Abstract
Liquid storage is the primary preservation method in the swine breeding industry because of its advantages over cryopreservation. Calcium (Ca2+), a key regulator of cell physiology, plays a crucial role during liquid preservation. Sarcoplasmic/Endoplasmic Reticulum Ca2+ ATPases (SERCA) belong to a family of P-type ATPases that regulate Ca2+ homeostasis within cells and have been previously described to play a function in the sperm of various mammalian species. Herein, we hypothesized that SERCA2 is present in pig sperm and is involved in the resilience of this cell to liquid preservation at 17 °C. For this purpose, sperm were incubated with different concentrations of thapsigargin (Thg; 0, 5, 25, and 50 µM) and stored at 17 °C for ten days. The presence and localization of SERCA2 were evaluated using immunoblotting and immunofluorescence, respectively. On days 0, 4, and 10, sperm motility was assessed using a computer-assisted sperm analysis (CASA) system, and sperm viability, membrane lipid disorder, acrosome integrity, mitochondrial membrane potential (MMP), and intracellular levels of Ca2+, superoxides and total reactive oxygen species (ROS) were evaluated by flow cytometry. We localized SERCA2 in the acrosome and midpiece of pig sperm. Furthermore, inhibition of SERCA with Thg resulted in reduced sperm viability and membrane stability, and increased MMP, and Ca2+ and ROS levels. In conclusion, the activity of SERCA prevents the accumulation of intracellular Ca2+ in sperm, which is detrimental to sperm quality and function during liquid storage at 17 °C. We thus suggest that the function of SERCA is crucial for the preservation of pig semen.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-88012-5.
Keywords: Semen, Pig, Liquid storage, SERCA, Thapsigargin, Calcium
Subject terms: Animal biotechnology, Reproductive biology
Introduction
Sperm preservation before artificial insemination (AI) is a critical step in the swine breeding industry, as it is essential for maintaining the function and survival of the male gamete1. Although two main preservation methods - cryopreservation and liquid storage - are available, 99% of AI in swine utilize liquid-stored semen2. This preference for liquid sperm storage is due to its ability to maintain sperm quality and fertilizing ability better than cryopreservation, which is known to impair their functionality and reduce their reproductive performance3. The main limitation of liquid storage, however, is the restricted period over which sperm can be preserved. As pig sperm are susceptible to low temperatures4, storing is typically carried out at temperatures between 15 and 20 °C5. At this range, nevertheless, the extent to which sperm metabolism can be reduced is smaller than at lower temperatures like 4–5 °C, making the preservation of these cells more difficult.
Calcium (Ca2+) has consistently been shown to be crucial for sperm physiology6–9. Intracellular Ca2+ levels and Ca2+ channels have been reported to play a critical role in regulating mammalian sperm motility10,11. Moreover, not only is Ca2+ highly relevant for the induction and regulation of sperm capacitation and acrosome reaction in mammals12–15, but also for the activation of the oocyte upon fertilization16,17. Regarding sperm preservation, Ca2+ has been particularly related to the maintenance of sperm over liquid storage. Indeed, previous studies associated high intracellular Ca2+ levels with a decrease in sperm viability during preservation of pig sperm at 15–20 °C18,19. Besides, higher Ca2+ levels in pig sperm are correlated to a reduction in the litter size, notwithstanding the molecular mechanisms underlying this relationship need to be investigated further20.
Considering the vital role of Ca2+ in sperm physiology, the study of proteins that regulate Ca2+ in sperm is of high interest. Sarcoplasmic/Endoplasmic Reticulum Ca2+-ATPases (SERCA) is a family of Ca2+ pumps that, in somatic cells, transport Ca2+ from the cytosol to the endoplasmic reticulum21. Three genes encode this protein family: SERCA1, SERCA2 and SERCA3, with the second one being expressed in most tissues. Although mature sperm do not have endoplasmic reticulum, the presence of SERCA2 was described in human, bovine and mouse sperm22, particularly in the acrosome and midpiece. Yet, and to the best of our knowledge, its presence in pig sperm has not been interrogated.
Thapsigargin (Thg), a drug compound derived from the plant Thapsia garganica, is a well-known, specific inhibitor of SERCA family isoforms23. Incubation of cancer cells with Thg was found to induce cell death through the depletion of endoplasmic reticulum Ca2+ stores24. Regarding its effect on sperm, Thg was observed to boost intracellular Ca2+ in humans, which was seen to result from the depletion of internal Ca2+ stores and the opening of Ca2+ channels of the plasma membrane. This was reported to lead to an increase in the occurrence of the acrosome reaction and a decrease in sperm motility25–27. In pigs, Thg was established to increase intracellular Ca2+, especially in fresh compared to cryopreserved sperm28. Furthermore, incubation with Thg was seen to induce head-to-head agglutination, which would suggest that SERCA activity is involved in capacitation-like changes mediated by Ca2+29. Despite this, no previous study has investigated the presence of SERCA in pig sperm, nor has it evaluated how inhibiting SERCA through Thg affects their resilience to liquid storage.
In light of the aforementioned, two objectives were set in the present work: (1) to determine the presence and localization of SERCA2 in pig sperm; and (2) to elucidate the role of SERCA, by their inhibition with Thg, in the maintenance of sperm quality and functionality during liquid storage at 17 °C. We hypothesize that SERCA are present in pig sperm and are involved in keeping them in good shape over preservation at 17 °C.
Results
SERCA2 is present in pig sperm and localizes at the acrosome and the midpiece
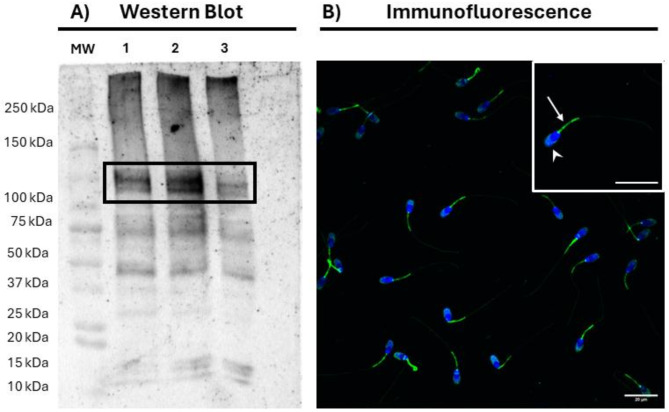
Immunoblotting with the anti-SERCA2 antibody revealed three predominant bands of 50, 75 and 110 kDa (Fig. 1A). The specificity of the SERCA2-bands was confirmed through a competition assay with the recombinant protein, and a negative control lacking the primary antibody (Supplementary Fig. S1). Neither in the competition assay with the recombinant protein nor in the negative control were the 75 kDa and 110 kDa bands present, whereas that of 50 kDa was observed in the two controls.
Fig. 1.
Presence and localization of SERCA2 in pig sperm. (a) Representative immunoblotting of SERCA2 in pig sperm after incubation with the anti-SERCA2 antibody. MW: Molecular Weight; 1–3 correspond to different samples. The black box indicates the SERCA2 band. (b) Representative immunofluorescence analysis of SERCA2 in pig sperm. Sperm nuclei are shown in blue (DAPI), whereas SERCA2 is shown in green. The white arrow shows the localization of SERCA2 in the midpiece, whereas arrowheads show its presence in the acrosome. Scale bars: 20 μm.
