Abstract
Saccharomyces cerevisiae adapts to osmotic stress through the activation of a conserved high-osmolarity growth (HOG) mitogen-activated protein (MAP) kinase pathway. Transmission through the HOG pathway is very well understood, yet other aspects of the cellular response to osmotic stress remain poorly understood, most notably regulation of actin organization. The actin cytoskeleton rapidly disassembles in response to osmotic insult and is induced to reassemble only after osmotic balance with the environment is reestablished. Here, we show that one of three MEK kinases of the HOG pathway, Ssk2p, is specialized to facilitate actin cytoskeleton reassembly after osmotic stress. Within minutes of cells' experiencing osmotic stress or catastrophic disassembly of the actin cytoskeleton through latrunculin A treatment, Ssk2p concentrates in the neck of budding yeast cells and concurrently forms a 1:1 complex with actin. These observations suggest that Ssk2p has a novel, previously undescribed function in sensing damage to the actin cytoskeleton. We also describe a second function for Ssk2p in facilitating reassembly of a polarized actin cytoskeleton at the end of the cell cycle, a prerequisite for efficient cell cycle completion. Loss of Ssk2p, its kinase activity, or its ability to localize and interact with actin led to delays in actin recovery and a resulting delay in cell cycle completion. These unique capabilities of Ssk2p are activated by a novel mechanism that does not involve known components of the HOG pathway.
INTRODUCTION
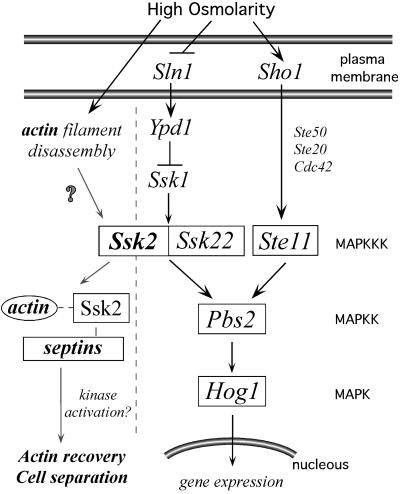
Mitogen-activated protein (MAP) kinase cascades play a critical role in all eukaryotes for adaptation to hyperosmotic stress (Gustin et al., 1998). In mammalian cells, components of the JNK and p38 pathways are believed to be involved in recovery from osmotic insult (Takekawa et al., 1997), whereas in the eukaryotic model, Saccharomyces cerevisiae, the high-osmolarity growth (HOG) pathway has been shown to be critical for adaptation to osmotic stress. In yeast, two plasma membrane proteins, Sln1p and Sho1p (Figure 1), activate the HOG pathway. Signals from Sln1p and Sho1p are transmitted through two independent branches of the HOG pathway that converge to activate the MAPKK Pbs2p (Maeda et al., 1995; Posas and Saito, 1997), which in turn phosphorylates and activates the MAPK Hog1p (Brewster et al., 1993). Phosphorylated Hog1p accumulates in the nucleus and induces the expression of genes involved in glycerol synthesis (Gustin et al., 1998). A resulting increase in intracellular glycerol concentration allows the cell to achieve osmotic balance with the external environment. Only disruption of both the Sho1 and Sln1 branches of the HOG pathway leads to osmosensitivity (Maeda et al., 1995).
Figure 1.
The S. cerevisiae HOG signal transduction pathway.
The focus of this work is on Ssk2p, one of two MAPKKKs of the Sln1p branch of the HOG pathway. The plasma membrane sensor Sln1p in association with Ypd1p forms a two-component, histidine phospho-relay that phosphorylates Ssk1p (Maeda et al., 1994; Posas et al., 1996). If the external osmolarity rises, the phospho-relay reaction is inhibited, and unphosphorylated Ssk1p accumulates. Unphosphorylated Ssk1p binds to the N-terminus of the MAPKKK Ssk2p and activates Ssk2p to autophosphorylate on Thr1460 (Posas and Saito, 1998). On autophosphorylation, Ssk2p becomes activated to phosphorylate Pbs2p, the downstream MAPKK of the HOG pathway. Ssk2p has a close homolog, Ssk22p, which is redundant with Ssk2p for phosphorylation/activation of Pbs2p (Maeda et al., 1995; Posas and Saito, 1998). Osmotic regulation is important to all eukaryotic cells; therefore, this pathway is highly conserved. In fact, Ssk2p shares strong similarity with the human MEK kinase MTK1, which can substitute for Ssk2p in the HOG pathway of yeast (Takekawa et al., 1997).
In addition to activation of glycerol synthesis, hyperosmotic stress also provokes a transient disassembly of the yeast actin cytoskeleton leading to a cell cycle delay (Chowdhury et al., 1992). The actin cytoskeleton is critically involved in survival of osmotic stress, because mutations in actin (Wertman et al., 1992) or in many actin-associated proteins cause osmosensitivity (Botstein et al., 1997). In yeast cells, actin filaments form two discrete polarized structures: cortical patches and actin cables. Actin cables are oriented along the long axis of budding cells, frequently terminating and attaching to polarized cortical patches (Amberg, 1998). This orientation is necessary to direct polarized secretion and polarized cell growth. Late in the cell cycle, actin patches reorient to the mother-bud neck and redirect polarized growth to the neck leading to septation and cell separation. Hyperosmotic shock causes a rapid disassembly of actin cables, followed by depolarization of actin patches from both the bud and the mother-bud neck. Depolarization of the actin cytoskeleton leads to a transient cell cycle arrest; after ∼1 h, the cells reassemble a polarized actin cytoskeleton, and polarized growth resumes (Chowdhury et al., 1992; Brewster and Gustin, 1994). The mechanisms regulating dynamic changes in actin cytoskeleton organization on osmotic shock are still not understood. However, work presented here suggests that Ssk2p is critically involved in actin reassembly after osmotic stress.
In this regard, Ssk2p has two related functions in mediating actin recovery from stress. First, on osmotic shock or damage to the actin cytoskeleton, Ssk2p associates stoichiometrically with actin and relocates from the cytosol to the septin cytoskeleton of the bud neck. This sensor function is activated by a novel mechanism (possibly actin disassembly itself) that does not involve other components of the HOG pathway or the kinase functions of Ssk2p. Subsequently, Ssk2p is required for timely reassembly of the actin cytoskeleton in the neck and resumption of the cell cycle. The role of Ssk2p in actin cytoskeleton recovery does require Ssk2p localization to the neck, its interaction with actin, and the kinase activity of Ssk2p.
MATERIALS AND METHODS
Yeast Strains, Media, and Genetic Methods
Strains used in this study are listed in Table 1. Replacement of the genomic copy of SSK2 with an allele expressing Ssk2p fused at its C terminus to green fluorescent protein (GFP) (TYY14) was done as described (Longtine et al., 1998). Deletion mutants were prepared by replacement of the chromosomal copies of the genes in diploid strain FY86 × FY23 with the URA3 gene by double-fusion PCR (Amberg et al., 1995b). The sequences of primers used for the SSK2 deletion were as follows: 5′-T G A A A A G A G T G A T A A A G G T G G G-3′ and 5′-G T C G T G A C T G G G A A A A C C C T G G C G A T G A C T G C C T C A T C T T G G A G-3′, 5′-T C C T G T G T G A A A T T G T T A T C C G C T G G T T G A G C T G T T G A T G G A T C-3′ and 5′-G C G A G C G T T T T A C A T T A A T C C-3′. The sequences of primers used for the SSK22 deletion were as follows: 5′-C A C T G G A T A T G A C G A T G A T G A T G C-3′ and 5′-G T C G T G A C T G G G A A A A C C C T G G C G G G A A C T A C C T T T T A T A G C C A C-3′, 5′-T C C T G T G T G A A A T T G T T A T C C G C T T A G C A T T T G G C A A C T C A G A G-3′ and 5′-G A G A T A G C A C C G G G A A C T C T C A G G-3′. The sequences of primers used for the SSK1 deletion were as follows: 5′-G C C C C T G G A T T G A A T C T T T G A C-3′ and 5′-G T C G T G A C T G G G A A A A C C C T G G C G G T C T A T T C G T A G C C A A A C C T-3′, 5′-T C C T G T G T G A A A T T G T T A T C C G C T C A A A T A G A A T T G T G A G T T T G G-3′ and 5′-G A C T G C G A T G T G T T C A T C C A T G-3′. The sequences of primers used for the PBS2 deletion were as follows: 5′-A G T G A G C G A T T T C G T G A G C-3′ and 5′-G T C G T G A C T G G G A A A A C C C T G G C G C T C A T G G A G A C T G A G G T T A G C-3′, 5′-T C C T G T G T G A A A T T G T T A T C C G C T C A T A T G G G T G G T T T A T A G C G-3′ and 5′-A A A G C A C C C A C A A G C A C A C T-3′. Gene disruption was verified by PCR analysis of chromosomal DNA. The haploid ssk2Δ ssk22Δ (TYY9B) strain was obtained by mating the single-mutant TYYD5B and TYY3D and tetrad dissection. The haploid strain sho1Δ/ssk2Δ/ssk22Δ (TYY10) was obtained by mating TYY9B and the sho1Δ haploid strain (TYY12) and tetrad dissection. The haploid strain sho1Δ/sln1Δ/ssk1Δ (TYY11) was obtained by mating the sho1Δ (TYY12) and the sln1Δ/ssk1Δ (TYY13) haploid strains and tetrad dissection. Standard yeast media and genetic procedures were as described (Rose et al., 1990). To induce osmotic shock, cells were grown in YPD medium for 6 h, and an equal volume of 1.8 M NaCl in YPD was added to a final concentration of 0.9 M NaCl. To examine septin dependence for Ssk2p localization on osmotic shock, the wild-type strain (FY86) and mutant cdc12–6 strain (CS01B), each expressing a GFP-Ssk2p fusion protein from plasmid pTY111U, were grown for 6 h at 25°C, osmotically stressed with 0.9 M NaCl, and then transferred to 37°C for 20 min. In each case, ≥100 cells were counted. Data from three or more independent experiments were used to calculate statistical errors.
