Abstract
We recently purified the rat liver hyaluronan receptor for endocytosis (HARE) and found abundant expression of 175- and ∼300-kDa HARE species in sinusoidal endothelial cells of the liver, spleen, and lymph nodes. We report herein the first cloning and functional expression of the rat 175-kDa HARE. Peptide sequences were obtained from the purified 175-kDa HARE, and degenerate oligonucleotide primers were designed for reverse transcription-polymerase chain reaction and cDNA cloning. Results of 5′-rapid amplification of cDNA ends, Northern analysis, N-terminal sequence, and antibody reactivity analyses indicated the absence of mRNA directly encoding the 175-kDa HARE. This protein is most likely derived from a larger precursor. Accordingly, we constructed an artificial 4.7-kb cDNA encoding the 1431 amino acid 175-kDa HARE. The predicted type I membrane protein has a mass of 156,393 Da and a pI of 7.86. The 175-kDa HARE cDNA, fused to the N-terminal leader sequence of the Ig κ-chain, was transfected transiently into COS-7 cells and stably into SK-Hep-1 cells, respectively, to assess hyaluronan or hyaluronic acid (HA)-binding activity and endocytosis. In both cases, HARE expression and HA-binding activity were detected. Furthermore, stable SK-175HARE cells demonstrated specific endocytosis of 125I-HA and receptor recycling. Fluorescence-activated cell sorting analysis confirmed that recombinant HARE was expressed on the cell surface and that fluorescent HA uptake was inhibited by a specific blocking monoclonal antibody against HARE. Additionally, HARE was substantially colocalized with clathrin, but not with internalized HA that was delivered to lysosomes. The results confirm that recombinant 175-kDa HARE is an authentic endocytic receptor for HA and that this receptor can function independently of the ∼300-kDa HARE. HARE is the first functionally identified member of a protein family that shares a similar organization of Fasciclin, epidermal growth factor-like, Xlink, and transmembrane domains.
INTRODUCTION
Hyaluronan or hyaluronic acid (HA) was discovered and named >68 years ago by Meyer and Palmer (1934) and then shown by many other investigators to be a common, ubiquitous component of essentially all extracellular matrixes (ECMs) in vertebrates. HA is a linear polymer composed of the repeating disaccharide unit 2-deoxy, 2-acetamido-d-glucopyranosyl-β(1,4)-d-glucuronopyranosyl-β(1,3). Although its structure is simple, HA influences many cell functions and behaviors, including cell migration, differentiation, and phagocytosis (Laurent and Fraser, 1992; Turley, 1992; Knudson and Knudson, 1993; Toole, 1997; Abatangelo and Weigel, 2000). HA is an important molecule in development (Gakunga et al., 1997; Toole, 1997), wound healing (Weigel et al., 1986; Burd et al., 1991; Chen and Abatangelo, 1999), angiogenesis (West et al., 1985; Deed et al., 1997; Rahmanian et al., 1997), and tumor growth and metastasis (Csoka et al., 1997; Delpech et al., 1997). The ability of HA to form large aggregates by binding to ECM proteoglycans, such as aggrecan and perlecan, is necessary for normal tissue differentiation (Vertel et al., 1994; Handler et al., 1997).
Previously, most investigators believed that the physiological function of HA in the ECM was only structural or physical. However, HA is now also recognized as a pharmacologically active signaling molecule. Numerous cell types respond physiologically to HA of different sizes. In particular, small, but not large, HA stimulates angiogenesis (West et al., 1985; Deed et al., 1997; Rahmanian et al., 1997) and small, not large, HA stimulates activated macrophages to induce the expression of a large number of genes (Horton et al., 1998, 2000).
The total body content of HA in a 70-kg human is roughly 15 g, and the turnover of HA is up to 5 g/d (Laurent and Fraser, 1991). HA and chondroitin sulfate are components of the ECM in all vertebrate tissues and are continuously synthesized and degraded in tissues throughout the body. For example, ∼50% of the body's total HA is in skin and this HA has a metabolic half-life of <1.5 d (Tammi et al., 1991). There are fewer studies of chondrotin sulfate turnover, but this is thought to occur in parallel with HA turnover via a similar, if not identical mechanism. This makes physiological sense, because HA, chondroitin sulfate, and other glycosaminoglycans are all released at the same time from ECM after the cleavage of HA and proteoglycans, especially aggregating proteoglycans associated with HA. In mammals, sinusoidal liver endothelial cells (LECs) express a recycling endocytic receptor that removes both HA and chondroitin sulfate from the circulation by endocytosis via the clathrin-coated pit pathway (Raja et al., 1988; Smedsrod et al., 1988; McGary et al., 1989; Laurent and Fraser, 1992).
In previous studies to characterize the rat LEC HA receptor, we identified two large membrane proteins of 175 and ∼300 kDa that were specifically labeled with a photoaffinity derivative of HA (Yannariello-Brown et al., 1992a) and that retained specific HA-binding activity in a novel ligand blot assay after SDS-PAGE (Yannariello-Brown et al., 1996). We finally achieved the first purification of these two putative HA receptors of 175 and ∼300 kDa from isolated rat LECs using a specific monoclonal antibody (mAb) against the 175-kDa protein (Zhou et al., 1999). A major goal in the present study was to determine whether these two proteins are able to function independently as HA receptors or whether they must both be present in a larger functional complex. We also demonstrated previously that these HA receptors are expressed not only in rat liver sinusoids but also in the venous sinuses of the red pulp in spleen and the medullary sinuses of lymph nodes (Zhou et al., 2000). Because this receptor internalizes HA by the rapid coated pit-mediated endocytosis pathway (Smedsrod et al., 1988) and its tissue distribution is not unique to liver, it was renamed hyaluronan receptor for endocytosis (HARE).
Based on elegant studies by Laurent and Fraser (1992), we now understand the basic mechanism for HA turnover in mammals, including humans. When [3H]HA is injected intravenously into mice (Fraser et al., 1983) or rabbits (Fraser et al., 1981), it is rapidly removed from the blood and concentrated predominantly in liver, and to a lesser extent, in spleen and lymph nodes. The present model of total body HA turnover in mammals is that large native HA molecules (up to ∼107 Da) in the ECM are partially degraded to large fragments of ∼106 Da that are released from the matrix into lymphatic vessels and then flow to lymph nodes. The removal and complete degradation of this HA and chondroitin sulfate then occurs via clearance systems in different tissues that use the recently identified HARE (Zhou et al., 1999, 2000). The first clearance site is in the lymphatic system, particularly lymph nodes, which account for ∼85% of HA and chondroitin sulfate turnover, and the second clearance site is in the LECs of liver, accounting for ∼15% of the total body HA and chondroitin sulfate turnover. HARE in spleen apparently accounts only for a small percentage of HA turnover.
Despite the turnover of up to 5 g of HA per day, the HA clearance systems using HARE in lymph nodes and liver keep the normal steady-state concentration of HA in blood very low (i.e., 10–100 ng/ml). Clearance of the circulating HA from lymph and blood is likely to be very important for normal health, because the viscosity of these fluids could increase to dangerous levels if the concentration of HA was allowed to increase, particularly if the HA was of high molecular weight (i.e., >106) as found in lymph fluid. Elevated serum HA levels are found in several disease conditions, such as liver cirrhosis (Lai et al., 1998; Yamada et al., 1998), rheumatoid arthritis (Manicourt et al., 1999), scleroderma (Freitas et al., 1996), psoriasis (Lundin et al., 1985), and some cancers (Thylen et al., 1999).
In this study we used degenerate oligonucleotide primers, based on peptide sequences of the purified rat 175-kDa HARE, for reverse transcription-polymerase chain reaction (RT-PCR) analysis, cDNA library screening, and 5′-rapid amplification of cDNA ends (RACE) analysis, to clone and express for the first time a functional 175-kDa HARE.
MATERIALS AND METHODS
Materials and Buffers
The original TA cloning kit, pTrcHis2-TOPO TA cloning kit, TOPO XL PCR cloning kit, pcDNA3.1, pSecTag2 vector, and TOP10F′ electrocompetent cells were from Invitrogen (Carlsbad, CA). TRIzol reagent, Platinum Taq DNA Polymerase High Fidelity, Thermoscript RT-PCR System kit, G418, and trypsin were purchased from Invitrogen. EndoFree Plasmid Maxi kit, QIAprep spin miniprep kit, and QIAquick Gel Extraction kit were from QIAGEN (Valencia, CA). QuikHyb Hybridization Solution, XL1-Blue MRF′ Escherichia coli supercompetent cells, pfuTurbo DNA polymerase, TaqPlus Long PCR System, λZAP Express vector, and random primer labeling kit were from Stratagene (La Jolla, CA). Nitrocellulose membranes (Protran 0.1 μm), polyvinylidene difluoride, and nylon membrane filters (NYTRAN) were from Schleicher & Schuell (Keene, NH). Marathon cDNA Amplification kit was from CLONTECH (Palo Alto, CA). Digoxigenin High Prime Labeling and Detection kit, RNA molecular weight marker, and FuGENE 6 transfection reagent were from Roche Applied Science (Indianapolis, IN). Thermo Sequenase Radiolabeled Terminator Cycle Sequencing kit was purchased from U.S. Biochemical (Cleveland, OH). Enhanced avian RT-PCR kit was from Sigma-Aldrich (St. Louis, MO). PolyATtract mRNA Isolation kit was from Promega (Madison, WI). Restriction enzymes were purchased from MBI Fermentas (St. Leon-Rot, Germany). Custom oligonucleotides were synthesized by The Great American Gene Co (Ramona, CA). and are shown in Table 1. [32P]dCTP was from Amersham Biosciences (Piscataway, NJ). Goat anti-mouse IgG (H+L) conjugated to rhodamine red or Alexa-Fluor 488 was from Molecular Probes (Eugene, OR). Custom antibodies to synthetic peptides were prepared in sheep or goat by Bethyl Laboratories (Montgomery, TX). 125I-HA (∼70 kDa) was prepared from aminohexyl-HA derivatives, modified only at the reducing ends, as described previously (Raja et al., 1984). Fluorescent-HA (fl-HA), which was prepared by reaction of aminohexyl-HA with the succinimidyl ester of rhodamine green (Molecular Probes), was a generous gift from Dr. Carl T. McGary (HealthEast Care Sys, St. Paul, MN). Saline sodium citrate (SSC) contains 150 mM sodium chloride and 15 mM sodium citrate, pH 7.0. Hanks' balanced salt solution was prepared according to the Grand Island Biologicals formulation (Invitrogen, New York, NY; formulation is online at www.invitrogen.com).