Immunofluorescence analysis revealed that, in pig sperm, SERCA2 is primarily localized in the acrosome and midpiece (Fig. 1B). Besides, weaker SERCA2 expression was observed in the post-acrosomal region of some sperm cells. No SERCA2 signal was detected in the competition assay with the recombinant protein or the negative control (Supplementary Fig. S2).
Inhibition of SERCA compromises plasma membrane and acrosome integrity during liquid storage of pig sperm
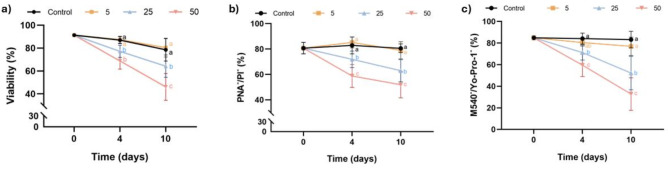
The effects of inhibiting SERCA with Thg during liquid preservation at 17 °C of pig semen are shown in Fig. 2 and Supplementary File 2. Regarding sperm viability, and as Fig. 2a shows, the blockage of SERCA with 25 µM and 50 µM Thg led to a decrease in the percentage of viable sperm (P < 0.05; SYBR-14+/PI−) after 4 and 10 days of storage at 17 °C. Concerning acrosome integrity (Fig. 2b), inhibiting SERCA with 25 µM and 50 µM Thg reduced (P < 0.05) the percentage of viable sperm with an intact acrosome (PNA−/PI−) after 4 and 10 days of storage. Similar results were observed in the case of lipid membrane stability, as the inhibition of SERCA activity with Thg (25 µM and 50 µM) decreased the percentage of viable sperm (P < 0.05) with high lipid membrane stability (M540−/Yo-Pro-1−) after 4 and 10 days of storage (Fig. 2c). Noticeably, in the three variables, the effects were similar and dose-dependent, with no change at 5 µM and the most significant impact at 50 µM.
Fig. 2.
Effect of inhibiting SERCA activity with Thg on the plasma membrane and acrosome integrity, and lipid membrane stability. (a) Viable sperm (SYBR-14+/PI−, %); (b) viable sperm with an intact acrosome (PNA−/PI−, %); and (c) viable sperm with high lipid membrane stability (M540−/Yo-Pro-1−, %) after 0, 4 and 10 days of liquid storage at 17 °C in the presence (5, 25 and 50 µM) or absence (0 µM; control) of Thg. Different letters (a-c) indicate significant differences between experimental groups at a given time point. Results are expressed as the mean ± SEM (n = 7).
Inhibition of SERCA with Thg increases ROS levels in liquid-stored sperm
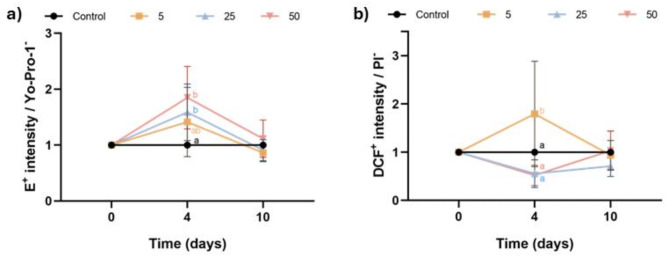
Intracellular levels of superoxides and total ROS were assessed using a double-staining with HE/Yo-Pro-1 and H2DCFDA/PI, respectively. Figure 3a shows the E+ intensity in viable sperm, normalized to the control. Incubation with 25 µM and 50 µM Thg significantly increased intracellular superoxide levels after 4 days of storage (P < 0.05); these differences were not observed at day 10. Regarding total ROS levels, Fig. 3b depicts the DCF+ fluorescence intensity, normalized to the control. Inhibiting SERCA with 5 µM Thg, but not with higher concentrations of this molecule (25 µM or 50 µM), increased the levels of total ROS after 4 days of storage (P < 0.05). This effect, however, was not observed after 10 days of storage.
Fig. 3.
Effect of inhibiting SERCA activity with Thg on reactive oxygen species (ROS) levels. (a) E+ intensity in viable sperm (Yo-Pro-1−); and (b) DCF+ intensity in viable sperm (PI−) after 0, 4 and 10 days of liquid storage at 17 °C in the presence (5, 25 and 50 µM) or absence (0 µM; control) of Thg. Different letters (a-c) indicate significant differences between experimental groups at a given time point. Results are expressed as the mean ± SEM (n = 7).
Inhibition of SERCA with thapsigragin increases mitochondrial activity and Ca2+ levels in liquid-stored sperm
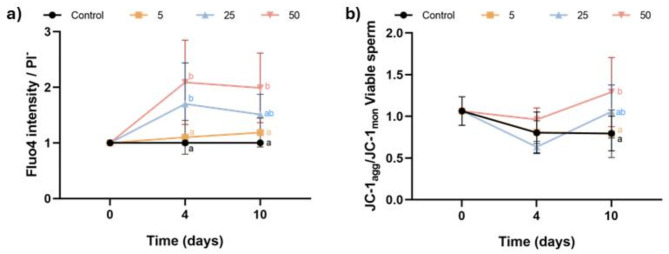
Intracellular Ca2+ levels were measured by the intensity of Fluo4 in viable sperm, normalized to the control (Fig. 4a). Incubation with 25 µM and 50 µM Thg resulted in a significant increase in intracellular Ca2+ levels at day 4, with only the highest concentration maintaining this effect at day 10 (P < 0.05). On the other hand, mitochondrial membrane potential was assessed using a double staining protocol with JC-1 and LD. The results, shown in Fig. 4b, were expressed as the ratio between JC-1agg and JC-1mon. Incubation of pig sperm with the highest concentration of Thg (50 µM) significantly raised the mitochondrial membrane potential at both days 4 and 10 (P < 0.05), whereas the other two concentrations (5 µM and 25 µM) had no effect (P > 0.05).
Fig. 4.
Effect of inhibiting SERCA activity with Thg on intracellular calcium (Ca2+) and mitochondrial membrane potential (MMP). (a) Fluo4 intensity in viable sperm (PI−); and b) ratio between JC-1agg and JC-1mon in viable sperm (LD−) after 0, 4 and 10 days of liquid storage at 17 °C in the presence (5, 25 and 50 µM) or absence (0 µM; control) of Thg. Different letters (a-c) indicate significant differences between experimental groups at a given time point. Results are expressed as the mean ± SEM (n = 7).
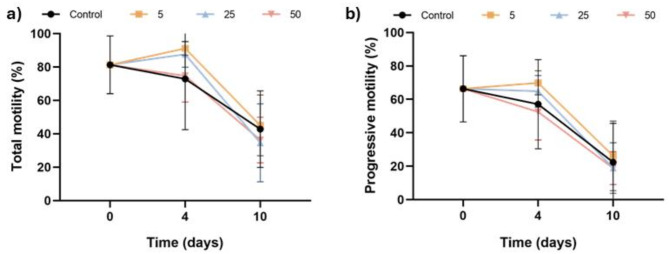
Sperm motility and kinematics are not affected by SERCA inhibition during liquid storage of pig sperm
Figure 5 illustrates the percentages of total motile sperm (Fig. 5a) and progressively motile sperm (Fig. 5b). Incubation with Thg did not affect these percentages at any of the time points and concentrations assessed (P > 0.05). Furthermore, Table 1 displays the impact of inhibiting SERCA on the kinematic parameters of pig sperm during liquid storage at 17 °C. No significant differences were observed between experimental groups for any of the analyzed parameters (P > 0.05).
