Abstract
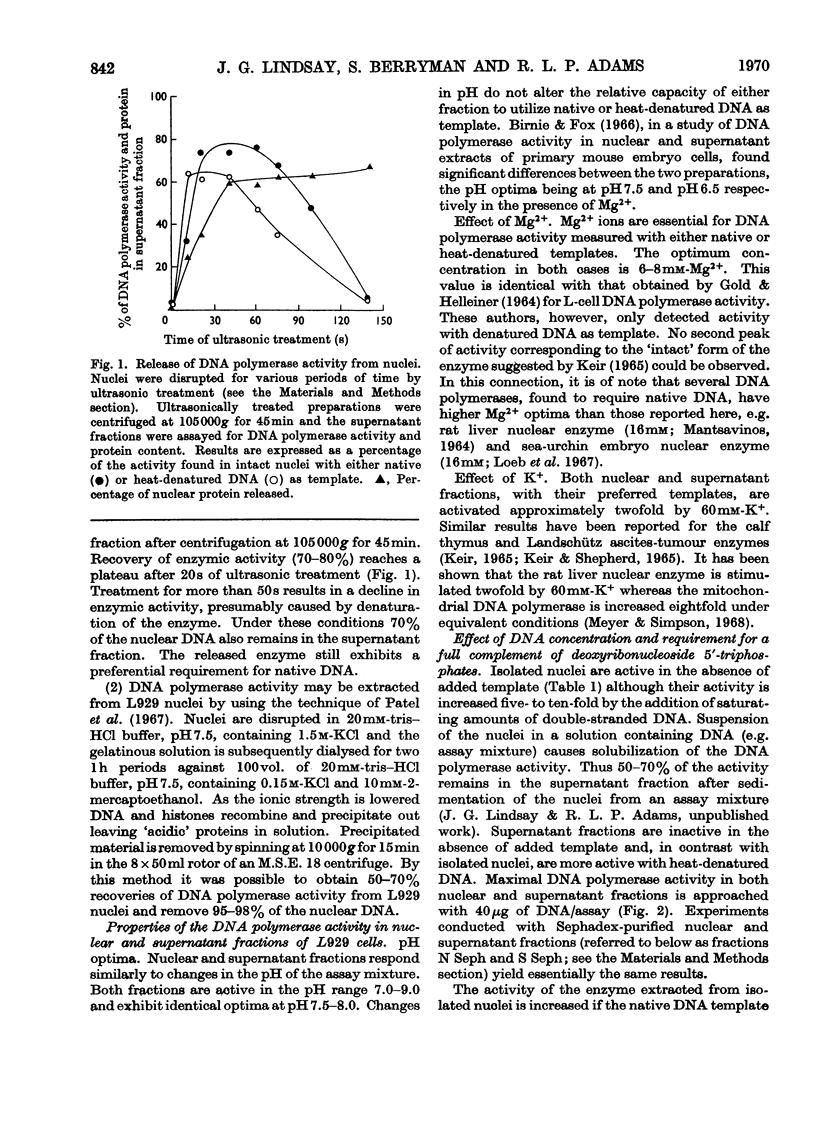
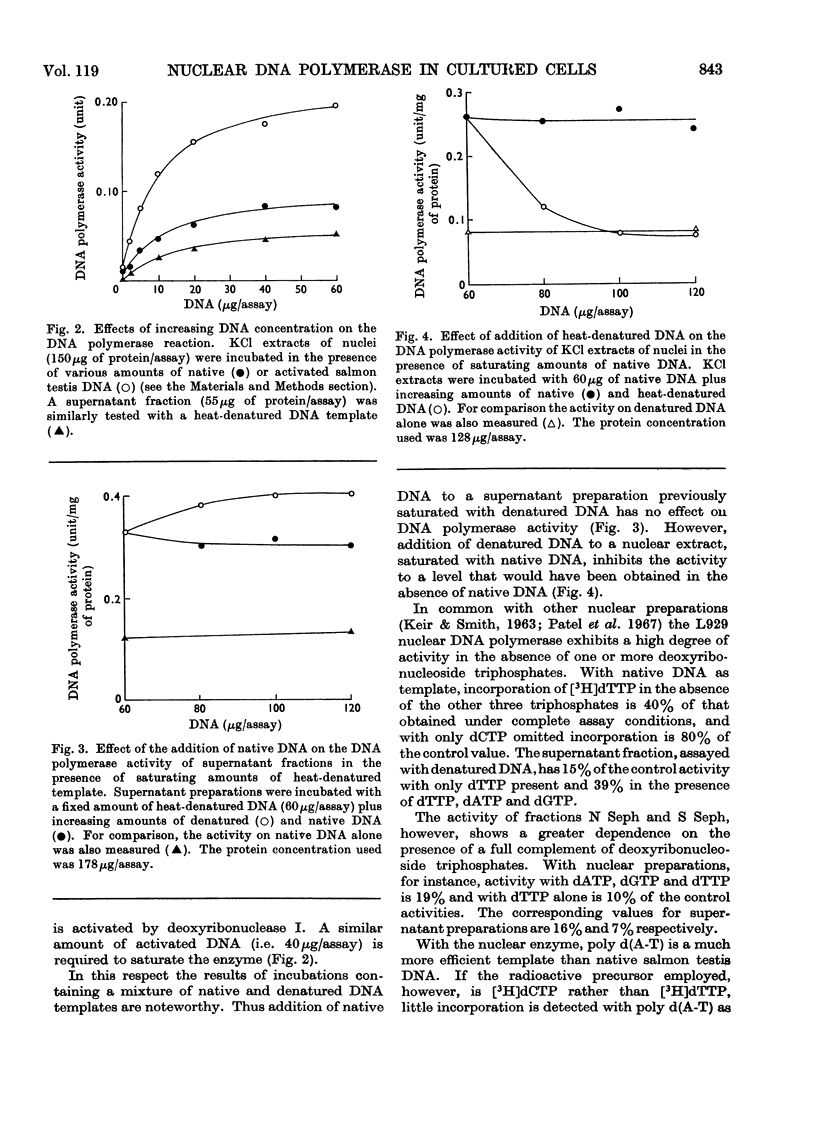
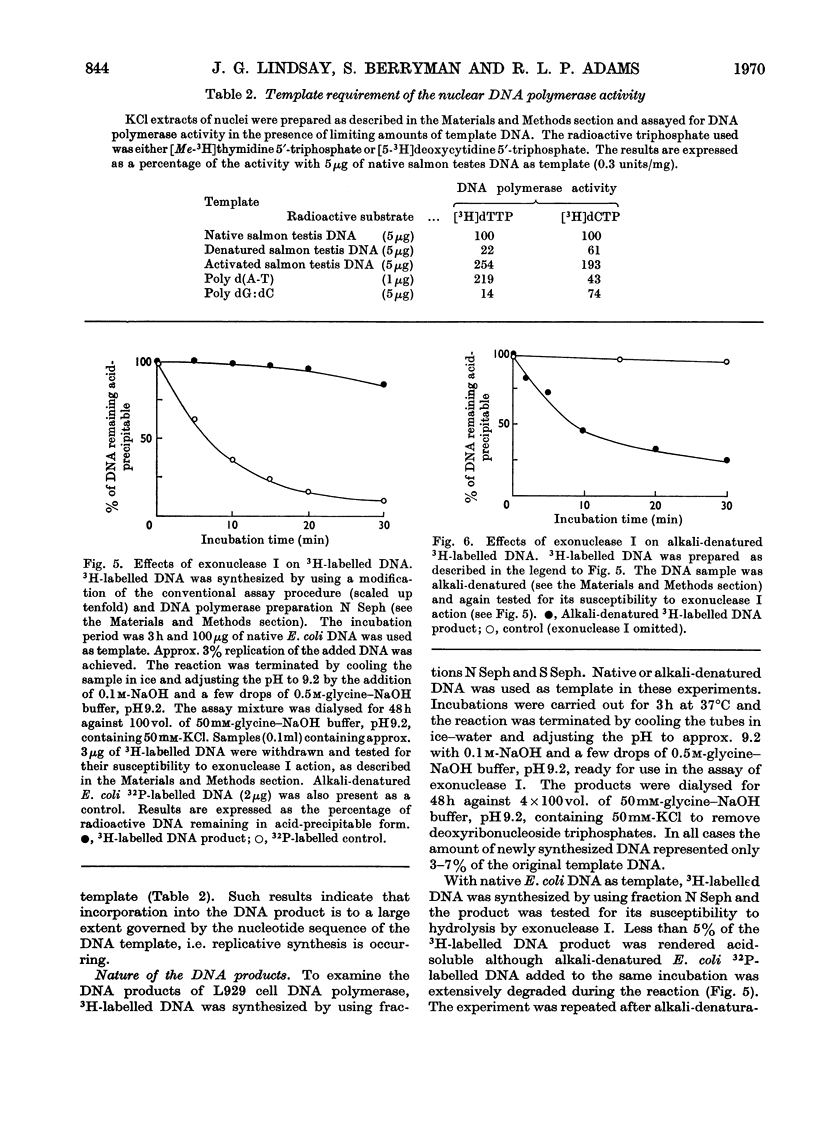
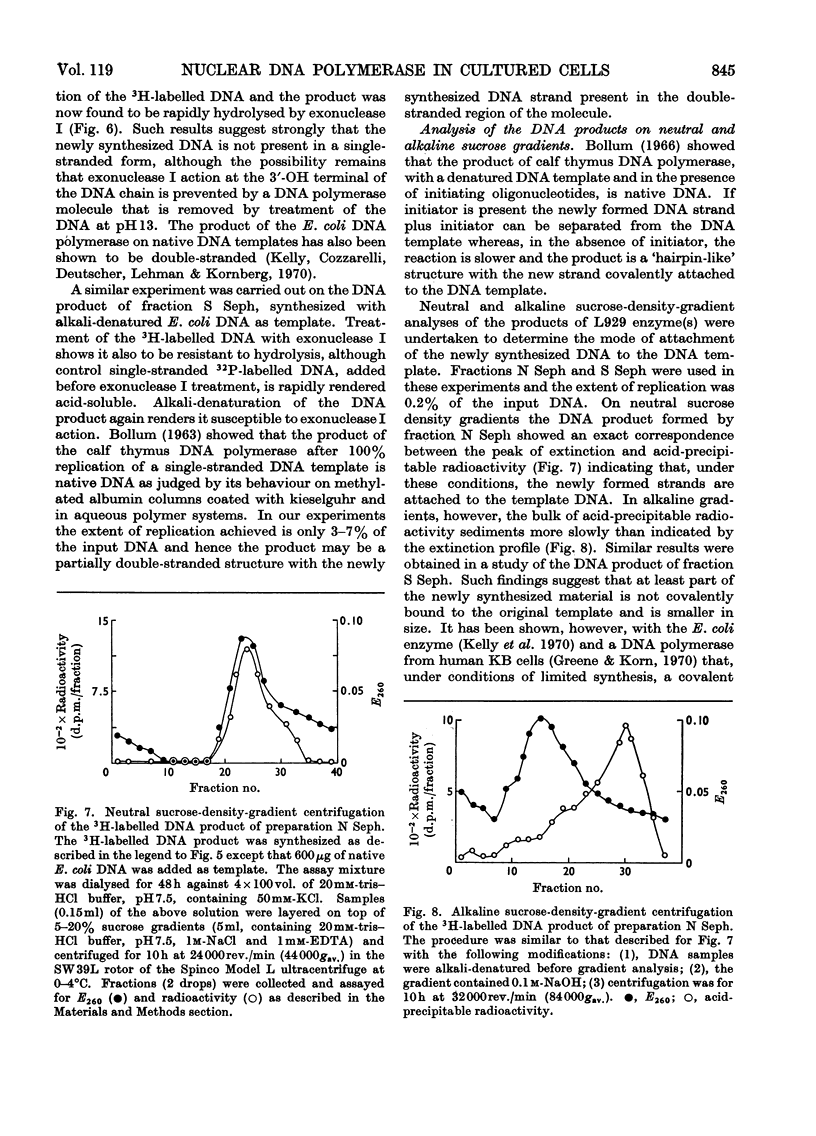
1. DNA polymerase activity is present in both nuclear and supernatant fractions prepared from rapidly dividing L929 mouse cells. 2. Nuclear preparations are 2–5 times more active with added native DNA as template and the supernatant fractions show an equivalent preference for heat-denatured DNA. 3. Isolated nuclei can carry on limited DNA synthesis in the absence of added template but are stimulated five- to ten-fold by addition of 50μg of native DNA per assay. 4. DNA polymerase activity can be released from intact nuclei by ultrasonic treatment or by extraction with 1.5m-potassium chloride. 5. The activities in nuclear and supernatant fractions, with their preferred templates, respond similarly to changes in pH and Mg2+ and K+ concentrations. 6. Maximal enzyme activity is approached with 40μg of DNA per assay and activation of the DNA template by treatment with deoxyribonuclease does not decrease the amount of DNA required to reach saturation. 7. The nuclear enzyme, incubated with native DNA, is markedly inhibited by the addition of heat-denatured DNA to the assay. In contrast, the supernatant DNA polymerase activity on denatured templates is not affected by the presence of native