Table 1.
Summary of oligonucleotides and amino acid sequences derived from peptides of the rat 175-kDa HARE protein
| HARE Peptide designation | Amino acid sequence | Start-end residue number |
|---|---|---|
| GT-68 | PLGQYK | 1070–1075 |
| GT-81 | AYPTTYASQK | 1120–1129 |
| GT-123 | VLQDLTTVAANHGYTK | 604–619 |
| GT-139 | QLYVNEAPIDYTNVATDK | 103–120 |
| GT-208 | LAGPGPFTVFAPLSSSFNHEPR | 488–509 |
| Peptide 1 | DILRYHVVLGEK | 62–73 |
| Peptide 3 | VLEIQK | 129–134 |
| Peptide 5 | LEALPEQQDFLFNQDNK | 651–668 |
| N-terminal | SLPSLLTRLEQMPDYSIF (major) | 1–18 |
| N-terminal | XXVIHGLEKVXXIQKNR (minor) | 122–136 |
| Oligonucleotides | |
|---|---|
| Designation | Sequence |
| 81R | 5′-GCRTAIGTNGTNGGRTANGC |
| 123R | 5′-TAICCRTGRTTNGCNGCNAC |
| 123F | 5′-GTNGCNGCNAAYCAYGGITA |
| 208F | 5′-CCNTTYACNGTNTTYGCICC |
| GSP-1R | 5′-CTCCAAACACGGGTTGATTTC |
| GSP-1R (AsnI) | 5′-CTCCAAACACGGATTAATTTC |
| GSP-1F (AsnI) | 5′-GAAATTAATCCGTGTTTGGAG |
| GSP-2R | 5′-TGGGGTGGTTCTTTTACAGTC |
| GSP-5F (EcoRI) | 5′-TGGTGGAATTCTTTACCAAGTCTACTCACC |
| GSP-GT81R | 5′-GGCATACGTAGTCGGGTAGGC |
The two rat HARE proteins were purified from isolated LECs after extraction and immunoaffinity chromatography by using anti-rat 175-kDa HARE mAbs. The immunoaffinity-purified proteins were subjected to SDS-PAGE, and the gels were either stained with Coomassie blue to identify the proteins to be excised for determination of internal peptide sequences after trypsin digestion or the proteins in the gel were electrotransferred to a polyvinylidene difluoride membrane for determination of N-terminal sequence. As described in MATERIALS AND METHODS, the purified 175-kDa HARE protein band was excised and sent to the Harvard Microchemistry Facility (GT peptides) and a partially purified preparation was sent to the Rockefeller University Microchemistry Facility (peptides 1, 3, and 5) for determination of internal protein sequences after trypsin digestion. The location of each peptide sequence found in the deduced protein sequence of the 175-kDa HARE protein (based on its cDNA) is indicated by the amino acid position number for the starting and ending residues in the 175-kDa HARE protein sequence. The one-letter code for amino acids is used; X indicates unknown residues. One-letter abbreviations conform to the IUB group codes for oligonucleotides. Amino acid sequences used to design forward (F) and reverse (R) oligonucleotide primers are shown in boldface. Restriction sites introduced in the indicated oligonucleotides are shown in italics and boldface.
Purification and Sequencing of HARE
Rat LEC HARE was purified from membrane extracts by affinity chromatography with RCA-I agarose and then mAb-30 Sepharose as described previously (Zhou et al., 1999). The purified proteins were reduced, subjected to SDS-PAGE, and stained with Coomassie blue. The 175-kDa HARE protein band was excised and sent to Dr. William Lane (Harvard Microchemistry Facility, Cambridge, MA) for internal peptide sequence analysis after trypsin digestion. Additionally, the 175-kDa protein was partially purified by two-dimensional electrophoresis (isoelectric focusing followed by SDS-PAGE), and the appropriate protein zones were sent to the Rockefeller University Microchemistry Facility for internal sequence analysis of tryptic peptides. N-Terminal sequence analysis of immunoaffinity-purified 175-kDa HARE was performed by Dr. Ken W. Jackson (Molecular Biology Resource Facility, William K. Warren Medical Research Institute, University of Oklahoma Health Sciences Center, Oklahoma City, OK). The resulting amino acid sequences of peptides (Table 1) obtained by the Harvard Facility (GT-68, GT-81, GT-123, GT-139, and GT-208) and the Rockefeller University Facility (peptides 1, 3, and 5) were used to design degenerate oligonucleotide primers for RT-PCR analysis and to confirm isolation of the correct cDNA.
RT-PCR Analysis
Total RNA from isolated rat LECs was prepared using TRIzol reagent, and the mRNA was isolated from total RNA by using a polyATtract mRNA Isolation kit following the manufacturers' recommended protocols. First-strand cDNA was synthesized using the Thermoscript RT-PCR system from Invitrogen with random hexameric oligonucleotides or oligo(dT)20. The PCR reactions were carried out with incubation at 94°C for 2 min; 30 cycles of 45°C for 30 s, 72°C for 6 min, and 94°C for 30 s; and one cycle of 45°C for 30 s and 72°C for 15 min with degenerate oligonucleotide primers (Table 1) based on a particular unique peptide sequence. The PCR products were cloned into pCR2.1 or pTrcHis2 expression vector by using TA cloning kits from Invitrogen.
cDNA Library Screening
An endothelial cell cDNA expression library was prepared from LEC mRNA in λZAP Express by Stratagene. Approximately 2.4 × 105 plaque-forming units were screened with two digoxigenin-labeled cloned RT-PCR products produced with primer pair 208F-123R (370 base pairs) and 123F-81R (1500 base pairs). All screening was performed on duplicate nitrocellulose filters. The nitrocellulose membranes were denatured in 1.5 M NaCl, 0.5 M NaOH for 2 min, neutralized in 1.5 M NaCl, 0.5 M Tris, pH 8.0, for 5 min, rinsed with 0.2 M Tris, pH 7.5, in 2× SSC for 30 s, and then baked at 80°C for 1 h. After prehybridization at 45°C for 30 min in QuikHyb Hybridization Solution, the membranes were allowed to hybridize overnight at 45°C with a mixture of the two digoxigenin-labeled probes. The membranes were then washed twice in SSC containing 0.05% SDS at room temperature, followed by two washes in 0.1× SSC containing 0.1% SDS at 45°C. The positive λZAP-Express bacteriophage was identified and purified, and the cloned DNA inserts were excised in vivo into PBK-CMV phagemid by using ExAssist helper phage and XLOLR bacterial cells as recommended in the manufacturer's manual. The phagemid DNAs were purified and the inserts were sequenced.
Northern Blot Analysis
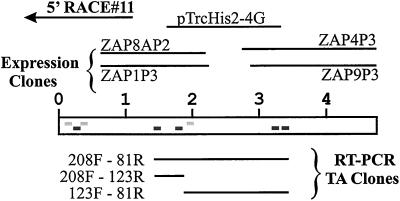
Total RNA and mRNA were isolated from rat LECs as described above. RNA (20 μg/lane) or mRNA (1 μg/lane) samples in 20 mM morpholinopropanesulfonic acid, 5 mM sodium acetate, pH 7.0, and 1 mM EDTA, containing 6% (vol/vol) formaldehyde and 50% (vol/vol) formamide were heated to 65°C for 5 min, placed immediately on ice, and then electrophoresed on a 0.8% agarose gel in 20 mM morpholinopropanesulfonic acid, 5 mM sodium acetate, pH 7.0, 1 mM EDTA, and 2% (vol/vol) formaldehyde. The RNA was transferred overnight to nylon membranes by the capillary method by using 5× SSC. The membranes were treated as described above and then hybridized at 45°C with three 32P-labeled DNA probes, prepared by the random primer extension method (Taylor et al., 1976). The probes were made from the inserts of clones 5′-RACE#11 (1108 base pairs), ZAP1P3 (1216 base pairs), and ZAP9P3 (1979 base pairs) and were located at the 5′ end, the middle, and the 3′ end of the 175-kDa HARE nucleotide sequence, respectively (Figure 1).
Figure 1.
Assembly of the 4.7-kb cDNA encoding the rat 175-kDa HARE. Peptide sequences obtained from the purified HARE by the Harvard Microchemistry (dark gray) or the Rockefeller facilities (light gray) are indicated by rectangles at the corresponding nucleotide positions. The scale units indicated are in kilobases. The ZAP1P3, ZAP8AP2, ZAP4P3, and ZAP9P3 clones were isolated by screening the λ-ZAP Express cDNA library with specific PCR-generated probes. The clone 5′-RACE#11 was obtained from 5′-RACE by using a marathon cDNA amplification kit (CLONTECH). All other clones were obtained by RT-PCR by using degenerate oligonucleotides primers based on peptide sequences (Table 1) and cloned into the pCR 2.1 vector or pTrcHis2 expression vector.
5′-RACE Analysis
The 5′ end of the 175-kDa HARE cDNA was obtained using a Marathon cDNA amplification kit from CLONTECH for 5′-RACE analysis. A gene-specific primer (GSP), oligonucleotide GSP-1R, was used for first-strand cDNA synthesis. After second-strand cDNA synthesis by the method of Gubler and Hoffman (1983), a library of adaptors (CLONTECH) was ligated to the double-strand cDNA. DNAs were amplified by PCR with adaptor-ligated double-strand cDNA as the template with primer GSP-2R and the adaptor primer from the Marathon kit as the primer pair. PCR conditions were as follows: 94°C for 2 min; 30 cycles of 45°C for 30 s, 72°C for 6 min, and 94°C for 30 s; and 1 cycle of 45°C for 30 s and 72°C for 15 min. The PCR products were then cloned into pCR2.1 by using the TA cloning kit from Invitrogen, and colonies were screened by PCR by using GSP-2R and the adaptor primer. Plasmid DNAs from positive clones were purified with QIAprep spin plasmid kits and the inserts were sequenced.
