Abstract
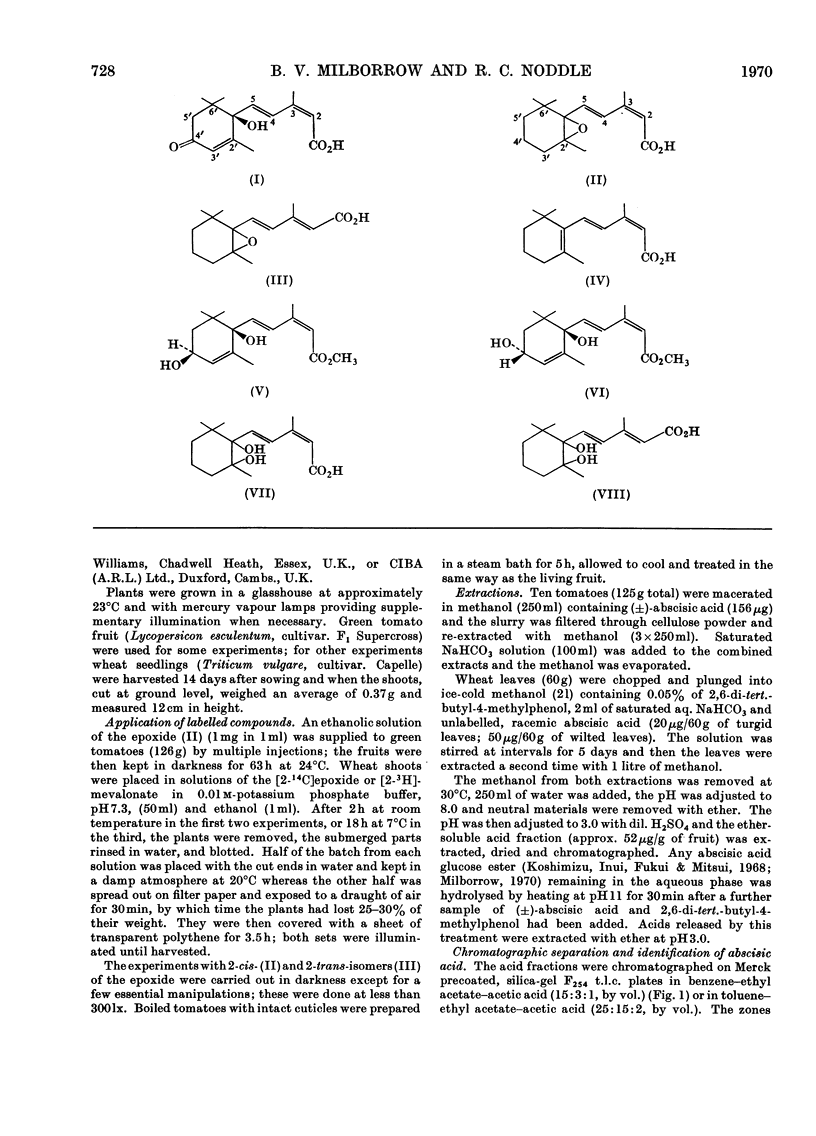
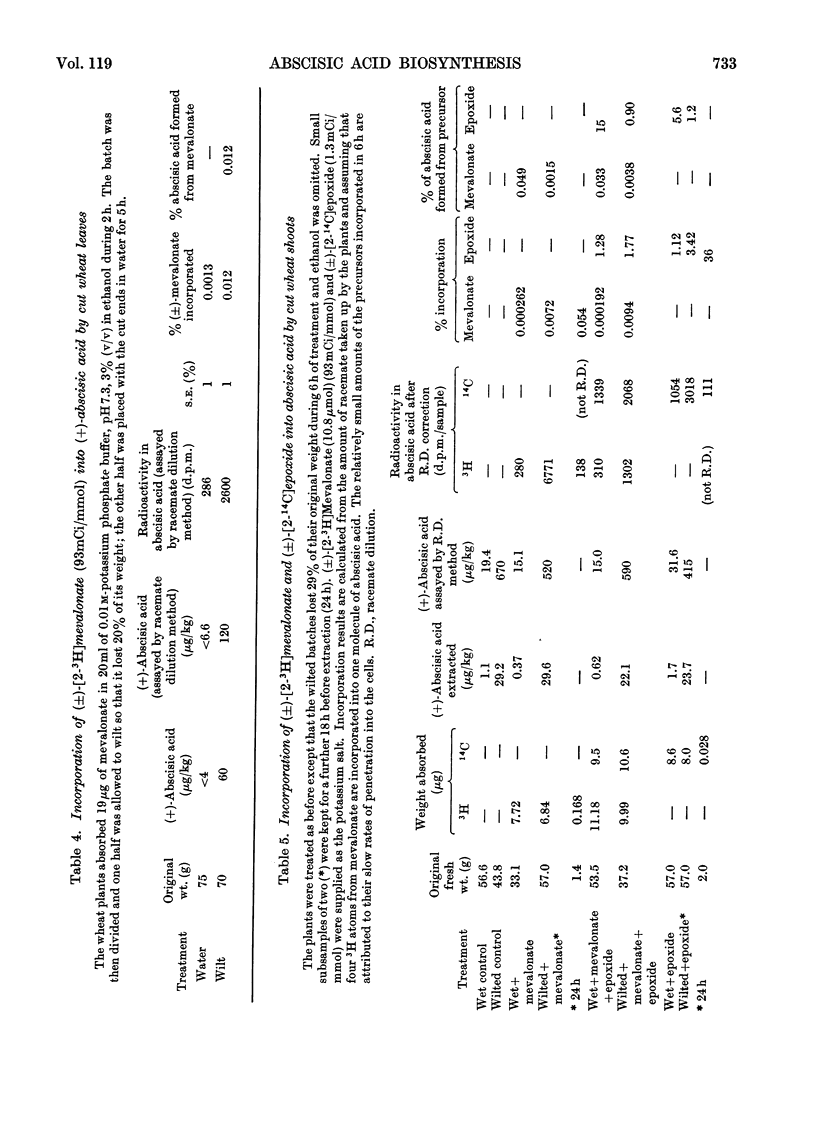
(±)-5-(1,2-Epoxy-2,6,6-trimethylcyclohexyl) -3-methyl[2-14C]penta-cis-2-trans-4-dienoic acid is converted into abscisic acid by tomato fruit in 1.8% yield (or 3.6% of one enantiomer if only one is utilized) and 15% of the abscisic acid is derived from the precursor. The 2-trans-isomer is not converted. The amounts of [2-3H]mevalonate incorporated into abscisic acid have shown that the 40-times higher concentration of (+)-abscisic acid in wilted wheat leaves in comparison with unwilted ones reported by Wright & Hiron (1969) arises by synthesis. The conversion of (±)-5-(1,2-epoxy-2,6,6-trimethylcyclohexyl) -3-methyl-[2-14C]penta-cis-2-trans-4-dienoic acid into abscisic acid by wheat leaves is also affected in the same way by wilting and it is concluded from this that the epoxide or a closely related compound derived from it is on the biosynthetic pathway leading to abscisic acid. The oxygen of the epoxy group was shown, by 18O-labelling, to become the oxygen of the tertiary hydroxyl group of abscisic acid.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- De Koning A. J. Estimation of sphingomyelin. Nature. 1965 May 15;206(985):715–716. doi: 10.1038/206715b0. [DOI] [PubMed] [Google Scholar]
- Noddle R. C., Robinson D. R. Biosynthesis of abscisic acid: incorporation of radioactivity from [2-14C]mevalonic acid by intact fruit. Biochem J. 1969 May;112(4):547–548. doi: 10.1042/bj1120547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D. R., Ryback G. Incorporation of tritium from [(4R)-4-3H]mevalonate into abscisic acid. Biochem J. 1969 Aug;113(5):895–897. doi: 10.1042/bj1130895. [DOI] [PMC free article] [PubMed] [Google Scholar]