Table 1.
Yeast strains
| Strain | Genotype | Source |
|---|---|---|
| Y187 | MATα gal 4 gal 80 his3 trp1 ade2 ura3 leu2 GAL–lacZ | Bai and Elledge |
| Y190 | MATα gal4 gal80 his3 trp1 ade2 ura3 leu2 URA3::GAL lacZ LYS2::GAL-HIS3 cyh r | Bai and Elledge |
| FY86 | MATα ura3-52 leu2 his3 | Winston |
| FY23 | MATα trp1·63 ura3 leu2 | Winston |
| TYYD5B | MATα ssk2::URA3 his3 | This study |
| TYY7D | MATα ssk1::URA3 his3 | This study |
| TYY3D | MATα ssk22::URA3 trp1 | This study |
| TYY9B | MATα ssk2::URA3 ssk22::URA3 trp1 | This study |
| TYY1B | MATα pbs2::URA3 his3 | This study |
| TYY10 | MATα ssk2::URA3 ssk22::URA3 sho1::URA3 trp1 | This study |
| TYY11 | MATα ssk1::URA3 sln1::URA3 sho1::URA3 trp1 | This study |
| TYY12 | MATα sho1::URA3 his3 | This study |
| TYY13 | MATα ssk1::URA3 sln1::URA3 trp1 | This study |
| TYY14 | MATα Ssk2-GFP his3 | This study |
| 3482 | MATα his3 leu2 met15 ura3 cdc10::KAN | Research Genetics |
| CS01B | MATα cdc12-6 leu2 ura3 | Haarer |
Plasmid Constructions and DNA Manipulations
Plasmids used in this study are listed in Table 2. General cloning methods were as described previously (Maniatis et al., 1989). For the GFP-Ssk2p expression construct (pTY111), the SSK2 coding region was PCR-amplified from S. cerevisiae genomic DNA with primers TYO-Ssk2-HindIII (5′-C G C G A A G C T T G A T G T C G C A T T C A G A C T A C-3′) and TYO-Ssk2-NheI (5′-C G G G C T A G C C T A C T C T C T T T C C T C A G-3′). The PCR fragment was digested with HindIII and NheI and cloned as a fusion to the C-terminus of the GFP coding region in low-copy Cen vector pTD125 (Doyle and Botstein, 1996), which had been digested with HindIII and XbaI. The construct pTY119 coding for GFP-Ssk2ΔLD (localized domain) was generated by double-fusion PCR with primers TYO-Ssk2-HindIII and TYO-NoFrag1–2 (5′-C T C G T C A G C G C T C A T A T T A T C G T C A T C T G A A A A C T G A G T A T T G A A-3′), and TYO-Ssk2-NheI and TYO-NoFrag1–3 (5′-A A T A C T C A G T T T T C A G A T G A C G A T A A T A T G A G C G C T G A C G A G G C T -3′).
Table 2.
Plasmids
| Plasmid | Description | Source |
|---|---|---|
| pTD125 | URA3 CEN ACT1p-GFP-MCS-ACT1t | Doyle et al., 1996 |
| pMF5 | LEU2 GAL4 AD-ssk2-AIR (aa 323–1032) | This study |
| p366 | LEU2 CEN | Hieter |
| pTY111U | GFP-SSK2 URA3 | This study |
| pTY111La | GFP-SSK2 LEU2 | This study |
| pTY112U | GFP-SSK2(T1460A) URA3 | This study |
| pTY112La | GFP-SSK2(T1460A) LEU2 | This study |
| pTY113U | GFP-SSK2(K1295N) URA3 | This study |
| pTY113La | GFP-SSK2(K1295N) LEU2 | This study |
| pTY114 | GFP-SSK2(aa 1–1032) | This study |
| pTY115 | GFP-SSK2(aa 323–1579; K1295N) | This study |
| pTY116 | GFP-SSK2(aa 850–1579; K1295N) | This study |
| pTY117 | GFP-SSK2(aa 323–1032) | This study |
| pEG(kt) | URA3 CEN GAL10p-ACT1t | Mitchell et al., 1993 |
| pTY118 | GST-SSK2 based on pEG(kt) | This study |
| pTY119U | GFP-SSK2ΔLD (aa 426–466Δ) URA3 | This study |
| pTY119La | GFP-SSK2ΔLD (aa 426–466Δ) LEU2 | This study |
| pTY119L(K/N) | GFP-SSK2ΔLD (aa 426–466Δ; K1295N)LEU2 | This study |
| pTY120 | GST-SSK2ΔLD (aa 426–466Δ) | This study |
| pSE1111 | LEU2 GAL4 AD-SNF4 | Elledge |
| pSE1112 | TRP1 GAL4 DBD-SNF1 | Elledge |
| pDab7 | TRP1 GAL4 DBD-ACT1 | Amberg et al., 1995b |
| pTY121 | LEU2 GAL4 AD-ssk2-AIRΔLD (aa 323–425+467–1032) | This study |
| pTY122 | TRP1 GAL4 DBD-CDC11 | This study |
| pTY123 | TRP1 GAL4 DBD-CDC10 | This study |
| pRS315 | LEU2 CEN GFP-CDC3 | Haarer |
These plasmids were constructed by homologous recombination between the pTD125-based constructs and p366.
