wilkinson, D. S. et al. Phosphorylation of LC3 by the Hippo kinases STK3/STK4 is essential for autophagy. Mol. Cell57, 55–68 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galluzzi, L. & Green, D. R. Autophagy-Independent Functions of the Autophagy Machinery. Cell177, 1682–1699 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan, J., Zhang, Q., Chen, S., Yan, M. & Yue, L. LC3-associated phagocytosis in bacterial infection. Pathogens11, 863 (2022). [DOI] [PMC free article] [PubMed]
- 18.Schmid, D., Pypaert, M. & Münz, C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity26, 79–92 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romao, S. et al. Autophagy proteins stabilize pathogen-containing phagosomes for prolonged MHC II antigen processing. J. Cell Biol.203, 757–766 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanjuan, M. A. et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature450, 1253–1257 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Peña-Martinez, C., Rickman, A. D. & Heckmann, B. L. Beyond autophagy: LC3-associated phagocytosis and endocytosis. Sci. Adv.8, eabn1702 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kondo, N., Ueda, Y. & Kinashi, T. Low-affinity LFA1-dependent outside-in signaling mediates avidity modulation via the Rabin8-Rab8 axis. PNAS Nexus3, pgae332 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Capece, T. et al. A novel intracellular pool of LFA-1 is critical for asymmetric CD8(+) T cell activation and differentiation. J. Cell Biol.216, 3817–3829 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ringer, P. et al. Multiplexing molecular tension sensors reveals piconewton force gradient across talin-1. Nat. Methods14, 1090–1096 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Chiou, S. H. et al. Pancreatic cancer modeling using retrograde viral vector delivery and in vivo CRISPR/Cas9-mediated somatic genome editing. Genes Dev.29, 1576–1585 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang, B., Johansen, K. H. & Schwartzberg, P. L. Efficient CRISPR/Cas9-mediated mutagenesis in primary murine T lymphocytes. Curr. Protoc. Immunol.124, e62 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roy, N. H. et al. LFA-1 signals to promote actin polymerization and upstream migration in T cells. J. Cell Sci.133, jcs248328 (2020). [DOI] [PMC free article] [PubMed]
- 28.Morishita, H. & Mizushima, N. Diverse cellular roles of autophagy. Annu. Rev. Cell Dev. Biol.35, 453–475 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Egan, D. F. et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science331, 456–461 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nwadike, C., Williamson, L. E., Gallagher, L. E., Guan, J. L. & Chan, E. Y. W. AMPK inhibits ULK1-dependent autophagosome formation and lysosomal acidification via distinct mechanisms. Mol. Cell Biol.38, e00023-18 (2018). [DOI] [PMC free article] [PubMed]
- 31.Mizushima, N. & Yoshimori, T. How to interpret LC3 immunoblotting. Autophagy3, 542–545 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Kumar, A. V., Mills, J. & Lapierre, L. R. Selective Autophagy Receptor p62/SQSTM1, a Pivotal Player in Stress and Aging. Front Cell Dev. Biol.10, 793328 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaizuka, T. et al. An autophagic flux probe that releases an internal control. Mol. Cell64, 835–849 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Nieto-Torres, J. L., Leidal, A. M., Debnath, J. & Hansen, M. Beyond autophagy: the expanding roles of ATG8 proteins. Trends Biochem. Sci.46, 673–686 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Durgan, J. et al. Non-canonical autophagy drives alternative ATG8 conjugation to phosphatidylserine. Mol. Cell81, 2031–2040.e2038 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasegawa, J. et al. A Role of Phosphatidylserine in the Function of Recycling Endosomes. Front Cell Dev. Biol.9, 783857 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uchida, Y. et al. Intracellular phosphatidylserine is essential for retrograde membrane traffic through endosomes. Proc. Natl Acad. Sci. USA108, 15846–15851 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee, S. et al. Transport through recycling endosomes requires EHD1 recruitment by a phosphatidylserine translocase. EMBO J.34, 669–688 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujita, N. et al. The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Mol. Biol. Cell19, 2092–2100 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fletcher, K. et al. The WD40 domain of ATG16L1 is required for its non-canonical role in lipidation of LC3 at single membranes. EMBO J37, e97840 (2018). [DOI] [PMC free article] [PubMed]
