Abstract
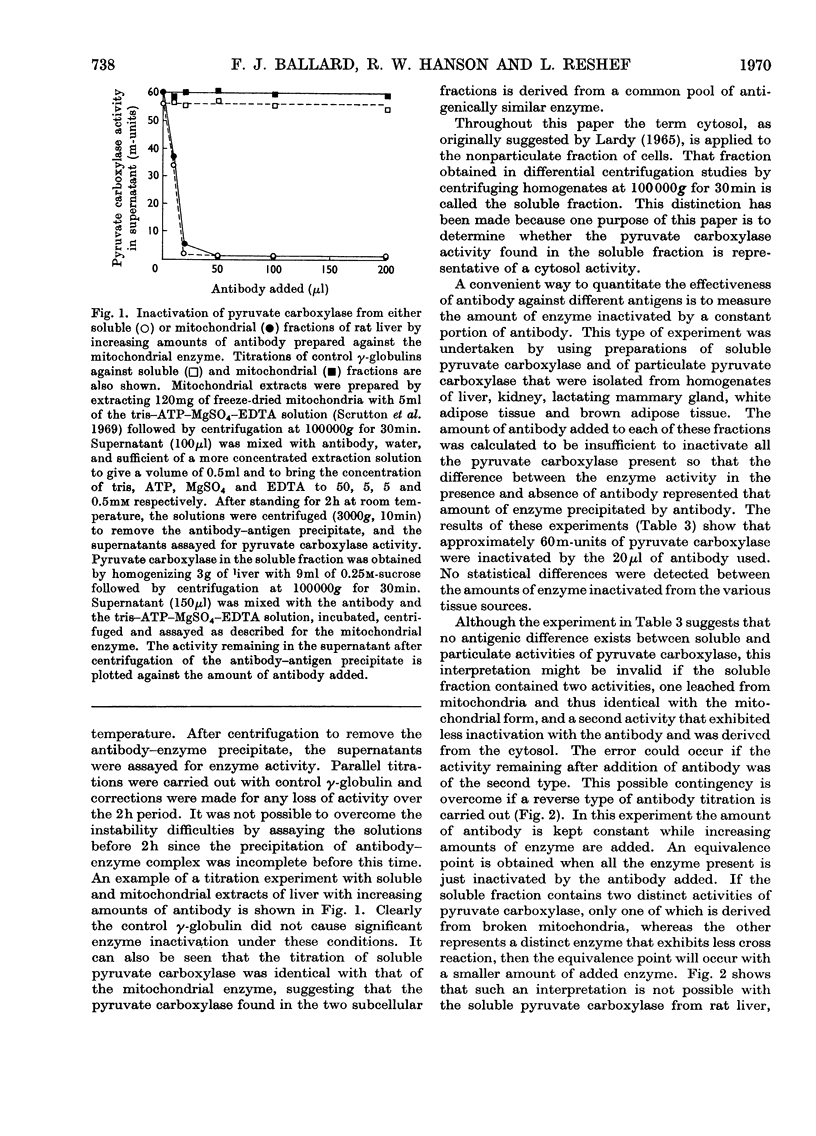
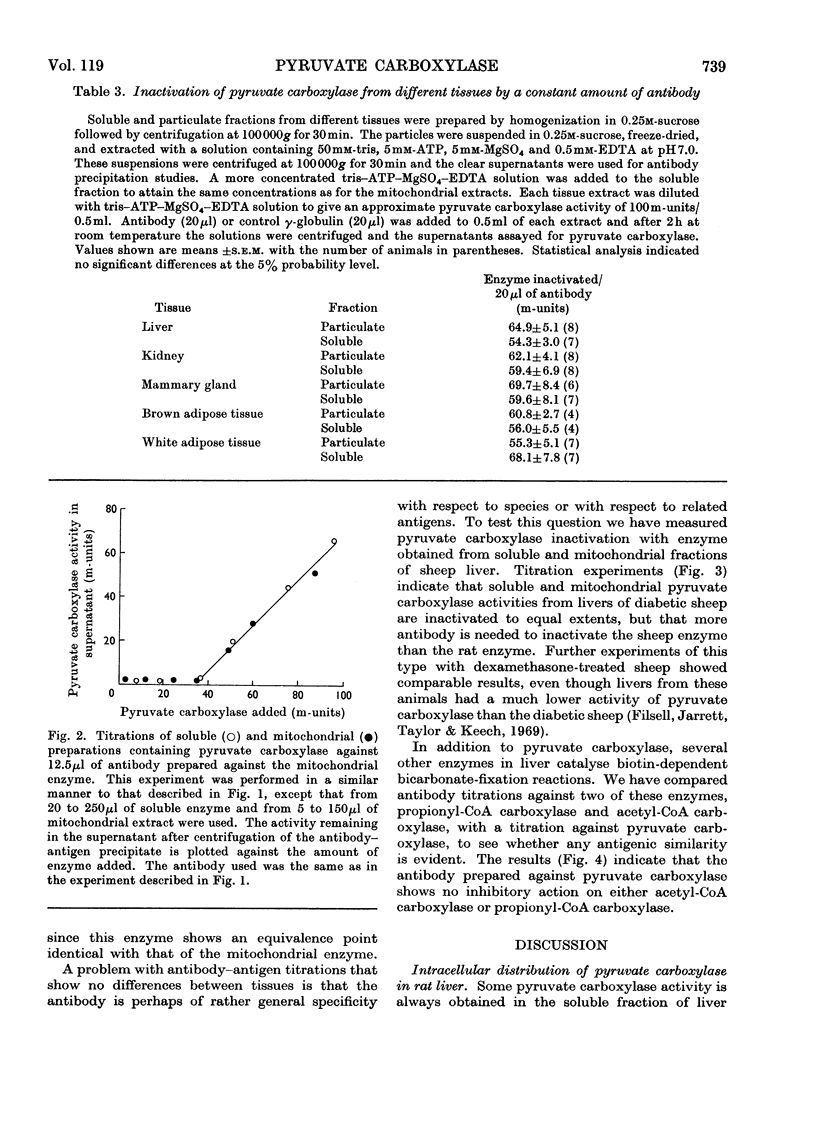
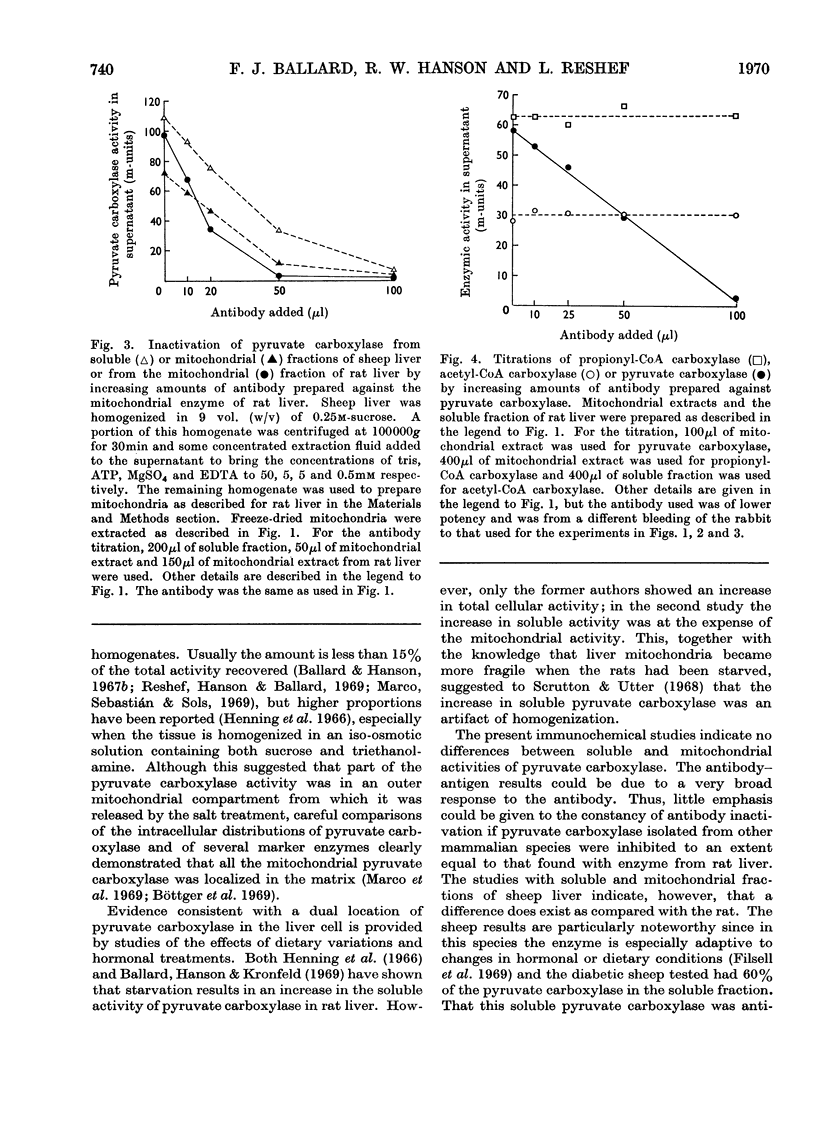
1. Pyruvate carboxylase (EC 6.4.1.1), purified from rat liver mitochondria to a specific activity of 14 units/mg, was used for the preparation of antibodies in rabbits. 2. Tissue distribution studies showed that pyruvate carboxylase was present in all rat tissues that were tested, with considerable activities both in gluconeogenic tissues such as liver and kidney and in tissues with high rates of lipogenesis such as white adipose tissue, brown adipose tissue, adrenal gland and lactating mammary gland. 3. Immunochemical titration experiments with the specific antibodies showed no differences between the inactivation of pyruvate carboxylase from mitochondrial or soluble fractions of liver, kidney, mammary gland, brown adipose tissue or white adipose tissue. 4. The antibodies were relatively less effective in reactions against pyruvate carboxylase from sheep liver than against the enzyme from rat tissues. 5. Pyruvate carboxylase antibodies did not inactivate either propionyl-CoA carboxylase or acetyl-CoA carboxylase from rat liver. 6. It is concluded that pyruvate carboxylase in lipogenic tissues is similar antigenically to the enzyme in gluconeogenic tissues and that the soluble activities of pyruvate carboxylase detected in many rat tissues do not represent discrete enzymes but are the result of mitochondrial damage during tissue homogenization.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballard F. J., Hanson R. W., Kronfeld D. S. Gluconeogenesis and lipogenesis in tissue from ruminant and nonruminant animals. Fed Proc. 1969 Jan-Feb;28(1):218–231. [PubMed] [Google Scholar]