For the construct pTY114, which codes for GFP-Ssk2p (aa 1–1032), the SSK2 coding region was PCR-amplified with primers TYO-Ssk2-HindIII and TYO-pMF5-NheI (5′-C G G G C T A G C G T C A C A T T G G T A T T C T A A G G-3′). For the construct pTY115, which codes for GFP-Ssk2p (aa 323-1579), the SSK2 coding region was PCR-amplified with primers TYO-pMF5-HindIII (5′-C G C G A A G C T T G A C G T C G A G C A C A A A A A A T-3′) and TYO-Ssk2-NheI. For the construct pTY116, which codes for GFP-Ssk2p (aa 850-1579), the SSK2 coding region was PCR-amplified from genomic DNA with primers TYO-Frag2-HindIII (5′-C G C G A A G C T T G G A A A T T A A T A A C A G T C T G A C-3′) and TYO-Ssk2-NheI. For the construct pTY117, which codes for GFP-Ssk2p (aa 323-1032), the SSK2 coding region was PCR-amplified from genomic DNA with primers TYO-pMF5-HindIII and TYO-Ssk2-NheI. The PCR fragments were digested with HindIII and NheI and ligated into HindIII/NheI-digested plasmid pTD125. The construct pTY112U coding for GFP-Ssk2pThr1460/Ala was generated by PCR-directed mutagenesis with primers 5′-A T G T A C A T A G G A G C T C C C A T C A T G-3′ and 5′-C A T G A T G G G A G C T C C T A T G T A C A T-3′ (the mutated residues are italicized). The construct pTY113U coding for GFP-Ssk2pLys1295Arn was generated by PCR-directed mutagenesis with primers 5′-G C T A T C T T G A A T A T T G A T T T C G T T G A C T G C T A A A A T C T C A C C-3′ and 5′-G G T G A G A T T T T A G C A G T C A A C G A A A T C A A T A T T C A A G A T A G C-3′. Because the Thr1460/Ala mutation introduced a SacI site and the Lys1295Asn introduced a HincII site, each mutation was verified by DNA restriction digestion. For the GST-Ssk2p expression construct (pTY118), the SSK2 coding region was PCR-amplified from genomic DNA with primers TYO-Ssk2-XmaI (5′-G C C C C C C G G G A A T G T C G C A T T C A G A C T A C) and TYO-Ssk2-HindIII (5′-G G G A A G C T T C T A C T C T C T T T C C T C A G T). The PCR fragment was digested with XmaI and HindIII and cloned as a fusion to the C-terminal coding region of the GST gene in plasmid pEG(kt) (Mitchell et al., 1993), which had been digested with SmaI and HindIII. For the GST-Ssk2ΔLD expression construct (pTY120), the SSK2ΔLD coding region was PCR-amplified from plasmid pTY119 with primers TYO-Ssk2-XmaI and TYO-Ssk2-HindIII and cloned in pEG(kt) as described above. For the construct pTY121, which codes for the ΔLD actin interacting region of Ssk2p (AIRΔLD, aa 323–426 + 467-1032) fused to the GAL4 activation domain, the AIRΔLD coding region was PCR-amplified from plasmid pTY119U with primers TYO-pMF5–2H-XmaI (5′-G A G G C C C C G G G A A C G T C G A G C A C A A A A A A T A A T G) and TYO-pMF5–2H-XhoI (5′-G A G T C C T C G A G C T A G T C A C A T T G G T A T T C T A A G). The PCR fragments were digested with XmaI and XhoI and ligated into XmaI/XhoI-digested plasmid pACTII. For the construct pTY122, which codes for the GAL4 DNA binding domain (DBD)-Cdc11, the CDC11 coding region was PCR-amplified from genomic DNA with primers TY-2HCdc11-NdeI (5′-G A T G G C C A T A T G A T G T C C G G A A T A A T T G A C) and TY-2HCdc11-SalI (5′-G A G G C G T C G A C T C A T T C T T C C T G T T T G A T T T T). The PCR fragments were digested with NdeI and SalI and ligated into NdeI/SalI-digested plasmid pAS1-CYH2 (S. Elledge, unpublished observations). For the construct pTY123, which codes for GAL4 DBD-Cdc10, the CDC10 coding region was PCR-amplified from genomic DNA with primers TYO-2H-Cdc10-NcoI (5′-G A T G G C C A T G G C A A T G G A T C C T C T C A G C T C A G T A) and TYO-2H-Cdc10-SalI (5′-G A G G C G T C G A C T C A A C G T T G A A T G G C G T T G C T). The PCR fragments were digested with NcoI and SalI and ligated into NcoI/SalI-digested plasmid pAS1-CYH2.
Microscopy, Rhodamine-Phalloidin, and DAPI Staining
GFP-Ssk2p localization was visualized without fixation on a Zeiss Axioskop (Carl Zeiss, Inc., Oberkochen, Germany) with a Plan-APOCHROMAT 100X/1.4NA objective. Images were captured with a SPOT2 camera (Diagnostic Instruments, Inc.) and visualized in Adobe Photoshop (Adobe Systems, Inc.) Rhodamine-phalloidin staining of actin was performed as described (Bi et al., 1998). DNA was visualized by adding 4′,6-diamidino-2-phenylindole (DAPI; Sigma Chemical Co., St. Louis, MO) in mounting medium for fixed cells. To determine whether cells had completed cytokinesis, cells were fixed with 3.7% formaldehyde for 2 h at 30°C and washed twice with phosphate-buffered saline, and then their cell walls were digested with 0.5 mg/ml Zymolase (US Biological, MA) for 60 min at 37°C.
Cell Synchronization and Latrunculin Treatment
To examine localization of GFP-Ssk2p in the absence of F-actin, cells in YPD medium were treated with 200 μM latrunculin A (Lat A; from stock solution 20 mM in dimethyl sulfoxide) for 20 min. The same volume of dimethyl sulfoxide was added to the control cell culture. For mitotic arrest assays, cells at a density of 5 × 106 to 1 × 107 cells/ml were grown on selective synthetic medium in the presence of 15 mg/ml hydroxyurea (HU) for 3 h at 30°C. Cells were pelleted, washed once with YPD to remove residual HU, and released into fresh YPD. After a 1-h recovery, an equal volume of 1.8 M NaCl in YPD was added to a final concentration of 0.9 M NaCl. Cells were fixed in 3.7% formaldehyde before osmotic stress and 60, 90, 120, 150, and 180 min after osmotic stress. In each case, >100 cells were counted. Numbers reported are averages calculated from 2–5 independent experiments.
Two-Hybrid Analyses
The two-hybrid analyses were performed as described (Amberg et al., 1995). Strain Y190 was transformed with constructs encoding fusions of actin (pDab7), the septins Cdc10 (pTY123) or Cdc11 (pTY122), and Snf1 (pSE1112) to the GAL4 DBD. Strain Y187 was transformed with constructs encoding fusions of the actin interacting region of Ssk2p (ssk2-AIR, aa 323-1032, pMF5), ssk2-AIR ΔLD (aa 323–425 + 467-1032, pTY121), or Snf4p (pSE1111) to the GAL4 activation domain (AD). After transformation, strains Y190 and Y187 were mated and plated onto SC-Trp-Leu medium. The selected diploids, carrying both DBD and AD fusions, were plated on SD + 10 μg/ml adenine plus 100 mM 3-aminotriazole (Sigma Chemical Co.) to assess activation of the HIS3 reporter.
Coprecipitation Assay
Cells were grown on selective synthetic medium in the presence of 2% galactose to a density of 1 × 107 cells/ml. The cultures before and 15 min after osmotic shock (0.9 M NaCl) were cooled on ice and harvested by centrifugation. Cells were resuspended in buffer A (50 mM Tris-HCl, pH 7.5, 15 mM EDTA, 2 mM dithiothreitol, 0.1% Triton X-100, 1 mM PMSF, 2 mM benzamidine, 5 μg/ml leupeptin, 1 μg/ml pepstatin A, 5 μg/ml aprotinin, 2 μg/ml chymostatin, 2.5 μg/ml antipain, 100 mM NaCl) and lysed by use of glass beads. Cell extract (750 μl in buffer A) was incubated with 100 μl of glutathione-Sepharose beads for 50 min at 4°C. The beads were washed 5 times with 1 ml of buffer A, resuspended in reducing sample buffer, and separated by electrophoresis through a 10% SDS-polyacrylamide gel. To determine stoichiometry of the Ssk2p–actin interaction, known amounts of purified GST and actin were analyzed in Western assays in parallel with GST pull-down samples. Immunoblotting was done with an anti-GST monoclonal antibody (mAb) at a 1:500 dilution (Amersham Biosciences, Arlington Heights, IL) and the mouse anti-actin mAb C4 at a 1:400 dilution (ICN Biomedicals, Inc., Cleveland, OH). In parallel, protein gels were stained with Ruby Red dye, and protein bands were visualized and quantified by use of Fluor-S system software (Bio-Rad, Richmond, CA) to determine relative amount of proteins before and after transfer to nitrocellulose.
RESULTS
Ssk2p Interacts With Actin in a Two-Hybrid Assay
A yeast genomic library (James et al., 1996) was screened, with the two-hybrid system, for actin-interacting proteins leading to the identification of the MAPKKK Ssk2p. A fragment of SSK2 encoding amino acids 323-1032 (ssk2-AIR) was found to interact specifically with actin (see Figure 5B). Note that this region of Ssk2p is upstream of, and does not overlap with, the kinase domain of Ssk2p (Figure 2D) but does partially overlap with the binding region for Ssk1p (aa 294–413), an activator of the Ssk2p kinase. Interestingly, an analogous fragment of the functionally redundant and homologous MAPKKK SSK22 (aa 141–787) was not able to interact with actin in the two-hybrid system.
