Abstract
CD98, an early marker of T-cell activation, is an important regulator of integrin-mediated adhesion events. Previous studies suggest that CD98 is coupled to both cellular activation and transformation and is involved in the pathogenesis of viral infection, inflammatory disease, and cancer. Understanding of the molecular mechanisms underlying CD98 activity may have far-reaching practical applications in the development of novel therapeutic strategies in these disease states. Using small cell lung cancer cell lines, which are nonadherent, nonpolarized, and highly express CD98, we show that, in vitro, under physiological conditions, CD98 is constitutively associated with β1 integrins regardless of activation status. Cross-linking CD98 with the monoclonal antibody 4F2 stimulated phosphatidylinositol (PI) 3-kinase, PI(3,4,5)P3, and protein kinase B in the absence of integrin ligation or extracellular matrix engagement. Furthermore, cross-linking CD98 promoted anchorage-independent growth. Using fibroblasts derived from β1 integrin null stem cells (GD25), wild-type GD25β1, or GD25 cells expressing a mutation preventing β1 integrin-dependent FAK phosphorylation, we demonstrate that a functional β1 integrin is required for CD98 signaling. We propose that by cross-linking CD98, it acts as a “molecular facilitator” in the plasma membrane, clustering β1 integrins to form high-density complexes. This results in integrin activation, integrin-like signaling, and anchorage-independent growth. Activation of PI 3-kinase may, in part, explain cellular transformation seen on overexpressing CD98. These results may provide a paradigm for events involved in such diverse processes as inflammation and viral-induced cell fusion.
INTRODUCTION
CD98 is a disulfide-linked 125-kDa heterodimeric type II transmembrane glycoprotein composed of a glycosylated 85-kDa heavy chain (designated CD98) and a nonglycosylated 40-kDa light chain. Early studies of peripheral blood T lymphocytes implicated CD98 in the regulation of cellular activation but did not define a specific function for this antigen (Haynes et al., 1981). CD98 is expressed ubiquitously and highly conserved between species. Although it is expressed at low levels on the surface of quiescent cells CD98 expression is rapidly up-regulated after cellular activation (Azzarone et al., 1985; Suomalainen, 1986; Parmacek et al., 1989). For instance, CD98 is strongly expressed on human embryonic and newborn fibroblasts but expression gradually diminishes from 100 to 1% on fibroblasts from normal adults (Azzarone et al., 1985). CD98 is reconstituted to high levels on many tumor cell types (Bellone et al., 1989; Dixon et al., 1990) and furthermore, overexpression of CD98 on NIH3T3 cells has been shown to result in cellular transformation (Hara et al., 1999). These expression patterns suggest that the function of CD98 is coupled to cellular activation, although a definitive role and mechanism of action have yet to be described.
Previous studies suggested that an intracellular signaling pathway mediates the action of CD98 (Warren et al., 1996; Okamoto et al., 1997; Tabata et al., 1997). Tyrosine kinase inhibitors inhibit CD98 activity in hematopoietic cells, suggesting that tyrosine kinase activation may be an early signal transduction pathway activated by CD98 (Warren et al., 1996). There is also evidence that CD98 is involved in the regulation of intracellular calcium concentration through the Na+/Ca2+ exchanger, although its effect seems to be cell type specific (Michalak et al., 1986; Posillico et al., 1987; Freidman et al., 1994). Although six alternative CD98 associated light chains have been identified to date, four of which are associated with L-type amino acid transport activity (Verrey et al., 1999), there seems to be only a single heavy chain, which has been demonstrated to act as a unique and highly specific regulator of integrin affinity (Fenczik et al., 1997). It has been shown previously that β1 integrin-mediated adhesion of the SCLC cell line H345 to fibronectin and laminin can be markedly up-regulated by cross-linking CD98 (Fenczik et al., 1997). In addition, CD98 has been shown to stimulate adhesion of breast cancer cells to laminin via the integrin α3β1 (Chandrasekaran et al., 1999).
Monocytes/macrophages are a key cell type in the control of inflammatory processes and CD98 has a critical role in the functional reprogramming of monocyte behavior. Anti-CD98 monoclonal antibody (mAb) promotes monocyte–monocyte interactions that ultimately lead to polykaryon (multinucleated giant cell) formation, a phenotype associated with chronic inflammatory conditions. Compelling evidence also exists for a connection between CD98 and virus-induced cell fusion. Antibodies to the heavy chain of CD98 promote cell fusion induced by Newcastle disease virus and by the gp160 envelope glycoprotein of human immunodeficiency virus (Ito et al., 1992; Ohgimoto et al., 1995).
In vitro binding studies have shown that CD98 heavy chain interacts specifically with the integrin β1A but not with the muscle-specific variant β1D, or the leukocyte-specific β7 cytoplasmic domain (Zent et al., 2000). Because β1A integrins are mostly expressed basolaterally in polarized cells, and β1D and β7 are expressed in nonpolarized cells, it has been speculated that interactions between CD98 and β1 integrins may influence the polarization state of epithelial cells (Merlin et al., 2001). Integrins are required for normal epithelial development, and abnormal regulation of integrin function may result in deranged growth control and altered epithelial cell function.
Thus, mounting evidence suggests that CD98 may be important in cancer, inflammation, and viral disease through its effects on cellular activation and integrin-mediated adhesion. Therefore, an understanding of the mechanisms and biological relevance of CD98 function may have far-reaching practical applications for the development of novel therapies in these disease states. The aim of this study was to explore the molecular mechanisms by which CD98 regulates integrin activation and integrin-mediated adhesion events as well as cellular activation and transformation.
MATERIALS AND METHODS
Cell Culture and Antibody Production
NCI-H69, NCI-H345, and NCI-H510 human SCLC cell lines were purchased from the American Type Culture Collection (Rockville, MD.). All SCLC cell lines were maintained in RPMI 1640 containing 25 mM HEPES supplemented with 10% (vol/vol) fetal bovine serum (FBS) (heat-inactivated at 57°C for 1 h). Before experimentation, SCLC cells were removed from culture medium, washed twice, and cultured for 24 h in either serum-free medium or in quiescent medium. Serum-free medium was made up of RPMI 1640 medium containing 25 mM HEPES supplemented with 30 nM selenium, 5 μg/ml insulin, 10 μg/ml transferrin, and 0.25% (wt/vol) bovine serum albumin (BSA) (SITA). Quiescent medium was made up of RPMI 1640 medium containing 25 mM HEPES supplemented with 0.25% BSA. All media contained 50 U/ml penicillin, 50 μg/ml streptomycin, and 5 μg/ml l-glutamine, and all cell lines were maintained in a humidified atmosphere of 5% CO2, 95% air at 37°C. Chinese hamster ovary (CHO)-K1 cells were obtained from the American Type Culture Collection and were grown in DMEM containing 10% FBS, 1% nonessential amino acids, 5 μg/ml glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin. The cell lines GD25, GD25β1A, and GD25β1AY783/795F have been described previously (Fassler et al., 1995; Sakai et al., 1998). The GD25 cells are fibroblasts derived from β1 null stem cells. The GD25β1A and GD25β1AY783/795F mutant cell lines were derived from GD25 cells upon stable transfection with cDNAs encoding the wild-type and mutated murine integrin subunit β1A, respectively. GD25β1AY783/795F cells have been shown to have a defect in β1 integrin-dependent FAK phosphorylation and activation (Wennerberg et al., 2000). GD25 cells were grown in DMEM containing 10% FBS, 5 μg/ml glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin; GD25β1A and GD25β1AY783/795F cells were grown in the same medium containing 10 μg/ml puromycin for selection. Transient transfection of the GD25 cell lines with full-length human CD98 was undertaken using LipofectAMINE Plus (Invitrogen, Groningen, The Netherlands) as per manufacturer's instructions. Under optimal conditions a transfection efficiency of at least 60% was achieved in each cell line. Control cells were transfected with control vector pcDNA 3.1.
The hybridoma cell lines 4F2 (C13) and TS2.16.2.1 were purchased from the American Type Culture Collection and cultured in DMEM containing 15% FBS, 50 U/ml penicillin, 50 μg/ml streptomycin, and 2 mM l-glutamine and OPI media supplement (Sigma-Aldrich, St. Louis, MO). Secreted antibody was purified using protein A affinity chromatography. F(ab′)2 fragments were prepared by pepsin digestion of purified 4F2 IgG (2 mg/ml) for 6 h at 37°C. Digestion was terminated by adding 1.5 M Tris pH 8.8 to achieve a final pH 7.5. F(ab′)2 fragments were dialyzed against 20 mM Tris/0.14 M NaCl pH 7.5, and Fab fragments were produced by addition of l-cysteine to a final concentration of 10 mM. Digestion was terminated by the addition of iodoacetamide. Fab fragments were purified on protein A-Sepharose columns. Fab fragments were characterized by SDS-PAGE and exhibited characteristic mobility. After characterization by FACSCalibur (BD Biosciences, San Jose, CA), 4F2 and 4F2-Fab antibodies were routinely used at a final concentration of 20 μg/ml.