- 41.Behrends, C., Sowa, M. E., Gygi, S. P. & Harper, J. W. Network organization of the human autophagy system. Nature466, 68–76 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nieto-Torres, J. L. et al. LC3B phosphorylation regulates FYCO1 binding and directional transport of autophagosomes. Curr. Biol.31, 3440–3449.e3447 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hesson, L., Dallol, A., Minna, J. D., Maher, E. R. & Latif, F. NORE1A, a homologue of RASSF1A tumour suppressor gene is inactivated in human cancers. Oncogene22, 947–954 (2003). [DOI] [PubMed] [Google Scholar]
- 44.Hopkins, S. C., Vale, R. D. & Kuntz, I. D. Inhibitors of kinesin activity from structure-based computer screening. Biochemistry39, 2805–2814 (2000). [DOI] [PubMed] [Google Scholar]
- 45.Raiborg, C. et al. Repeated ER-endosome contacts promote endosome translocation and neurite outgrowth. Nature520, 234–238 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Heckmann, B. L., Boada-Romero, E., Cunha, L. D., Magne, J. & Green, D. R. LC3-associated phagocytosis and inflammation. J. Mol. Biol.429, 3561–3576 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bitra, A., Sistla, S., Mariam, J., Malvi, H. & Anand, R. Rassf proteins as modulators of Mst1 kinase activity. Sci. Rep.7, 45020 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jatana, N., Ascher, D. B., Pires, D. E. V., Gokhale, R. S. & Thukral, L. Human LC3 and GABARAP subfamily members achieve functional specificity via specific structural modulations. Autophagy16, 239–255 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng, X. et al. Structural basis of FYCO1 and MAP1LC3A interaction reveals a novel binding mode for Atg8-family proteins. Autophagy12, 1330–1339 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wesch, N., Kirkin, V. & Rogov, V. V. Atg8-family proteins-structural features and molecular interactions in autophagy and beyond. Cells9, 2008 (2020). [DOI] [PMC free article] [PubMed]
- 51.Olsvik, H. L. et al. FYCO1 contains a C-terminally extended, LC3A/B-preferring LC3-interacting region (LIR) motif required for efficient maturation of autophagosomes during basal autophagy. J. Biol. Chem.290, 29361–29374 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Magné, J. & Green, D. R. LC3-associated endocytosis and the functions of Rubicon and ATG16L1. Sci. Adv.8, eabo5600 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu, X. & Mullins, R. D. LC3 and STRAP regulate actin filament assembly by JMY during autophagosome formation. J. Cell Biol.218, 251–266 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baisamy, L., Cavin, S., Jurisch, N. & Diviani, D. The ubiquitin-like protein LC3 regulates the Rho-GEF activity of AKAP-Lbc. J. Biol. Chem.284, 28232–28242 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Itoh, T., Kanno, E., Uemura, T., Waguri, S. & Fukuda, M. OATL1, a novel autophagosome-resident Rab33B-GAP, regulates autophagosomal maturation. J. Cell Biol.192, 839–853 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gustafsson, N. et al. Fast live-cell conventional fluorophore nanoscopy with ImageJ through super-resolution radial fluctuations. Nat. Commun.7, 12471 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Austen, K. et al. Extracellular rigidity sensing by talin isoform-specific mechanical linkages. Nat. Cell Biol.17, 1597–1606 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Naviaux, R. K., Costanzi, E., Haas, M. & Verma, I. M. The pCL vector system: rapid production of helper-free, high-titer, recombinant retroviruses. J. Virol.70, 5701–5705 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gu, B., Posfai, E. & Rossant, J. Efficient generation of targeted large insertions by microinjection into two-cell-stage mouse embryos. Nat. Biotechnol.36, 632–637 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The data are available within the Supplementary Information and Source Data file. Both the Supplementary Information and the Source Data files are provided with this paper. Source data are provided with this paper.