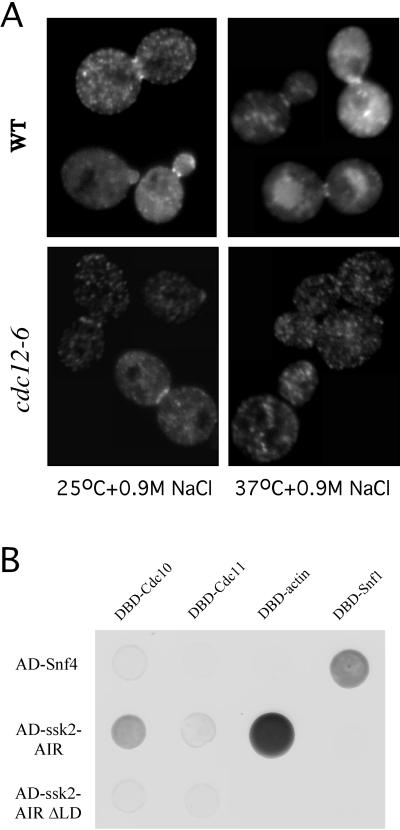
Figure 5.
The septin cytoskeleton is involved in neck localization of Ssk2p. (A) Wild-type (WT) strain (FY86) and cdc12–6 strain (CS01B) expressing GFP-Ssk2p from plasmid pTY111U were osmotically stressed with 0.9 M NaCl for 20 min at 25°C (left) and for 20 min after a shift to 37°C (right). GFP-Ssk2p localization was examined by fluorescence microscopy. (B) Strain Y190 was transformed with constructs encoding fusions of actin (pDab7), the septins Cdc10 (pTY123) or Cdc11 (pTY122), and Snf1 (pSE1112) to the GAL4 DBD. Strain Y187 was transformed with constructs encoding fusions of the ssk2-AIR (pMF5), ssk2-AIR ΔLD (pTY121), or Snf4p (pSE1111) to the GAL4 AD. Diploids carrying both DBD and AD fusions were selected on SC-Trp-Leu medium, then plated onto SD + 10 μg/ml adenine plus 100 mM 3-aminotriazole (Sigma Chemical Co.) to assess activation of the HIS3 reporter.
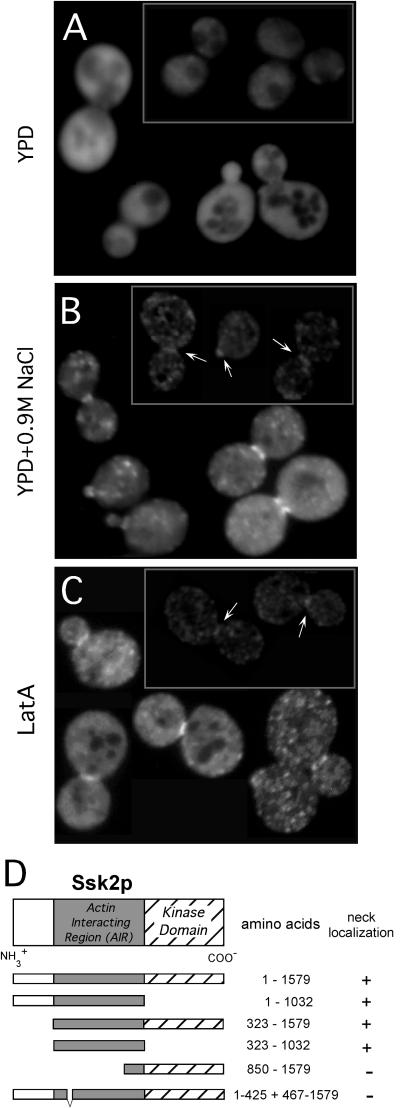
Figure 2.
Osmotic shock and actin depolymerization induce Ssk2p to localize to the neck and cortex of small buds. (A–C) Wild-type strain FY86 was transformed with plasmid pTY111U expressing GFP-Ssk2p, and the cells were examined by fluorescence microscopy. Distribution of GFP-Ssk2p in living cells is shown under (A) normal osmotic conditions, (B) 15 min after osmotic shock, and (C) 20 min after Lat A treatment. (A, Inset) Strain TYY14 carrying a genomic insertion of GFP fused to the C-terminal coding sequence of SSK2 was grown under normal osmotic conditions or (B, inset) osmotically stressed for 15 min and visualized by fluorescence microscopy. (D) The domains and subclones of SSK2 are shown schematically on the left with their precise amino acid positions indicated on the right. The ability of the subclones to translocate to the neck in response to osmotic shock is indicated by a +, and the inability to translocate to the neck is indicated by a −.
Ssk2p Rapidly Localizes to the Mother-Bud Neck in Response to Osmotic Shock
The Ssk2p-actin interaction suggested a potential role for Ssk2p in the cytoskeleton. Therefore, we next asked whether Ssk2p associates with cytoskeletal structures in vivo. First, we replaced the genomic copy of SSK2 with an allele that expressed Ssk2p fused at its C-terminus to GFP. This allele was found to be functional for transmission within the HOG pathway (our unpublished observations). In low-osmotic medium (YPD), Ssk2p-GFP was localized throughout the cytosol as visualized by fluorescence microscopy (Figure 2A, inset). However, within 5 min of application of osmotic stress (0.9 M NaCl), the fusion protein localized to the bud neck, to the cortex of very small buds, and in punctate spots (Figure 2B, inset). Expression from the integrated allele was quite low, making visualization difficult. Therefore, we constructed a fusion of GFP to the N-terminus of full-length Ssk2p, under the control of the actin promoter on a low-copy Cen vector (pTY111; all subsequent GFP-Ssk2p experiments were done with this low-copy vector). This construct was also able to complement an ssk2Δ allele (see Figure 3A), did not induce any adverse effects on cells, and led to an approximately sixfold increase in expression as assayed in Western blots with anti-GFP antibodies (our unpublished observations). As for the C-terminally tagged and integrated version, on low-osmotic medium, GFP-Ssk2p was uniformly distributed throughout the cytoplasm (Figure 2A). After a shift into 0.9 M NaCl, localization of GFP-Ssk2p expressed from the plasmid was similar to that observed for the integrated version (Figure 2B). Of osmotically stressed wild-type cells, 82 ± 2% showed accumulation of GFP-Ssk2p in the bud neck and small bud cortex. Localization of GFP-Ssk2p to the neck, to the small bud, and into punctate spots was transient; ∼1 h after osmotic stress, the protein was once again uniformly distributed in the cytosol (our unpublished observations). Note that spot localization of Ssk2p was quite variable between experiments and between cells within experiments but that neck and small bud cortex localization was very consistent.
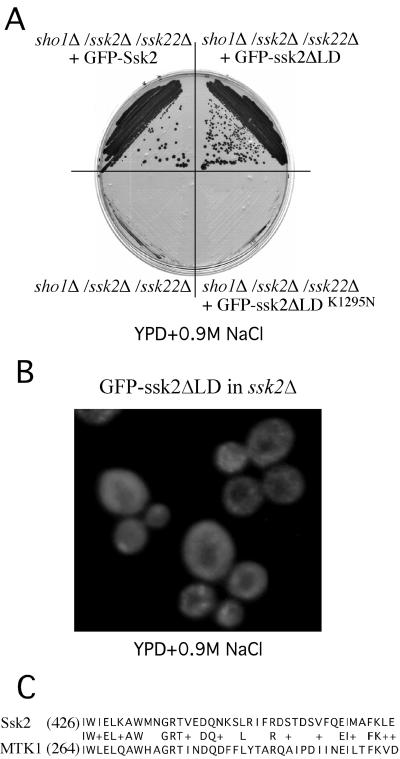
Figure 3.
The Ssk2ΔLD mutant is functional in the HOG pathway but is defective for neck localization. (A) The sho1Δ/ssk2Δ/ssk22Δ strain (TYY10) was transformed with plasmids expressing GFP-Ssk2 (pTY111L), GFP-Ssk2ΔLD (pTY119L), or GFP-Ssk2ΔLDK1295N [pTY119L(K/N)] and grown on solid YPD + 0.9 M NaCl medium. (B) The distribution of GFP-Ssk2ΔLD in wild-type FY86 cells is shown after 15 min of osmotic stress. (C) Sequence comparison between the LD of Ssk2p (aa 426–466) and an N-terminal fragment of human MTK1. + indicates a conservative amino acid substitution.