Flow Cytometry
Aliquots of 5 × 105 cells were washed and resuspended in 100 μl of phosphate-buffered saline (PBS) containing 0.2% (wt/vol) BSA and 0.1% sodium azide (PBS+). Incubation with 4F2 (for CD98) and 9EG7 (for β1 integrin) (BD PharMingen, San Diego, CA) was performed for 30 min at room temperature followed by two washes with PBS+. Samples were then incubated with species appropriate fluorescein isothiocyanate (FITC)-conjugated secondary antibody (1:40) (DAKO, Bucks, United Kingdom) for 30 min at 4°C and again washed twice in PBS. Samples were finally resuspended in PBS and analyzed by flow cytometry by using FACSCalibur (BD Biosciences). Control IgG2a and IgG1 antibodies for 4F2 and 9EG7, respectively, were used as indicated in figure legends.
Confocal Immunofluorescence
The following primary monoclonal antibodies were used: 4F2 conjugated with Alexa-Fluor 568 (Molecular Probes, Leiden, The Netherlands) (designated 4F2-AR), K20-FITC (DAKO, Glostrup, Denmark), 4B4 (Beckman Coulter, Fullerton, CA), TS2/16 conjugated with FITC (Sigma-Aldrich) (designated TS2/16-FITC), and CD71-FITC (BD Biosciences). The following secondary and tertiary antibodies were used: to amplify the FITC signal, Alexa-Fluor 488 rabbit anti-fluorescein and Alexa-Fluor 488 goat anti-rabbit IgG (Molecular Probes); to localize 4B4, Alexa-Fluor 488 goat anti-mouse IgG (Molecular Probes). Mouse IgG1 and IgG2A antibodies directed against Aspergillus niger glucose oxidase (DAKO, Bucks, United Kingdom) were used as negative controls.
To assess the native state of CD98 and β1 integrin, SCLC cells were plated onto glass coverslips and fixed with 3% paraformaldehyde. Formaldehyde groups were quenched by immersing the coverslips in 50 mM NH4Cl. Nonspecific binding sites were then blocked using 0.2% fish skin gelatin in PBS. Cells were then incubated sequentially with 1) 4F2-AR and K20-FITC or IgG1 and IgG2A negative control antibodies, 2) secondary anti-fluorescein, and 3) tertiary anti-rabbit IgG. To assess the effect on colocalization of cross-linking CD98 with mAb 4F2, primary antibody incubation with 4F2-AR and K20-FITC was carried out before fixation. Secondary and tertiary antibody labeling was performed as described above. To assess the effect of β1 integrin function-stimulating or β1 integrin function-blocking antibodies, cells were incubated with TS2/16-FITC and 4B4, respectively, before fixation, and subsequent secondary and tertiary antibody labeling. In these latter experiments, incubation with 4F2-AR was performed last of all, after secondary and tertiary labeling of the β1 integrin. As a further negative control, localization of 4F2-AR and β1 integrin was compared with that of the transferrin receptor (CD71), by using an isotype-matched (IgG2A) mouse anti-human CD71 antibody (CD71-FITC). In all experiments cells were gently washed twice with PBS between steps. Finally, cells were mounted from distilled water in Mowiol. Confocal microscopy was performed with a TCS NT confocal microscope system (Leica, Heidelburg, Germany) and image analysis was performed using TCS NT software (Leica).
Coimmunoprecipitation
Cells (∼30 × 106) were washed with Hanks' buffered salt solution, before being lysed using 20 mM HEPES, 150 mM NaCl, 2 mM MgCl2, 0.5 mM CaCl2, 1% 3-[(3-cholamidopropyl)dimethylammonio]propanesulfonate, and Complete protease inhibitor cocktail (Roche Applied Science, Mannheim, Germany). Lysates were clarified by centrifugation at 13,000 rpm. To remove nonspecific contaminants, lysates were then incubated with 30 μl of albumin-agarose (Sigma-Aldrich) for 30 min. After further centrifugation, the residual lysates were incubated overnight with 2 μg of either 4F2, mouse anti-human CD71 as a negative control antibody, K20 (DAKO, Bucks, United Kingdom), or a mouse IgG1 negative control antibody (DAKO, Bucks, United Kingdom). Protein G-agarose (50 μl) was used to precipitate the antibodies. Agarose beads were then washed twice in 20 mM HEPES, 150 mM NaCl, 2 mM MgCl2, and 0.5 mM CaCl2 before being boiled with SDS-PAGE sample buffer. Samples were run on 10% Sepharose gel and transferred to nitrocellulose membrane (Amersham Biosciences UK, Little Chalfont, Buckinghamshire, United Kingdom). After blocking with 5% nonfat milk, Western blots were probed with antibody to β1 integrin (Transduction Laboratories, Lexington, KY) and a secondary anti-mouse horseradish peroxidase conjugate (DAKO, Bucks, United Kingdom). Visualization was by enhanced chemiluminescence (Amersham Biosciences UK).
PI 3-Kinase Activity Assay
PI 3-kinase activity was measured as described previously (Moore et al., 1998). Briefly, cells were lysed using ice-cold lysis buffer containing 50 mM HEPES pH 7.4, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 10 mM sodium pyrophosphate, 100 mM sodium fluoride, 10% (vol/vol) glycerol, 1% (vol/vol) Triton X-100, 0.5 mM dithiothreitol, 1 mM sodium orthovanadate, 50 μM 4-(2-aminoethyl)benzenesulfonyl fluoride, 5 μg/ml leupeptin, 20 μg/ml aprotinin, and 10 μg/ml soybean trypsin inhibitor. PI 3-kinase was immunoprecipitated from protein-equilibrated cell lysates by using a specific p85α PI 3-kinase antibody (Upstate Biotechnology, Lake Placid, NY) and assayed for activity by using [γ-32P]ATP and phosphatidylinositol as substrate. 3-Phosphorylated lipids were resolved using thin layer chromatography, identified by autoradiography, and quantified by liquid scintillation counting. The identity of the PI(3)P was confirmed by monomethylamine deacylation and high-performance liquid chromatography analysis by using an SAX 5 column and (NH4)2HPO4 gradient and authentic tritiated standards as markers.
Radioligand Displacement Assay for Mass Measurement of PI(3,4,5)P3
PI(3,4,5)P3 levels were measured as described previously (van der Kaay et al., 1999). In brief, SCLC cells (5 × 106) were subjected to a standard Folch extraction, and lipid extracts containing PI(3,4,5)P3 were then subjected to alkaline hydrolysis, resulting in the release of the polar head group Ins(1,3,4,5)P4. The mass of Ins(1,3,4,5)P4 was measured by [3H]Ins(1,3,4,5)P4 displacement from a recombinant Ins(1,3,4,5)P4-GST binding protein, by using a calibration curve obtained using unlabeled Ins(1,3,4,5)P4 standards.
Protein Kinase B Activity Assay
Protein kinase B activity (PKB) was measured as described previously (Moore et al., 1998). In brief, PKB was immunoprecipitated from cell lysates by using an anti-PKB PH-domain antibody (Upstate Biotechnology) preconjugated to 5 μl of protein G-Sepharose. PKB activity was assayed by incubating washed immunoprecipitates with [γ-32P]ATP and Cross-tide (Upstate Biotechnology) as substrate. The assays were terminated by placing the assay mixture onto P8l Whatman chromatography paper (Whatman, Maidstone, United Kingdom) and washing four times with 0.5% (vol/vol) phosphoric acid and once with acetone. Radioactive incorporation was quantified by liquid scintillation counting.
Amino Acid Transport Assay
Cells (5 × 106) were washed twice and resuspended in amino acid-free and Na+-free uptake solution containing 100 mM choline chloride, 2 mM KCl, 1 mM MgCl2, 1 mM CaCl2, and 10 mM HEPES pH 7.5. After equilibration at 37°C for 30 min cells were treated for a further 10 min with or without 4F2 (20 μg/ml) in the presence or absence of 5 mM 2-amino-2-norbornanecarboxylic acid (BCH) (Sigma-Aldrich). After this, 2 μCi of l-[4,5-3H]leucine (82 Ci/mmol) containing 2 mM cold l-leucine was added to each tube and incubation continued for a further 30 min at 37°C. Cells were then placed on ice, pelleted, and washed three times with 1 ml of ice-cold wash buffer containing 80 μM choline chloride, 2 mM KCl, 1 mM MgCl2, 1 mM CaCl2, and 10 mM HEPES pH 7.5. The washed cells were then digested with 200 μl 0.2% SDS in 0.2 M NaOH for 1 h. Protein equilibrated aliquots of 100 μl were then added to scintillation fluid containing 100 μl of 0.2 M HCl and activity counted in a scintillation counter.