Fig. 5.
Effect of inhibiting SERCA activity with Thg on sperm motility. (a) Percentages of total motile sperm; and (b) percentages of progressively motile sperm after 0, 4 and 10 days of liquid storage at 17 °C in the presence (5, 25 and 50 µM) or absence (0 µM; control) of Thg. No significant differences between groups were observed Results are expressed as the mean ± SEM (n = 7).
Discussion
In pigs, semen preservation ahead of AI primarily relies on liquid storage due to the limitations of cryopreservation5. Ca2+ is a well-known regulator of sperm physiology, and its involvement in keeping sperm in good shape during liquid storage has been previously documented8,18,19. Consequently, studying the molecular mechanisms regulating Ca2+ homeostasis in pig sperm is particularly interesting. Related to this, SERCA are a family of pumps that mediate the intracellular Ca2+ transport21. While the presence of SERCA in the sperm of various mammalian species has been investigated, their localization and role in pig sperm have not been explored, despite their potential relevance for liquid preservation22. The present study, therefore, sought to determine the presence and localization of SERCA2 in pig sperm as well as elucidate the role of these Ca2+ pumps in maintaining their function and survival during liquid storage at 17 °C.
We first assessed the presence of SERCA2 in pig sperm through immunoblotting. Two SERCA2-specific bands of ~75 and ~110 kDa were observed in the blots. Besides, a ~50-kDa band was observed in the same blots, but it was determined to be nonspecific to SERCA2, as it also appeared in both the competition assay with the recombinant SERCA2 and the negative control without the primary antibody. It is worth noting that the ~110-kDa band coincides with the molecular weight of SERCA2, which is known to be between 110 and 115 kDa30. In contrast, the ~75-kDa band, though specific to SERCA2, has not been previously reported and could be an alternative isoform resulting from splicing events. Further studies are, however, necessary to confirm this suggestion. Remarkably, the presence of a 110-kDa band in porcine sperm aligns with previous studies that identified SERCA2 in human, bovine and murine sperm22. Taken together, these results suggest that SERCA2 is present and conserved across the sperm of different mammalian species.
Immunofluorescence analysis was carried out to localize SERCA2 in pig sperm, revealing that this protein resides in the acrosome and midpiece of pig sperm. This localization pattern agrees with previous findings in other mammalian species. In human sperm, SERCA2 exhibits a similar localization pattern as that observed in porcine sperm in the present study22. Conversely, in bovine and murine sperm, SERCA2 is localized in the acrosome but not in the midpiece22. These findings again suggest a conserved localization pattern for SERCA2 across mammalian sperm.
After confirming the presence and localizing SERCA2 in porcine sperm, we aimed to determine the role of SERCA in the maintenance of sperm quality during liquid storage at 17 °C; for this purpose, SERCA were specifically inhibited with Thg. One of the most notable effects of inhibiting SERCA during liquid preservation of pig sperm was the impairment of plasma and acrosome integrity, suggesting that the activity of these pumps is essential for maintaining sperm viability and keeping the acrosome intact over storage at 17 °C. Thapsigargin has been described to induce cell death through depleting Ca2+ in the endoplasmic reticulum of other cell types24,31. While mature sperm lack the endoplasmic reticulum, the negative impact of Thg on their viability has also been reported in human sperm27, likely due to increased intracellular Ca2+ levels. An overload of intracellular Ca2+ might lead to the activation of cell death pathways, thus compromising sperm viability32–34. Specifically, an increase in Fluo4 intensity has been previously associated with reduced sperm viability during liquid storage of pig sperm18, suggesting a critical relationship between Ca2+ homeostasis and sperm viability.
On the other hand, how inhibiting SERCA with Thg affects acrosome integrity has been extensively documented. In capacitated human and mouse sperm, Thg was identified as an inducer of the acrosome reaction25,35. This is consistent with the established role of the acrosome as an important Ca2+ reservoir26,36,37. Considering the localization pattern of SERCA2 described in this and other studies, one could suggest that the active Ca2+ transport into the acrosome is regulated by SERCA22. This hypothesis would be supported by a previous study showing that incubating non-capacitated human sperm with Thg increases the percentages of viable sperm undergoing acrosome reaction and those of non-viable sperm with an exocytosed acrosome27. The ability to induce the acrosome reaction in capacitated sperm is understood to rely upon the Ca2+ entry from the external medium26,38. Thus, one may reasonably suggest that SERCA play a role in preventing spontaneous acrosome exocytosis during liquid preservation of pig sperm by maintaining Ca2+ homeostasis.
Regarding the influence of SERCA activity in the architecture of the sperm plasma membrane, inhibition of SERCA with Thg resulted in a decrease in lipid membrane stability. While, to the best of our knowledge, there is no previous literature specifically addressing the effect of Thg on the lipid disorder of sperm plasmalemma, the decrease in lipid membrane stability observed herein after blocking the SERCA could be related to the detrimental impact on sperm viability and acrosome integrity mentioned above. M540 incorporation into the plasma membrane of mammalian sperm is associated to the first stages of sperm capacitation39, suggesting that inhibiting SERCA with Thg may trigger capacitation-like changes during liquid storage of pig sperm40. This aligns with our hypothesis that SERCA are critical to prevent premature sperm capacitation and acrosome exocytosis under these conditions. Nevertheless, further research is required to fully elucidate the connection between the inhibition of SERCA by Thg and the occurrence of capacitation-like changes.
One of the most notable effects of blocking SERCA on pig sperm was the increase in intracellular Ca2+ levels. It is well documented that inhibiting SERCA activity with Thg induces a rise in intracellular Ca2+ levels. Two mechanisms have been described for this effect. First, Thg leads to the depletion of intercellular Ca2+ stores26. Second, SERCA inhibition is also known to trigger Ca2+ entry from the extracellular medium in capacitated sperm27,38. As Fluo4 stains the overall intracellular Ca2+18, the increase observed in the present study could result from an extracellular Ca2+ influx. Hence, not only do SERCA regulate intracellular Ca2+ transport but they may also influence the capacitating extracellular Ca2+ uptake, suggesting a potential role in the activation of stored-operated Ca2+ entry (SOCE). Store-operated Ca2+, which are activated in somatic cells upon depletion of Ca2+ from the endoplasmic reticulum41, mediate capacitating Ca2+ entry from the extracellular medium42,43. While the presence of ORAI and STIM, which are key components of SOCE, has been identified in sperm44, further research is required to clarify the specific role these channels play in sperm function. As previously noted, Ca2+ is crucial in regulating sperm physiology under various conditions8,11,15. Hence, the observed intracellular Ca2+ overload could provide an explanation for the detrimental effects on sperm viability, membrane stability, and acrosome integrity.