Construction of a 175-kDa HARE cDNA with an N-Terminal Ig κ-Chain Leader Sequence
An 1152-base pair 5′ fragment of the 175-kDa HARE cDNA was amplified by RT-PCR using pfuTurbo polymerase with GSP-5F(EcoRI), which contains an EcoRI restriction site and encodes the N-terminal seven amino acids of the 175-kDa protein, and GSP-1R(AsnI), which contains two silent G→A mutations that create a AsnI restriction site. The PCR products were separated on a 1% agarose gel, and the 1.15-kb DNA band was excised and purified using a QIAquick kit. The DNA was cloned into the pSecTag2 B vector, which contains a murine Ig κ-chain leader sequence for protein secretion. The ligated plasmid was electrotransformed into TOP10F′ electrocompetent cells (Invitrogen), amplified by bacterial growth, and purified with a QIAprep spin miniprep kit. The NheI/AsnI fragment of this plasmid, which contains the upstream leader sequence, was then excised and purified. An interior 2226-base pair fragment of the 175-kDa HARE cDNA sequence was amplified by RT-PCR by using pfuTurbo DNA polymerase with GSP-1F(AsnI), which contains a silent C→T mutation to create a AsnI restriction site, and primer GSP-GT81R. The PCR products were separated on a 1.0% agarose gel and the excised 2.2-kb DNA band was purified using a QIAquick Gel Extraction kit and digested with AsnI and Eco52I (there is a Eco52I site within the 3′ approximate one-third of the HARE sequence, starting at nucleotide position 3329). The 1.5-kb insert from the ZAP9P3 clone, which contains the 3′ end of the 175-kDa HARE cDNA, including the polyA site, was amplified, purified, and cut with Eco52I and XhoI to give the third fragment, which contains 1378 base pairs. The three purified fragments of HARE cDNA were then simultaneously ligated with pcDNA3.1, which had been digested with NheI and XhoI, at a molecular ratio of 2:1 (insert:vector). The ligated DNA was electroporated into TOP10F′ electrocompetent cells, and colonies were screened by PCR and restriction enzyme digestion to identify full-length inserts. Plasmid DNA from positive clones was amplified in TOP10F′ bacteria and purified using endofree plasmid maxi kits, and the complete inserts were sequenced. The resulting plasmid containing the 4708-base pair cDNA encoding the 175-kDa HARE is designated p175HARE-κ.
Transient Expression of the 175-kDa HARE in COS-7 Cells
COS-7 cells were grown to ∼80% confluence in 35-mm culture dishes, by using DMEM containing 10% fetal calf serum, l-glutamine, and 100 U each of penicillin/streptomycin, and then transfected with the purified p175HARE-κ DNA (2 μg) by using 6 μl of FuGENE 6. At 40 h posttransfection, the cells were detached by treatment with 0.05% trypsin and 0.53 mM EDTA, collected, and washed two times with phosphate-buffered saline (PBS). The cells were extracted with Tris-buffered saline (TBS) containing 1% NP-40 and analyzed by SDS-PAGE. HARE expression was assessed by Western analysis with anti-HARE mAbs (Zhou et al., 2000), and expression of active HARE was assessed by a ligand blot assay using 125I-HA (Yannariello-Brown et al., 1996; Zhou et al., 1999).
Selection of Stable Tranfectants Expressing the 175-kDa HARE
SK-Hep-1 cells (American Type Culture Collection, Manassas, VA) were transfected with the purified p175HARE-κ DNA by using FuGENE 6 in 35-mm culture dishes. Twenty-four hours after transfection the cells were transferred to 100-mm dishes and grown in DMEM containing 10% fetal calf serum, l-glutamine, 100 U each of penicillin/streptomycin, and 0.4 mg/ml G418 for selection. After 15–20 d, antibiotic-resistant individual colonies were isolated using cloning rings and detached by treatment with 0.05% trypsin and 0.53 mM EDTA for 5 min at room temperature. Collected cells were expanded in 12-well plates to assess HARE protein expression and function by enzyme-linked immunosorbent assay, Western blot, and 125I-HA binding assays. Cultures that were positive in these assays were further purified by dilution cloning. Final clones are designated SK-175HARE-#.
Fluorescence-activated Cell Sorting (FACS) Analysis of 175-kDa HARE Expression
SK-175HARE cells were grown in tissue culture flasks until confluent, trypsinized, divided into 13- × 100-mm glass tubes, and incubated on ice for 1 h with TBS containing 1% bovine serum albumin (BSA) and 10% goat serum to block nonspecific binding sites. For the analysis of surface staining, the cells were then incubated with 1 μg/ml of the indicated mAbs on ice for 30 min with gentle agitation every ∼5 min. The cells were washed twice at 4°C by centrifugation for 3 min, resuspensed in PBS, and incubated with 4 μg/ml Alexa 488-conjugated goat anti-mouse secondary antibody for 45 min on ice. After two washes in PBS, the cells were resuspended in PBS, filtered through a 37-μm mesh to remove aggregates, and analyzed on an FACScalibur cytometer (BD Biosciences, San Jose, CA). For the analysis of fl-HA uptake, the cells were first incubated at 37°C for 30 min with 50 μg/ml of the indicated IgG or 100 μg/ml nonlabeled HA before adding fl-HA to a final concentration of 1.0 μg/ml. The cells were then incubated another 90 min at 37°C, washed, trypsinized, filtered, and analyzed as described above. Viable cells (10,000/sample) were selected for analysis by using appropriate gating settings.
Confocal Fluorescence Microscopy
SK-175HARE-34 cells were grown in Lab-Tek II chamber slides (Nalge-Nunc, Naperville, IL) to 75–80% confluence, washed in PBS, fixed on ice with 4% formaldehyde in PBS for 15 min, and washed in PBS. The rest of the protocol was also performed on ice. Cells were permeabilized with 0.1% Triton X-100 in PBS for 5 min and incubated in 10 mM HEPES pH 7.4, 150 mM NaCl, and 6.7 mM KCl containing 1.5% (wt/vol) BSA. A mix of four anti-HARE mAbs (nos. 30, 154, 174, and 235) at 1 μg/ml each and a rabbit anti-clathrin polyclonal antibody (diluted 1:500; Santa Cruz Biotechnology, Santa Cruz, CA) in buffer 1/BSA (Yannariello-Brown et al. 1992a) were incubated with the cells for 30 min. After washing in PBS, clathrin was detected using goat anti-rabbit IgG conjugated to Alexa-Flour 488 (diluted 1:500 in buffer 1/BSA), and HARE was detected with rhodamine red-X–labeled goat anti-mouse IgG (diluted 1:1000 in buffer1/BSA) obtained from Molecular Probes. The secondary antibodies were incubated for 15 min and then washed in PBS. Samples were mounted in Fluorsave (Calbiochem, San Diego, CA), and fluorescence was detected using a TCS NT laser confocal microscope (Leica, Deerfield, IL). Digital images were recorded using the TCS software package (Leica). To assess HA and lysosome colocalization, cells were incubated at 37°C with 1 μg/ml fl-HA in minimal essential medium for 1.5 h. In some samples specific uptake was assessed by adding 100 μg/ml unlabeled HA to the medium containing fl-HA. The medium was then aspirated and the cells were incubated for an additional 30 min at 37°C with Lysotracker (Molecular Probes) diluted 1 × 106 in minimal essential medium. The cells were then washed with PBS and fixed as described above. In the HARE and lysosome colocalization studies, after the cells were treated with Lysotracker they were fixed, permeabilized, and HARE was detected using Alexa-Flour 488 goat anti-mouse IgG (diluted 1:1000 in buffer 1/BSA) as described above.
General
Protein content was determined by the method of Bradford (1976) by using BSA as a standard. SDS-PAGE was performed according to the method of Laemmli (1970). Western blotting was performed as described by Burnette (1981) with minor modifications (Zhou et al., 2000). DNA sequencing was performed by the dideoxy nucleotide method (Sanger et al., 1977), either manually using the thermo sequenase radiolabeled terminator cycle sequencing kit or by the Department of Microbiology and Immunology Sequence Facility (University of Oklahoma Health Sciences Center) with automated DNA sequencers (model 377; Applied Biosystems, Foster City, CA; or ALF). 125I Radioactivity was measured using an Auto-Gamma Counting System (Packard Instrument Company, Downers Grove, IL). Digital images from confocal microscopy were processed in Excel or PowerPoint (Microsoft, Redmond, WA). Other digital images obtained by scanning blots or autoradiograms with a ScanMaker 9600 XL (MicroTek, Redondo Beach, CA) were processed using Visioneer Paperport, version 5.1, and then Corel Paint and Corel Draw, version 9.0.
RESULTS
Assembly of Rat 175-kDa HARE cDNA
Using a specific anti-175-kDa HARE mAb, we recently purified two rat liver HARE species that may be functional isoreceptors for HA (Yannariello-Brown et al., 1997; Zhou et al., 1999, 2000). The 175-kDa and ∼300-kDa HARE proteins are each able to bind 125I-HA in a ligand blot assay after nonreducing SDS-PAGE and electrotransfer and are immunologically related, because all mAbs raised against the 175-kDa HARE also recognize the ∼300-kDa HARE (Zhou et al., 2000). The 175-kDa HARE contains only one protein, whereas the ∼300-kDa HARE contains three disulfide-bonded subunits of ∼260, 230, and 97 kDa (Zhou et al., 1999). The 260- and 230-kDa subunits of the ∼300-kDa HARE are both recognized by the panel of anti-175-kDa HARE mAbs (Zhou et al., 2000).
The immunoaffinity purified 175-kDa HARE was reduced, resolved by SDS-PAGE, excised, and subjected to internal tryptic peptide analysis (Table 1). Primers were designed based on amino acid sequences of the resulting peptides and PCR fragments were generated, cloned, and used as probes to screen a custom-made λ-ZAP Express rat LEC cDNA library. Overlapping clones of various types were then used to assemble a partial cDNA (Figure 1) that encoded the peptides identified from tryptic digests of the purified 175-kDa HARE (Table 1). To verify the fidelity of key partial cDNA clones isolated from the library or by RT-PCR, we confirmed that these clones resulted in the expression, in transformed bacteria, of protein fragments that were recognized in Western analysis by one or more of our eight mAbs against the 175-kDa HARE (our unpublished data). For example, clones ZAP9P3 and ZAP4P3 showed reactive bands at 68 and 72 kDa, respectively, with mAb-159 and mAb-174. The cDNA assembled from the various positive clones, however, lacked 5′-upstream noncoding sequences, an initiating codon and a leader sequence for appropriate membrane insertion. When this partial cDNA was extended further upstream by 5′-RACE analysis, the resulting in-frame coding region was longer than anticipated for a glycoprotein of 185 kDa, which is the size of the 175-kDa HARE when reduced (Zhou et al., 1999, 2000).