Immunoblotting
Cell pellets were lysed at 4°C in PI 3-kinase lysis buffer for 30 min. Lysates were clarified by centrifugation at 13,000 × g for 10 min at 4°C. Samples (20 μg of protein) were solubilized in SDS-PAGE sample buffer and resolved on 10% gels. The proteins were transferred to nitrocellulose membranes, blocked using 3% (wt/vol) albumin in Tris-buffered saline/Tween (20 mM Tris-HCl pH 7.4, 150 mM NaCl, and 0.02% [vol/vol] Tween 20) overnight at 4°C and then incubated with anti-PKB or anti-phospho PKB (serine 473) antibody (New England Biolabs, Beverly, MA), anti-FAK or anti-phospho FAK antibody (Santa Cruz Biotechnology, Santa Cruz, CA), or goat anti-CD98 antibody (SC-7095) (Santa Cruz Biotechnology). Species appropriate horseradish peroxidase-conjugated antibodies (DAKO, Bucks, United Kingdom) were used for secondary labeling. Immunoreactive bands were identified using enhanced chemiluminescence (Amersham Biosciences UK) according to the manufacturer's instruction. Densitometry was performed using Grab-IT gel documentation system (Ultra Violet Products, Cambridge, United Kingdom). Using different exposures, blots were confirmed to be within the dynamic range of the film before analysis.
Clonogenic Assay
SCLC cells, 5 d postpassage, were washed and subsequently incubated in serum-free SITA medium for 24 h. Before experimentation cells were washed twice and resuspended in fresh SITA medium before being gently disaggregated as described above. Viability was determined by trypan blue exclusion on a hemocytometer. Cells (1 × 104) were mixed with SITA containing 0.3% (wt/vol) agarose in the presence or absence of agonist/antagonist and layered over a solid base of 0.5% (wt/vol) agarose in SITA. The cultures were incubated in a humidified atmosphere of 5% CO2, 95% air at 37°C for 21 d and then stained with the vital stain nitro-blue tetrazolium. Colonies of >16 cells were counted with a microscope.
Statistical Analysis
Data were analyzed by one-way analysis of variance with comparison between groups made using the Newman-Keuls procedure. P values < 0.05 were considered to be significant
RESULTS
CD98 Is Highly Expressed on SCLC Cells In Vitro
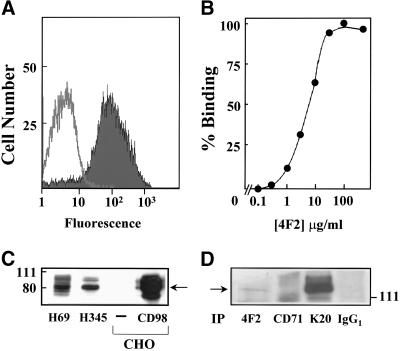
CD98 expression on SCLC cell lines in vitro was examined. CD98 expression was determined by flow cytometric analysis by using an indirect immunofluorescence technique with the mAb 4F2 directed against the heavy chain of CD98. Figure 1A shows CD98 expression on H69 SCLC cells. Mean fluorescence intensity was 79.4 arbitrary units compared with an IgG2A isotype-matched control of 14.2 arbitrary units. When H69 SCLC cells were incubated in the presence of increasing concentrations of 4F2, a dose-dependent increase in binding was seen with a half-maximal value of 6.5 μg/ml (Figure 1B). Saturated binding occurred between 10 and 30 μg/ml (Figure 1B).
Figure 1.
CD98 expression in SCLC cells. (A) CD98 expression in H69 SCLC cells was determined by flow cytometric analysis by using an indirect immunofluorescence technique with a mAb (4F2) to the heavy chain of CD98. (B) Dose response for binding of mAb 4F2 to H69 SCLC cells. (C) Western blot analysis of whole cell lysates that had been probed with goat anti-CD98 polyclonal antibody to show CD98 expression on H69 and H345 SCLC cells, native CHO cells, and CHO cells that had been transiently transfected with CD98. (D) CD98 and β1 integrin coimmunoprecipitate in SCLC cells. H69 SCLC whole cell lysates were incubated with antibodies against CD98 (4F2) (lane 1), transferrin receptor (CD71) (lane 2), β1 integrin (K20) (lane 3), and an IgG1 negative control antibody (lane 4), as described in MATERIALS AND METHODS. Immunoprecipitates were Western blotted and probed for β1 integrin. All data shown are representative of two to three independent experiments.
We confirmed CD98 expression in H69 SCLC cells by using Western blot analysis of whole cell lysates that had been probed with an antibody for CD98 and showed a strong band at ∼85 kDa corresponding with the heavy chain of CD98. Similar levels of expression of CD98 were also seen in all other SCLC cell lines examined, including H345 (Figure 1C) and H510, LS274, LS310, WX330, and GLC19 (our unpublished data). For control purposes, expression of CD98 in our SCLC cell lines was compared with that in CHO-K1 cells. This cell line normally expresses CD98 at low levels but can be transfected with human CD98, resulting in cells that show high expression. CD98 transfected and untransfected CHO cells were used as positive and negative controls respectively (Figure 1C).
CD98 and β1 Integrins Coimmunoprecipitate
CD98 was immunoprecipitated from H69 SCLC whole cell lysates by using 4F2 and appropriate control antibodies as described in MATERIALS AND METHODS. Western blots were probed for β1 integrin. Figure 1D shows that upon immunoprecipitation by 4F2, a band was visualized at ∼130 kDa (lane 1). This was not seen in immunoprecipitation with CD71 control antibody (lane 2), or with control IgG1 antibody (lane 4), but was clearly seen with immunoprecipitation by the β1 integrin antibody K20 (lane 3). This band corresponds to β1 integrin.
CD98 and β1 Integrins Are Constitutively Associated
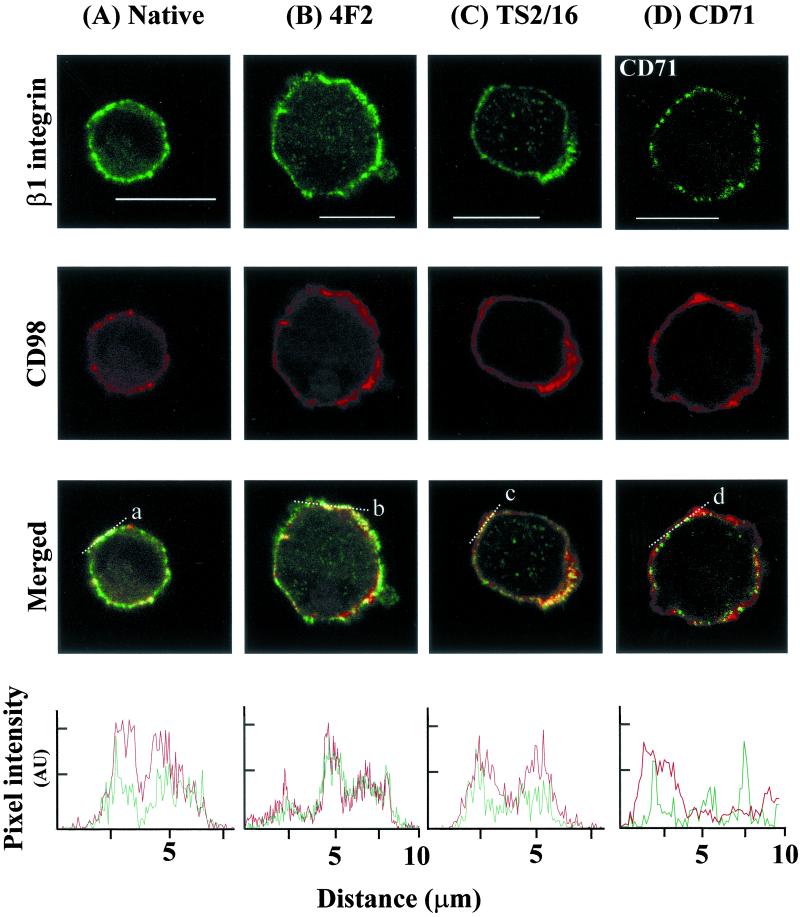
We used dual label confocal immunofluorescence to examine the physical relationship between β1 integrins and CD98 in vivo in nonpolarized, nonadherent H69 cells. In the unstimulated native state, CD98 (labeled by 4F2) and β1 integrin (labeled by K20 mAb) were colocalized in the plasma membrane of the cell (Figure 2A). When 4F2 was used to cross-link and stimulate CD98 before fixation and antibody labeling, colocalization was again seen (Figure 2B). Furthermore, when cells were incubated with the β1 integrin-stimulating antibody (TS2/16) before fixation, colocalization between β1 integrin and CD98 was still apparent (Figure 2C). Colocalization was still present in the presence of a β1 integrin function-blocking antibody (4B4) (our unpublished data). However, no colocalization was seen between CD98 and another constitutively expressed plasma membrane protein, the transferrin receptor (CD71) (Figure 2D), nor between CD71 and β1 integrin (our unpublished data), confirming that the relationship between CD98 and β1 integrin is specific. IgG1 and IgG2A negative control antibodies for β1 and CD98, respectively, revealed no evidence of nonspecific antibody binding (our unpublished data). These data demonstrate that CD98 and β1 integrins are physically colocalized, regardless of integrin activation state and cell polarization. In addition, these results suggest that β1 integrins and CD98 can form high-density complexes within the plasma membrane.
