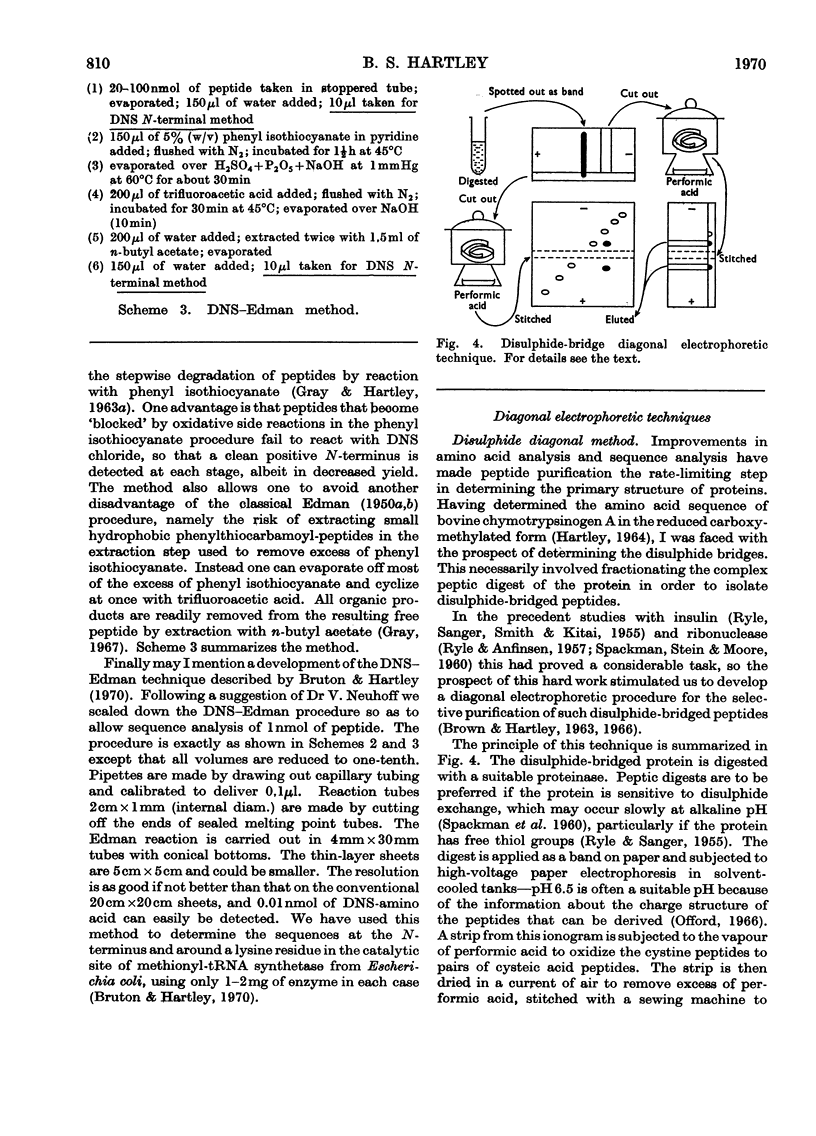
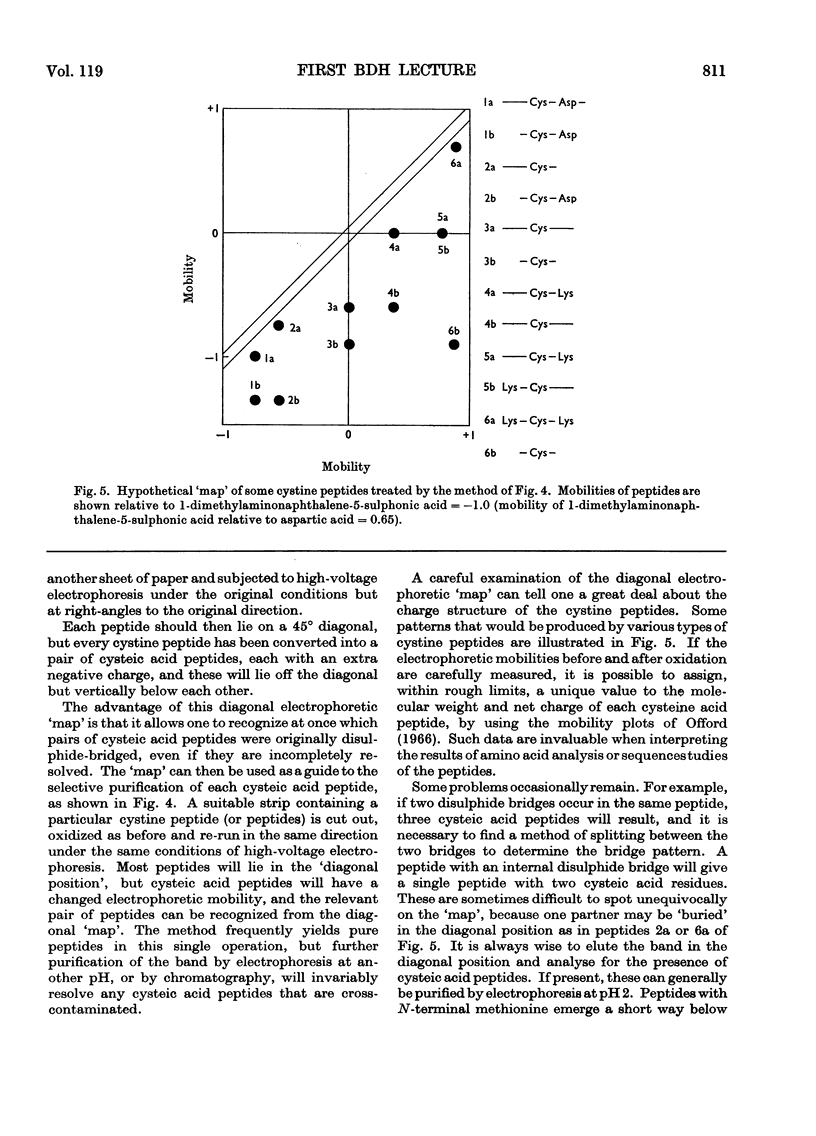
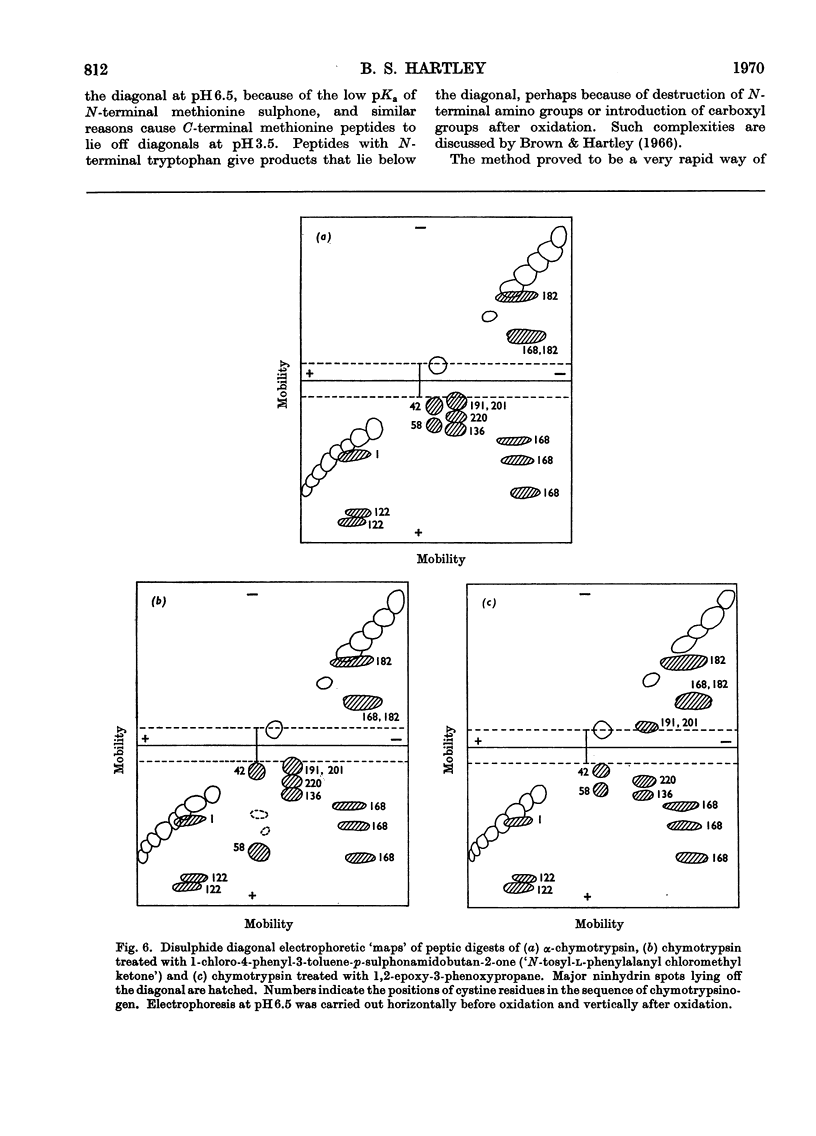
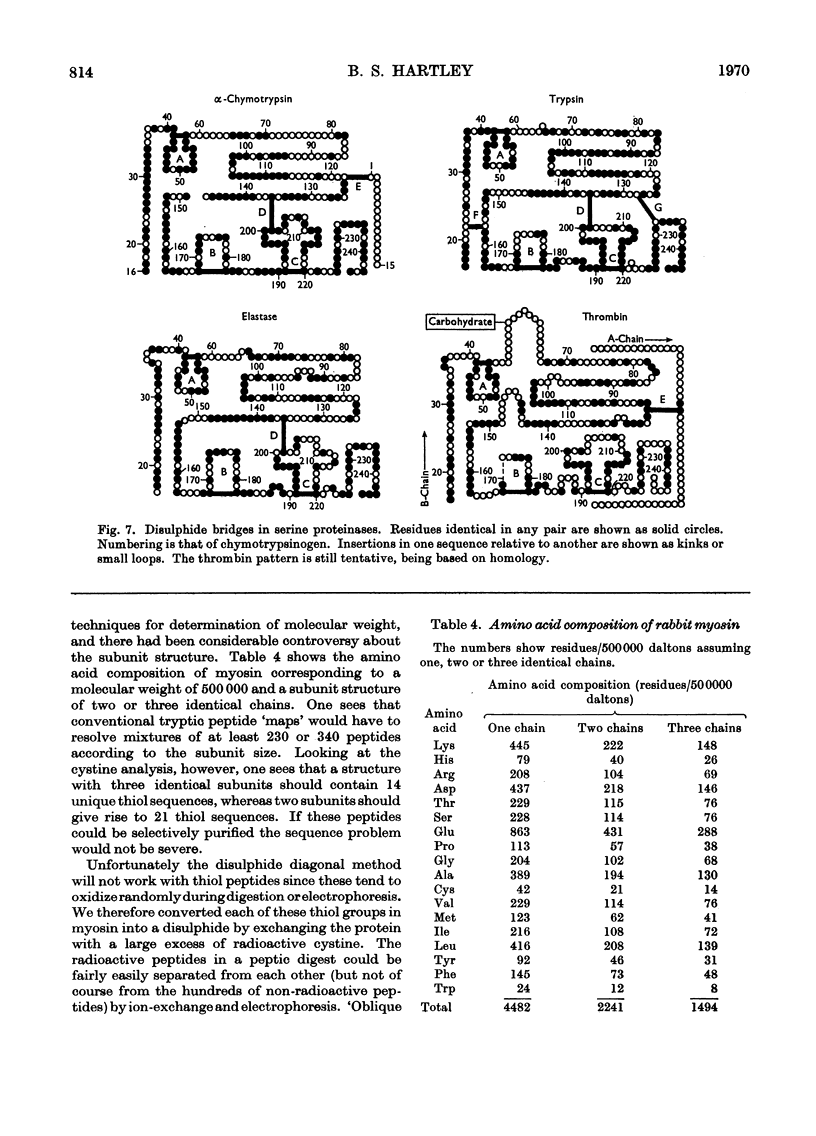
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayliss R. S., Knowles J. R., Wybrandt G. B. An aspartic acid residue at the active site of pepsin. The isolation and sequence of the heptapeptide. Biochem J. 1969 Jun;113(2):377–386. doi: 10.1042/bj1130377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. R., Hartley B. S. Location of disulphide bridges by diagonal paper electrophoresis. The disulphide bridges of bovine chymotrypsinogen A. Biochem J. 1966 Oct;101(1):214–228. doi: 10.1042/bj1010214. [DOI] [PMC free article] [PubMed] [Google Scholar]
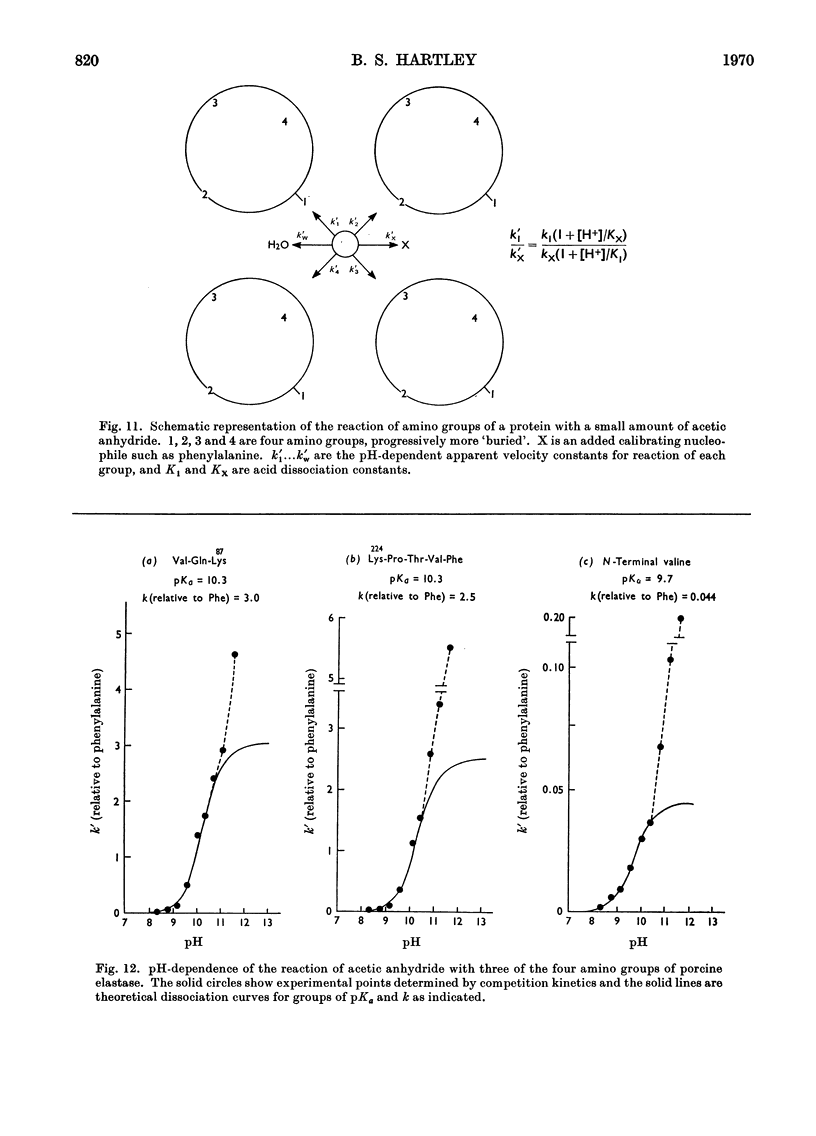
- Brown J. R., Kauffman D. L., Hartley B. S. The primary structure of porcine pancreatic elastase. The N-terminus and disulphide bridges. Biochem J. 1967 May;103(2):497–507. doi: 10.1042/bj1030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruice T. C., Pandit U. K. INTRAMOLECULAR MODELS DEPICTING THE KINETIC IMPORTANCE OF "FIT" IN ENZYMATIC CATALYSIS. Proc Natl Acad Sci U S A. 1960 Apr;46(4):402–404. doi: 10.1073/pnas.46.4.