Abstract
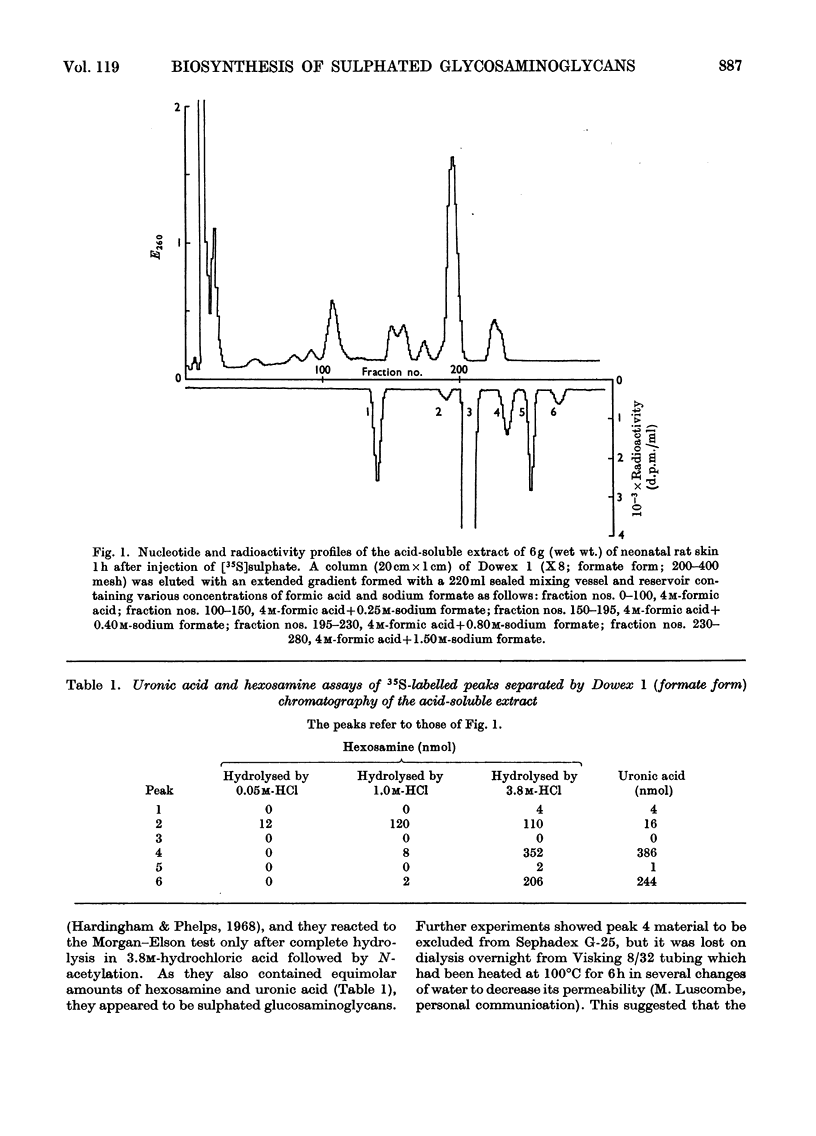
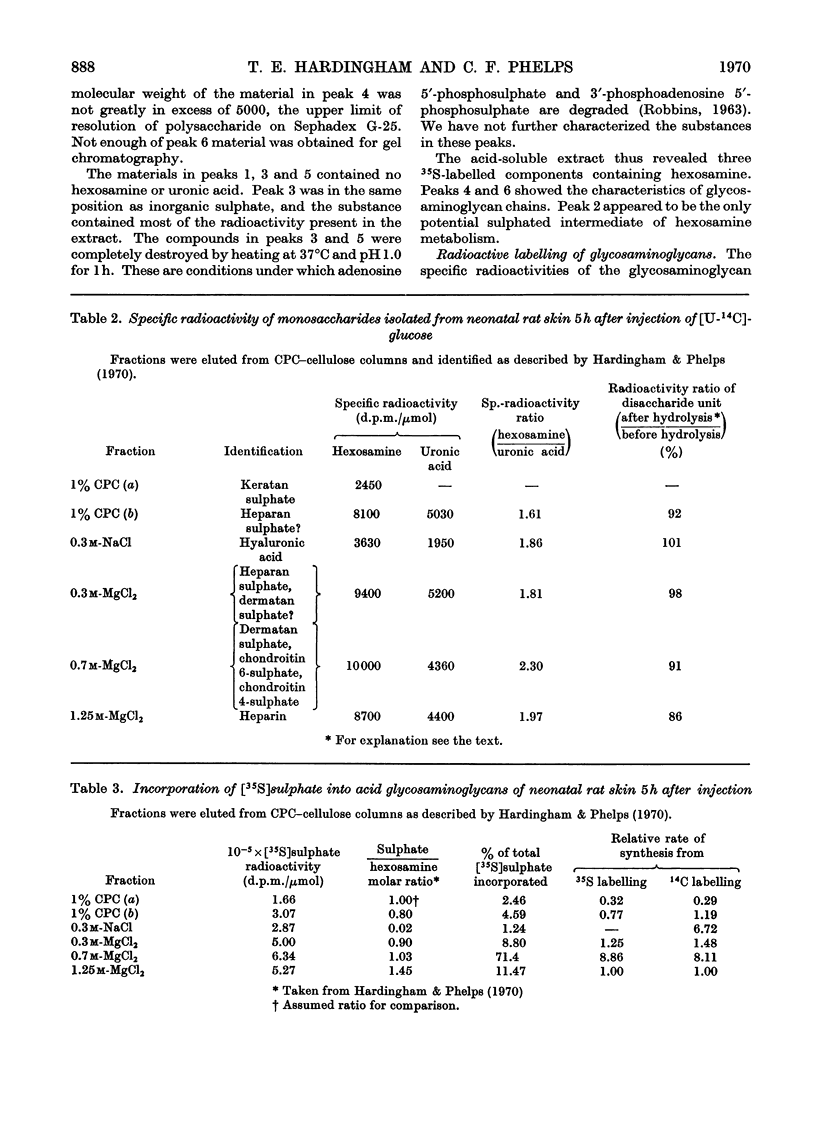
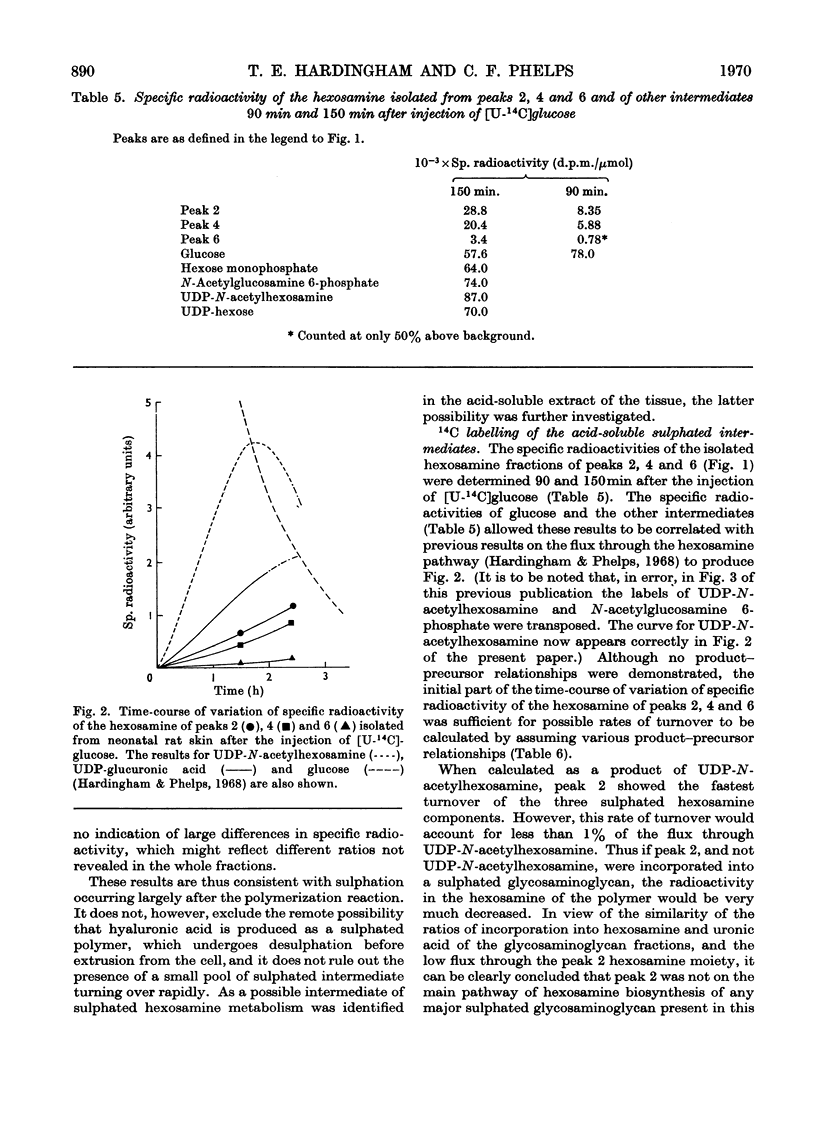
1. The incorporation of [35S]sulphate in vivo into the acid-soluble intermediates extracted from young rat skin showed three sulphated hexosamine-containing components. 2. The rates of synthesis of these components were determined in vivo by measuring the incorporation of radioactivity from [U-14C]glucose into their isolated hexosamine moieties. 3. The incorporation of radioactivity from [U-14C]glucose into the isolated hexosamine and uronic acid moieties of the acid glycosaminoglycans was also measured. These results, combined with those obtained on the intermediary pathways of hexosamine and uronic acid biosynthesis previously determined in this tissue, indicated that the acid-soluble sulphated hexosamine-containing components were not precursors of the sulphated hexosamine found in the acid glycosaminoglycans. 4. The rates of synthesis of the acid glycosaminoglycan fractions were calculated from the incorporation of radioactivity from [U-14C]glucose into the hexosamine moiety. The sulphated components containing principally dermatan sulphate, chondroitin 6-sulphate and in smaller amounts, chondroitin 4-sulphate, heparan sulphate and heparin appeared to be turning over about twice as rapidly as hyaluronic acid and about four times as rapidly