Abstract
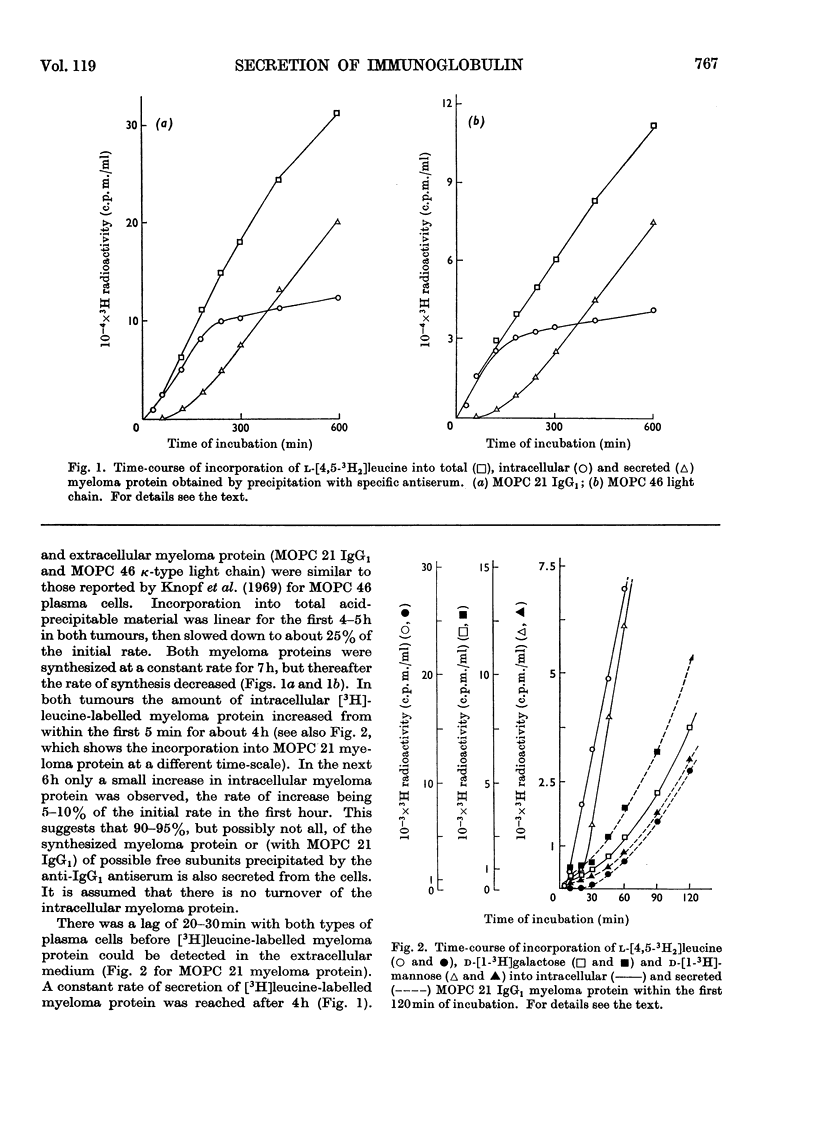
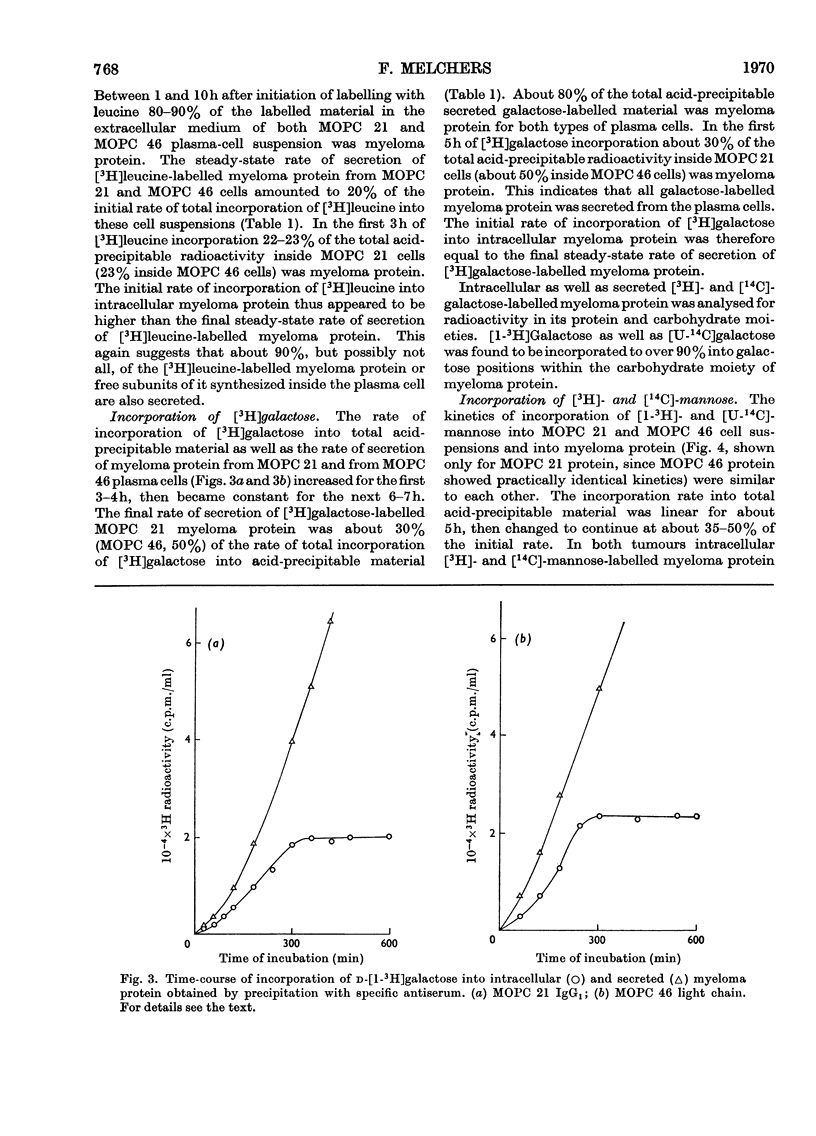
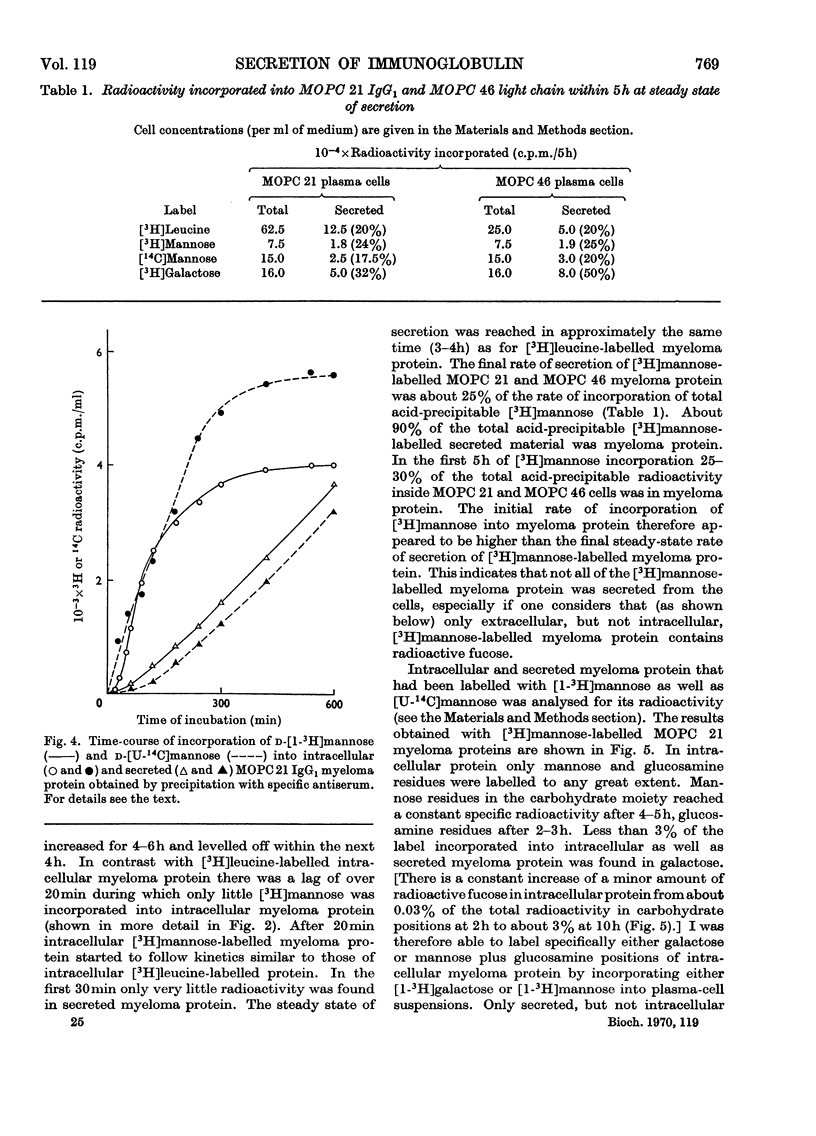
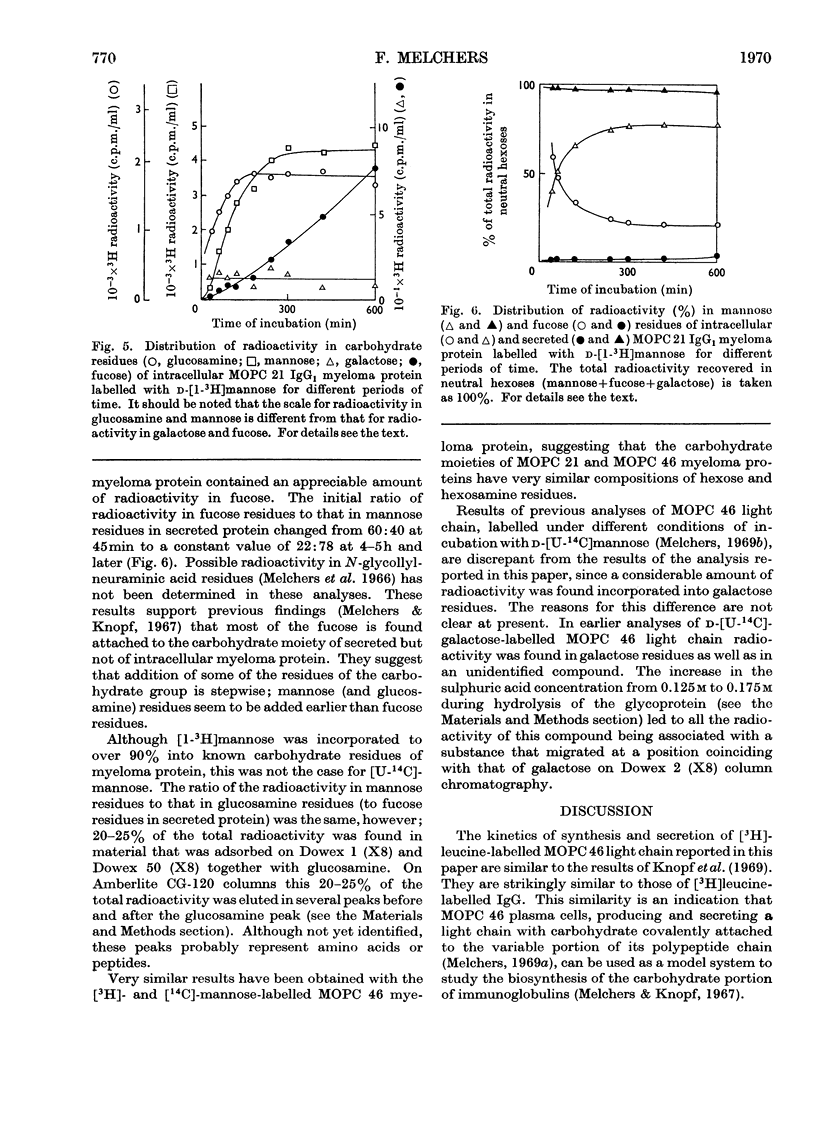
The kinetics of incorporation of leucine, galactose and mannose into intracellular and secreted myeloma protein, MOPC 21 IgG1 and MOPC 46 κ-type light chain, by cell suspensions of two myeloma plasma-cell tumours, MOPC 21 and MOPC 46, were similar. Radioactive galactose was incorporated to over 90% into galactose residues of intracellular and secreted protein, mannose to over 90% into glucosamine and mannose residues of intracellular protein and to over 90% into glucosamine, mannose and fucose residues of secreted protein, but not into galactose residues. The results show that specific residues in the carbohydrate portion of myeloma proteins can be labelled by specific radioactive monosaccharides, and suggest that fucose residues are added, while myeloma protein is in its final stage of secretion from the plasma cell. The kinetics of incorporation indicate at least three sequential precursor–product relationships between different intracellular forms and the secreted form of myeloma protein.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Choules G. L., Zimm B. H. An acrylamide gel soluble in scintillation fluids: its application to electrophoresis at neutral and low pH. Anal Biochem. 1965 Nov;13(2):336–344. doi: 10.1016/0003-2697(65)90202-2. [DOI] [PubMed] [Google Scholar]