Ssk2p Kinase Activation Is Not Involved in Regulated Localization of Ssk2p
To map the region of Ssk2p involved in neck localization, we constructed truncation mutants of SSK2 fused to GFP (Figure 2D). We discovered that the actin-interacting region (aa 323-1032) of Ssk2p is necessary for neck localization. Sequence comparisons within this region of Ssk2p showed that amino acids 426–466 of Ssk2p are particularly well conserved with MTK1 (Figure 3C), a human homolog of Ssk2p that functions as a MEK kinase in the p38 stress-response pathway (Takekawa et al., 1997). A GFP-ssk2ΔLD mutant lacking these 40 amino acids (pTY119) was not able to localize to the neck and bud cortex after osmotic stress (Figure 3B). Note that this mutant could complement the osmotic defect of the sho1Δ/ssk2Δ/ssk22Δ strain (TYY10), proving that its kinase domain is functional and that it is capable of being bound and activated by Ssk1p (Figure 3A). Because the Ssk1p binding site lies immediately N-terminal to the LD motif, this small deletion does not appear to lead to a general disruption in the structure of the ssk2p N-terminal domain.
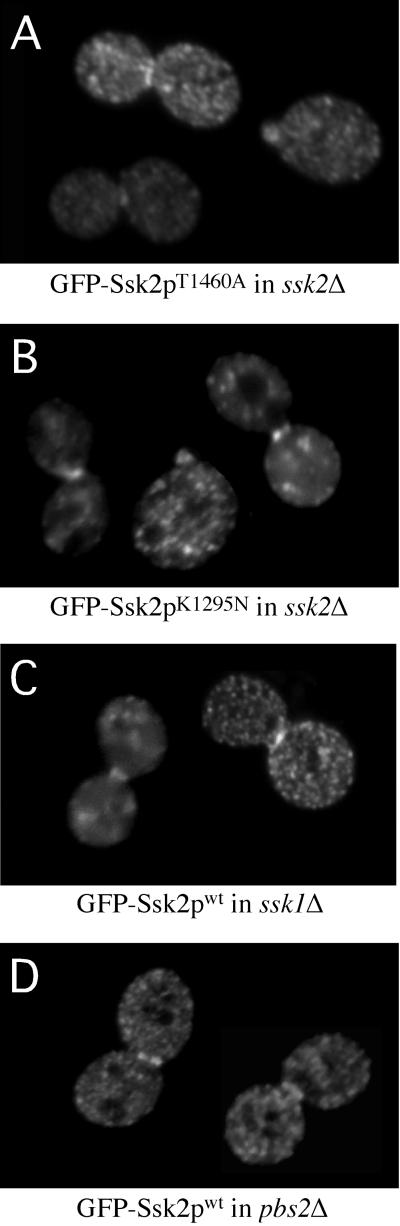
The preceding results suggested that the kinase domain appears to have little role in osmotically regulated localization of Ssk2p. To confirm this observation, we examined the localization of ssk2p mutants defective for both autophosphorylation and kinase activity. We found that an Ssk2p mutant unable to be phosphorylated (Thr1460/Ala) and a kinase-dead mutant of Ssk2p (Lys1295Asn) (Posas and Saito, 1998) localized to the neck and bud cortex on osmotic shock in 79 ± 3.6% and 79 ± 2.0% of the cells, respectively, compared with 82 ± 2% for wild-type GFP-Ssk2p (Figure 4, A and B). Therefore, neither autophosphorylation as induced by Ssk1p binding (or by any other means) nor activation of the kinase activity of Ssk2p is required for regulation of Ssk2p localization.
Figure 4.
Activation of the HOG pathway is not required for neck localization of Ssk2p. The ssk2Δ strain TYYD5B was transformed with (A) plasmid pTY112L, expressing GFP-Ssk2pT1460A, or (B) plasmid pTY113L, expressing GFP-Ssk2pK1295N. (C) ssk1Δ strain TYY7D and (D) pbs2Δ strain TYY1B were transformed with plasmid pTY112L expressing wild-type GFP-Ssk2p. Cells were osmotically stressed with 0.9 M NaCl for 15 min, and localization of the GFP-Ssk2p fusion proteins was examined by fluorescence microscopy.
We next examined whether transmission through the HOG pathway (Figure 1) is required for translocation of Ssk2p to the neck and bud cortex. We disrupted the pathway immediately upstream of Ssk2p by knocking out SSK1 (TYY7D) and found that Ssk2p still translocates to the neck in high-osmotic conditions in 77 ± 5% of cells (Figure 4C). Pbs2p has also been shown to localize to the neck in osmotically stressed cells and could therefore play a role in neck localization of Ssk2p (Reiser et al., 2000). However, in the pbs2Δ strain (TYY1B), GFP-Ssk2p moved to the neck and cortex of small buds on application of osmotic shock in 75 ± 1% of cells (Figure 4D). This also rules out the possibility of feedback regulation by the HOG pathway as a mechanism for regulating the initial movement of Ssk2p to the neck. Interestingly, GFP-Ssk2p remained in the neck for ≥3–4 h in the pbs2Δ strain after osmotic shock, whereas in wild-type cells, GFP-Ssk2p was uniformly distributed in the cytoplasm 1 h after osmotic shock (our unpublished observations). This suggests that relocation of Ssk2p to the cytoplasm requires that the cells achieve osmotic balance with the environment.
We have also examined Ssk2p localization in cells lacking both putative membrane sensors of the HOG pathway, Sho1p and Sln1p (the sho1Δ/sln1Δ/ssk1Δ strain, TYY11), and found no defect in osmotically induced Ssk2p translocation (81 ± 2.5% cells showed neck-localized Ssk2p). Collectively, these results suggest that activation of the HOG pathway either upstream or downstream of Ssk2p plays no role in translocation of Ssk2p to the neck or bud.
The Septin Cytoskeleton Is Involved in Neck Localization of Ssk2p
Many regulators of yeast cell polarity, including several kinases, localize to the mother-bud neck in a septin-dependent manner (Field and Kellogg, 1999). To examine septin dependence for GFP-Ssk2p localization, we expressed GFP-Ssk2p (pTY111) in a wild-type strain (FY86) and in a strain (CS01B) carrying a temperature-sensitive allele (cdc12–6) in one of the major septin genes CDC12 (Figure 5). It has been observed that mutants carrying this allele rapidly lose septin neck filaments (Kim et al., 1991). At permissive temperature (25°C), 77 ± 5% and 76 ± 4% of cells from both the wild-type and cdc12–6 cultures, respectively, displayed neck localization of GFP-Ssk2p on osmotic shock (Figure 5A). The cultures were shifted to 37°C for 20 min, and at the same time, they were subjected to osmotic stress (0.9 M NaCl) with prewarmed medium. After 20 min, neck localization of GFP-Ssk2p was observed in only 17 ± 5% of the septin mutant cells, compared with 75 ± 4% of the wild-type cells (Figure 5A). In parallel experiments, GFP-tagged Cdc3, expressed from plasmid (pRS315), localized to the neck in ∼20% of osmotically stressed cdc12–6 cells shifted to 37°C for 20 min, thus suggesting that septin cytoskeleton disassembly is incompletely penetrant in the cdc12–6 strain in this relatively short time course. Nonetheless, our observations indicate that an intact septin cytoskeleton is required for efficient neck localization of Ssk2p.
To gain potential insights into the mechanism of Ssk2p localization to the neck cytoskeleton, we tested the ability of the N-terminal AIR (aa 323-1032) of Ssk2p to interact with the septin proteins in a two-hybrid assay. We found that the Ssk2p AIR could not interact with septins Cdc3p or Cdc12p (our unpublished data) but is able to interact with the septin Cdc10p (Figure 5B). Deletion of the LD (aa 426–466) in the Ssk2p AIR resulted in loss of its ability to interact with Cdc10p in a two-hybrid assay (Figure 5B). In addition, neck localization of GFP-Ssk2p was observed in only 15 ± 5% of osmotically stressed cdc10Δ cells, compared with 75 ± 4% of the wild-type control cells (FY86). One possible explanation for residual neck localization of GFP-Ssk2p in cdc10Δ cells could be the ability of Ssk2p to interact with another septin, Cdc11p. Activation of two-hybrid reporters was weaker for the Ssk2p-Cdc11p interaction than for the Ssk2p-Cdc10p interaction but greater than that observed for the negative controls (Cdc11-Snf4) (Figure 5B). These results suggest that an intact septin cytoskeleton and Cdc10p in particular are involved in neck localization of Ssk2p.