The purified 175-kDa HARE is a broad, rather than well focused, band in SDS-PAGE, suggesting that it contains species of heterogeneous size. Although some size heterogeneity is expected because the two HARE species are glycoproteins with ≥25 kDa of N-linked oligosaccharides (Zhou et al., 1999), another reason could be that the purified protein was either randomly or specifically cleaved by proteases. To test this latter hypothesis we performed NH2-terminal sequence analysis on the affinity-purified 175-kDa HARE and discovered two distinct termini corresponding to regions of the encoded deduced protein that were 122 amino acids apart (Table 1 and Figure 2). Previous NH2-terminal sequencing attempts, before having the partial cDNA sequence, had not yielded interpretable data, because the yields were relatively low and a unique sequence was not obtained. The deduced protein sequence information, however, enabled us to identify a major and a minor NH2-terminal sequence beginning at amino acid 1 (SLPSL… ) and 122 (VIHGL… ), respectively.
Figure 2.
Nucleic acid and deduced amino acid sequences of the 4.7-kb cDNA encoding the rat 175-kDa HARE. The artificial cDNA containing 4708 nucleotides encodes a 1431-amino acid recombinant 175-kDa HARE protein, whose deduced amino acid sequence begins with a serine. Amino acid sequences verified by peptide sequence analysis of the purified HARE are underlined, and the two N-terminal peptides found in the purified protein are underlined and in italics. Putative N-glycosylation sites are in boldface, and Cys residues are highlighted in boldface and italics. Three alternative N-glycosylation sites of the type N-X-C are located at N135, N218, and N930. The predicted transmembrane domain of the type I membrane protein is underlined and in boldface. The three shaded regions in the cytoplasmic domain are potential motifs for targeting the receptor to clathrin-coated pits. Potential HA-binding motifs of the type B-X7-B, which are in the predicted extracellular domain, are enclosed in boldface brackets.
The above-mentioned results indicated that the 175-kDa HARE protein could be derived by proteolytic processing and led us to consider the possibility that there is no mRNA species directly encoding the protein, but rather that it is encoded by a much larger mRNA for the ∼300-kDa HARE, whose protein product is then proteolytically processed to the 175-kDa HARE. Consistent with this interpretation, Northern analysis, using mRNA from rat LECs and probes from either the 5′ end, the middle, or the 3′ end of the cDNA (Figure 2) revealed a major ∼10-kb band (Figure 3). We did not observe a separate mRNA species in the range of ∼6–8 kb, which might be the expected size range of the 175-kDa HARE transcript if it was encoded directly.
Figure 3.
Northern blot analysis of rat LEC RNA with 175-kDa HARE cDNA probes. Total RNA (lanes 1) or mRNA (lanes 2) samples prepared from isolated rat LECs were subjected to formamide gel electrophoresis, transferred to nylon membranes, and processed as described in MATERIALS AND METHODS. The membranes were allowed to hybridize separately with three different 32P-labeled DNA probes, 5′RACE#11 (A), ZAP1P3 (B), or ZAP9P3 (C), which are located at the 5′ end, middle, or 3′ end of the 175-kDa HARE cDNA sequence, respectively.
Domain Structure and Characteristics of the Deduced 175-kDa HARE
The cDNA sequence presented in Figure 2 encodes a 1431-amino acid protein that starts with the Ser residue that was identified as the major NH2 terminus. The deduced protein contains all five internal tryptic peptide sequences derived from the purified 175-kDa HARE protein, as well as three additional internal peptides obtained from a partially purified HARE preparation (Table 1). The protein is predicted to be a type I membrane protein (Figure 4), with a large NH2-terminal extracellular domain (1322–1324 residues depending on the particular prediction program used), a single transmembrane domain (∼L1323–A1343), and a small COOH-terminal cytoplasmic domain (∼88 amino acids). The exact boundaries predicted for the transmembrane domain of HARE are somewhat uncertain; they vary by two or three amino acids on both sides of the predicted domain, depending on the algorithm used. For example, the programs TMPred, TMHMM, and PSORTII, respectively, predict a transmembrane domain between residues 1327–1347, 1325–1347, and 1327–1343. The predicted mass of the protein is 156,393 Da and the predicted isoelectric point is pH 7.86. The ectodomain contains 15 typical putative N-glycosylation sites (excluding one N-P-S sequon and three atypical N-X-C sites), and two cysteine-rich regions. The extracellular domain has multiple motifs and subdomains with homology to regions identified in other receptors and matrix molecules. Multiple epidermal growth factor (EGF)-like, βIgH3, and Fasciclin domains, as well as one delta serrate ligand domain are also organized throughout the extracellular domain of the 175-kDa HARE. In addition, a 93-amino acid region near the membrane junction (Gly1063–Tyr1155) is homologous to the mammalian proteoglycan extracellular Xlink domain and the HA-binding domain of the link protein (e.g., 40 and 35% identical, respectively, to these domains in human CD44 and bovine aggrecan). Thirteen potential HA-binding motifs of the B-X7-B type, where B is Lys or Arg (Yang et al. 1994), are also present in the 175-kDa HARE ectodomain.
Figure 4.
Domain structure of the 175-kDa HARE protein. The scheme depicts the organization of multiple protein domains, within the 1431 amino acid HARE protein, that are identified by predictive search programs such as SMART (Schultz et al., 1998), CD-search, and other sites linked to ExPASy or National Center for Biotechnology Information. TM, transmembrane domain; E2, Ea, and Ec represent, respectively, EGF-2, laminin-like EGF, and EGF-Ca2+ domains; potential N-linked glycosylation sites of the type N-X-T/S are indicated by the inverted Y symbols. A leucine zipper motif is also present at L587–L608 and a weakly homologous metallothionein domain is at G345–T411.
The 175-kDa HARE Is Derived from a Larger Protein
The mRNA, partial cDNA, amino acid sequence, and mAb reactivity data are all consistent with the hypothesis that there is a precursor relationship among the 260- and 230-kDa subunits of the 300-kDa HARE and the 175-kDa HARE protein. To test this possibility, we examined the reactivity of these three HARE proteins with two different polyclonal anti-peptide antibodies. One antibody was raised against a sequence within the rat 175-kDa protein shown in Figure 2 (PKCPLKSKGVKK773) and the other antibody was raised against a 16-amino acid putative coding region (TVLVPSRRAFEDMDQNK) that begins 107 amino acids upstream of the SLP… sequence identified as the amino-terminal start of the purified rat 175-kDa HARE. There was no prior information about whether this putative protein region is expressed. However, if all three HARE proteins are derived from a larger precursor, then our prediction was that the former antibody should recognize all three proteins, whereas the latter antibody would recognize only the two larger proteins but not the 175-kDa protein. This was the result obtained (Figure 5), which strongly supports the conclusion that the 175-kDa HARE is indeed derived from one of the larger HARE proteins of the 300-kDa HARE. Peptide mapping of the three purified HARE proteins will ultimately be required to confirm this conclusion.
Figure 5.
An in-frame region upstream of the 175-kDa cDNA encodes amino acid sequences present in the larger HARE subunits. LECs were lysed in Laemmli (1970) buffer containing 5% β-mercaptoethanol and samples were subjected to SDS-PAGE and electrotransfer. Nitrocellulose strips were cut and incubated with lane 1, a mixture of eight mAbs that recognize all three HARE proteins (i.e., the 175-kDa HARE and the 260- and 230-kDa subunits of the 300 HARE complex); lane 2, preimmune goat IgG; lane 3, goat IgG (Ab2 in the diagram) raised against a 16-amino acid putative coding region (TVLVPSRRAFEDMDQNK−91) upstream of the amino terminal start of the purified rat 175-kDa protein; lane 4, preimmune sheep IgG; and lane 5, sheep IgG (Ab1 in the diagram) raised against a peptide corresponding to the sequence PKCPLKSKGVKK773 within the rat 175-kDa protein. Strips were washed and incubated with the appropriate secondary antibody-alkaline phosphatase conjugates and substrates for color development.
Expression of a Functional HA Receptor for Endocytosis from the 175-kDa HARE cDNA
To verify that we have cloned a bone fide cDNA for the 175-kDa HARE, we performed HA-binding and internalization studies by using transfected COS-7 or SK-Hep-1 cells expressing the 175-kDa protein. Because there is no natural mRNA directly coding for the 175-kDa HARE protein, we constructed an artificial cDNA that encodes the open reading frame (ORF) for the 175-kDa HARE fused at the 5′ end to a short region of the Ig κ-light chain sequence containing a start codon and a membrane insertion signal or leader sequence (Figure 6). Transient transfection of this cDNA into COS-7 cells, yielded a protein of the expected size that was recognized in Western blots by the specific anti-HARE mAbs and that bound 125I-HA specifically in the ligand blot assay (Figure 7).
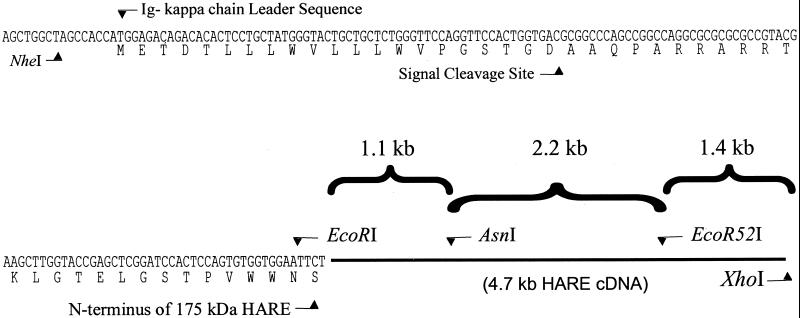
Figure 6.
Schematic map of the recombinant 175-kDa HARE construct. A 5′-end fragment (1.1 kb) of the 175-kDa HARE open reading frame was amplified by RT-PCR with primers containing EcoRI sites, and cloned in-frame with the Ig κ-chain leader sequence of the pSecTag2 vector. The DNA insert was cut with NheI and AsnI and then cloned into pcDNA3.1 together with two other 175-kDa HARE cDNA fragments of 2.2 and 1.4 kb, which were derived from the RT-PCR and cDNA library screenings, respectively, and which encode the remainder of the 175-kDa HARE protein. The fragments were digested with different restriction enzymes as indicated and assembled as described in MATERIALS AND METHODS.