DNA. 8. The nuclear enzyme exhibits high activity in the absence of one or more deoxyribonucleoside triphosphates but this is much diminished after partial purification of the enzyme by precipitation at pH5 and fractionation on Sephadex G-200 columns. 9. The 3H-labelled DNA products formed by Sephadex-purified nuclear and supernatant fractions, with their preferred templates, were found to be resistant to treatment with exonuclease I. Alkali-denaturation of the 3H-labelled DNA products rendered them susceptible to attack by exonuclease I. 10. Analysis of the products on alkaline sucrose density gradients suggests that the newly synthesized material may not be covalently bound to the original DNA template. 11. By using their preferred templates the specific activity of supernatant fractions varies markedly with the position of the cells in the cell-cycle, but the specific activity of nuclear fractions varies only slightly.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APOSHIAN H. V., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. IX. The polymerase formed after T2 bacteriophage infection of Escherichia coli: a new enzyme. J Biol Chem. 1962 Feb;237:519–525. [PubMed] [Google Scholar]
- Adams R. L., Abrams R., Lieberman I. Deoxycytidylate synthesis and entry into the period of deoxyribonucleic acid replication in rabbit kidney cells. J Biol Chem. 1966 Feb 25;241(4):903–905. [PubMed] [Google Scholar]