Actin Cytoskeleton Disassembly Induces Translocation of Ssk2p to the Neck and Bud Cortex
Osmotic stress induces a rapid disassembly of the actin cytoskeleton (Chowdhury et al., 1992). Therefore, we asked whether actin disassembly in the absence of osmotic stress was sufficient to induce Ssk2p translocation to the neck. Lat A was used to induce rapid and complete disassembly of the actin cytoskeleton (Ayscough et al., 1997) in cells (FY86) expressing GFP-Ssk2p (pTY111). Within 2 min of Lat A treatment, GFP-Ssk2p localized in an apparently identical manner to osmotically stressed cells (Figure 2C). Furthermore, these results show that the Ssk2p-containing spots are not actin cortical spots, because Lat A is known to lead very rapidly to the complete loss of actin cortical patches (Ayscough et al., 1997).
Actin Damage Induced by Osmotic Stress Induces Ssk2p to Complex with Actin
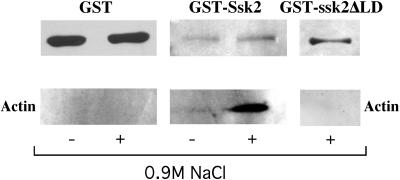
The preceding data show that Ssk2p can alter its cellular location in response to actin damage. We reasoned that one mechanism for Ssk2p to respond to actin damage would be for the kinase to form a complex with actin specifically under these conditions. Because the expression of Ssk2-GST from the endogenous promoter was too low to detect in pull-down assays, GST-Ssk2p was expressed from a high-copy, 2-μm plasmid under the control of the galactose-inducible GAL1/10 promoter (pTY118). Overexpression of GST-Ssk2p from this plasmid did not induce an observable mutant phenotype (our unpublished observations). GST-Ssk2p was precipitated from cell extracts with glutathione-agarose beads, and a Western assay was used to probe for the presence of actin (Figure 6). In normal osmotic conditions, actin was barely detectable in the GST-Ssk2p precipitate. However, 15 min after a shift into high-osmotic medium, actin coprecipitated with GST-Ssk2p. Purified GST and actin of known quantities were used in a parallel titration and Western assay to determine the amounts of GST-Ssk2p and actin in the immunoprecipitates and their relative stoichiometries. This analysis indicated that ∼1.6 actin subunits came down with each molecule of GST-Ssk2p. However, transfer of the large GST-Ssk2p protein (217 kDa) was less efficient than transfer of GST (26 kDa); pretransfer and posttransfer Ruby Red staining of the gels and densitometry analysis indicated that ∼50% of the GST-Ssk2p transferred. Therefore, within the errors of the procedure, it appears that Ssk2p is in an ∼1:1 complex with actin after osmotic stress.
Figure 6.
Osmotic shock induces Ssk2p to interact with actin. JTY142 strain was transformed with plasmids expressing GST [pEG(kt); left], GST-Ssk2p (pTY118; middle), and GST-Ssk2ΔLD (pTY120; right). Cells were incubated in the presence (+) or absence (−) of 0.9 M NaCl for 15 min; cell extracts were prepared, and the complex was precipitated with glutathione-Sepharose beads. Western blot assays were performed to detect actin with a mouse anti-actin mAb (bottom) and GST with an anti-GST antibody (top).
We further investigated the correlation between Ssk2p translocation to the neck and complex formation with actin by examining the ability of the localization-defective, ssk2ΔLD mutant to interact with actin during osmotic stress. GST-ssk2ΔLD (pTY120) was expressed at comparable levels to the wild-type fusion but failed to bring down any detectable actin (Figure 6). This result suggests not only that there is a temporal relationship between localization and actin binding but also that these two phenomena may be functionally coupled as well.
Ssk2p Promotes Actin Cytoskeleton Reassembly After Osmotic Stress
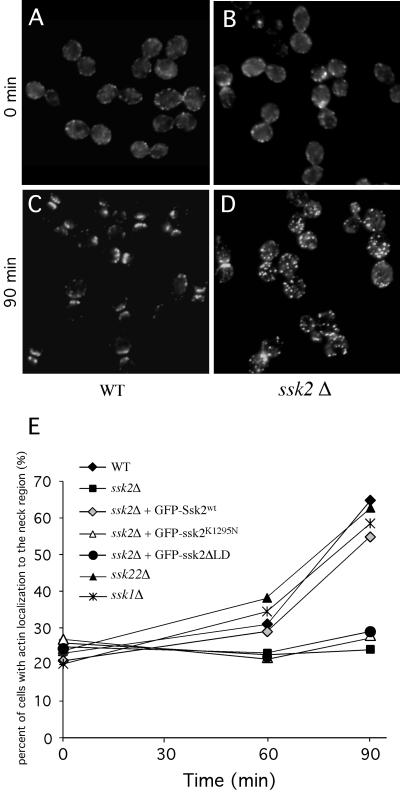
Given the prominent neck localization of Ssk2p during recovery from osmotic stress, we surmised that the kinase might have a role in recovery of the neck cytoskeleton, septation, and cell separation after osmotic stress. First, we examined the behavior of the actin cytoskeleton in osmotically stressed wild-type cells (FY86) synchronized at late stages of the cell cycle. The cells were synchronized in S-phase with HU for 3 h, washed, allowed to recover from drug treatment for 1 h, treated with 0.9 M NaCl, and stained with rhodamine-phalloidin over time to examine the organization of the actin cytoskeleton. One hour after removal of the HU and just before the application of osmotic stress (time 0), wild-type cells had not initiated actin repolarization (Figure 7A). However, 90 min after osmotic stress, there was an impressive repolarization of filamentous actin into two broad bands on either side of the neck in 65% of the cells (Figure 7C).
Figure 7.
Ssk2p promotes actin repolarization to the mother-bud neck after osmotic stress. Wild-type (WT) (FY86), ssk2Δ (TYYD5B), ssk1Δ (TYY7D), and ssk22Δ (TYY3D) strains were synchronized with HU, then osmotically stressed with 0.9 M NaCl and stained with rhodamine-phalloidine. Actin cytoskeleton organization is shown in (A, C) wild-type FY86 cells and (B, D) ssk2Δ cells before osmotic stress (time 0) (A, B) and 90 min after osmotic stress (C, D). (E) The percentages of cells with actin polarized to the neck before osmotic shock (time 0) vs. 60 and 90 min after osmotic shock are plotted for the wild-type FY86, ssk2Δ, ssk1Δ, and ssk22Δ strains or the ssk2Δ strain, expressing GFP-Ssk2pwt (pTY111L), GFP-Ssk2pK1295N (pTY113L), or GFP-Ssk2ΔLD (pTY119L). n ≥ 100. Averages reported were calculated from 2–5 independent experiments.
In contrast, ssk2Δ cells were delayed for actin reassembly in this assay: 90 min after osmotic stress, only 23% of the ssk2Δ cells showed any significant accumulation of actin near the mother-bud neck (Figure 7, D and E). By 120 min, only 36% of the ssk2Δ cells were able to repolarize their cortical actin cytoskeleton to the neck. The ssk22Δ (TYY3D) and the ssk1Δ (TYY7D) strains were not defective for actin recovery (Figure 7E), suggesting that Ssk2p has a specific function in actin recovery and that this function, as for localization, is not activated by Ssk1p.
This led us to investigate whether Ssk2p kinase activity is necessary to facilitate actin recovery. The ssk2Δ strain (TYYD5B) expressing GFP-Ssk2pwt (pTY111L) was able to recover as well as the wild-type strain (Figure 7E). However, a comparable construct expressing the kinase-dead variant GFP-ssk2Lys1295Asn (pTY113L) failed to complement the actin recovery defects of the ssk2Δ allele, indicating that activation of Ssk2p kinase activity is required (Figure 7E). GFP-ssk2ΔLD (pTY119L), the localization and actin-binding–deficient mutant, also failed to complement actin recovery in the ssk2Δ strain (Figure 7E). The latter result is critically important, because it suggests that neck localization and actin binding by Ssk2p is required for the protein to perform its subsequent functions related to actin reassembly in the neck.