as the small keratan sulphate fraction. The relative rates of synthesis of the sulphated glycosaminoglycans were calculated from the incorporation of [35S]sulphate and were in agreement with those from 14C-labelling studies.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- D'ABRAMO F., LIPMANN F. The formation of adenosine-3'-phosphate-5'-phosphosulfate in extracts of chick embryo cartilage and its conversion into chondroitin sulfate. Biochim Biophys Acta. 1957 Jul;25(1):211–213. doi: 10.1016/0006-3002(57)90452-3. [DOI] [PubMed] [Google Scholar]
- DAVIDSON E. A., SMALL W., PERCHEMLIDES P., BAXLEY W. Age-dependent metabolism of connective-tissue polysaccharides. Biochim Biophys Acta. 1961 Jan 1;46:189–190. doi: 10.1016/0006-3002(61)90663-1. [DOI] [PubMed] [Google Scholar]
- DODGSON K. S. Determination of inorganic sulphate in studies on the enzymic and non-enzymic hydrolysis of carbohydrate and other sulphate esters. Biochem J. 1961 Feb;78:312–319. doi: 10.1042/bj0780312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOOD T. A., BESSMAN S. P. DETERMINATION OF GLUCOSAMINE AND GALACTOSAMINE USING BORATE BUFFERS FOR MODIFICATION OF THE ELSON-MORGAN AND MORGAN-ELSON REACTIONS. Anal Biochem. 1964 Nov;9:253–262. doi: 10.1016/0003-2697(64)90183-6. [DOI] [PubMed] [Google Scholar]