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton C. J., Hartley B. S. Sub-unit structure and specificity of methionyl-transfer-ribonucleic acid synthetase from Escherichia coli. Biochem J. 1968 Jun;108(2):281–288. doi: 10.1042/bj1080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
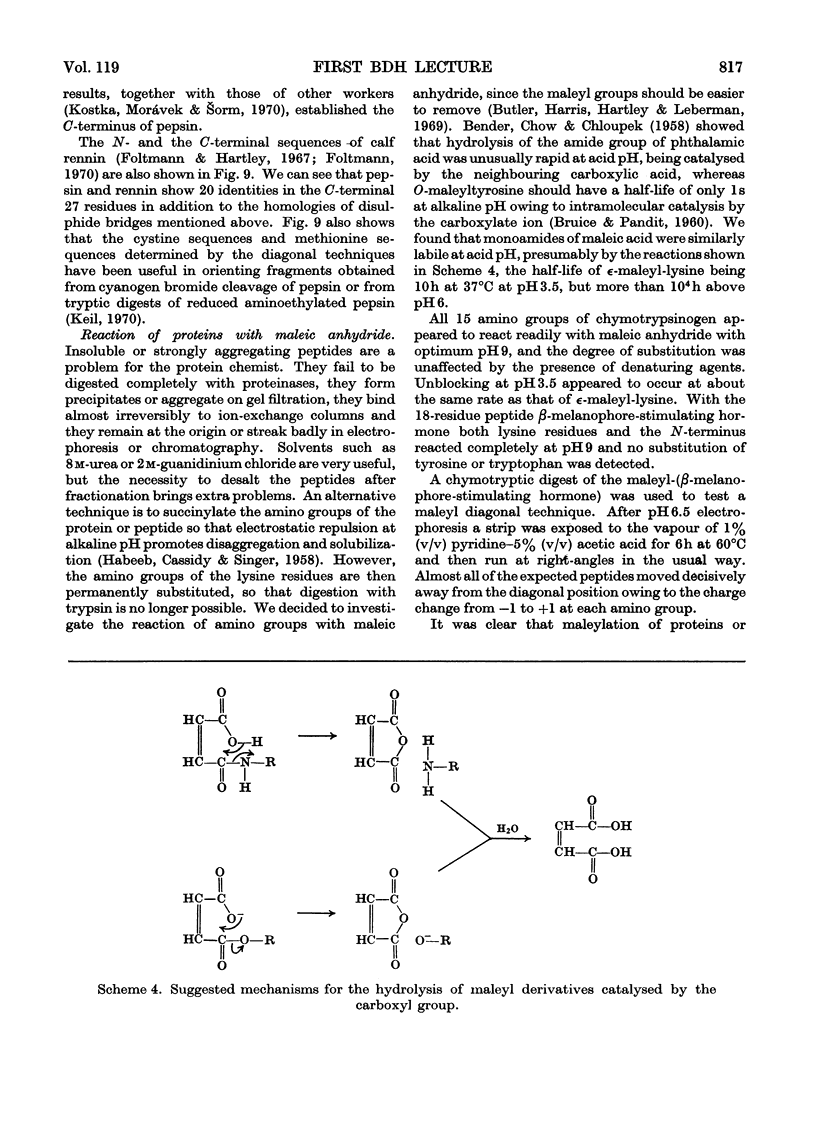
- Butler P. J., Harris J. I., Hartley B. S., Lebeman R. The use of maleic anhydride for the reversible blocking of amino groups in polypeptide chains. Biochem J. 1969 May;112(5):679–689. doi: 10.1042/bj1120679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALAM D. H., WALEY S. G. Acidic peptides of the lens. 8. S-(alpha beta-dicarboxyethyl) glutathione. Biochem J. 1963 Feb;86:226–231. doi: 10.1042/bj0860226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowshaw K., Jessup S. J., Ramwell P. W. Thin-layer chromatography of 1-dimethylaminonaphthalene-5-sulphonyl derivatives of amino acids present in superfusates of cat cerebral cortex. Biochem J. 1967 Apr;103(1):79–85. doi: 10.1042/bj1030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon H. B., Perham R. N. Reversible blocking of amino groups with citraconic anhydride. Biochem J. 1968 Sep;109(2):312–314. doi: 10.1042/bj1090312. [DOI] [PMC free article] [PubMed] [Google Scholar]