Ssk2p Promotes Cell-Cycle Completion After Osmotic Stress
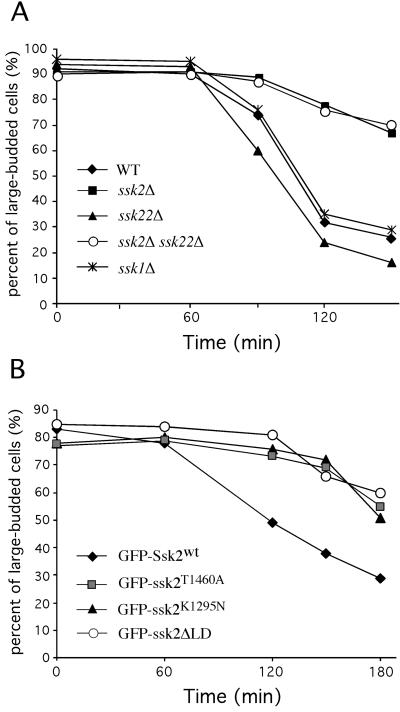
A failure to repolarize actin to the neck region at the end of the cell cycle would be expected to lead to defects in cell separation. Indeed, after 2 h in osmotic medium, 49% of cells in asynchronous ssk2Δ culture (TYYD5B) were large-budded, up from 31% in a nonstressed cell population. In contrast, we observed only 18% of large-budded cells in the asynchronous wild-type strain (FY86), down from 32% before osmotic shock. The reduction in large-budded cells observed in the osmotically stressed wild-type culture was attributed to the accumulation of small-budded cells (43%). These small-budded cells are most likely derived from the population of cells previously arrested in the large-budded state.
To investigate this question further, we examined cell cycle progression in HU synchronized/osmotically stressed wild-type (FY86) and ssk2Δ (TYYD5B) strains (as described above). At time 0 (just before osmotic stress) and during the first hour after osmotic stress, nearly 100% of the cells in both cultures had large buds (Figure 8A). By 120 min, ∼50% of the wild-type cells had completed cell division, whereas the ssk2Δ cells remained arrested as large-budded cells. Even after 3 h in osmotic medium, 60% of the ssk2Δ culture remained as large-budded cells compared with 25% of the wild-type control.
Figure 8.
Ssk2p promotes cell cycle completion after osmotic stress. Wild-type (WT) strain (FY86) and congenic deletion strains (ssk2Δ, TYYD5B; ssk22Δ, TYY3D; ssk1Δ, TYY7D; ssk2Δ/ssk22Δ, TYY9B) were synchronized by HU treatment and osmotically stressed with 0.9 M NaCl. (A) The percentages of large-budded cells in the wild-type and deletion strains before osmotic shock (time 0) vs. 60, 90, 120, and 150 min after osmotic shock are plotted. (B) The percentages of large-budded cells in ssk2Δ strain expressing GFP-Ssk2p (pTY111L), GFP-Ssk2pT1460A (pTY112L), GFP-Ssk2pK1295N (pTY113L), and GFP-Ssk2ΔLD (pTY119L) before osmotic shock (time 0) vs. 60, 120, 150, and 180 min after osmotic shock are plotted. n ≥ 100. Averages were calculated from 2–5 independent experiments.
Almost all large-budded ssk2Δ cells were separable by Zymolase treatment, indicating that the cells had successfully completed cytokinesis. Therefore, we believe that Ssk2p promotes cell division by facilitating cell separation.
Note that both the wild-type and ssk2Δ strains treated with HU without osmotic stress started cycling with the same kinetics (our unpublished observations), indicating that the observed differences were not caused by differences in HU sensitivity. Accurate nuclear segregation was also completed in both cell cultures, as evidenced by DAPI staining (our unpublished observations).
As was observed for actin recovery, the ssk22Δ (TYY3D) and ssk1Δ (TYY7D) strains were not defective for completion of cell division (Figure 8A). However, activation of Ssk2p kinase activity was required, because both the kinase-dead and phosphorylation-defective mutants failed to support timely cell cycle completion (Figure 8B). Furthermore, the ssk2ΔLD mutant was also delayed for cell cycle completion (Figure 8B). Therefore, we conclude that actin recovery facilitated by Ssk2p late in the cell cycle is important for efficient completion of cell division during osmotic stress.
DISCUSSION
We have described two novel activities of Ssk2p, a conserved MEK kinase of the HOG stress-response pathway of S. cerevisiae. Ssk2p rapidly responds to osmotic stress by complexing with actin and translocating to the septin neck cytoskeleton. Mutational analysis suggests that actin binding and localization to the neck are coupled activities of Ssk2p. Ssk2p remains in the neck until the recovery phase, during which a kinase activity of Ssk2p is necessary to facilitate efficient reassembly of the actin cytoskeleton in cells arrested late in the cell cycle. Although this report is focused on late stages of the cell cycle, we have found that Ssk2p is similarly required for actin polarization and bud emergence at early stages of the cell cycle (manuscript in preparation).
What Regulates Ssk2p Localization?
Several kinases in yeast have been found to use the septin cytoskeleton for localization to the neck region, including Gin4, Kcc4, and Hsl1 (Field and Kellogg, 1999). Therefore, it was not particularly surprising to find that Ssk2p displays septin-dependent neck localization as well. What makes Ssk2p different is that its localization is regulated by osmotic stress and/or disruption of the actin cytoskeleton, whereas localization of these other kinases is largely regulated by the cell cycle. Because Ssk1p has been shown to activate autophosphorylation of Ssk2p (Posas and Saito, 1998) in response to osmotic stress and its binding region overlaps with the AIR of Ssk2p, it was an obvious candidate for the regulation of Ssk2p localization. However, we observed accumulation of Ssk2p in the neck in the ssk1Δ strain on osmotic shock (Figure 4C). In addition, phosphorylation-defective and kinase-dead mutants of Ssk2p were able to translocate to the neck on osmotic shock (Figure 4, A and B). Therefore, we can eliminate the possibility that autophosphorylation or phosphorylation of any known downstream substrates plays a role in the regulation of Ssk2p localization.
Several observations suggest to us that actin binding to Ssk2p may regulate its neck localization. Actin depolymerization caused by Lat A treatment in the absence of any alterations in external osmolarity can induce Ssk2p to move to the neck (Figure 2C). We also found that GFP-Ssk2ΔLD, a mutant lacking a region of high conservation with the human homolog MTK1 (aa 426–466), was not able to interact with the septin Cdc10p in the two-hybrid system (Figure 5B), did not localize to the neck (Figure 3B), and did not interact with actin in vivo (Figure 6), suggesting that these capabilities may be coupled. Temporally, actin binding and neck localization are coupled, because they both occur within minutes of an osmotic insult to the cell. One model that could account for these observations would posit that actin monomer interacts with Ssk2p, inducing neck localization by activating a septin-interacting site (Figure 1). Stress and Lat A treatment, by causing a catastrophic disassembly of actin filaments, would lead to a rise in free actin monomer levels and activation of Ssk2p localization to the neck.
In an alternative model, Ssk2p localization to the neck could be activated by an unknown mechanism, and localization, perhaps mediated by a septin interaction, would activate the actin-interacting activity of Ssk2p. Such a model does not provide an easy explanation for the Lat A result or justify the actin interaction. Because only part of the Ssk2p pool is located on the neck, this model could not account for the 1:1 stoichiometry of the Ssk2-actin interaction either. It is also possible that the ability to localize and interact with actin may not be coupled; although the ssk2ΔLD is lacking only 40 amino acids, it may affect two separable activities.
How Does Ssk2p Promote Actin Reassembly?
Our results have shown that ∼1 h after osmotic stress has induced Ssk2p localization to the neck cytoskeleton, the Ssk2p kinase plays another role facilitating actin polymerization in the neck region, leading to reestablishment of a polarized actin cytoskeleton late in the cell cycle. In the absence of Ssk2p, actin recovery is delayed and asynchronous, leading to delays in cell separation. We have shown that Ssk1p is not required for promotion of actin recovery but that phosphorylation on Thr1460 of Ssk2p and its kinase activity are required. Although there is a rather long delay between localization/actin binding and facilitation of actin reassembly, the early events are required, because the ssk2ΔLD mutant cannot interact with actin, localize to the neck, or mediate actin reassembly. Importantly, the ssk2ΔLD mutant can be activated by Ssk1p and is a functional kinase within the traditional HOG pathway. Therefore, we believe that neck localization and/or the actin interaction are critical for activation of Ssk2p interactions with unique substrates involved in promoting actin cytoskeleton reassembly.
One potential substrate of the Ssk2p kinase is actin itself. It has been shown previously that phosphorylation of Dictyostelium actin plays a role in assembly/disassembly of the actin cytoskeleton (Furuhashi and Hatano, 1990; Howard et al., 1993). In addition, it was recently found that yeast actin is phosphorylated (Futcher et al., 1999). Perhaps Ser/Thr phosphorylation of actin by Ssk2p facilitates the polymerization of actin filaments in the neck area by inducing F-actin nucleation. Alternatively, Ssk2p may phosphorylate and activate regulators of actin polymerization, such as the Arp2/3 complex and the Aip3p/Bni1p complex (Evangelista et al., 2001), or may phosphorylate and inactivate G-actin–sequestering proteins.