- Friedman D. L., Mueller G. C. A nuclear system for DNA replication from synchronized HeLa cells. Biochim Biophys Acta. 1968 Jul 23;161(2):455–468. doi: 10.1016/0005-2787(68)90122-6. [DOI] [PubMed] [Google Scholar]
- Furlong N. B. Effects of oligonucleotides on the Walker 256 carcinosarcoma DNA polymerase reaction. Biochim Biophys Acta. 1966 Mar 21;114(3):491–500. doi: 10.1016/0005-2787(66)90098-0. [DOI] [PubMed] [Google Scholar]
- GOLD M., HELLEINER C. W. DEOXYRIBONUCLEIC ACID POLYMERASE IN L CELLS. I. PROPERTIES OF THE ENZYME AND ITS ACTIVITY IN SYNCHRONIZED CELL CULTURES. Biochim Biophys Acta. 1964 Feb 17;80:193–203. [PubMed] [Google Scholar]
- Greene R., Korn D. Partial purification and characterization of deoxyribonucleic acid polymerase from KB cells. J Biol Chem. 1970 Jan 25;245(2):254–261. [PubMed] [Google Scholar]
- Iwamura Y., Ono T., Morris H. P. The heterogeneity of DNA polymerases in rat liver and hepatomas. Cancer Res. 1968 Dec;28(12):2466–2476. [PubMed] [Google Scholar]
- KEIR H. M., SHEPHERD J. B. THIOL GROUPS IN DEOXYRIBONUCLEIC ACID NUCLEOTIDYLTRANSFERASE. Biochem J. 1965 May;95:483–489. doi: 10.1042/bj0950483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEIR H. M., SMITH M. J. Characteristics of the DNA nucleotidyltransferase activity in non-aqueous type calf-thymus nuclei. Biochim Biophys Acta. 1963 Apr 30;68:589–598. doi: 10.1016/0006-3002(63)90188-4. [DOI] [PubMed] [Google Scholar]