Figure 7.
Western blot and 125I-HA ligand blot analysis of recombinant 175-kDa HARE expression in COS-7 cells. COS-7 cells were transfected with two different clones containing the 175-kDa HARE cDNA (lanes 1 and 2) fused at its 5′ end with the Ig κ-heavy chain leader sequence. Lanes 3 are mock-transfected COS-7 cells, and lanes 4 are a positive control containing rat LEC extract. Expression of the 175-kDa HARE was analyzed by SDS-PAGE of cells extracts with (A) and without (B and C) reduction followed by transfer to nitrocellulose. The blots were incubated with 2 μg/ml 125I-HA with (B) or without (A and C) excess unlabeled HA (300 μg/ml) to assess total and nonspecific binding, respectively. After ligand blotting (top), the membrane was subjected to Western analysis (bottom) as described in MATERIALS AND METHODS. As previously observed for the native LEC protein (Zhou et al., 1999), the recombinant HARE is inactivated by reduction. The apparent difference in HA-binding activity between the recombinant and native 175-kDa HARE is due to a difference in protein loaded (see Figure 8).
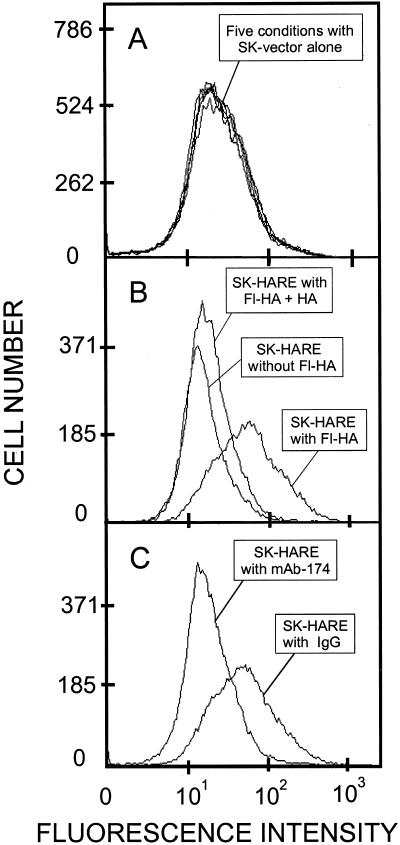
We then used the same p175HARE-κ vector to generate stable cell lines expressing HARE after antibiotic selection of transfected SK-Hep-1 cells. This cell line was chosen because it does not express any detectable endogenous HA receptors capable of specific 125I-HA binding or endocytosis, and does not show reactivity with the anti-HARE mAbs in Western blots. Seven independent clones were selected, all of which had essentially identical characteristics with respect to 175-kDa HARE expression and function. The recombinant 175-kDa HARE expressed by these cells and the purified rat LEC protein were essentially identical in their ability to bind 125I-HA in the ligand blot assay (Figure 8). FACS analysis showed that the recombinant HARE protein was present at the cell surface (Figure 9). Specific mAbs against the 175-kDa HARE bound to cells expressing recombinant HARE, but not to SK-Hep-1 parental cells (our unpublished data) or cells transfected with vector alone. The internalization of fl-HA by SK-175HARE cells was specific and was mediated by the recombinant 175-kDa HARE as judged by its competition with an excess of unlabeled HA (Figure 10B), its inhibition by ∼98% with mAb-174 (Figure 10C), and the lack of fl-HA uptake by SK-Hep-1 cells or cells transfected with vector alone (Figure 10A).
Figure 8.
Comparison of HA binding by the native and recombinant 175-kDa HARE proteins. Membranes from isolated LECs (lanes 1 and 2) and SK-175HARE-34 cells (lanes 3 and 4) were solubilized in TBS containing 0.5% NP-40 plus protease inhibitors and HARE proteins were immunoprecipitated using mAb-30 coupled to Sepharose. The proteins were eluted with sample buffer (Laemmli, 1970), subjected to SDS-PAGE and electrotransfer, and the nitrocellulose was incubated overnight in TBS containing 0.1% Tween 20. Ligand blotting with 1 μg/ml 125I-HA (lanes 1 and 3 from autoradiogram) was performed as described in MATERIALS AND METHODS. The same blots were then incubated in TBS containing 1% BSA and subjected to Western analysis (lanes 2 and 4) by using a mixture of eight mAbs against HARE. A series of dilutions verified that the Western staining responses for both samples were proportional to protein load and were not saturated. The open and solid arrows indicate, respectively, the ∼300- and 175-kDa HARE species. The HA-binding intensity relative to the Western staining of the 175-kDa HARE was essentially the same from LECs and the stable cells.
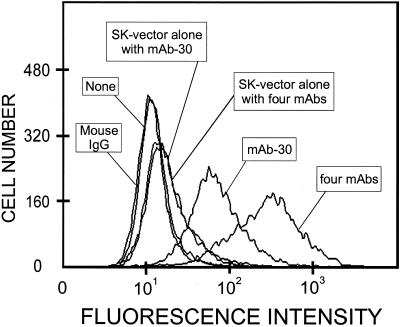
Figure 9.
Cell surface expression of the recombinant 175-kDa HARE in stably transfected cells. After blocking nonspecific binding sites, SK-175HARE cells or SK-Hep-1 cells transfected with vector alone were incubated, as indicated, with either nothing, 1 μg/ml mAb-30, 1 μg/ml mouse IgG, or a mixture of four mAbs (nos. 30, 154, 174, and 235 each at 1 μg/ml) as described in MATERIALS AND METHODS. The cells were washed, incubated with Alexa 488-conjugated secondary antibody for 45 min on ice, and processed for FACS analysis.
Figure 10.
FACS analysis of fl-HA uptake in SK-175HARE cells mediated by the 175-kDa HARE. SK-Hep-1 cells transfected with vector alone (A) or SK-175HARE-34 cells (B and C) were grown to confluence in six-well tissue culture plates, washed, and preincubated at 37°C, as indicated in the figure, with no addition or nonlabeled HA (B) or mouse IgG or mAb-174 (C) followed by fl-HA as described in MATERIALS AND METHODS. The same five conditions were used in A.
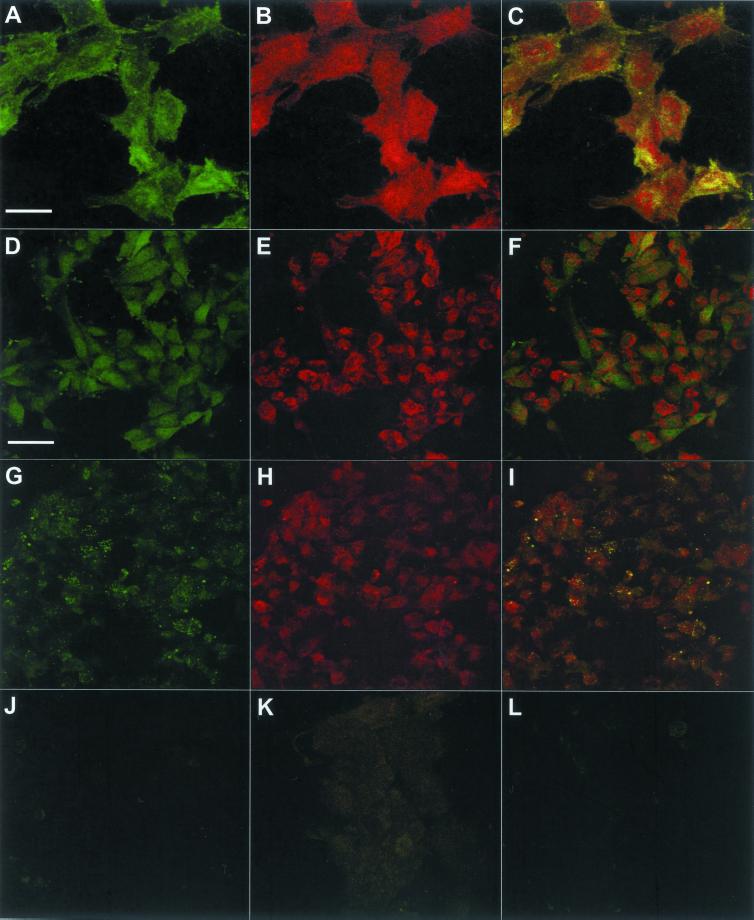
Confocal fluorescence microscopy was then used to assess the cellular distribution of HARE and internalized HA in SK-175HARE cells (Figure 11). As expected for a recycling receptor mediating endocytosis via coated pits, much of the cellular clathrin was colocalized with HARE (Figure 11, A–C), whereas most of the intracellular HARE staining was not present in clathrin-containing compartments, which is typical for an endocytic, recycling receptor (Mellman, 1996). HARE was not targeted to lysosomes as a consequence of mediating HA uptake (Figure 11, D–F), although internalized HA was delivered to lysosomes as assessed by its colocalization with the Lysotracker dye (Figure 11, G–I). The internalization of fl-HA was virtually eliminated by a large excess of unlabeled HA (Figure 11J). A variety of controls showed no significant fluorescence, including SK-175HARE cells treated with mouse (our unpublished data) or rabbit IgG (Figure 11K), and SK-Hep-1 cells (our unpublished data) or cells transfected with vector alone (Figure 11L) incubated with fl-HA.
Figure 11.
Confocal microscopy of the 175-kDa HARE in SK-175HARE cells. The cellular distributions of the recombinant HARE, fl-HA, clathrin, and lysosomes were determined in SK-175HARE-34 cells as described in MATERIALS AND METHODS. (A–C) Colocalization of clathrin (A) and HARE (B) in the overlay picture (C). The different distribution patterns of HARE (D) and Lysotracker (E) in cells incubated with unlabeled HA are shown in the overlay picture (F). (I) Colocalization pattern of fl-HA (G) and Lysotracker (H). The effect of excess unlabeled HA on the uptake of fl-HA is shown in J. The background staining of SK-175HARE cells with rabbit IgG is shown in K. (L) Anti-HARE staining of SK-Hep-1 cells stably transfected with the backbone plasmid (containing no cDNA insert). The bar in A (20 μm) applies to A–C, and the bar in D (50 μm) applies to D–L.