- Ballard F. J., Hanson R. W. Phosphoenolpyruvate carboxykinase and pyruvate carboxylase in developing rat liver. Biochem J. 1967 Sep;104(3):866–871. doi: 10.1042/bj1040866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard F. J., Hanson R. W. Purification of phosphoenolpyruvate carboxykinase from the cytosol fraction of rat liver and the immunochemical demonstration of differences between this enzyme and the mitochondrial phosphoenolpyruvate carboxykinase. J Biol Chem. 1969 Oct 25;244(20):5625–5630. [PubMed] [Google Scholar]
- Böttger I., Wieland O., Brdiczka D., Pette D. Intracellular localization of pyruvate carboxylase and phosphoenolpyruvate carboxykinase in rat liver. Eur J Biochem. 1969 Mar;8(1):113–119. doi: 10.1111/j.1432-1033.1969.tb00503.x. [DOI] [PubMed] [Google Scholar]
- FLATT J. P., BALL E. G. STUDIES ON THE METABOLISM OF ADIPOSE TISSUE. XV. AN EVALUATION OF THE MAJOR PATHWAYS OF GLUCOSE CATABOLISM AS INFLUENCED BY INSULIN AND EPINEPHRINE. J Biol Chem. 1964 Mar;239:675–685. [PubMed] [Google Scholar]
- GRIMM F. C., DOHERTY D. G. Properties of the two forms of malic dehydrogenase from beef heart. J Biol Chem. 1961 Jul;236:1980–1985. [PubMed] [Google Scholar]
- Gevers W. The regulation of phosphoenolpyruvate synthesis in pigeon liver. Biochem J. 1967 Apr;103(1):141–152. doi: 10.1042/bj1030141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gul B., Dils R. Pyruvate carboxylase in lactating rat and rabbit mammary gland. Biochem J. 1969 Feb;111(3):263–271. doi: 10.1042/bj1110263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson R. W., Ballard F. J. The relative significance of acetate and glucose as precursors for lipid synthesis in liver and adipose tissue from ruminants. Biochem J. 1967 Nov;105(2):529–536. doi: 10.1042/bj1050529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning H. V., Stumpf B., Ohly B., Seubert W. On the mechanism of gluconeogenesis and its regulation. 3. The glucogenic capacity and the activities of pyruvate carboxylase and PEP-carboxylase of rat kidney and rat liver after cortisol treatment and starvation. Biochem Z. 1966 Apr 27;344(3):274–288. [PubMed] [Google Scholar]