Although how blocking SERCA affects mitochondrial activity was not previously interrogated, our findings indicated that inhibiting the activity of these pumps during liquid storage increases the MMP of pig sperm. In addition to all the aforementioned, Ca2+ is known to be an important regulator of mitochondrial activity, acting as a secondary messenger in various mitochondrial functions, including ATP production45. While, to the best of our knowledge, the current literature does not establish a direct link between high intracellular Ca2+ levels and elevated MMP in sperm, the results of the present study suggest that Ca2+ homeostasis could influence the mitochondrial activity in pig sperm. Nevertheless, further research is required to elucidate this association and the molecular mechanisms involved in this regulation. Concerning the effect of inhibiting SERCA on ROS levels, the incubation of sperm with Thg increased both superoxide and total ROS levels, but this was observed only at day 4 of storage. This effect could be tightly related to the previously described increase in MMP, as mitochondria are widely understood as a major source of ROS in sperm46,47. The observed rise in mitochondrial activity could thus be responsible for the increased intracellular ROS levels.
Finally, blocking SERCA activity during liquid storage had no effect on sperm motility, as neither the percentages of total and progressively motile sperm nor kinematic parameters were affected by the different concentrations of Thg. These findings contrasted with a previous study in humans, where incubation of sperm with Thg resulted in a rapid reduction of sperm motility27. The decrease in motility was attributed to an overload of intracellular Ca2+, which may inhibit sperm motility48. Despite observing an increase in intracellular Ca2+ in our study, the same impact on sperm motility was not detected. This would open the possibility that, in liquid-stored sperm - and in contrast to capacitated sperm where the PKC pathway plays a vital role -, the function of Ca2+ in the regulation of sperm motility is different. Nevertheless, further research is needed to better understand the relationship between Ca2+ and motility in liquid-stored sperm, as well as the role of SERCA.
Conclusions
In conclusion, this study has confirmed the presence and reported the localization of SERCA2 in pig sperm, which resides in the acrosome and the midpiece. Furthermore, it has demonstrated that Ca2+ homeostasis during liquid storage is essential for preserving sperm function and survival, and that SERCA are crucial for that homeostasis, given their relevance for sperm viability and acrosome integrity and because they prevent excessive mitochondrial activity and ROS levels. Our study contributes to increase the knowledge base on the molecular mechanisms regulating the maintenance of sperm during liquid preservation. Moreover, it opens the door to further research, investigating whether semen aging during liquid preservation is, among other factors, caused by impaired SERCA function. This highlights the need to explore other proteins involved in the regulation of intracellular Ca2+.
Methods
Samples and experimental design
Semen samples from seven healthy and sexually mature boars were used in the present work. Samples were provided by an artificial insemination center (Grup Gepork, S.A.; Masies de Roda, Spain), which fed animals under controlled conditions and held adequate permissions to sell seminal doses. As the authors of this study did not manipulate any animal and seminal doses were commercial, no approval from an Ethics committee was required. Seminal doses were diluted to 33 × 106 sperm per mL in a final volume of 90 mL of a commercial extender (Vitasem LD; Magapor, S.L.; Zaragoza, Spain). Samples were then cooled to 17 °C and transported to our laboratory within the first 24 h post-collection. Upon arrival, every sample was divided into four aliquots and subsequently incubated at 17 °C for ten days with different concentrations of Thg (0, 5, 25, and 50 µM). Sperm parameters were assessed on days 0, 4, and 10.
Sperm motility
Sperm motility was evaluated using a computer-assisted sperm analysis (CASA) system, consisting of a phase-contrast microscope (Olympus BX41; Olympus, Tokyo, Japan) equipped with a camera and the Integrated Sperm Analysis System (ISAS; V1.0; Proiser, S.L.; Valencia, Spain) software. Samples were incubated at 38 °C for 5 min and placed in a pre-warmed Leja chamber (Leja Products BV; Nieuw-Vennep, The Netherlands). A total of 1,000 sperm per sample were captured. Percentages of total motile sperm (%) and progressively motile sperm (%) were recorded for each sample, as well as the following kinematic parameters: curvilinear velocity (VCL, µm/s), straight-line velocity (VSL, µm/s); average-path velocity (VAP, µm/s), linearity (LIN, %), straightness (STR, %), wobble (WOB, %), amplitude of lateral head displacement (ALH, µm) and beat-cross frequency (BCF, Hz). Sperm with a VAP equal to or greater than 10 μm/s were considered motile and those with an STR equal to or higher than 45% were considered progressively motile. Twenty-five images per second were captured, and settings were configured as follows: only particles between 10 and 80 µm2 were analyzed, connectivity was set to 11, and at least 10 images were required to calculate the ALH.
Flow cytometry
A CytoFlex flow cytometer (Beckman Coulter; Brea, CA, USA) was used to evaluate sperm functionality parameters: sperm viability (SYBR-14/propidium iodide [PI]), lipid membrane disorder (merocyanine 540 [M540]/Yo-Pro-1), acrosome integrity (Arachis hypogaea peanut lectin [PNA]/PI), mitochondrial membrane potential (JC-1/[ LIVE/DEAD™ Fixable Far Red]), intracellular superoxide levels (dihydroethidium [HE]/Yo-Pro-1), intracellular total ROS levels (dichlorodihyodrofluorescin diacetate [H2DCFDA]/PI) and intracellular Ca2+ levels (Fluo4-AM/PI).
The Forward Scatter Detector (FSD) and the Side Scatter Detector (SSD) were used to gate the sperm population. All fluorochromes were excited at 488 nm, except LIVE/DEAD™ Fixable Far Red (LD), which was excited at 638 nm. The fluorescence from SYBR-14, Yo-Pro-1, PNA-FITC, DCF, Fluo4, and JC-1 monomers (JC-1mon) was detected through the FITC channel (524/40), whereas that from JC-1 aggregates (JC-1agg) and E was collected through the PE channel (585/42). The fluorescence emitted by M540 was detected by the ECD channel (610/20), and that from LD by the APC channel (660/20). The fluorescence emitted by PI was collected through the PC5.5 channel (690/50). A minimum of 5,000 spermatozoa were analyzed per sample.
Sperm viability (SYBR-14/PI)
Sperm viability was evaluated by assessing the integrity of the plasma membrane, following the protocol of Garner & Johnson49. For this purpose, the LIVE/DEAD viability kit (Molecular Probes; Eugene, OR, US) was used. Samples were stained with SYBR-14 (31.5 nmol/L) and PI (7.6 µmol/L) and incubated at 38 °C for 10 min in the dark. While SYBR-14 is a counterstain, binding to the nuclei of viable and non-viable sperm, PI can only penetrate sperm with damaged plasma membrane49. The percentage of SYBR-14-positive and PI-negative (SYBR-14+/PI−) sperm, which corresponded to viable sperm, was recalculated after subtracting the percentage of debris particles (SYBR-14−/PI−).