Figure 2.
β1 integrin and CD98 are colocalized in SCLC cells. H69 cells were incubated with antibodies against β1 integrin and CD98 as described in MATERIALS AND METHODS. White bars, 10 μm. (A–C) Localization of β1 (green) and CD98 (red) in a single confocal plane under either (A) native conditions, (B) stimulation by 4F2 cross-linking, and (C) β1 integrin stimulation by using mAb TS2/16. In the “merged” panel, areas of colocalization of β1 integrin and CD98 appear yellow. (D) Localization of the transferrin receptor was defined by use of mAb CD71-FITC (green) and CD98 (red) by 4F2-AR. No colocalization between CD98 and CD71 was seen (merged panel). Bottom, analysis of β1 integrin and CD98 localization along the lines a, b, and c. In contrast, analysis along line d shows no colocalization between CD98 and CD71.
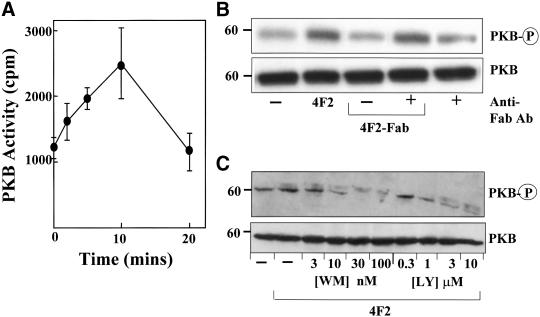
Cross-Linking CD98 Stimulates PI 3-Kinase Activity and PI(3,4,5)P3 Formation
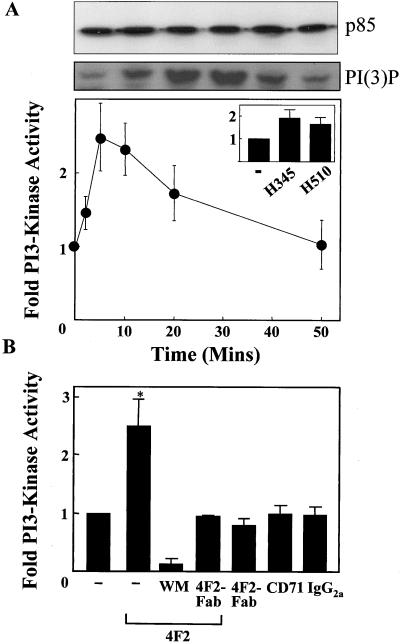
The mechanism of action of CD98 is poorly understood. The cytoplasmic tail of CD98 seems to be critical for its action and there is some evidence to suggest that CD98 is involved in the regulation of intracellular signaling. Therefore, the effect of cross-linking CD98 on intracellular signaling was examined. To specifically examine the effect of cross-linking CD98 the mAb to the heavy chain of CD98 (4F2) was used. 4F2 did not stimulate mobilization of intracellular calcium in fura-2–loaded SCLC cells (our unpublished data). Because PI 3-kinase plays a key role in integrin activation, cellular activation, and transformation (Carpenter and Cantley, 1996) we examined the effect of 4F2 on PI 3-kinase activity in SCLC cells. PI 3-kinase activity from p85α immunoprecipitates was measured as described in MATERIALS AND METHODS. Confirmation of equal amounts of PI 3-kinase loading was obtained by probing Western blots of p85α immunoprecipitates with p85 PI 3-kinase antibody (Figure 3A, top). Figure 3A, bottom, shows that 4F2 (20 μg/ml) caused a 2.5-fold increase in PI 3-kinase activity, first evident within 2 min, maximal at 5 min, and returning to baseline by 50 min. Similar results were found with the H345 and H510 cell lines (Figure 3A, inset). PI 3-kinase activation by 4F2 could be blocked by preincubation with the PI 3-kinase inhibitor wortmannin or monovalent Fab′-4F2 fragments (Figure 3B). In addition, the Fab-4F2 monovalent fragments alone did not have any effect on PI 3-kinase activation, confirming that cross-linking of CD98 is required for PI 3-kinase activation. Saturating concentrations of an IgG2a negative control antibody (directed against Aspergillus niger glucose oxidase) or an IgG2A antibody (10 μg/ml) to the CD71 transferrin receptor, which is highly expressed on SCLC cells (EC50 of 1.4 μg/ml to H69 SCLC cells), had no effect on PI 3-kinase activity (Figure 3B). Again equal loading of p85 PI 3-kinase immunoprecipitates was confirmed for all conditions as outlined above (our unpublished data).
Figure 3.
Cross-linking CD98 activates PI 3-kinase. (A) Time course of PI 3-kinase activation by 4F2 (20 μg/ml). PI 3-kinase was immunoprecipitated from H69 SCLC cell lysates by using an anti p85α-SH3 antibody. PI 3-kinase was assayed using phosphatidylinositol as substrate. 3-Phosphorylated lipids were resolved using thin layer chromatography, identified by autoradiography, and quantified by liquid scintillation counting. An autoradiograph showing the 3-phosphorylated reaction product [PI(3)P] is shown for a typical experiment. Equal loading of PI 3-kinase was confirmed by probing a Western blot of p85α immumoprecipitates with p85 PI 3-kinase antibody (top). Inset, effect of 4F2 (20 μg/ml) at 5 min on PI 3-kinase activity in H345 and H510 SCLC cells (−, diluent only). (B) Cross-linking of CD98 is required for PI 3-kinase activation. H69 cells were treated with 4F2 (20 μg/ml), 4F2-Fab (20 μg/ml), IgG2A control antibody (20 μg/ml), or antibody to CD71 (10 μg/ml), having been pretreated with wortmannin (100 nM), 4F2-Fab (20 μg/ml), or diluent as indicated. PI 3-kinase activity was assayed as described in MATERIALS AND METHODS. All data are the mean of three to four independent experiments performed in triplicate ± SEM. *p < 0.05 compared with control cells.
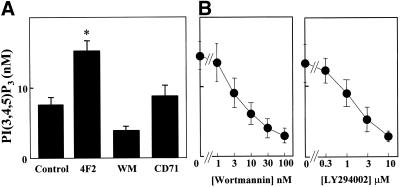
Although PI 3-kinase can phosphorylate PI, PI(4)P, and PI(4,5)P2 in vitro, PI(4,5)P2 is believed to be the preferred substrate in vivo, generating the second messenger PI(3,4,5)P3. Therefore, we measured PI(3,4,5)P3 levels by using a radioisotope dilution assay as described previously (van der Kaay et al., 1999). Cross-linking of CD98 with 4F2 resulted in a significant increase in PI(3,4,5)P3 compared with control cells (Figure 4A). This effect could be blocked in a dose-dependent manner by preincubation with the PI 3-kinase inhibitors wortmannin (IC50 of 3.2 nM) and LY294002 (IC50 of 1.4 μM) (Figure 4B). Again, the anti-CD71 mAb had no effect on PI(3,4,5)P3 levels.
Figure 4.
Cross-linking CD98 stimulates PI(3,4,5)P3. (A) H69 cells were treated with either diluent (control), 4F2 (20 μg/ml for 10 min), wortmannin (WM) (100 nM, 10 min) followed by 4F2 (20 μg/ml for 10 min), or antibody to CD71 (10 μg/ml for 10 min). PI(3,4,5)P3 levels were measured by an isotope dilution assay as described in MATERIALS AND METHODS. (B) H69 SCLC cells were pretreated with increasing concentrations of the PI 3-kinase inhibitors wortmannin or LY294002 as shown, followed by 4F2 (20 μg/ml for 10 min) and PI(3,4,5)P3 levels measured. All data are the mean of three independent experiments performed in triplicate ± SEM. *p < 0.05 compared with control cells.