All the stable cell lines expressing HARE were also able to mediate the continuous endocytosis of 125I-HA at 37°C (our unpublished data). HA uptake, assessed by competition with unlabeled HA, was ∼90% specific and the amount of HA uptake in ∼10 h was 14-times the surface receptor content (i.e., the number of HA binding sites per cell). HARE protein was not degraded during this period, based on Western analysis, indicating that the recombinant 175-kDa HARE is a recycling endocytic receptor. Preliminary results (Weigel, Zhou, and Weigel, unpublished data) confirm that the internalized HA is degraded after endocytosis mediated by the recombinant 175-kDa HARE. We also assessed the sensitivity of 125I-HA uptake to hyperosmolarity, because doubling the osmolarity of the medium by the addition of sucrose disrupts clathrin recycling and coated pit formation (Oka et al., 1989) and blocks ≥90% of the specific 125I-HA accumulation by LECs (McGary et al., 1989). Hyperosmolar treatment of SK-175HARE clones 36 and 27 inhibited their specific endocytosis of 125I-HA (37°C; 4 h) by 90 and 81%, respectively. These above-mentioned results affirm that the 175-kDa HARE is responsible for the observed specific HA binding and internalization in the SK-175HARE transfectants and that endocytosis of HA occurs via the coated pit pathway.
DISCUSSION
Because it is nonimmunogenic and has special viscoelastic and rheological properties in solution, HA is used in many clinical applications, and its medical uses are growing rapidly. For example, high molecular weight HA preparations are routinely used in ophthalmic surgeries (Goa and Benfield, 1994) and to treat patients with osteoarthritis or rheumatoid arthritis by intraarticular injection (Manek and Lane, 2000; Rosier and O'Keefe, 2000). Due to its use in such a wide array of medical applications, it is important that we understand the biological effects of exogenously administered HA and how its turnover and clearance from the body is regulated. Clearance of the endogenous circulating HA from lymph and blood is also likely to be very important for normal health, because the viscosity of these fluids would rapidly increase to dangerous levels if HA was allowed to accumulate, particularly if it was of high molecular weight as found in lymph fluid (>106). HARE is abundantly expressed in the sinusoids of liver and lymphatic tissues (Zhou et al., 2000), which is a localization ideally suited for keeping the level of systemic HA low.
Our results indicate that the native rat 175-kDa HARE protein is most likely derived from the proteolytic processing of a larger protein in LECs. Although this cannot be unequivocally proven until this larger protein is identified and shown to generate the 175-kDa HARE species, the following results indicate that the precursor protein is one of the two large subunits of the ∼300-kDa HARE (Zhou et al., 1999). First, the 260- and 230-kDa subunits of the ∼300-kDa HARE are immunologically related to the 175-kDa HARE, because they cross-react with all mAbs against the 175-kDa HARE (Zhou et al., 2000) and with the anti-peptide antibody used herein (Figure 5). Second, the 175-kDa HARE does not have a unique N terminus (Table 1), indicating that it is sensitive to one or more cellular proteases. Third, the mRNA encoding the 175-kDa HARE is longer than expected for this size protein. Fourth, our present partial cDNA for the HARE protein encodes >200 amino acids upstream of the N-terminal Ser of the functional 175-kDa HARE. Finally, the two largest HARE proteins were reactive with an antibody against a predicted amino acid sequence upstream of the cDNA region encoding the native 175-kDa HARE. The latter result, in particular, strongly supports the proteolytic processing model. We conclude that the 260-kDa subunit (or its precursor) is the initial gene product, from which both the 230- and 175-kDa proteins are then derived by proteolysis.
The 175-kDa HARE protein is a functional HA receptor when expressed from a synthetic cDNA. This recombinant 175-kDa HARE mediated HA endocytosis through the coated pit pathway in the absence of the ∼300-kDa HARE complex. Although it is possible that the 175-kDa HARE might interact with other protein(s) in LECs to achieve an even more rapid internalization, this HARE is nonetheless an independent functional receptor. The two HARE species can, therefore, be viewed as structurally related HA isoreceptors. Two HA receptors (i.e., the 175- and ∼300-kDa HARE proteins) may be necessary to mediate the efficient uptake and degradation of HA in mammals because of the broad molecular mass range of HA present in tissues throughout the body. The two receptors present in liver, spleen, and lymph node could have different preferences for the size of the HA with which they interact.
Based on the SMART program of Schultz et al. (1998), the large extracellular domain of the 175-kDa HARE (∼S1–L1324) is predicted to contain four Fasciclin-like domains, a delta serrate ligand domain, a Link domain, and at least 11 EGF-like domains arranged in two clusters. These two clusters, which are cysteine-rich, are separated by a ∼360 amino acid cysteine-poor region. The Fasciclin-like domains are related to a family of three Fasciclins, which are Ig-like cell adhesion molecules expressed on a subset of axons during neuronal development in insects (Kose et al., 1997). Among the numerous EGF-like domains are laminin-like, Ca2+-binding, EGF-1, and EGF-2 domains. Previous studies of HARE function in isolated rat LECs showed that HA binding and endocytosis do not require Ca2+ or other divalent cations (Yannariello-Brown et al., 1992b). Most of these EGF-like domains are only partial, but several have the pattern of six cysteines needed for the typical organization and folding of this domain (Selander-Sunnerhagen et al., 1992).
The 93-residue Link domain is a good candidate for an HA-binding region within the extracellular domain of HARE, but it is likely that multiple, as yet unidentified, non-Link HA-binding domains are present in HARE as well. Day, Jackson, and colleagues have investigated the structural requirements for HA-binding activity of Link domains from various HA-binding proteins (Bajorath et al., 1998; Banerji et al., 1998; Kahmann et al., 2000). Although each active Link domain engages HA by critical contacts with a distinct set of amino acids that form a long pocket on the surface of the protein, there are common residues or residues with comparable characteristics at equivalent positions in these HA-binding regions. Most of the proteins containing Link domains are in the ECM and can form stable multivalent networks with HA through these Link interactions, even although the binding affinity for a single HA-Link domain interaction is relatively weak. Because the efficient endocytic clearance of HA requires a high-affinity interaction with HARE, we expect that the extracellular domain of HARE will contain other HA-binding regions (e.g., the multiple B-X7-B motifs are potential HA-binding sites).
The cytoplasmic domain of HARE (∼Y1344–R1431) contains many possible phosphorylation sites: two Tyr, nine Ser, two His, and 11 Thr residues, although only residues S1378, S1392, T1354, T1380, T1396, and T1410 are predicted (by NetPhos 2.0) to be phosphorylated. No PEST motifs for rapid degradation, or consensus sequences for O-glycosylation by GlcNAc are present. As expected, for an endocytic receptor, the cytoplasmic domain contains several candidate motifs for targeting the protein to clathrin-coated pits. The sequence YSYFRL1349, which is at the junction between the predicted transmembrane and cytoplasmic domains, contains an interesting overlapping combination of two ΦXXB motifs, where Φ is either tyrosine or phenylalanine, X can be any amino acid and B is a hydrophobic residue with a bulky side chain. Similar overlapping motifs are responsible for coated pit targeting of the low density lipoprotein (LDL), mannose, and cation-dependent mannose 6-phosphate receptors (Mellman, 1996), all of which are recycling, endocytic clearance receptors. In addition, a third candidate ΦXXB motif is present at FQRF1359 and a dileucine motif occurs at LL1370.
The domain organization of HARE is different from that of the other well-characterized HA-binding proteins or HA receptors, including intercellular adhesion molecule-1, RHAMM (CD168), CD44, TSG-6, Link protein, and LYVE-1 (Banerji et al., 1999; Abatangelo and Weigel, 2000). For example, HARE and LYVE-1, which is a CD44-family member, are unrelated except in their homologous Xlink domains. Although LYVE-1 is also found in LECs (Carreira et al., 2001), our preliminary results indicate that LYVE-1 and HARE are distributed very differently within rat LECs. An earlier identification of ICAM-1 as the endocytic HA receptor in LECs was later acknowledged to be an artifact (McCourt and Gustafson, 1997). HARE is distinct from all other cell surface HA receptors because it is an endocytic, recycling receptor that mediates the rapid and efficient endocytosis of both HA and chondroitin sulfate via the clathrin-coated pit pathway. Liver may also contain a scavenger receptor able to internalize HA (McCourt et al., 1999).
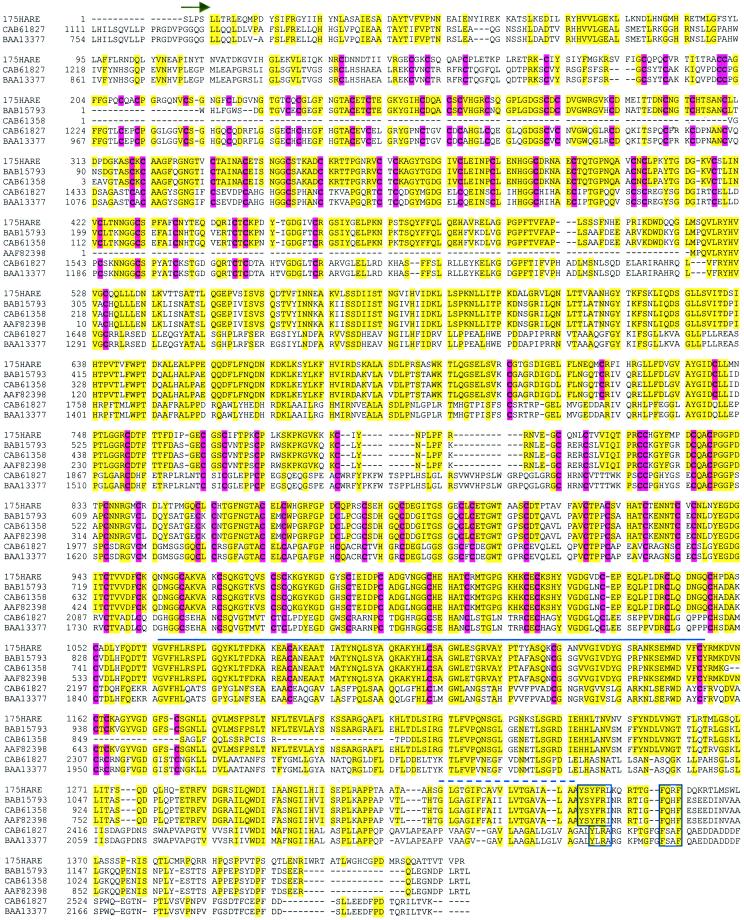
A BLAST search of the protein database found five related sequences that share a high level of identity with the rat 175-kDa HARE (Figure 12). There are two subfamilies of related proteins, all of which are human, each with a distinct pattern of conserved sequences. The three deposited sequences most related to HARE (CAB61358, BAB15793, and AAF82398) represent putative proteins of unknown function, with no evidence that they are expressed. The latter deduced protein was designated FELL, a CD44-like precursor, because it contains Fasciclin, EGF-like, and Link domains. The remaining two sequences (CAB61827 encoding stabilin-1 and BAA13377) are more related to each other than to HARE and the three sequences noted above. The BAA13377 mRNA sequence was found in endothelial cells, but expression of the protein was not verified and possible HA-binding activity was not determined (Tsifrina et al., 1999). The critical question of whether the other putative proteins are expressed and whether they are able to bind HA, remains to be tested. Nonetheless, it is clear that these putative HA-binding proteins are highly related to the HARE protein reported herein.