- DE PETRIS S., KARLSBAD G., PERNIS B. Localization of antibodies in plasma cells by electron microscopy. J Exp Med. 1963 May 1;117:849–862. doi: 10.1084/jem.117.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eylar E. H. On the biological role of glycoproteins. J Theor Biol. 1966 Jan;10(1):89–113. doi: 10.1016/0022-5193(66)90179-2. [DOI] [PubMed] [Google Scholar]
- FLEISCHMAN J. B., PAIN R. H., PORTER R. R. Reduction of gamma-globulins. Arch Biochem Biophys. 1962 Sep;Suppl 1:174–180. [PubMed] [Google Scholar]
- HELMREICH E., KERN M., EISEN H. N. The secretion of antibody by isolated lymph node cells. J Biol Chem. 1961 Feb;236:464–473. [PubMed] [Google Scholar]
- Herscovics A. Biosynthesis of thyroglobulin. Incorporation of [1-14C] galactose, [1-14C] manose and [4,5-3H2] leucine into soluble proteins by rat thyroids in vitro. Biochem J. 1969 May;112(5):709–719. doi: 10.1042/bj1120709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOLLES P., SAMOUR D., LEDERER E. ISOLEMENT DE FRACTIONS PEPTIDO-GLYCOLIPIDIQUES 'A PARTIR DES CIRES D DE MYCOBACT'ERIES BOVINES, ATYPIQUES, AVIAIRES ET SAPROPHYTES. Biochim Biophys Acta. 1963 Oct 29;78:342–350. doi: 10.1016/0006-3002(63)91644-5. [DOI] [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Intracellular transport of secretory proteins in the pancreatic exocrine cell. I. Role of the peripheral elements of the Golgi complex. J Cell Biol. 1967 Aug;34(2):577–596. doi: 10.1083/jcb.34.