Cross-Linking CD98 Activates Protein Kinase B
In integrin signal transduction, PKB has been identified as a key downstream effector of PI 3-kinase (Bellacosa et al., 1991). Therefore, the effect of 4F2 on PKB activity was examined using an in vitro kinase assay as described in MATERIALS AND METHODS. Figure 5A shows that cross-linking CD98 with 4F2 antibody stimulated PKB activity in a time-dependent manner. An increase in PKB activity was first evident at 2 min, maximal by 10 min, and had returned to baseline by 20 min. This magnitude of response, and time course, was very similar to that seen for PI 3-kinase activation. Again, no effect on PKB activity was seen after incubation of SCLC cells with either Fab′-4F2 for 10 min or control antibodies. These results were confirmed by Western blotting by using a phospho-PKB antibody that recognizes phosphorylation of PKB at serine 473. Figure 5B shows that 4F2 caused an increase in phosphorylation of PKB compared with untreated control or Fab′-4F2–treated cells. Furthermore, an anti-mouse IgG F(ab′)2 fragment-specific antibody (Jackson Immunoresearch, West Grove, PA) was able to restore PKB phosphorylation in Fab′-4F2–treated cells confirming that cross-linking of CD98 is required for downstream signaling. The anti-mouse IgG F(ab′)2 fragment-specific antibody alone had no effect on PKB phosphorylation. We have also shown that 4F2-induced PKB phosphorylation can be inhibited in a dose-dependent manner by the PI 3-kinase inhibitors wortmannin and LY294002 (Figure 5C). Therefore, cross-linking CD98, in addition to activating PI 3-kinase, also activates its principal downstream effector, PKB. We have also shown that cross-linking CD98 with 4F2 promotes FAK phosphorylation in SCLC cells and CD98-transfected CHO cells; furthermore, 4F2 stimulation promotes mitogen-activated protein (MAP) kinase activation in SCLC cells. Moreover, cross-linking CD98 had no effect on either H-Ras or R-Ras GTP loading/activation (Rintoul, Mackinnon, and Sethi, unpublished data). Signaling via FAK, PI 3-kinase, and MAP kinase without activating Ras is a feature of integrin signaling (King et al., 1997). Taken together, these results suggest that cross-linking CD98 promotes integrin-like signaling.
Figure 5.
Cross-linking CD98 activates PKB. (A) Time course of PKB activation by 4F2. PKB was immunoprecipitated from H69 cell lysates by using an anti-PKB PH-domain antibody. PKB activity was assayed as described in MATERIALS AND METHODS. The figure shows data from a representative experiment performed in triplicate. (B) Western blot of PKB and phospho-PKB. H69 SCLC cells were incubated in the presence or absence of 20 μg/ml 4F2 or 4F2-Fab for 10 min or 4F2-Fab (20 μg/ml) for 10 min followed by an anti-mouse IgG F(ab′)2 fragment specific antibody (20 μg/ml) for 20 min (anti-Fab antibody). Western blots were probed with an antibody, which recognizes PKB or phosphorylation of PKB at serine 473 (PKB-P). (C) H69 SCLC cells were pretreated with increasing concentrations of the PI 3-kinase inhibitors LY294002 or wortmannin as shown, followed by 4F2 (20 μg/ml for 10 min) and Western blots were probed with an antibody, which recognizes PKB or phosphorylation of PKB at serine 473 (PKB-P). Representative blots from experiments performed two to three times are shown.
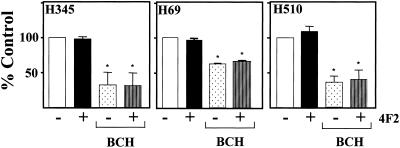
Cross-Linking CD98 Has No Effect on Amino Acid Transport
The light chain of CD98 has been demonstrated to function as an L-type amino acid transporter. We examined whether cross-linking CD98 with mAb 4F2 had any effect on amino acid transport. Using an assay that measures tritiated leucine uptake into cells, Figure 6 shows that there is no demonstrable effect of 4F2 on amino acid transport, in the presence or absence of the L-system amino acid transport blocking agent BCH. Furthermore, BCH had no effect on PI 3-kinase activation by 4F2 (our unpublished data). These data show that the effects of 4F2 on integrin-like signaling are independent of amino acid transport.
Figure 6.
Cross-linking CD98 does not effect amino acid transport. H69, H345, and H510 SCLC cells in amino acid- and Na+-free buffer were treated for 10 min with or without 4F2 (20 μg/ml) in the presence or absence of 5 mM BCH. [3H]Leucine uptake was measured as described in MATERIALS AND METHODS. Data are expressed as percentage of control cells and are the means ± SEM of three independent experiments performed in triplicate. *p < 0.05 compared with control cells.
Cross-Linking CD98 Promotes Anchorage-independent Growth
PI 3-kinase and PKB activity play a central role in regulating cell survival, transformation, and promoting anchorage-independent growth (Khwaja et al., 1997). The ability to form colonies in agarose semisolid medium is a hallmark of the transformed phenotype. There is a positive correlation between the cloning efficiency of cells and the histological involvement and invasiveness of the tumor in specimens taken from SCLC (Carney et al., 1980). Therefore, we examined the functional consequences of cross-linking CD98 with 4F2 in SCLC cells.
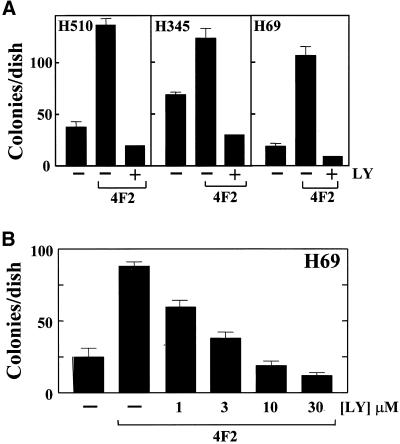
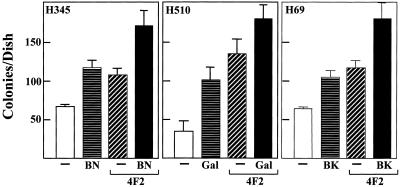
Cross-linking CD98 markedly enhanced the ability of SCLC cell lines H69, H510, and H345 cells to form colonies in semisolid agarose (Figure 7A). SCLC basal colony growth can be stimulated 200–300% by the addition of 20 μg/ml 4F2. LY294002 inhibited 4F2-stimulated colony formation in a dose-dependent manner (IC50 of 2.1 μM) (Figure 7B), suggesting that PI 3-kinase activity is required for the CD98 stimulation of anchorage-independent growth in SCLC cells. Furthermore, addition of maximal clonal-stimulating concentrations of neuropeptide growth factors bombesin (10 nM), galanin (50 nM), and bradykinin (10 nM) in H345, H510, and H69 cell lines, respectively, caused an additive stimulation of clonal growth (Figure 8).
Figure 7.
Cross-linking CD98 stimulates SCLC clonal growth. (A) H69, H345, and H510 SCLC cells were plated in SITA medium containing 0.3% agarose, in the presence or absence of 4F2 (20 μg/ml) and LY294002 (10 μM) (LY) as shown, over a base of 0.5% agarose in culture medium as described in MATERIALS AND METHODS. After 21 d, colonies of >120 μm (16 cells) were counted. (B) Dose response of H69 SCLC cell clonal growth to 4F2 (20 μg/ml) in the presence of increasing concentrations of LY294002 (LY) as shown. Results are expressed as mean ± SEM colonies/dish of two independent experiments performed in triplicate.
Figure 8.
Effect of cross-linking CD98 on neuropeptide-stimulated clonal growth in SCLC cells. H345, H510, and H69 SCLC cells were plated in SITA medium containing 0.3% agarose, in the presence or absence of either bombesin (10 nM) (BN), galanin (50 nM) (Gal), bradykinin (10 nM) (BK), and 4F2 (20 μg/ml), over a base of 0.5% agarose in culture medium as described in MATERIALS AND METHODS. After 21 d, colonies of >120 μm (16 cells) were counted. Results are expressed as mean ± SEM colonies/dish of three independent experiments performed in triplicate.