Lipid membrane stability (M540/Yo-Pro-1)
Lipid membrane stability was determined following the protocol of Rathi et al.50, with minor modifications. Briefly, samples were stained with M540 (2.5 µmol/L) and Yo-Pro-1 (25 nmol/L) at 38 °C in the dark for 10 min. M540 can intercalate into the membrane and emit red fluorescence in conditions of elevated membrane lipid disorder51. Data were corrected by subtracting the percentage of debris particles (SYBR-14−/PI−) from the double-negative quadrant (M540−/Yo-Pro-1−); the percentages of the four sperm subpopulations were then recalculated. The percentage of viable sperm with low membrane lipid disorder (M540−/Yo-Pro-1−), known as lipid membrane stability, was the main parameter.
Acrosome integrity (PNA-FITC/PI)
To determine the integrity of the acrosomal membrane, a co-staining with PNA-FITC and PI was performed as described by Nagy et al.52. Samples were stained with PNA-FITC (1.17 µmol/L) and PI (5.6 µmol/L) and subsequently incubated at 38 °C in the dark for 10 min. PNA is a lectin that specifically binds the β-galactose residues of the inner acrosomal membrane, which are exposed after the acrosome reaction or when the membrane is not intact; this lectin thus allows for the evaluation of acrosomal integrity53. Data were corrected by subtracting the percentage of debris particles (SYBR-14−/PI−) from the double-negative quadrant (PNA-FITC−/PI−); the percentages of the four sperm subpopulations were then recalculated. The percentage of viable sperm with an intact acrosome membrane (PNA-FITC−/PI−) was used to assess the acrosome integrity.
Intracellular superoxide levels
Intracellular superoxide levels were assessed by following the protocol of Guthrie & Welch54. Briefly, sperm were stained with HE (5 µmol/L) and Yo-Pro-1 (31.25 nmol/L) at 38 °C in the dark for 20 min. HE is oxidized into E+ by superoxide ions, emitting red fluorescence. Data were corrected by subtracting the percentage of debris particles (SYBR-14−/PI−) from the double-negative quadrant (E−/Yo-Pro-1−); the percentages of the four sperm subpopulations were then recalculated. The fluorescence intensity of E+ in viable sperm (Yo-Pro-1−) was used to evaluate the intracellular superoxide levels. Results are expressed as the ratio between the fluorescence intensity of E+ normalized by its respective control.
Intracellular total ROS levels (H2DCFDA/PI)
Sperm were stained with H2DCFDA and PI, following the protocol of Guthrie & Welch54. Briefly, sperm were incubated with H2DCFDA (100 µmol/L) at 38 °C in the dark for 20 min. Subsequently, samples were incubated with PI (12 µmol/L) for 5 min under the same conditions. In the presence of ROS within the cells, H2DCFDA is oxidized to DCF+, which emits red fluorescence. Data were corrected by subtracting the percentage of debris particles (SYBR-14−/PI−) from the double-negative quadrant (DCF−/PI−); the percentages of the four sperm subpopulations were then recalculated. The DCF+ intensity in viable sperm was used to assess total ROS levels. Results are expressed as the ratio between the fluorescence intensity of DCF+ normalized by its respective control.
Intracellular Ca2+ levels (Fluo4-AM/PI)
Intracellular levels of Ca2+ were assessed as described by Harrison et al.55, with minor modifications. Samples were co-stained with Fluo4-AM (1.17 µmol/L) and PI (5.6 µmol/L) at 38 °C in the dark for 10 min. Data were corrected by subtracting the percentage of debris particles (SYBR-14−/PI−) from the double-negative quadrant (Fluo4−/PI−); the percentages of the four sperm subpopulations were subsequently recalculated. The intensity of Fluo4 fluorescence in viable sperm was used to evaluate the intracellular Ca2+ levels, and was expressed as a ratio relative to the corresponding control for each sample.
Mitochondrial membrane potential (JC-1/LIVE/DEAD™ Fixable Far Red)
The evaluation of the mitochondrial membrane potential (MMP) was performed following the protocol described by Llavanera et al.56, with minor modifications. Briefly, sperm were stained with JC-1 (750 nmol/L) and LD (1:8,000; v: v) before incubation at 38 °C in the dark for 30 min. At high MMP, JC-1 forms aggregates that emit orange fluorescence (JC-1agg), whereas at low MMP, JC-1 remains in its monomeric form, emitting green fluorescence (JC-1mon). The ratio between JC-1agg and JC-1mon in viable sperm (LD−) was calculated and used for assessing the MMP.
Immunoblotting
Sperm were centrifuged at 4,000×g for 5 min, resuspended in PBS, and again centrifuged at the same conditions. The resulting pellets were stored at -80 °C until further use. Upon thawing, sperm were resuspended in 500 µL of lysis buffer (RIPA buffer; Sigma) and then incubated for 45 min, with vortexing every 5 min to facilitate the lysis of cells. Samples were subsequently centrifuged at 14,000×g and 4 °C for 20 min, and the total protein content was quantified using a commercial kit (BioRad; Richmond, CA, USA). Fifteen µL of protein extracts were resuspended in 4× Laemmli Redcutor Buffer (v: v) supplemented with β-mercaptoethanol (10%, v: v), and samples were heated at 95 °C for 5 min. Afterward, samples were loaded in 8–16% Mini-PROTEAN TGX Stain-Free Gels (BioRad), and electrophoresis was conducted at 150 V for 60 min. The Stain-Free method was used to quantify the total protein content in the gel through a G: BOX Chemi XL system (Syngene, Frederick, MD, US). Next, proteins from the gel were transferred onto a PVDF low fluorescence membrane by a Trans-Blot Turbo system (BioRad). Following this, membranes were incubated with blocking buffer (5% BSA in TBS) in agitation for 1 h. Next, membranes were incubated with a primary rabbit anti-SERCA2 antibody (ref. ab3625; Abcam; 1:10,000, v: v) at 4 °C in agitation overnight. Thereafter, membranes were washed thrice in 1× TBS-Tween 20, and incubated with a secondary goat anti-rabbit antibody (ref. P0448; Dako; 1:10,000; v: v) in agitation for 1 h. Finally, membranes were washed five times in 1× TBS-Tween 20 and bands were visualized using a chemiluminescent substrate in a G: BOX Chemi XL system (Syngene).
To confirm the specificity of the primary antibody, a competition assay using a recombinant SERCA2 protein (ref. H00000488-P01; Novus Biologicals; 1:1, v: v, regarding the primary antibody) was run. Besides, a negative control by incubating an additional membrane solely with the secondary antibody was included as a second specificity test for the primary antibody.
Immunofluorescence
The localization of SERCA2 in pig sperm was determined by immunofluorescence. For this purpose, samples were diluted to a concentration of 5 × 106 sperm per mL in PBS, and centrifuged at 600×g for 5 min and resuspended in PBS twice. Subsequently, sperm were fixed with 4% paraformaldehyde in PBS (Thermofisher, Kandel, Germany) for 20 min, centrifuged at 600×g for 5 min and resuspended in PBS. For each sample, a 30 µL- drop was smeared on a separate slide. Sperm were permeabilized in PBS containing 1% Triton X-100 at room temperature for 30 min, and then blocked with 0.02 M glycine in PBS at room temperature for 20 min. Next, samples were washed once with PBS for 5 min, and then incubated with the primary rabbit anti-SERCA2 antibody (ref. ab3625; Abcam; 1:200; v: v) diluted in PBS containing 0.1% BSA, at room temperature overnight. Slides were washed with PBS for 5 min and incubated with a secondary donkey anti-rabbit Alexa Fluor 488 (A21206; ThermoFisher; 1:200; v: v), diluted in PBS containing 0.1% BSA at room temperature for 45 min. Finally, slides were washed five times with PBS and prepared with a mounting medium containing DAPI (Dako, Santa Clara, CA, USA). Samples were observed under a confocal laser-scanning microscope (CLSM, Nikon A1R; Nikon Corp., Tokyo, Japan) with preset acquisition settings, and the images captured were analyzed with the Fiji ImageJ software57.