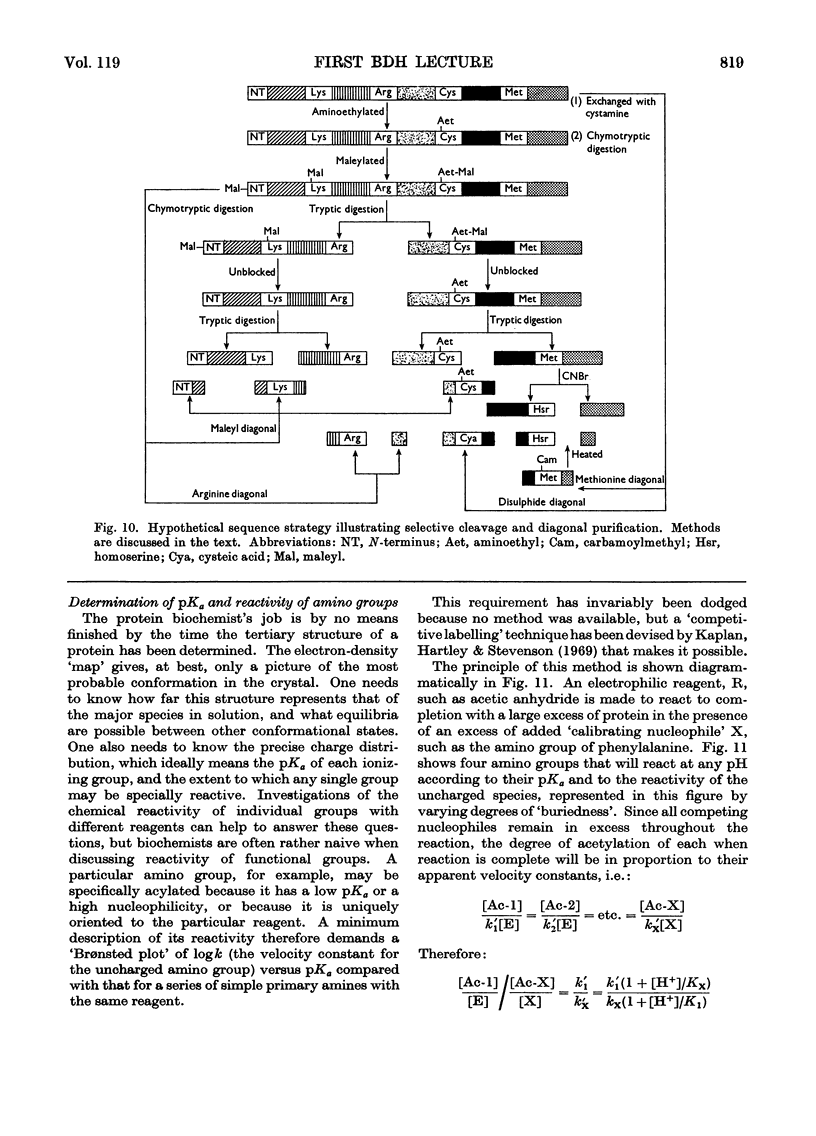
- Foltmann B., Hartley B. S. The disulphide bridges and soluble tryptic peptides of calf rennin. Biochem J. 1967 Sep;104(3):1064–1074. doi: 10.1042/bj1041064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltmann B. The N-terminal and C-terminal amino acid sequence of calf rennin. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):147–151. doi: 10.1098/rstb.1970.0017. [DOI] [PubMed] [Google Scholar]
- Freedman M. H., Grossberg A. L., Pressman D. The effects of complete modification of amino groups on the antibody activity of antihapten antibodies. Reversible inactivation with maleic anhydride. Biochemistry. 1968 May;7(5):1941–1950. doi: 10.1021/bi00845a044. [DOI] [PubMed] [Google Scholar]
- GRAY W. R., HARTLEY B. S. THE STRUCTURE OF A CHYMOTRYPTIC PEPTIDE FROM PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:379–380. doi: 10.1042/bj0890379. [DOI] [PubMed] [Google Scholar]
- GROSS E., WITKOP B. Nonenzymatic cleavage of peptide bonds: the methionine residues in bovine pancreatic ribonuclease. J Biol Chem. 1962 Jun;237:1856–1860. [PubMed] [Google Scholar]