CD98 Signaling Is β1 Integrin and FAK dependent
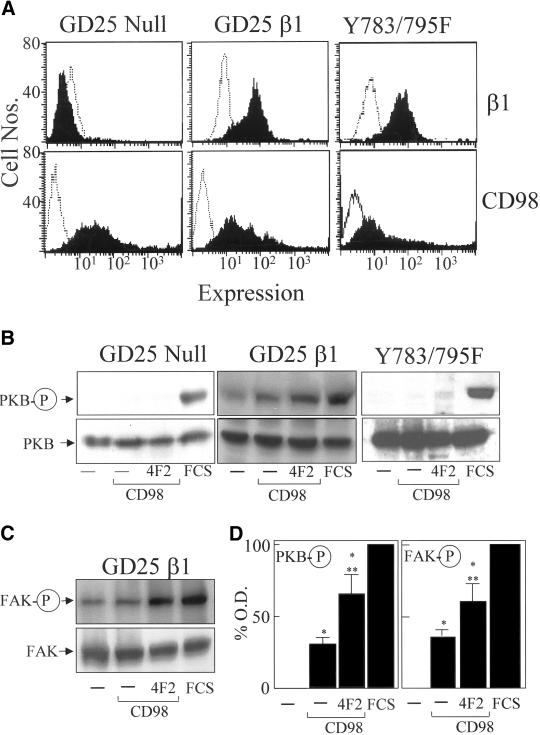
The colocalization of CD98 and β1 integrins and CD98-induced integrin-like signaling and functional effects in SCLC cells led us to examine whether the presence of β1 integrin was necessary for CD98 signaling. We used GD25 fibroblasts derived from β1 null ES cells and GD25β1A and GD25β1AY783/795F mutant cell lines, derived from GD25 cells upon stable transfection with cDNAs encoding the wild-type β1A, and β1A integrin subunit with point mutations Y783/795F, respectively. GD25β1AY783/795F cells have been shown to have a defect in β1 integrin-dependent FAK activation (Wennerberg et al., 2000). Using flow cytometry, we were unable to detect any endogenous CD98 expression in these cell lines (our unpublished data). Therefore, each cell line was transiently transfected with full-length human CD98. Transfection efficiency of ∼50–60% was achieved in all cell lines as described in MATERIALS AND METHODS. Control cells received control vector pcDNA 3.1. β1 integrin and CD98 expression were confirmed for each cell line by using flow cytometry (Figure 9A). CD98 signaling was examined by determining PKB phosphorylation on Western blotting. In the wild-type GD25β1A cells, overexpression of CD98 promoted constitutive PKB and FAK phosphorylation (Figure 9, B and C). Cross-linking CD98 with 4F2 caused a further modest increase in PKB phosphorylation. However, CD98 overexpression in the GD25β1-null cells did not stimulate PKB phosphorylation, confirming that β1 integrin expression was necessary for CD98 signaling. Furthermore, overexpression of CD98 in GD25β1AY783/795F cells did not stimulate PKB phosphorylation (Figure 9B). The results for the GD25β1 cells were quantified by densitometry (Figure 9D). Phospho PKB and phospho FAK expression were significantly higher in the CD98 transfected cells compared with control cells and furthermore cross-linking with 4F2 caused a significant increase in phospho PKB and phospho FAK expression over the CD98 transfected cells. These results suggest that CD98 signaling through the PI 3-kinase/PKB pathway is dependent on a functional β1 integrin and may be FAK dependent.
Figure 9.
CD98 signaling is β1 integrin and FAK dependent. (A) Flow cytometric analysis of β1 integrin and CD98 expression (solid black line) and IgG1 and IgG2a negative control antibodies respectively (hatched line) in GD25 β1 integrin null cells, wild-type GD25 β1 integrin cells, and mutant GD25Y783/795F cells. (B) GD25 β1 integrin null, wild-type GD25β1, and GD25Y783/795F cells were transiently transfected with CD98 or control vector and treated in the presence or absence of 4F2 (20 μg/ml) or fetal calf serum (FCS) (10% for 10 min) as positive control. Cell lysates were Western blotted and probed with an antibody, which recognizes PKB or phosphorylation of PKB at serine 473 (PKB-P). (C) Wild-type GD25β1 were transiently transfected with CD98 or control vector and treated in the presence or absence of 4F2 (20 μg/ml) or FCS as described above. Cell lysates were Western blotted and probed with an antibody, that recognizes FAK or phosphorylated FAK. Representative blots from experiments performed in duplicate, three times, are shown. (D) Densitometric analysis of GD25β1 phospho PKB, and phospho FAK blots was undertaken as described in MATERIALS AND METHODS. Results are expressed as percentage maximum expression (FCS). *p < 0.05 compared with control cells; **p < 0.05 compared with CD98-transfected cells.
DISCUSSION
In this study, we have examined the interaction between CD98 and β1 integrin in nonpolarized, nonadherent cells, which are transformed and lack an organized cytoskeleton. We demonstrate the following: 1) CD98 and β1 integrin are constitutively colocalized, regardless of integrin activation state and cell polarization. 2) Cross-linking CD98 can stimulate integrin-like signaling in the absence of integrin ligation or extracellular matrix engagement and furthermore can promote anchorage-independent growth. 3) The presence of β1 integrin is necessary for CD98 intracellular signaling.
Recent work suggests that CD98 may be important in cancer, inflammation, and viral disease through its effects on cellular activation and integrin-mediated adhesion. Despite being implicated in an extraordinary diversity of functions its mechanism of action has not been fully elucidated. There is now good evidence that the heavy chain, which dimerizes with up to six alternative light chains, is important in regulating amino acid transport (Quackenbush et al., 1987; Teixeira et al., 1987; Warren et al., 1996; Kanai et al., 1998; Mastroberardino et al., 1998; Nakamura et al., 1999). Furthermore, there is evidence that CD98 plays an important role in regulating integrin affinity (Fenczik et al., 1997). Although a functional association between CD98 and β1 integrin in the cell membrane has been described previously (Fenczik et al., 1997; Warren et al., 2000; Cho et al., 2001), until recently there was no clear data showing physiological interaction between CD98 and β1 integrin. Recently, Zent et al. (2000) have shown that CD98 can associate with isolated cytoplasmic portions of some β1 integrin isoforms. Using an anchorage-dependent intestinal epithelial cell layer model, Merlin et al. (2001) demonstrated that CD98 could be coimmunoprecipitated with both β1 integrin and the L-amino acid transporter-2) and that all three proteins were polarized to the basolateral domain. Furthermore, Kolesnikova et al. (2001) have recently shown that CD98 constitutively and specifically associated with various β1 integrin subunits. Our findings confirm and extend these results and show that on anchorage-independent nonpolarized cells, CD98 and β1 integrin coimmunoprecipitate and under physiological conditions are colocalized irrespective of activation state. This finding has implications for our understanding of the mechanisms underlying cell migration and adhesion that are central not only to tumor metastasis but also to a range of physiological processes such as embryogenesis, hemostasis, and the immune response. Cross-linking CD98 has been shown to stimulate β1-dependent tumor cell adhesion to extracellular matrix proteins (Fenczik et al., 1997; Chandrasekaran et al., 1999). Although ubiquitous, CD98 expression is up-regulated on a variety of anchorage-independent cell types, including hematopoietic cells and most human tumor cells (Azzarone et al., 1985). This up-regulation may be important for modulating the changes in integrin affinity and avidity state required to regulate the processes of adhesion and de-adhesion required for cell migration.
The second major finding from this study is that cross-linking CD98 stimulates PI 3-kinase, as well as its product PI(3,4,5)P3, and its downstream effector, PKB. Until now, the mechanism of action of CD98 has been unclear. It has been shown previously that tyrosine kinase activation mediates CD98 induced homotypic aggregation of lymphoid progenitor cells (Ito et al., 1992; Warren et al., 1996). We examined PI 3-kinase as a likely candidate for CD98-mediated intracellular signaling because it can be activated by the majority of receptors with intrinsic or associated tyrosine kinase activity and by receptors linked to heterotrimeric G proteins.
The finding that treatment with 4F2 activated PI 3-kinase, PI(3,4,5)P3, and PKB in a PI 3-kinase-dependent manner suggests that cross-linking CD98 promotes “integrin-like” intracellular signaling. We confirmed that cross-linking of CD98 was necessary, by showing that monoclonal 4F2-Fab fragments, which are unable to cross-link CD98, did not have any effect on kinase activation. However, cross-linking of 4F2-Fab with an anti-Fab antibody restored CD98 signaling capability. Furthermore, preincubation with 4F2-Fab antibody blocked subsequent 4F2 stimulation of PI 3-kinase supporting this hypothesis. Importantly, IgG2A isotype matched control antibody to the transferrin receptor (CD71), which is well expressed on SCLC cells, failed to activate PI 3-kinase or increase PI(3,4,5)P3 levels. Taken together, these results indicate that it is the specific cross-linking of CD98 heavy chain that is required for activation of intracellular signaling. Cross-linking the heavy chain of CD98 had no effect on amino acid transport. In addition, the use of the L-system amino acid transport blocking agent BCH had no effect on 4F2-mediated intracellular signaling, suggesting that CD98 light chain, which has been implicated in amino acid transport, does not play a direct role in this process. This is entirely in keeping with previous published results that have shown that integrin activation by CD98 is independent of its amino acid transport function (Zent et al., 2000; Merlin et al., 2001).