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Intracellular transport of secretory proteins in the pancreatic exocrine cell. II. Transport to condensing vacuoles and zymogen granules. J Cell Biol. 1967 Aug;34(2):597–615. doi: 10.1083/jcb.34.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Role of the Golgi complex in the intracellular transport of secretory proteins. Proc Natl Acad Sci U S A. 1966 Feb;55(2):424–431. doi: 10.1073/pnas.55.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. H., Shome B., Liao T. H., Pierce J. G. Analysis of neutral sugars by gas-liquid chromatography of alditol acetates: application to thyrotropic hormone and other glycoproteins. Anal Biochem. 1967 Aug;20(2):258–274. doi: 10.1016/0003-2697(67)90031-0. [DOI] [PubMed] [Google Scholar]
- Knopf P. M., Parkhouse R. M., Lennox E. S. Biosynthetic units of an immunoglobulin heavy chain. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2288–2295. doi: 10.1073/pnas.58.6.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers F., Lennox E. S., Facon M. A carbohydrate-containing mouse light chain-protein. Biochem Biophys Res Commun. 1966 Jul 20;24(2):244–251. doi: 10.1016/0006-291x(66)90727-3. [DOI] [PubMed] [Google Scholar]
- Melchers F. The attachment site of carbohydrate in a mouse immunoglobulin light chain. Biochemistry. 1969 Mar;8(3):938–947. doi: 10.1021/bi00831a026. [DOI] [PubMed] [Google Scholar]