- HABEEB A. F., CASSIDY H. G., SINGER S. J. Molecular structural effects produced in proteins by reaction with succinic anhydride. Biochim Biophys Acta. 1958 Sep;29(3):587–593. doi: 10.1016/0006-3002(58)90016-7. [DOI] [PubMed] [Google Scholar]
- HARTLEY B. S. AMINO-ACID SEQUENCE OF BOVINE CHYMOTRYPSINOGEN-A. Nature. 1964 Mar 28;201:1284–1287. doi: 10.1038/2011284a0. [DOI] [PubMed] [Google Scholar]
- HARTLEY B. S., MASSEY V. The active centre of chymotrypsin. I. Labelling with a fluorescent dye. Biochim Biophys Acta. 1956 Jul;21(1):58–70. doi: 10.1016/0006-3002(56)90093-2. [DOI] [PubMed] [Google Scholar]
- Hartley B. S., Brown J. R., Kauffman D. L., Smillie L. B. Evolutionary similarities between pancreatic proteolytic enzymes. Nature. 1965 Sep 11;207(5002):1157–1159. doi: 10.1038/2071157a0. [DOI] [PubMed] [Google Scholar]
- Keil B. II. Acid proteinases. Chemical studies of porcine pepsin. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):125–133. doi: 10.1098/rstb.1970.0015. [DOI] [PubMed] [Google Scholar]
- Kostka V., Morávek L., Sorm F. Amino acid sequence of C-terminal fragment of hog pepsin. Eur J Biochem. 1970 Apr;13(3):447–454. doi: 10.1111/j.1432-1033.1970.tb00948.x. [DOI] [PubMed] [Google Scholar]