Figure 12.
Alignment of the rat 175-kDa HARE deduced amino acid sequence with a family of hypothetical protein sequences of unknown function. Sequences were aligned with DNASIS (version 2.50), saved as a text file, and edited in Microsoft Word. The hypothetical protein sequences, all of which are human, are designated by their GenBank protein accession numbers. Our deposited sequences for the rat 175-kDa HARE (rHARE) are under accession numbers AY007370 and AAG13634 for the nucleic acid and protein sequences, respectively. Further details about this family are in the text. The recombinant 175-kDa HARE that was constructed to demonstrate the functionality of this receptor starts with Ser (arrow). Residues in HARE identical to one or more of the other sequences are shaded in yellow. Conserved cysteine residues are in boldface and shaded red. The residues under the solid bold line are identified as an extracellular Link domain (Xlink), a putative HA-binding domain. The dashed line is above the single putative transmembrane domain in each protein. Regions within boxes denote candidate ϕXXB motifs for targeting to coated pits.
In an ongoing study (Zhou, McGary, Weigel, Saxena, and Weigel, unpublished data), we have affinity purified the human HARE proteins and identified a partial human cDNA, represented in part by accession number BAB15793, as the human HARE homolog. A striking feature of this HARE family is that virtually all of the cysteine residues within the predicted extracellular domains are absolutely conserved (Figure 12). This suggests that the overall folding and organization of the extracellular domains of these proteins are the same. In addition, family members have the same overall domain organization including the Xlink domain and a single predicted transmembrane region. Although the cytoplasmic domains of the two HARE subfamilies are the most divergent regions, the candidate φXXB domains for targeting to coated pits are, nonetheless, highly conserved. Based on the overall similarities in their extracellular and cytoplasmic domains, we suggest that these proteins constitute a family of membrane-bound receptors, with the 175-kDa HARE as the prototype and first functionally identified member. The members of this family may all be able to bind and internalize HA, chondroitin sulfate, or even other glycosaminoglycans through the clathrin-coated pit pathway.
A ∼2.5-kb portion of the rat HARE ORF maps to a region of the mouse genome spanning ∼190 kb, deposited as a putative gene of unknown expression or function. This mouse HARE gene (accession no. AC025501.3) contains numerous introns and short exons. Although the orientation and relative position of many HARE exons (assigned when the sequence was compiled) seem to be incorrect throughout this region, there is >90% identity between the mouse and rat nucleotide sequences over >2 kb of coding region. The complete gene may, therefore, be >500 kb. The present report is the first functional identification of the protein encoded by this gene. A candidate human HARE gene (Zhou, McGary, Weigel, Saxena, and Weigel, unpublished data), located on chromosome 12 (accession no. NT_024383.2), has a similar organization to the mouse gene.
We conclude that the rat 175-kDa HARE is a bone fide endocytic receptor for HA, capable of functioning independently of the ∼300-kDa HARE. Although it is possible that the 175- and ∼300-kDa HARE species could also function together as a large complex, it is apparently not necessary for these two HAREs to be present in the same cell to create a specific functional HA receptor. Therefore, the 175- and ∼300-kDa HAREs are most likely independent isoreceptors for HA.
ACKNOWLEDGMENTS
We thank Drs. Judy Yannariello-Brown and Paul DeAngelis for helpful discussions, Leona Medved for help preparing the manuscript, Dr. Carl T. McGary for the fl-HA, and Anil Singh for the preparation of LECs and technical assistance. We gratefully acknowledge the assistance of Jim Henthorn and the Flow and Image Cytometry Laboratory supported by the Warren Medical Research Institute. Accession numbers for the nucleic acid and protein sequences reported herein are in the GenBank database under AY007370 and AAG13634, respectively. This research was supported by National Institute of General Medical Sciences grant GM-35978 from the National Institutes of Health.
Abbreviations used:
- ECM
extracellular matrix
- FACS
fluorescence-activated cell sorting
- fl-HA
fluorescent-HA
- GSP
gene-specific primer
- HA
hyaluronic acid, hyaluronate, hyaluronan
- HARE
hyaluronan receptor for endocytosis
- LEC
liver endothelial cell
- mAb
monoclonal antibody
- ORF
open reading frame
- PBS
phosphate-buffered saline
- RT-PCR reverse transcription-polymerase chain reaction
SSC, saline sodium citrate
- TBS
Tris-buffered saline
Footnotes
DOI: 10.1091/mbc.02–03–0048.
REFERENCES
- Abatangelo G, Weigel PH, editors. New Frontiers in Medical Sciences: Redefining Hyaluronan. Amsterdam: Elsevier Science B.V; 2000. [Google Scholar]
- Bajorath J, Greenfield B, Munro SB, Day AJ, Aruffo A. Identification of CD44 residues important for hyaluronan binding and delineation of the binding site. J Biol Chem. 1998;273:338–343. doi: 10.1074/jbc.273.1.338. [DOI] [PubMed] [Google Scholar]
- Banerji S, Day AJ, Kahmann JD, Jackson DG. Characterization of a functional hyaluronan-binding domain from the human CD44 molecule expressed in Escherichia coli. Protein Expr Purif. 1998;14:371–381. doi: 10.1006/prep.1998.0971. [DOI] [PubMed] [Google Scholar]
- Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burd DAR, Greco RM, Regauer S, Longaker MT, Siebert JW, Garg HG. Hyaluronan and wound healing: a new perspective. Br J Plast Surg. 1991;44:579–584. doi: 10.1016/0007-1226(91)90093-y. [DOI] [PubMed] [Google Scholar]
- Burnette WN. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate–polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chen WY, Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999;7:79–89. doi: 10.1046/j.1524-475x.1999.00079.x. [DOI] [PubMed] [Google Scholar]
- Csoka TB, Frost GI, Stern R. Hyaluronidases in tissue invasion. Invasion Metastasis. 1997;17:297–311. [PubMed] [Google Scholar]
- Deed R, Rooney P, Kumar P, Norton JD, Smith J, Freemont AJ, Kumar S. Early-response gene signaling is induced by angiogenic oligosaccharides of hyaluronan in endothelial cells. Inhibition by non-angiogenic, high-molecular-weight hyaluronan. Int J Cancer. 1997;71:251–256. doi: 10.1002/(sici)1097-0215(19970410)71:2<251::aid-ijc21>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Delpech B, Girard N, Bertrand P, Courel MN, Chauzy C, Delpech A. Hyaluronan: fundamental principles and applications in cancer. J Intern Med. 1997;242:41–8. doi: 10.1046/j.1365-2796.1997.00172.x. [DOI] [PubMed] [Google Scholar]
- Fraser JR, Appelgren LE, Laurent TC. Tissue uptake of circulating hyaluronic acid. A whole body autoradiographic study. Cell Tissue Res. 1983;233:285–293. doi: 10.1007/BF00238296. [DOI] [PubMed] [Google Scholar]
- Fraser JR, Laurent TC, Pertoft H, Baxter E. Plasma clearance, tissue distribution and metabolism of hyaluronic acid injected intravenously in the rabbit. Biochem J. 1981;200:415–424. doi: 10.1042/bj2000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas JP, Filipe P, Emerit I, Meunier P, Manso CF, Guerra Rodrigo F. Hyaluronic acid in progressive systemic sclerosis. Dermatology. 1996;192:46–49. doi: 10.1159/000246314. [DOI] [PubMed] [Google Scholar]
- Gakunga P, Frost G, Shuster S, Cunha G, Formby B, Stern R. Hyaluronan is a prerequisite for ductal branching morphogenesis. Development. 1997;124:3987–3997. doi: 10.1242/dev.124.20.3987. [DOI] [PubMed] [Google Scholar]
- Goa KL, Benfield P. Hyaluronic acid. A review of its pharmacology and use as a surgical aid in ophthalmology, and its therapeutic potential in joint disease and wound healing. Drugs. 1994;47:536–566. doi: 10.2165/00003495-199447030-00009. [DOI] [PubMed] [Google Scholar]
- Gubler U, Hoffman BJ. A simple and very efficient method for generating cDNA libraries. Gene. 1983;25:263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Handler M, Yurchenco PD, Iozzo RV. Developmental expression of perlecan during murine embryogenesis. Dev Dyn. 1997;210:130–145. doi: 10.1002/(SICI)1097-0177(199710)210:2<130::AID-AJA6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Horton MR, McKee CM, Bao C, Liao F, Farber JM, Hodge-DuFour J, Pure E, Oliver BL, Wright TM, Noble PW. Hyaluronan fragments synergize with interferon-gamma to induce the C-X-C chemokines Mig and interferon-inducible protein-10 in mouse macrophages. J Biol Chem. 1998;273:35088–35094. doi: 10.1074/jbc.273.52.35088. [DOI] [PubMed] [Google Scholar]
- Horton MR, Olman MA, Bao C, White KE, Choi AM, Chin BY, Noble PW, Lowenstein CJ. Regulation of plasminogen activator inhibitor-1 and urokinase by hyaluronan fragments in mouse macrophages. Am J Physiol Lung Cell Mol Physiol. 2000;279:707–715. doi: 10.1152/ajplung.2000.279.4.L707. [DOI] [PubMed] [Google Scholar]