- Glaser L., Brown D. H. THE ENZYMATIC SYNTHESIS IN VITRO OF HYALURONIC ACID CHAINS. Proc Natl Acad Sci U S A. 1955 May 15;41(5):253–260. doi: 10.1073/pnas.41.5.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Phelps C. F. The glycosaminoglycans of neonatal rat skin. Biochem J. 1970 May;117(5):813–818. doi: 10.1042/bj1170813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Phelps C. F. The tissue content and turnover rates of intermediates in the biosynthesis of glycosaminoglycans in young rat skin. Biochem J. 1968 Jun;108(1):9–16. doi: 10.1042/bj1080009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBSON B., DAVIDSON E. A. Biosynthesis of uronic acids by skin enzymes. I. Uridine diphosphate-D-glucuronic acid-5-epimerase. J Biol Chem. 1962 Mar;237:638–642. [PubMed] [Google Scholar]
- Jeffrey P. L., Rienits K. G. An improved method for the isolation of hexuronic acid from chondroitin sulphate preparations. Biochim Biophys Acta. 1967 Jun 13;141(1):179–181. doi: 10.1016/0304-4165(67)90258-9. [DOI] [PubMed] [Google Scholar]
- MARKOVITZ A., CIFONELLI J. A., DORFMAN A. The biosynthesis of hyaluronic acid by group A Streptococcus. VI. Biosynthesis from uridine nucleotides in cell-free extracts. J Biol Chem. 1959 Sep;234:2343–2350. [PubMed] [Google Scholar]