- Matthews B. W., Sigler P. B., Henderson R., Blow D. M. Three-dimensional structure of tosyl-alpha-chymotrypsin. Nature. 1967 May 13;214(5089):652–656. doi: 10.1038/214652a0. [DOI] [PubMed] [Google Scholar]
- Milstein C., Pink J. R. Structure and evolution of immunoglobulins. Prog Biophys Mol Biol. 1970;21:209–263. doi: 10.1016/0079-6107(70)90026-x. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Perham R. N. A diagonal paper-electrophoretic technique for studying amino acid sequences around the cysteine and cystine residues of proteins. Biochem J. 1967 Dec;105(3):1203–1207. doi: 10.1042/bj1051203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perham R. N., Jones G. M. The determination of the order of lysine-containing tryptic peptides of proteins by diagonal paper electrophoresis. A carboxyl-terminal sequence for pepsin. Eur J Biochem. 1967 Jul;2(1):84–89. doi: 10.1111/j.1432-1033.1967.tb00110.x. [DOI] [PubMed] [Google Scholar]
- Pink J. R., Milstein C. Inter heavy-light chain disulphide bridge in immune globulins. Nature. 1967 Apr 1;214(5083):92–94. doi: 10.1038/214092a0. [DOI] [PubMed] [Google Scholar]
- RAFTERY M. A., COLE R. D. Tryptic cleavage at cysteinyl peptide bonds. Biochem Biophys Res Commun. 1963 Mar 25;10:467–472. doi: 10.1016/0006-291x(63)90381-4. [DOI] [PubMed] [Google Scholar]