Our results demonstrate that the presence of wild-type β1 integrin is necessary for CD98 signaling. The role of β1 integrins in CD98 signaling was examined. CD98 was transfected into fibroblasts derived from β1 null stem cells (GD25), GD25 cells expressing wild-type β1, or GD25 cells expressing β1 integrin subunits with point mutations of the cytoplasmic domain Y783/Y795F, which impairs FAK tyrosine phosphorylation and activation in response to β1-dependent adhesion compared with wild type. Overexpression of CD98 in the wild-type β1 integrin cells was sufficient to promote PKB and FAK phosphorylation. This was also observed in CD98-transfected CHO cells. Overexpression of CD98 is sufficient to rescue β1 integrin from tac-β1-mediated integrin suppression (Fenczik et al., 1997), and transform NIH3T3 cells (Hara et al., 1999). We propose that this is due to clustering of overexpressed CD98. Further cross-linking of CD98 with 4F2 caused an additional significant increase in both PKB and FAK phosphorylation. The fact that FAK phosphorylation is promoted by cross-linking CD98 in GD25 β1 wild-type cells but not in GD25 cells expressing the β1 Y783/Y795F mutant integrin, suggests that CD98 signaling may be mediated through a β1 integrin/FAK axis. However, it is possible that the β1 Y783/Y795F mutant integrin is defective in other signals in addition to FAK. GD25 cells express other integrins apart from β1 that can activate FAK (e.g., β3 integrins). Overexpressing (and cross-linking) CD98 in the null cells did not stimulate PKB or FAK phosphorylation, suggesting that there is some specificity toward β1 integrins in stimulating signal transduction. Indeed, in vitro binding studies have shown that CD98 heavy chain interacts specifically with the integrin β1A but not with β1D or β7 cytoplasmic domain (Zent et al., 2000). In addition, there may also be α subunit specificity, for example CD98-induces clustering of α3β1 but not α4β1 (Kolesnikova et al., 2001).
We have also shown that cross-linking CD98 promoted anchorage-independent growth. The ability of cells to grow in soft agar is a feature of anchorage independence and pathognomonic of the transformed phenotype, correlating with tumorigenicity and invasiveness of the tumor (Carney et al., 1980). PI 3-kinase acting through PKB has been shown to promote anchorage-independent growth (Moore et al., 1998). There is now increasing evidence that CD98 has oncogenic potential. Hara et al., (1999) showed that human CD98 transfected NIH3T3 cells were capable of anchorage-independent growth and that CD98 transfected clones led to tumor development in athymic mice. Our results showing that cross-linking CD98 promotes anchorage-independent growth supports this idea. It has previously been shown that constitutively active PI 3-kinase can transform chick embryo fibroblasts (Chang et al., 1997) and that a mutant p85 can transform fibroblasts in vitro (Jimenez et al., 1998). Furthermore, integrin-mediated PI 3-kinase activation seems to be important for cell migration and can promote carcinoma invasion (Keely et al., 1997; Renshaw et al., 1997). Constitutive activation of PI 3-kinase as a result of overexpression of CD98 may explain the transformation seen in CD98-transfected NIH3T3 cells.
Full oncogenic transformation is believed to require both serum- and anchorage-independent growth (Schwartz, 1997). SCLC cell growth is promoted by multiple autocrine and paracrine growth loops involving calcium mobilizing neuropeptides (Sethi and Rozengurt, 1991), which activate MAP kinase through G protein-coupled receptors (Seufferlein and Rozengurt, 1996). Recent work suggests that for full activation of the MAP kinase pathway by growth factors an integrin-mediated cosignal is required (Renshaw et al., 1997). The finding that CD98 augments neuropeptide-mediated SCLC colony growth supports this hypothesis. Therefore, we propose that cross-linking CD98 mimics integrin-dependent signal transduction and may facilitate neuropeptide-mediated cell growth.
It now seems that CD98 is a multifunctional molecule: cell fusion regulator, amino acid transporter, comitogen, and now an integrin regulator and an oncogenic protein. In summary, we propose that by cross-linking CD98, it acts as a “molecular facilitator” in the plasma membrane, clustering β1 integrins to form high-density complexes. This results in integrin activation and adhesion, integrin signaling, and anchorage-independent growth. In particular, CD98 has been shown to stimulate β1 integrin-mediated cell adhesion to extracellular matrix (Fenczik et al., 1997). We propose that this may occur as a result of both affinity and avidity changes. CD98 has been shown to reverse tac-β1 suppression, increasing integrin affinity; this may occur as a result of cell signaling and/or conformational changes. In addition, the clustering of integrins by CD98 will increase binding avidity. Further understanding of the mechanism of action of CD98 may facilitate our knowledge of the mechanisms that regulate cell adhesion, migration, and metastasis. Furthermore, this information may provide a paradigm for events involved in such diverse processes as inflammation and viral-induced cell fusion.
ACKNOWLEDGMENTS
We gratefully acknowledge Linda Sharp (University of Edinburgh Medical School confocal facility) for expert technical assistance and Judith Gordon (University of Edinburgh Medical School) for secretarial support. This work was supported by the Medical Research Council, UK (Clinical Training Fellowships to R.C.R. and R.C.B.) and the Wellcome Trust.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–11–0530. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–11–0530.
REFERENCES
- Azzarone B, Malpiece Y, Zaech P, Moretta L, Fauci A, Suarez H. Analysis of the expression of the 4F2 surface antigen in normal and neoplastic fibroblastic human cells of embryonic and adult origin. Exp Cell Res. 1985;159:451–462. doi: 10.1016/s0014-4827(85)80018-5. [DOI] [PubMed] [Google Scholar]
- Bellacosa A, Testa JR, Staal SP, Tsichlis PN. A retroviral oncogene, Akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991;254:274–277. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- Bellone G, Alloatti G, Levi R, Geuna M, Tetta C, Peruzzi L, Letarte M, Malavasi F. Identification of a new epitope of the 4F2/44D7 molecular complex present on sarcolemma and isolated cardiac fibers. Eur J Immunol. 1989;19:1–8. doi: 10.1002/eji.1830190102. [DOI] [PubMed] [Google Scholar]
- Carney DN, Gazdar AF, Minna JD. Positive correlation between histological tumor involvement and generation of tumor cell colonies in agarose in specimens taken directly from patients with small-cell carcinoma of the lung. Cancer Res. 1980;40:1820–1823. [PubMed] [Google Scholar]
- Carpenter CL, Cantley LC. Phosphoinositide kinases. Curr Opin Cell Biol. 1996;8:153–158. doi: 10.1016/s0955-0674(96)80060-3. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran S, Guo NH, Rodrigues RG, Kaiser J, Roberts DD. Proadhesive and chemotactic activities of thrombospondin-1 for breast carcinoma cells are mediated by α3β1 integrin and regulated by insulin-like growth factor-1 and CD98. J Biol Chem. 1999;274:11408–11416. doi: 10.1074/jbc.274.16.11408. [DOI] [PubMed] [Google Scholar]
- Chang HW, Aoki M, Fruman D, Auger KR, Bellacosa A, Tsichlis PN, Cantley LC, Roberts TM, Vogt PK. Transformation of chicken cells by the gene encoding the catalytic subunit of PI 3-kinase. Science. 1997;276:1848–1850. doi: 10.1126/science.276.5320.1848. [DOI] [PubMed] [Google Scholar]
- Cho JY, Fox DA, Horejsi V, Sagawa K, Skubitz KM, Katz DR, Chain B. The functional interactions between CD98, β-1 integrins and Cd147 in the induction of U937 homotypic aggregation. Blood. 2001;98:374–382. doi: 10.1182/blood.v98.2.374. [DOI] [PubMed] [Google Scholar]
- Dixon WT, Sikora LK, Demetrick DJ, Jerry LM. Isolation and characterization of a heterodimeric surface antigen on human melanoma cells and evidence that it is the 4F2 cell activation/proliferation molecule. Int J Cancer. 1990;45:59–68. doi: 10.1002/ijc.2910450113. [DOI] [PubMed] [Google Scholar]
- Fassler R, Pfaff M, Murphy J, Noegel AA, Johansson S, Timpl R, Albrecht R. Lack of β1 integrin gene in embryonic stem cells affects morphology, adhesion, and migration but not integration into the inner cell mass of blastocysts. J Cell Biol. 1995;128:979–988. doi: 10.1083/jcb.128.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenczik CA, Sethi T, Ramos JW, Hughes PE, Ginsberg MH. Complementation of dominant suppression implicates CD98 in integrin activation. Nature. 1997;390:81–85. doi: 10.1038/36349. [DOI] [PubMed] [Google Scholar]