- Mathews M. B. The interaction of collagen and acid mucopolysaccharides. A model for connective tissue. Biochem J. 1965 Sep;96(3):710–716. doi: 10.1042/bj0960710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meezan E., Davidson E. A. Mucopolysaccharide sulfation in chick embryo cartilage. I. Properties of the sulfation system. J Biol Chem. 1967 Apr 25;242(8):1685–1689. [PubMed] [Google Scholar]
- Picard J., Gardais A. Nucléotides-sulfates du tissu conjonctif. I. Incorporation in vivo de radiosulfate dans les sulfonucléotides du cartilage de l'aorte et de l'isthme d'OVIDUCTE. Bull Soc Chim Biol (Paris) 1967;49(12):1689–1705. [PubMed] [Google Scholar]
- SCHILLER S., MATHEWS M. B., CIFONELLI J. A., DORFMAN A. The metabolism of mucopolysaccharides in animals. III. Further studies on skin utilizing C14-glucose, C14-acetate, and S35-sodium sulfate. J Biol Chem. 1956 Jan;218(1):139–145. [PubMed] [Google Scholar]
- STROMINGER J. L. Uridine diphosphate acetylglucosamine phosphate and uridine diphosphate acetylgalactosamine sulfate. Biochim Biophys Acta. 1955 Jun;17(2):283–285. doi: 10.1016/0006-3002(55)90367-x. [DOI] [PubMed] [Google Scholar]
- Silbert J. E., DeLuca S. Biosynthesis of chondroitin sulfate. 3. Formation of a sulfated glycosaminoglycan with a microsomal preparation from chick embryo cartilage. J Biol Chem. 1969 Feb 10;244(3):876–881. [PubMed] [Google Scholar]
- YEMM E. W., WILLIS A. J. The estimation of carbohydrates in plant extracts by anthrone. Biochem J. 1954 Jul;57(3):508–514. doi: 10.1042/bj0570508. [DOI] [PMC free article] [PubMed] [Google Scholar]