- Kalf G. F., Ch'ih J. J. Purification and properties of deoxyribonucleic acid polymerase from rat liver mitochondria. J Biol Chem. 1968 Sep 25;243(18):4904–4916. [PubMed] [Google Scholar]
- Kelly R. B., Cozzarelli N. R., Deutscher M. P., Lehman I. R., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXXII. Replication of duplex deoxyribonucleic acid by polymerase at a single strand break. J Biol Chem. 1970 Jan 10;245(1):39–45. [PubMed] [Google Scholar]
- LEHMAN I. R., NUSSBAUM A. L. THE DEOXYRIBONUCLEASES OF ESCHERICHIA COLI. V. ON THE SPECIFICITY OF EXONUCLEASE I (PHOSPHODIESTERASE). J Biol Chem. 1964 Aug;239:2628–2636. [PubMed] [Google Scholar]
- LEHMAN I. R. The deoxyribonucleases of Escherichia coli. I. Purification and properties of a phosphodiesterase. J Biol Chem. 1960 May;235:1479–1487. [PubMed] [Google Scholar]
- LITTLEFIELD J. W., McGOVERN A. P., MARGESON K. B. Changes in the distribution of polymerase activity during DNA synthesis in mouse fibroblasts. Proc Natl Acad Sci U S A. 1963 Jan 15;49:102–107. doi: 10.1073/pnas.49.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb L. A., Mazia D., Ruby A. D. Priming of DNA polymerase in nuclei of sea urchin embryos by native DNA. Proc Natl Acad Sci U S A. 1967 Mar;57(3):841–848. doi: 10.1073/pnas.57.