- Kahmann JD, O'Brien R, Werner JM, Heinegard D, Ladbury JE, Campbell ID, Day AJ. Localization and characterization of the hyaluronan-binding site on the link module from human TSG-6. Structure Fold Des. 2000;8:763–774. doi: 10.1016/s0969-2126(00)00163-5. [DOI] [PubMed] [Google Scholar]
- Knudson CB, Knudson W. Hyaluronan-binding proteins in development, tissue homeostasis, and disease. FASEB J. 1993;7:1233–1241. [PubMed] [Google Scholar]
- Kose H, Rose D, Zhu X, Chiba A. Homophilic synaptic target recognition mediated by immunoglobulin-like cell adhesion molecule Fasciclin III. Development. 1997;124:4143–4152. doi: 10.1242/dev.124.20.4143. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai KN, Szeto CC, Lam CWK, Lai KB, Wong TYH, Leung JCK. Increased ascitic level of hyaluronan in liver cirrhosis. J Lab Clin Med. 1998;131:354–359. doi: 10.1016/s0022-2143(98)90186-x. [DOI] [PubMed] [Google Scholar]
- Laurent TC, Fraser JRE. In: In: Degradation of Bioactive Substances: Physiology and Pathophysiology. Henriksen JH, editor. Boca Raton, FL: CRC Press; 1991. pp. 249–265. [Google Scholar]
- Laurent TC, Fraser JRE. Hyaluronan. FASEB J. 1992;6:2397–2404. [PubMed] [Google Scholar]
- Lundin A, Engstrom-Laurent A, Hallgren R, Michaelsson G. Circulating hyaluronate in psoriasis. Br J Dermatol. 1985;112:663–671. doi: 10.1111/j.1365-2133.1985.tb02334.x. [DOI] [PubMed] [Google Scholar]
- Manek NJ, Lane NE. Osteoarthritis: current concepts in diagnosis and management. Am Fam Physician. 2000;61:1795–1804. [PubMed] [Google Scholar]
- Manicourt DH, Poilvache P, Nzeusseu A, van Egeren A, Devogelaer JP, Lenz ME, Thonar EJ. Serum levels of hyaluronan, antigenic keratan sulfate, matrix metalloproteinase 3, and tissue inhibitor of metalloproteinases 1 change predictably in rheumatoid arthritis patients who have begun activity after a night of bed rest. Arthritis Rheum. 1999;42:1861–1869. doi: 10.1002/1529-0131(199909)42:9<1861::AID-ANR10>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- McCourt PAG, Gustafson S. On the adsorption of hyaluronan and ICAM-1 to modified hydrophobic resins. Int J Biochem Cell Biol. 1997;29:1179–1189. doi: 10.1016/s1357-2725(97)00058-7. [DOI] [PubMed] [Google Scholar]
- McCourt PA, Smedsrod BH, Melkko J, Johansson S. Characterization of a hyaluronan receptor on rat sinusoidal liver endothelial cells and its functional relationship to scavenger receptors. Hepatology. 1999;30:1276–1286. doi: 10.1002/hep.510300521. [DOI] [PubMed] [Google Scholar]
- McGary CT, Raja RH, Weigel PH. Endocytosis of hyaluronic acid by rat liver endothelial cells. Evidence for receptor recycling. Biochem J. 1989;257:875–884. doi: 10.1042/bj2570875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- Meyer K, Palmer JW. The polysaccharide of the vitreous humor. J Biol Chem. 1934;107:629–634. [Google Scholar]
- Mouta Carreira CM, Nasser SM, di Tomaso E, Padera TP, Boucher Y, Tomarev SI, Jain RK. LYVE-1 is not restricted to the lymph vessels: expression in normal liver blood sinusoids and down-regulation in human liver cancer and cirrhosis. Cancer Res. 2001;61:8079–8084. [PubMed] [Google Scholar]
- Oka JA, Christensen MD, Weigel PH. Hyperosmolarity inhibits galactosyl receptor-mediated but not fluid phase endocytosis in isolated rat hepatocytes. J Biol Chem. 1989;264:12016–12024. [PubMed] [Google Scholar]
- Rahmanian M, Pertoft H, Kanda S, Christofferson R, Claesson-Welsh L, Heldin P. Hyaluronan oligosaccharides induce tube formation of a brain endothelial cell line in vitro. Exp Cell Res. 1997;237:223–230. doi: 10.1006/excr.1997.3792. [DOI] [PubMed] [Google Scholar]
- Raja RH, LeBoeuf R, Stone G, Weigel PH. Preparation of alkylamine and 125I-radiolabeled derivatives of hyaluronic acid uniquely modified at the reducing end. Anal Biochem. 1984;139:168–177. doi: 10.1016/0003-2697(84)90402-0. [DOI] [PubMed] [Google Scholar]
- Raja RH, McGary CT, Weigel PH. Affinity and distribution of surface and intracellular hyaluronic acid receptors in isolated rat liver endothelial cells. J Biol Chem. 1988;263:16661–16668. [PubMed] [Google Scholar]
- Rosier RN, O'Keefe RJ. Hyaluronic acid therapy. Instr Course Lect. 2000;49:495–502. [PubMed] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J, Milpetz F, Bork P, Ponting CP. SMART, a simple modular architecture research tool: identification of signaling domains. Proc Natl Acad Sci USA. 1998;95:5857–5864. doi: 10.1073/pnas.95.11.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selander-Sunnerhagen M, Ullner M, Persson E, Teleman O, Stenflo J, Drakenberg T. How an epidermal growth factor (EGF)-like domain binds calcium. High resolution NMR structure of the calcium form of the NH2-terminal EGF-like domain in coagulation factor X. J Biol Chem. 1992;267:19642–19649. doi: 10.2210/pdb1ccf/pdb. [DOI] [PubMed] [Google Scholar]
- Smedsrod B, Malmgren M, Ericsson J, Laurent TC. Morphological studies on endocytosis of chondroitin sulfate proteoglycan by rat liver endothelial cells. Cell Tissue Res. 1988;253:39–45. doi: 10.1007/BF00221737. [DOI] [PubMed] [Google Scholar]
- Tammi R, Saamanen AM, Maibach HI, Tammi M. Degradation of newly synthesized high molecular mass hyaluronan in the epidermal and dermal compartments of human skin in organ culture. J Invest Dermatol. 1991;97:126–130. doi: 10.1111/1523-1747.ep12478553. [DOI] [PubMed] [Google Scholar]
- Taylor JM, Illmensee R, Summers J. Efficient transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976;442:324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Thylen A, Wallin J, Martensson G. Hyaluronan in serum as an indicator of progressive disease in hyaluronan-producing malignant mesothelioma. Cancer. 1999;86:2000–2005. [PubMed] [Google Scholar]
- Toole BP. Hyaluronan in morphogenesis. J Intern Med. 1997;242:35–40. doi: 10.1046/j.1365-2796.1997.00171.x. [DOI] [PubMed] [Google Scholar]
- Tsifrina E, Ananyeva NM, Hastings G, Liau G. Identification and characterization of three cDNAs that encode putative novel hyaluronan-binding proteins, including an endothelial cell-specific hyaluronan receptor. Am J Pathol. 1999;155:1625–1633. doi: 10.1016/S0002-9440(10)65478-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley EA. Molecular mechanisms of cell motility. Cancer Metastasis Rev. 1992;11:1–3. doi: 10.1007/BF00047598. [DOI] [PubMed] [Google Scholar]
- Vertel BM, Grier BL, Li H, Schwartz NB. The chondrodystrophy, nanomelia: biosynthesis and processing of the defective aggrecan precursor. Biochem J. 1994;301:211–216. doi: 10.1042/bj3010211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel PH, Fuller GM, LeBoeuf RD. A model for the role of hyaluronic acid and fibrin in the early events during the inflammatory response and wound healing. J Theoret Biol. 1986;119:219–234. doi: 10.1016/s0022-5193(86)80076-5. [DOI] [PubMed] [Google Scholar]
- West DC, Hampson IN, Arnold F, Kumar S. Angiogenesis induced by degradation products of hyaluronic acid. Science. 1985;14:1324–1326. doi: 10.1126/science.2408340. [DOI] [PubMed] [Google Scholar]
- Yamada M, Fukuda Y, Nakano I, Katano Y, Takamatsu J, Hayakawa T. Serum hyaluronan as a marker of liver fibrosis in hemophiliacs with hepatitis C virus-associated chronic liver disease. Acta Hematol. 1998;99:212–216. doi: 10.1159/000040841. [DOI] [PubMed] [Google Scholar]
- Yang B, Yang BL, Savani RC, Turley EA. Identification of a common hyaluronan binding motif in the hyaluronan binding proteins RHAMM, CD44 and link protein. EMBO J. 1994;13:286–296. doi: 10.1002/j.1460-2075.1994.tb06261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannariello-Brown J, Frost SF, Weigel PH. Identification of the Ca(+2)-independent endocytic hyaluronan receptor in rat liver sinusoidal endothelial cells using a photoaffinity cross-linking reagent. J Biol Chem. 1992a;267:20451–20456. [PubMed] [Google Scholar]
- Yannariello-Brown J, McGary CT, Weigel PH. The endocytic hyaluronan receptor in rat liver sinusoidal endothelial cells is Ca(+2)-independent and distinct from a Ca(+2)-dependent hyaluronan binding activity. J Cell Biochem. 1992b;48:73–80. doi: 10.1002/jcb.240480111. [DOI] [PubMed] [Google Scholar]
- Yannariello-Brown J, Zhou B, Ritchie D, Oka JA, Weigel PH. A novel ligand blot assay detects different hyaluronan-binding proteins in rat liver hepatocytes and sinusoidal endothelial cells. Biochem Biophys Res Commun. 1996;218:314–319. doi: 10.1006/bbrc.1996.0055. [DOI] [PubMed] [Google Scholar]
- Yannariello-Brown J, Zhou B, Weigel PH. Identification of a 175-kDa protein as the ligand-binding subunit of the rat liver sinusoidal endothelial cell hyaluronan receptor. Glycobiology. 1997;7:15–21. doi: 10.1093/glycob/7.1.15. [DOI] [PubMed] [Google Scholar]
- Zhou B, Oka JA, Singh A, Weigel PH. Purification and subunit characterization of the rat liver endocytic hyaluronan receptor. J Biol Chem. 1999;274:33831–33834. doi: 10.1074/jbc.274.48.33831. [DOI] [PubMed] [Google Scholar]
- Zhou B, Weigel JA, Fauss L, Weigel PH. Identification of the hyaluronan receptor for endocytosis (HARE) J Biol Chem. 2000;275:37733–37741. doi: 10.1074/jbc.M003030200. [DOI] [PubMed] [Google Scholar]