To confirm the primary antibody’s specificity, a competition assay was performed using a recombinant SERCA2 protein (ref. H00000488-P01; Novus Biologicals; 2 times in excess with respect to the primary antibody). Additionally, a negative control lacking the primary antibody and incubated solely with the secondary antibody was included. Brightness and contrast were uniformly adjusted across all captured images.
Statistical analyses
Statistical analyses were conducted with SPSS Ver. 27.0 (IBM Corp., Armonk, NY, USA), and results were plotted using GraphPad Prism v.8 (GraphPad software, La Jolla, CA, USA). Data normality and homoscedasticity were tested through the Shapiro-Wilk and Levene tests, respectively. The effects of inhibiting SERCA with Thg on the resilience of sperm to liquid preservation at 17 °C were determined through a linear mixed model. The intra-subjects factor was the time of storage (0, 4, and 10 days), and the inter-subjects factor was the Thg concentration (0, 5, 25, and 50 µM). Next, pairwise comparisons were established with the post-hoc Bonferroni test. The level of significance was set at P ≤ 0.05. Data are represented as the mean ± the standard error of the mean (SEM).
Table 1.
Effects of inhibiting SERCA with Thg (5, 25, and 50 µM) on the kinetic parameters of pig sperm stored at 17 °C for 10 days.
| Treatment | Day | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 4 | 10 | |||||||
| CTRL | CTRL | 5 µM | 25 µM | 50 µM | CTRL | 5 µM | 25 µM | 50 µM | |
| VCL (µm/s) | 84.84 ± 17.69 | 70.75 ± 22.63 | 86.66 ± 18.70 | 81.68 ± 17.03 | 74.33 ± 14.78 | 46.78 ± 25.03 | 50.69 ± 15.84 | 45.14 ± 16.03 | 46.97 ± 11.61 |
| VSL (µm/s) | 50.65 ± 8.91 | 38.54 ± 11.27 | 43.64 ± 6.30 | 40.40 ± 5.99 | 36.06 ± 5.84 | 20.07 ± 15.79 | 21.12 ± 12.57 | 20.39 ± 12.28 | 21.42 ± 7.91 |
| VAP (µm/s) | 64.71 ± 11.12 | 51.94 ± 17.33 | 61.81 ± 10.53 | 56.54 ± 7.67 | 48.94 ± 7.27 | 26.74 ± 15.05 | 28.89 ± 12.22 | 28.33 ± 14.86 | 27.88 ± 7.82 |
| LIN (%) | 61.05 ± 11.07 | 55.50 ± 12.11 | 51.78 ± 9.43 | 50.73 ± 8.98 | 49.42 ± 8.56 | 40.04 ± 11.05 | 38.58 ± 13.51 | 42.30 ± 11.59 | 44.73 ± 7.77 |
| STR (%) | 78.52 ± 7.29 | 75.22 ± 8.56 | 71.13 ± 6.59 | 71.64 ± 6.67 | 73.63 ± 4.99 | 69.48 ± 15.00 | 68.16 ± 15.32 | 69.98 ± 9.63 | 75.40 ± 9.68 |
| WOB (%) | 77.20 ± 8.08 | 73.21 ± 9.52 | 72.39 ± 8.53 | 70.46 ± 8.38 | 66.82 ± 8.22 | 56.96 ± 5.18 | 55.30 ± 8.17 | 59.83 ± 11.12 | 59.15 ± 4.66 |
| ALH (µm) | 2.63 ± 0.70 | 2.49 ± 0.66 | 2.84 ± 0.76 | 2.87 ± 0.69 | 2.79 ± 0.53 | 2.43 ± 0.80 | 2.49 ± 0.49 | 2.30 ± 0.58 | 2.72 ± 0.24 |
| BCF (Hz) | 8.48 ± 0.64 | 8.11 ± 1.79 | 8.87 ± 1.37 | 8.50 ± 0.82 | 7.67 ± 2.20 | 4.23 ± 1.81 | 4.85 ± 2.38 | 4.30 ± 2.65 | 4.77 ± 2.22 |
Data are shown as mean ± SEM (n = 7).
Abbreviations: CTRL = control; VCL = curvilinear velocity; VSL = straight-line velocity; VAP = average-path velocity; LIN = linearity; STR = straightness; WOB = wobble; ALH = amplitude of lateral head displacement; BCF = beat-cross frequency.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- AI
Artificial Insemination
- ALH
Amplitude of Lateral Head displacement
- BCF
Beat-Cross Frequency
- Ca2+
Calcium
- CASA
Computer-Assisted Sperm Analysis
- CLSM
Confocal Laser-Scanning Microscope
- FSD
Forward Scatter Detector
- H2DCFDA
Dichlorodihydrofluorescin diacetate
- HE
Dihydroethidium
- ISAS
Integrated Sperm Analysis System
- JC-1agg
JC-1 aggregates
- JC-1mon
JC-1 monomers
- LD
LIVE/DEAD™ Fixable Far Red
- LIN
Linearity
- M540
Merocyanine 540
- MMP
Mitochondrial Membrane Potential
- PI
Propidium Iodide
- PNA
Arachys hypogaea peanut lectin
- ROS
Reactive Oxygen Species
- SERCA
Sarcoplasmic/Endoplasmic Ca2+ ATPase
- SSD
Side Scatter Detector
- STR
Straightness
- Thg
Thapsigargin
- VAP
Average-path velocity
- VCL
Curvilinear velocity
- VSL
Straight-line velocity
- WOB
Wobble
Author contributions
FG, ML and MY conceived the research and designed the experiments. FG, JM-H and AP-B conducted laboratory analysis. FG, ML and MY participated in the discussion of data. FG wrote the Manuscript. ML and MY revised and edited the Manuscript. All authors contributed to the finalized Manuscript, read and approved the final version.
Funding
This study was supported by the Ministry of Science, Innovation and Universities, Spain (grants: PID2020-113320RB-I00 and PRE2021-098896), the European Union-Next Generation EU Funds (University of Murcia, Margarita Salas Scheme 181/MSJD/22), the Regional Government of Catalonia (2021-SGR-0900), and the Catalan Institution for Research and Advanced Studies (ICREA).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Marc Llavanera and Marc Yeste have share senior authorship.
Contributor Information
Marc Llavanera, Email: llavanera@biochem.mpg.de.
Marc Yeste, Email: marc.yeste@udg.edu.