- Rotman B., Papermaster B. W. Membrane properties of living mammalian cells as studied by enzymatic hydrolysis of fluorogenic esters. Proc Natl Acad Sci U S A. 1966 Jan;55(1):134–141. doi: 10.1073/pnas.55.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth D. S., Utsumi S. Structure at the hinge region in rabbit immunoglobulin-G. Nature. 1967 Oct 28;216(5113):332–335. doi: 10.1038/216332a0. [DOI] [PubMed] [Google Scholar]
- Swenson R. M., Kern M. Synthesis and secretion of gamma-globulin by lymph node cells. II. The intracellular segregation of amino acid-labeled and carbohydrate-labeled gamma-globulin. J Biol Chem. 1967 Jul 10;242(13):3242–3244. [PubMed] [Google Scholar]
- Swenson R. M., Kern M. The synthesis and secretion of gamma-globulin by lymph node cells. 3. The slow acquisition of the carbohydrate moiety of gamma-globulin and its relationship to secretion. Proc Natl Acad Sci U S A. 1968 Feb;59(2):546–553. doi: 10.1073/pnas.59.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGT M., DULBECCO R. Steps in the neoplastic transformation of hamster embryo cells by polyoma virus. Proc Natl Acad Sci U S A. 1963 Feb 15;49:171–179. doi: 10.1073/pnas.49.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walborg E. F., Jr, Christensson L., Gardell S. An ion-exchange column chromatographic method for the separation and quantitative analysis of neutral monosaccharides. Anal Biochem. 1965 Nov;13(2):177–185. doi: 10.1016/0003-2697(65)90187-9. [DOI] [PubMed] [Google Scholar]