- Freidman AW, Diaz LA, Jr, Moore S, Schaller J, Fox DA. The human 4F2 antigen: evidence for cryptic and noncryptic epitopes and for a role of 4F2 in human T lymphocyte activation. Cell Immunol. 1994;154:253–263. doi: 10.1006/cimm.1994.1075. [DOI] [PubMed] [Google Scholar]
- Hara K, Kudoh H, Enomoto T, Hashimoto Y, Masuko T. Malignant transformation of NIH3T3 cells by overexpression of early lymphocyte activation antigen CD98. Biochem Biophys Res Commun. 1999;262:720–725. doi: 10.1006/bbrc.1999.1051. [DOI] [PubMed] [Google Scholar]
- Haynes BF, Hemler ME, Mann D, Eisenbarth G, Shelhamer J, Mostowski HS, Thomas CA, Strominger JL, Fauci A. Characterization of a monoclonal antibody (4F2) that binds to human monocytes and to a subset of activated lymphocytes. J Immunol. 1981;126:1409–1414. [PubMed] [Google Scholar]
- Ito Y, Komada H, Kusagawa S, Tsurudome M, Matsumura H, Kawano M, Ohta H, Nishio M. Fusion regulation proteins on the cell surface: isolation and characterization of monoclonal antibodies which enhance giant polykaryocyte formation in Newcastle disease virus-infected cell lines of human origin. J Virol. 1992;66:5999–6007. doi: 10.1128/jvi.66.10.5999-6007.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez C, et al. Identification and characterization of a new oncogene derived from the regulatory subunit of phosphoinositide 3-kinase. EMBO J. 1998;17:743–753. doi: 10.1093/emboj/17.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98) J Biol Chem. 1998;273:23629–23632. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- Keely PJ, Westwick JK, Whitehead IP, Der CJ, Parise LV. Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature. 1997;390:632–636. doi: 10.1038/37656. [DOI] [PubMed] [Google Scholar]
- King WG, Mattaliano MD, Chan TO, Tsichlis PN, Brugge JS. Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/mitogen-activated protein kinase pathway activation. Mol Cell Biol. 1997;17:4406–4418. doi: 10.1128/mcb.17.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne PH, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnikova TV, Mannion B, Berditchevski F, Hemler ME. β1 integrins show specific association with CD98 protein in low density membranes. BMC Biochem. 2001;2:10. doi: 10.1186/1471-2091-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroberardino L, Spindler B, Pfeiffer R, Skelly PJ, Loffing J, Shoemaker CB, Verrey F. Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature. 1998;395:288–291. doi: 10.1038/26246. [DOI] [PubMed] [Google Scholar]
- Merlin D, Sitaraman S, Liu X, Eastburn K, Sun J, Kucharzik T, Lewis B, Madara JL. CD98-mediated links between amino acid transport and β-1 integrin distribution in polarized columnar epithelia. J Biol Chem. 2001;276:39282–39289. doi: 10.1074/jbc.M105077200. [DOI] [PubMed] [Google Scholar]
- Michalak M, Quackenbush EJ, Letarte M. Inhibition of Na+/Ca2+ exchanger activity in cardiac and skeletal muscle sarcolemmal vesicles by monoclonal antibody 44D7. J Biol Chem. 1986;261:92–95. [PubMed] [Google Scholar]
- Moore SM, Rintoul RC, Walker TR, Chilvers ER, Haslett C, Sethi T. The presence of a constitutively active phosphoinositide 3-kinase in small cell lung cancer cells mediates anchorage-independent proliferation via a protein kinase B and p70s6k-dependent pathway. Cancer Res. 1998;58:5239–5247. [PubMed] [Google Scholar]
- Nakamura E, Sato M, Yang H, Miyagawa F, Harasaki M, Tomita K, Matsuoka S, Noma A, Iwai K, Minato N. 4F2 (CD98) heavy chain is associated covalently with an amino acid transporter and controls intracellular trafficking and membrane topology of 4F2 heterodimer. J Biol Chem. 1999;274:3009–3016. doi: 10.1074/jbc.274.5.3009. [DOI] [PubMed] [Google Scholar]
- Ohgimoto S, Tabata N, Suga S, Nishio M, Ohta H, Tsurudome M, Komada H, Kawano M, Watanabe Y. Molecular characterization of fusion regulatory protein-1 (FRP-1) that induces multinucleate formation of monocytes and HIV gp160-mediated cell fusion. FRP-1 and 4F2/CD98 are identical. J Immunol. 1995;155:3585–3592. [PubMed] [Google Scholar]
- Okamoto K, Ohgimoto S, Nishio M, Tsurudome M, Kawano M, Komada H, Ito M, Sakakura Y, Ito Y. Paramyxovirus-induced syncytium cell formation is suppressed by a dominant negative fusion regulatory protein-1 (FRP-1)/CD98 mutated construct: an important role of FRP-1 in virus-induced cell fusion. J Gen Virol. 1997;78:775–783. doi: 10.1099/0022-1317-78-4-775. [DOI] [PubMed] [Google Scholar]
- Parmacek MS, Karpinski BA, Gottesdiener KM, Thompson CB, Leiden JM. Structure, expression and regulation of the murine 4F2 heavy chain. Nucleic Acids Res. 1989;17:1915–1931. doi: 10.1093/nar/17.5.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posillico JT, Srikanta S, Eisenbarth G, Quaranta V, Kajiji S, Brown EM. Binding of monoclonal antibody (4F2) to its cell surface antigen on dispersed adenomatous parathyroid cells raises cytosolic calcium and inhibits parathyroid hormone secretion. J Clin Endocrinol Metab. 1987;64:43–50. doi: 10.1210/jcem-64-1-43. [DOI] [PubMed] [Google Scholar]
- Quackenbush E, Clabby M, Gottesdiener KM, Barbosa J, Jones NH, Strominger JL, Speck S, Leiden JM. Molecular cloning of complementary DNAs encoding the heavy chain of the human 4F2 cell-surface antigen: a type II membrane glycoprotein involved in normal and neoplastic cell growth. [published erratum appears in Proc. Natl. Acad. Sci. USA (1987) 84, 8618] Proc Natl Acad Sci USA. 1987;84:6526–6530. doi: 10.1073/pnas.84.18.6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw MW, Ren XD, Schwartz MA. Growth factor activation of MAP kinase requires cell adhesion. EMBO J. 1997;16:5592–5599. doi: 10.1093/emboj/16.18.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Zhang Q, Fassler R, Mosher D. Modulation of β1A integrin functions by tyrosine residues in the β1 cytoplasmic domain. J Cell Biol. 1998;141:527–538. doi: 10.1083/jcb.141.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA. Integrins, oncogenes, and anchorage independence. J Cell Biol. 1997;139:575–578. doi: 10.1083/jcb.139.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi T, Rozengurt E. Multiple neuropeptides stimulate clonal growth of small cell lung cancer: effects of bradykinin, vasopressin, cholecystokinin, galanin, and neurotensin. Cancer Res. 1991;51:3621–3623. [PubMed] [Google Scholar]
- Seufferlein T, Rozengurt E. Galanin, neurotensin, and phorbol esters rapidly stimulate activation of mitogen-activated protein kinase in small cell lung cancer cells. Cancer Res. 1996;56:5758–5764. [PubMed] [Google Scholar]
- Suomalainen HA. The monoclonal antibodies Trop-4 and 4F2 detect the same membrane antigen that is expressed at an early stage of lymphocyte activation and is retained on secondary lymphocytes. J Immunol. 1986;137:422–427. [PubMed] [Google Scholar]
- Tabata N, et al. Protein tyrosine kinase activation provides an early and obligatory signal in anti-FRP-1/CD98/4F2 monoclonal antibody induced cell fusion mediated by HIV gp160. Med Microbiol Immunol. 1997;186:115–123. doi: 10.1007/s004300050053. [DOI] [PubMed] [Google Scholar]
- Teixeira S, Di Grandi S, Kuhn LC. Primary structure of the human 4F2 antigen heavy chain predicts a transmembrane protein with a cytoplasmic NH2 terminus. J Biol Chem. 1987;262:9574–9580. [PubMed] [Google Scholar]
- van der Kaay J, Cullen PJ, Downes CP. Phosphatidylinositol (3,4,5) trisphosphate mass measurement using a radioligand displacement assay. In: Bird IM, editor. Phospholipid Signaling Protocols. Totowa, NJ: Humana Press; 1999. pp. 109–125. [DOI] [PubMed] [Google Scholar]
- Verrey F, Jack DL, Paulsen IT, Saier MH, Pfeiffer R. New glycoprotein-associated amino acid transporters. J Membr Biol. 1999;172:181–192. doi: 10.1007/s002329900595. [DOI] [PubMed] [Google Scholar]
- Warren AP, Patel K, McConkey DJ, Palacios R. CD98: a type II transmembrane glycoprotein expressed from the beginning of primitive and definitive hematopoiesis may play a critical role in the development of hematopoietic cells. Blood. 1996;87:3676–3687. [PubMed] [Google Scholar]
- Warren AP, Patel K, Miyamoto Y, Wygant JN, Woodside DG, McIntyre BW. Convergence between CD98 and integrin-mediated T-lymphocyte co-stimulation. Immunology. 2000;99:62–68. doi: 10.1046/j.1365-2567.2000.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennerberg K, Armulik A, Sakai T, Karlsson M, Fassler R, Schaefer EM, Mosher D, Johansson S. The cytoplasmic tyrosines of integrin subunit β1 are involved in focal adhesion kinase activation. Mol Cell Biol. 2000;20:5758–5765. doi: 10.1128/mcb.20.15.5758-5765.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zent R, Fenczik CA, Calderwood DA, Liu S, Dellos M, Ginsberg MH. Class and splice variant-specific association of CD98 with integrin β cytoplasmic domains. J Biol Chem. 2000;275:5059–5064. doi: 10.1074/jbc.275.7.5059. [DOI] [PubMed] [Google Scholar]