Ssk2p localization occurs within minutes after osmotic shock, but promotion of actin recovery happens 1 h later, presumably when osmotic balance has been achieved. For example, in a pbs2Δ strain (which is unable to achieve osmotic balance), Ssk2p persists in the neck for many hours, yet actin fails to reassemble. Perhaps restoration of osmotic balance is a prerequisite for the accumulation of key substrates of Ssk2p or activation of the Ssk2p kinase for cytoskeletal substrates.
In summary, osmotic stress leads to translocation of Ssk2p in a novel Ssk1p-independent manner. Ssk2p localization to the neck is septin dependent and can be induced by actin depolymerization alone. Ssk2p appears to both sense actin integrity and promote reassembly of the actin cytoskeleton in the neck region, thus facilitating late cell cycle progression. These results provide the first insights into how components of the HOG pathway regulate actin reassembly during osmotic stress. Furthermore, Ssk2p is the first MAP kinase found to physically associate with actin and to regulate actin cytoskeleton organization. We have identified a 40-amino-acid motif of Ssk2p that is highly conserved with its human homolog, MTK1. This motif is required for localization of Ssk2p, the Ssk2p–actin interaction, and actin reassembly in osmotically stressed cells, suggesting that these unique capabilities of Ssk2p are likely to be conserved.
ACKNOWLEDGMENTS
We thank Brian Haarer, Mark Schmitt, and Patricia Kane for critical reading of our manuscript and members of the Amberg laboratory for support and encouragement. This research was supported by National Institutes of Health grant GM56189.
Abbreviations used:
- AIR
actin-interacting region
- AN
activation domain
- DAPI
4′,6-diamidino-2-phenylindole
- DBD
DNA-binding domain
- GFP
green fluorescent protein
- HOG
high-osmolarity growth
- HU
hydroxyurea
- Lat A
latrunculin A
- LD
localized domain
- mAb
monoclonal antibody
- MAP
mitogen-activated protein
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.02–01–0004. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.02–01–0004.
REFERENCES
- Amberg DC. Three-dimensional imaging of the yeast actin cytoskeleton through the budding cell cycle. Mol Biol Cell. 1998;9:3259–3262. doi: 10.1091/mbc.9.12.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amberg DC, Basart E, Botstein D. Defining protein interactions with yeast actin in vivo. Nat Struct Biol. 1995a;2:28–35. doi: 10.1038/nsb0195-28. [DOI] [PubMed] [Google Scholar]
- Amberg DC, Botstein D, Beasley EM. Precise gene disruption in Saccharomyces cerevisiae by double fusion polymerase chain reaction. Yeast. 1995b;11:1275–1280. doi: 10.1002/yea.320111307. [DOI] [PubMed] [Google Scholar]
- Ayscough KR, Stryker J, Pokala N, Sanders M, Crews P, Drubin DG. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J Cell Biol. 1997;137:399–416. doi: 10.1083/jcb.137.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi E, Maddox P, Lew DJ, Salmon ED, McMillan JN, Yeh E, Pringle JR. Involvement of an actomyosin contractile ring in Saccharomyces cerevisiae cytokinesis. J Cell Biol. 1998;142:1301–1312. doi: 10.1083/jcb.142.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein D, Amberg D, Mulholland J, Huffaker T, Adams A, Drubin D, Stearns T. In: The Yeast Cytoskeleton, vol. 1, The Molecular and Cellular Biology of the Yeast Saccharomyces. Pringle J, Broach J, Jones E, editors. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1997. [Google Scholar]
- Brewster JL, de Valoir T, Dwyer ND, Winter E, Gustin MC. An osmosensing signal transduction pathway in yeast. Science. 1993;259:1760–1763. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- Brewster JL, Gustin MC. Positioning of cell growth and division after osmotic stress required a MAP kinase pathway. Yeast. 1994;10:425–439. doi: 10.1002/yea.320100402. [DOI] [PubMed] [Google Scholar]
- Chowdhury S, Smith KW, Gustin MC. Osmotic stress and the yeast cytoskeleton: phenotype-specific suppression of an actin mutation. J Cell Biol. 1992;118:561–571. doi: 10.1083/jcb.118.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle T, Botstein D. Movement of yeast cortical actin cytoskeleton visualized in vivo. Proc Natl Acad Sci USA. 1996;93:3886–3891. doi: 10.1073/pnas.93.9.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista M, Pruyne D, Amberg DC, Boone C, Bretscher A. Formins direct Arp2/3-independent actin filament assembly to polarize cell growth in yeast. Nat Cell Biol. 2001;4:32–41. doi: 10.1038/ncb718. [DOI] [PubMed] [Google Scholar]
- Field CM, Kellogg D. Septins: cytoskeletal polymers or signaling GTPases? Trends Cell Biol. 1999;9:387–394. doi: 10.1016/s0962-8924(99)01632-3. [DOI] [PubMed] [Google Scholar]
- Furuhashi K, Hatano S. Control of actin filament length by phosphorylation of fragmin-actin complex. J Cell Biol. 1990;111:1081–1087. doi: 10.1083/jcb.111.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futcher B, Latter GI, Monardo P, McLaughlin CS, Garrels JI. A sampling of the yeast proteome. Mol Cell Biol. 1999;19:7357–7368. doi: 10.1128/mcb.19.11.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin MC, Albertyn J, Alexander M, Davenport K. MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1998;62:1264–1300. doi: 10.1128/mmbr.62.4.1264-1300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard PK, Sefton BM, Firtel RA. Tyrosine phosphorylation of actin in Dictyostelium associated with cell-shape changes. Science. 1993;259:241–244. doi: 10.1126/science.7678470. [DOI] [PubMed] [Google Scholar]
- James P, Halladay J, Craig EA. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics. 1996;144:1425–1436. doi: 10.1093/genetics/144.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HB, Haarer BK, Pringle JR. Cellular morphogenesis in the Saccharomyces cerevisiae cell cycle: localization of the CDC3 gene product and the timing of events at the budding site. J Cell Biol. 1991;112:535–544. doi: 10.1083/jcb.112.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine MS, McKenzie A, III, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR. Additional modules for versatile and economical P.C.R-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Maeda T, Takekawa M, Saito H. Activation of yeast PBS2 MAPKK by MAPKKKs or by binding of an SH3-containing osmosensor. Science. 1995;269:554–558. doi: 10.1126/science.7624781. [DOI] [PubMed] [Google Scholar]
- Maeda T, Wurgler-Murphy SM, Saito H. A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature. 1994;369:242–245. doi: 10.1038/369242a0. [DOI] [PubMed] [Google Scholar]
- Maniatis T, Fritsch EF, Sambrook J. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Mitchell DA, Marshall TK, Deschenes RJ. Vectors for the inducible overexpression of glutathione S-transferase fusion proteins in yeast. Yeast. 1993;9:715–722. doi: 10.1002/yea.320090705. [DOI] [PubMed] [Google Scholar]
- Posas F, Saito H. Osmotic activation of the HOG MAPK pathway via Ste11p MAPKKK: scaffold role of Pbs2p MAPKK. Science. 1997;276:1702–1705. doi: 10.1126/science.276.5319.1702. [DOI] [PubMed] [Google Scholar]
- Posas F, Saito H. Activation of the yeast SSK2 MAP kinase kinase kinase by the SSK1 two-component response regulator. EMBO J. 1998;17:1385–1394. doi: 10.1093/emboj/17.5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posas F, Wurgler-Murphy SM, Maeda T, Witten EA, Thai TC, Saito H. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell. 1996;86:865–875. doi: 10.1016/s0092-8674(00)80162-2. [DOI] [PubMed] [Google Scholar]
- Reiser V, Salah SM, Ammerer G. Polarized localization of yeast Pbs2 depends on osmostress, the membrane protein Sho1, and Cdc42. Nat Cell Biol. 2000;2:620–627. doi: 10.1038/35023568. [DOI] [PubMed] [Google Scholar]
- Rose MD, Winston F, Hieter P. Methods in Yeast Genetics: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1990. [Google Scholar]
- Takekawa M, Posas F, Saito H. A human homolog of the yeast Ssk2/Ssk22 MAP kinase kinase kinases, MTK1, mediates stress-induced activation of the p38 and JNK pathways. EMBO J. 1997;16:4973–4982. doi: 10.1093/emboj/16.16.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertman KF, Drubin DG, Botstein D. Systematic mutational analysis of the yeast ACT1 gene. Genetics. 1992;132:337–350. doi: 10.1093/genetics/132.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]