- RYLE A. P., ANFINSEN C. B. Studies on the disulfide bridges in ribonuclease. Biochim Biophys Acta. 1957 Jun;24(3):633–635. doi: 10.1016/0006-3002(57)90258-5. [DOI] [PubMed] [Google Scholar]
- RYLE A. P., SANGER F. Disulphide interchange reactions. Biochem J. 1955 Aug;60(4):535–540. [PMC free article] [PubMed] [Google Scholar]
- RYLE A. P., SANGER F., SMITH L. F., KITAI R. The disulphide bonds of insulin. Biochem J. 1955 Aug;60(4):541–556. doi: 10.1042/bj0600541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan T. G., Moore S., Stein W. H. Pepsin from pepsinogen. Preparation and properties. J Biol Chem. 1966 Nov 10;241(21):4940–4950. [PubMed] [Google Scholar]
- SCHOELLMANN G., SHAW E. Direct evidence for the presence of histidine in the active center of chymotrypsin. Biochemistry. 1963 Mar-Apr;2:252–255. doi: 10.1021/bi00902a008. [DOI] [PubMed] [Google Scholar]
- SPACKMAN D. H., STEIN W. H., MOORE S. The disulfide bonds of ribonuclease. J Biol Chem. 1960 Mar;235:648–659. [PubMed] [Google Scholar]
- STARK G. R., STEIN W. H. ALKYLATION OF THE METHIONINE RESIDUES OF RIBONUCLEASE IN 8 M UREA. J Biol Chem. 1964 Nov;239:3755–3761. [PubMed] [Google Scholar]