References
- 1.Waberski, D., Riesenbeck, A., Schulze, M., Weitze, K. F. & Johnson, L. Application of preserved boar semen for artificial insemination: Past, present and future challenges. Theriogenology137, 2–7. 10.1016/j.theriogenology.2019.05.030 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Yeste, M. Sperm cryopreservation update: Cryodamage, markers, and factors affecting the sperm freezability in pigs. Theriogenology85, 47–64. 10.1016/j.theriogenology.2015.09.047 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Yánez-Ortiz, I., Catalán, J., Rodríguez-Gil, J. E., Miró, J. & Yeste, M. Advances in sperm cryopreservation in farm animals: Cattle, horse, pig and sheep. Anim. Reprod. Sci.246, 106904. 10.1016/j.anireprosci.2021.106904 (2022). [DOI] [PubMed] [Google Scholar]
- 4.Pursel, V. G., Johnson, L. A. & Schulman, L. L. Effect of dilution, seminal plasma and incubation period on cold shock susceptibility of Boar Spermatozoa. J. Anim. Sci.37, 528–531. 10.2527/jas1973.372528x (1973). [DOI] [PubMed] [Google Scholar]
- 5.Yeste, M. State-of-the-art of boar sperm preservation in liquid and frozen state. Anim. Reprod.14, 69–81. 10.21451/1984-3143-AR895 (2017). [Google Scholar]
- 6.Alasmari, W. et al. Ca2+ signals generated by CatSper and Ca2+ stores regulate different behaviors in human Sperm*. J. Biol. Chem.288, 6248–6258. 10.1074/jbc.M112.439356 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brukman, N. G. et al. Tyrosine phosphorylation signaling regulates ca 2+ entry by affecting intracellular pH during human sperm capacitation. J. Cell. Physiol.234, 5276–5288. 10.1002/jcp.27337 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Mata-Martínez, E. et al. Role of calcium oscillations in sperm physiology. Biosystems209, 104524. 10.1016/j.biosystems.2021.104524 (2021). [DOI] [PubMed] [Google Scholar]
- 9.Sushadi, P. S. et al. Arresting calcium-regulated sperm metabolic dynamics enables prolonged fertility in poultry liquid semen storage. Sci. Rep.13, 21775. 10.1038/s41598-023-48550-2 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren, D. et al. A sperm ion channel required for sperm motility and male fertility. Nature413, 603–609. 10.1038/35098027 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakata, Y. et al. Ca v 2.3 (α 1E) ca 2+ channel participates in the control of sperm function. FEBS Lett.516, 229–233. 10.1016/S0014-5793(02)02529-2 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Dragileva, E., Rubinstein, S. & Breitbart, H. Intracellular Ca2+-Mg2+-ATPase regulates calcium influx and Acrosomal Exocytosis in Bull and Ram Spermatozoa1. Biol. Reprod.61, 1226–1234. 10.1095/biolreprod61.5.1226 (1999). [DOI] [PubMed] [Google Scholar]
- 13.Ho, H-C., Granish, K. A. & Suarez, S. S. Hyperactivated motility of bull sperm is triggered at the Axoneme by Ca2+ and not cAMP. Dev. Biol.250, 208–217. 10.1006/dbio.2002.0797 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Luconi, M., Krausz, C., Forti, G. & Baldi, E. Extracellular calcium negatively modulates tyrosine phosphorylation and tyrosine kinase activity during Capacitation of Human Spermatozoa1. Biol. Reprod.55, 207–216. 10.1095/biolreprod55.1.207 (1996). [DOI] [PubMed] [Google Scholar]
- 15.Shabtay, O. & Breitbart, H. CaMKII prevents spontaneous acrosomal exocytosis in sperm through induction of actin polymerization. Dev. Biol.415, 64–74. 10.1016/j.ydbio.2016.05.008 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Hachem, A. et al. PLCζ is the physiological trigger of the Ca2+ oscillations that induce embryogenesis in mammals but conception can occur in its absence. Development144, 2914–2924. 10.1242/dev.150227 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saunders, C. M. et al. PLCζ: a sperm-specific trigger of Ca2+ oscillations in eggs and embryo development. Development129, 3533–3544. 10.1242/dev.129.15.3533 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Garriga, F., Martínez-Hernández, J., Gener-Velasco, N., Rodríguez-Gil, J. E. & Yeste, M. Voltage-dependent anion channels are involved in the maintenance of pig sperm quality during liquid preservation. Theriogenology224, 26–33. 10.1016/j.theriogenology.2024.05.003 (2024). [DOI] [PubMed] [Google Scholar]
- 19.Pavaneli, A. P. P. et al. The presence of seminal plasma during liquid storage of pig spermatozoa at 17°C modulates their ability to elicit in vitro capacitation and trigger acrosomal exocytosis. Int. J. Mol. Sci.21, 4520. 10.3390/ijms21124520 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinart, E., Yeste, M., Puigmulé, M., Barrera, X. & Bonet, S. Acrosin activity is a suitable indicator of boar semen preservation at 17°C when increasing environmental temperature and radiation. Theriogenology80, 234–247. 10.1016/j.theriogenology.2013.04.001 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Periasamy, M. & Kalyanasundaram, A. SERCA pump isoforms: Their role in calcium transport and disease. Muscle Nerve. 35, 430–442. 10.1002/mus.20745 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Lawson, C., Dorval, V., Goupil, S. & Leclerc, P. Identification and localisation of SERCA 2 isoforms in mammalian sperm. MHR: Basic. Sci. Reproductive Med.13, 307–316. 10.1093/molehr/gam012 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Lytton, J., Westlin, M. & Hanley, M. R. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J. Biol. Chem.266, 17067–17071. 10.1016/S0021-9258(19)47340-7 (1991). [PubMed] [Google Scholar]
- 24.Sehgal, P. et al. Inhibition of the sarco/endoplasmic reticulum (ER) Ca2+-ATPase by thapsigargin analogs induces cell death via ER Ca2+ depletion and the unfolded protein response. J. Biol. Chem.292, 19656–19673. 10.1074/jbc.M117.796920 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meizel, S. & Turner, K. O. Initiation of the human sperm acrosome reaction by thapsigargin. J. Exp. Zool.267, 350–355. 10.1002/jez.1402670312 (1993). [DOI] [PubMed] [Google Scholar]
- 26.Rossato, M., Di Virgilio, F., Rizzuto, R., Galeazzi, C. & Foresta, C. Intracellular calcium store depletion and acrosome reaction in human spermatozoa: role of calcium and plasma membrane potential. Mol. Hum. Reprod.7, 119–128. 10.1093/molehr/7.2.119 (2001). [DOI] [PubMed] [Google Scholar]
- 27.Williams, K. M. & Ford, W. C. L. Effects of Ca-ATPase inhibitors on the intracellular calcium activity and motility of human spermatozoa. Int. J. Androl.26, 366–375. 10.1111/j.1365-2605.2003.00438.x (2003). [DOI] [PubMed] [Google Scholar]
- 28.Kim, J-C. et al. Effects of Cryopreservation on Ca2+ signals Induced by membrane depolarization, Caffeine, Thapsigargin and Progesterone in Boar Spermatozoa. Mol. Cells. 26, 558–565. 10.1016/S1016-8478(23)14037-4 (2008). [PubMed] [Google Scholar]