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANTSAVINOS R. STUDIES ON THE SYNTHESIS OF DEOXYRIBONUCLEIC ACID BY MAMMALIAN ENZYMES. I. INCORPORATION OF DEOXYRIBONUCLEOSIDE 5'-TRIPHOSPHATES INTO DEOXYRIBONUCLEIC ACID BY A PARTIALLY PURIFIED ENZYME FROM REGENERATING RAT LIVER. J Biol Chem. 1964 Oct;239:3431–3435. [PubMed] [Google Scholar]
- Mantsavinos R., Munson B. Studies on the synthesis of deoxyribonucleic acid by mammalian enzymes. II. An investigation of the primer requirements of partially purified regenerating rat liver deoxyribonucleic acid nucleotidyltransferase. J Biol Chem. 1966 Jun 25;241(12):2840–2844. [PubMed] [Google Scholar]
- Patel G., Howk R., Wang T. Y. Partial purification of DNA-polymerase from the non-histone chromatin proteins of rat liver. Nature. 1967 Sep 30;215(5109):1488–1489. doi: 10.1038/2151488a0. [DOI] [PubMed] [Google Scholar]
- Shepherd J. B., Keir H. M. Deoxyribonucleic acid nucleotidyltransferase from Landschütz ascites-tumour cells. Biochem J. 1966 May;99(2):443–453. doi: 10.1042/bj0990443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YONEDA M., BOLLUM F. J. DEOXYNUCLEOTIDE-POLYMERIZING ENZYMES OF CALF THYMUS GLAND. I. LARGE SCALE PURIFICATION OF TERMINAL AND REPLICATIVE DEOXYNUCLEOTIDYL TRANSFERASES. J Biol Chem. 1965 Aug;240:3385–3391. [PubMed] [Google Scholar]