- Shotton D. M., Hartley B. S. Amino-acid sequence of porcine pancreatic elastase and its homologies with other serine proteinases. Nature. 1970 Feb 28;225(5235):802–806. doi: 10.1038/225802a0. [DOI] [PubMed] [Google Scholar]
- Smillie L. B., Furka A., Nagabhushan N., Stevenson K. J., Parkes C. O. Structure of chymotrypsinogen B compared with chymotrypsinogen A and trypsinogen. Nature. 1968 Apr 27;218(5139):343–346. doi: 10.1038/218343a0. [DOI] [PubMed] [Google Scholar]
- Smillie L. B., Hartley B. S. Histidine sequences in the active centres of some 'serine' proteinases. Biochem J. 1966 Oct;101(1):232–241. doi: 10.1042/bj1010232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smillie L. B., Hartley B. S. The disulphide bridges of bovine chymotrypsinogen B. Biochem J. 1967 Dec;105(3):1125–1133. doi: 10.1042/bj1051125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth D. G., Blumenfeld O. O., Konigsberg W. Reactions of N-ethylmaleimide with peptides and amino acids. Biochem J. 1964 Jun;91(3):589–595. doi: 10.1042/bj0910589. [DOI] [PMC free article] [PubMed] [Google Scholar]
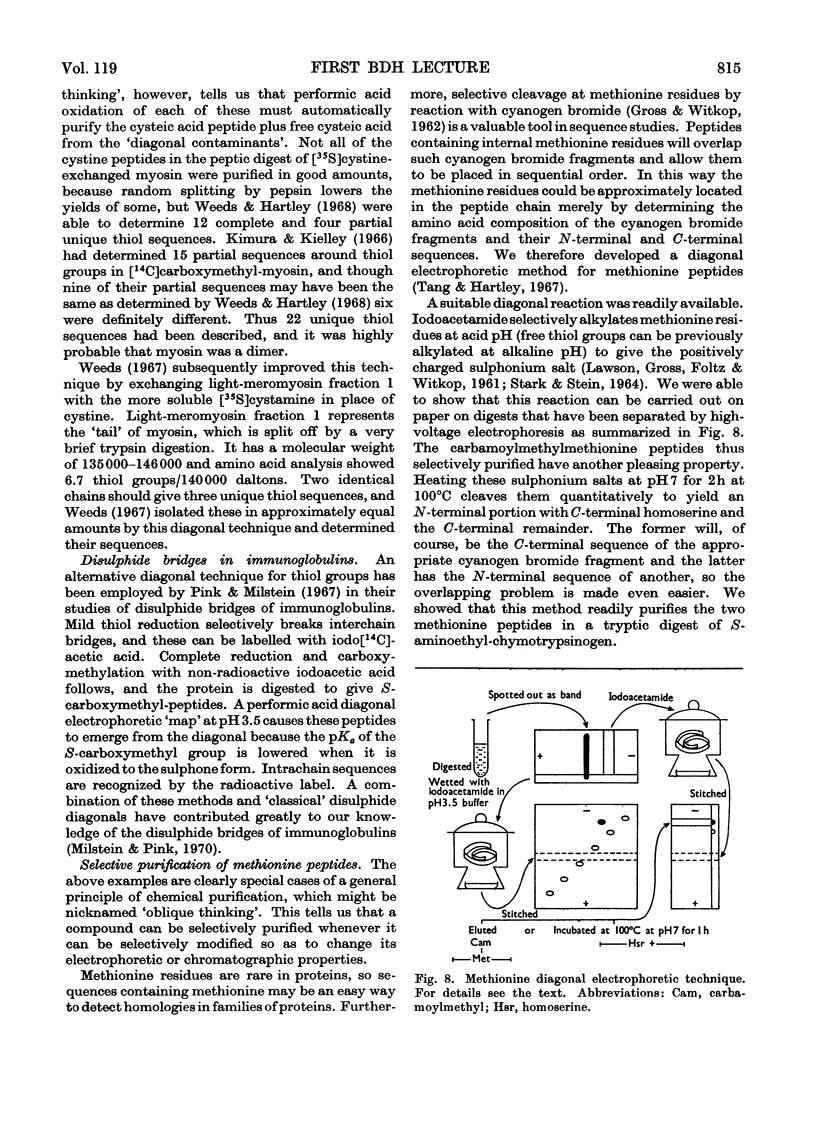
- Tang J., Hartley B. S. A diagonal electrophoretic method for selective purification of methionine peptides. Biochem J. 1967 Feb;102(2):593–599. doi: 10.1042/bj1020593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J., Hartley B. S. Amino acid sequences around the disulphide bridges and methionine residues of porcine pepsin. Biochem J. 1970 Jul;118(4):611–623. doi: 10.1042/bj1180611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER G. Polarization of the fluorescence of macromolecules. II. Fluorescent conjugates of ovalbumin and bovine serum albumin. Biochem J. 1952 May;51(2):155–167. doi: 10.1042/bj0510155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson H. C., Shotton D. M., Cox J. M., Muirhead H. Three-dimensional Fourier synthesis of tosyl-elastase at 3.5 å resolution. Nature. 1970 Feb 28;225(5235):806–811. doi: 10.1038/225806a0. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Hartley B. S. Selective purification of the thiol peptides of myosin. Biochem J. 1968 Apr;107(4):531–548. doi: 10.1042/bj1070531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeds A. G. The thiol sequences and sub-units of light meromyosin fraction 1. Biochem J. 1967 Sep;104(3):44P–44P. [PMC free article] [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]