- 29.Harayama, H., Okada, K. & Miyake, M. Involvement of cytoplasmic free calcium in boar sperm: Head-to-head agglutination induced by a cell-permeable cyclic adenosine monophosphate analog. J. Androl.24, 91–99 (2003). [PubMed] [Google Scholar]
- 30.Lytton, J. & MacLennan, D. H. Molecular cloning of cDNAs from human kidney coding for two alternatively spliced products of the cardiac Ca2+-ATPase gene. J. Biol. Chem.263, 15024–15031 (1988). [PubMed] [Google Scholar]
- 31.Jaskulska, A., Janecka, A. E. & Gach-Janczak, K. Thapsigargin—from traditional medicine to anticancer drug. Int. J. Mol. Sci.22, 4. 10.3390/ijms22010004 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bejarano, I. et al. Caspase 3 activation in human spermatozoa in response to hydrogen peroxide and progesterone. Fertil. Steril.90, 1340–1347. 10.1016/j.fertnstert.2007.08.069 (2008). [DOI] [PubMed] [Google Scholar]
- 33.Engel, K. M., Springsguth, C. H. & Grunewald, S. What happens to the unsuccessful spermatozoa? Andrology6, 335–344. 10.1111/andr.12467 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Keshtgar, S. & Ghani, E. Impact of calcium and reactive oxygen species on human sperm function: role of < scp > NOX5. Andrologia 54. 10.1111/and.14470 (2022). [DOI] [PubMed]
- 35.Llanos, M. N. Thapsigargin stimulates acrosomal exocytosis in hamster spermatozoa. Mol. Reprod. Dev.51, 84–91. https://doi.org/10.1002/(SICI)1098-2795(199809)51:1<84::AID-MRD10>3.0.CO;2-U (1998). [DOI] [PubMed] [Google Scholar]
- 36.Costello, S. et al. Ca2+-stores in sperm: Their identities and functions. Reproduction138, 425–437. 10.1530/REP-09-0134 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herrick, S. B. et al. The acrosomal vesicle of mouse sperm is a calcium store. J. Cell. Physiol.202, 663–671. 10.1002/jcp.20172 (2005). [DOI] [PubMed] [Google Scholar]
- 38.O’Toole, C. M. B., Arnoult, C., Darszon, A., Steinhardt, R. A. & Florman, H. M. Ca2+ entry through Store-operated channels in mouse sperm is initiated by Egg ZP3 and drives the Acrosome reaction. Mol. Biol. Cell.11, 1571–1584. 10.1091/mbc.11.5.1571 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bernecic, N. C., Gadella, B. M., Leahy, T. & de Graaf, S. P. Novel methods to detect capacitation-related changes in spermatozoa. Theriogenology137, 56–66. 10.1016/j.theriogenology.2019.05.038 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Harayama, H. Flagellar hyperactivation of bull and boar spermatozoa. Reprod. Med. Biol.17, 442–448. 10.1002/rmb2.12227 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prakriya, M. & Lewis, R. S. Store-operated calcium channels. Physiol. Rev.95, 1383–1436. 10.1152/physrev.00020.2014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strange, K., Yan, X., Lorin-Nebel, C. & Xing, J. Physiological roles of STIM1 and Orai1 homologs and CRAC channels in the genetic model organism Caenorhabditis elegans. Cell. Calcium. 42, 193–203. 10.1016/j.ceca.2007.02.007 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang, Y. et al. ORAI AND TRPC channels in the control of calcium entry signals in smooth muscle. Clin. Exp. Pharmacol. Physiol.35, 1127–1133. 10.1111/j.1440-1681.2008.05018.x (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis, F. M. et al. Male infertility in mice lacking the store-operated Ca2+ channel Orai1. Cell. Calcium. 59, 189–197. 10.1016/j.ceca.2016.02.007 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rossi, A., Pizzo, P. & Filadi, R. Calcium, mitochondria and cell metabolism: A functional triangle in bioenergetics. Biochimica et Biophysica Acta (BBA). Mol. Cell. Res.1866, 1068–1078. 10.1016/j.bbamcr.2018.10.016 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Sabeti, P., Pourmasumi, S., Rahiminia, T., Akyash, F. & Talebi, A. R. Etiologies of sperm oxidative stress. Int. J. Reprod. Biomed.14, 231–240. 10.29252/ijrm.14.4.231 (2016). [PMC free article] [PubMed] [Google Scholar]
- 47.Mateo-Otero, Y., Llavanera, M., Torres-Garrido, M. & Yeste, M. Embryo development is impaired by sperm mitochondrial-derived ROS. Biol. Res.57, 5. 10.1186/s40659-024-00483-4 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li, X. et al. Calcium regulates motility and protein phosphorylation by changing cAMP and ATP concentrations in boar sperm in vitro. Anim. Reprod. Sci.172, 39–51. 10.1016/j.anireprosci.2016.07.001 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Garner, D. L. & Johnson, L. A. Viability Assessment of mammalian sperm using SYBR-14 and Propidium Iodide1. Biol. Reprod.53, 276–284. 10.1095/biolreprod53.2.276 (1995). [DOI] [PubMed] [Google Scholar]
- 50.Rathi, R., Colenbrander, B., Bevers, M. M. & Gadella, B. M. Evaluation of in vitro capacitation of stallion spermatozoa1. Biol. Reprod.65, 462–470. 10.1095/biolreprod65.2.462 (2001). [DOI] [PubMed] [Google Scholar]
- 51.Williamson, P., Mattocks, K. & Schlegel, R. A. Merocyanine 540, a fluorescent probe sensitive to lipid packing. Biochimica et Biophysica Acta (BBA). Biomembr.732, 387–393. 10.1016/0005-2736(83)90055-X (1983). [DOI] [PubMed] [Google Scholar]
- 52.Nagy, S., Jansen, J., Topper, E. K. & Gadella, B. M. A triple-stain Flow Cytometric Method to assess plasma- and acrosome-membrane Integrity of Cryopreserved bovine sperm immediately after thawing in Presence of Egg-Yolk Particles1. Biol. Reprod.68, 1828–1835. 10.1095/biolreprod.102.011445 (2003). [DOI] [PubMed] [Google Scholar]
- 53.Mortimer, D., Curtis, E. F. & Miller, R. G. Specific labelling by peanut agglutinin of the outer acrosomal membrane of the human spermatozoon. Reproduction81, 127–135. 10.1530/jrf.0.0810127 (1987). [DOI] [PubMed] [Google Scholar]
- 54.Guthrie, H. D. & Welch, G. R. Determination of intracellular reactive oxygen species and high mitochondrial membrane potential in Percoll-treated viable boar sperm using fluorescence-activated flow cytometry1. J. Anim. Sci.84, 2089–2100. 10.2527/jas.2005-766 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Harrison, R. A. P., Mairet, B. & Miller, N. G. A. Flow cytometric studies of bicarbonate-mediated Ca2+ influx in boar sperm populations. Mol. Reprod. Dev.35, 197–208. 10.1002/mrd.1080350214 (1993). [DOI] [PubMed] [Google Scholar]
- 56.Llavanera, M. et al. Deactivation of the JNK pathway by GSTP1 is essential to maintain sperm functionality. Front. Cell. Dev. Biol. 9. 10.3389/fcell.2021.627140 (2021). [DOI] [PMC free article] [PubMed]
- 57.Schindelin, J. et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods. 9, 676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.