- KEECH D. B., UTTER M. F. PYRUVATE CARBOXYLASE. II. PROPERTIES. J Biol Chem. 1963 Aug;238:2609–2614. [PubMed] [Google Scholar]
- Katz J., Landau B. R., Bartsch G. E. The pentose cycle, triose phosphate isomerization, and lipogenesis in rat adipose tissue. J Biol Chem. 1966 Feb 10;241(3):727–740. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ling A. M., Keech D. B. Pyruvate carboxylase from sheep kidney. I. Purification and some properties of the enzyme. Enzymologia. 1966 Jun 30;30(6):367–380. [PubMed] [Google Scholar]
- Marco R., Sebastián J., Sols A. Location of the enzymes of the oxalacetate metabolic cross-roads in rat liver mitochondria. Biochem Biophys Res Commun. 1969 Mar 10;34(5):725–730. doi: 10.1016/0006-291x(69)90799-2. [DOI] [PubMed] [Google Scholar]
- NORDLIE R. C., LARDY H. A. Mammalian liver phosphoneolpyruvate carboxykinase activities. J Biol Chem. 1963 Jul;238:2259–2263. [PubMed] [Google Scholar]
- NORDLIE R. C., VARRICCHIO F. E., HOLTEN D. D. EFFECTS OF ALTERED HORMONAL STATES AND FASTING ON RAT-LIVER MITOCHONDRIAL PHOSPHOENOLOPYRUVATE CARBOXYKINASE LEVELS. Biochim Biophys Acta. 1965 Feb 15;97:214–221. doi: 10.1016/0304-4165(65)90085-1. [DOI] [PubMed] [Google Scholar]
- Scrutton M. C., Utter M. F. Pyruvate carboxylase. IX. Some properties of the activation by certain acyl derivatives of coenzyme A. J Biol Chem. 1967 Apr 25;242(8):1723–1735. [PubMed] [Google Scholar]
- UTTER M. F., KEECH D. B. PYRUVATE CARBOXYLASE. I. NATURE OF THE REACTION. J Biol Chem. 1963 Aug;238:2603–2608. [PubMed] [Google Scholar]


