Abstract
Introduction
The clinical landscape for the treatment of patients with chronic kidney disease (CKD) and type 2 diabetes (T2D) is rapidly evolving. As part of the FOUNTAIN platform (NCT05526157; EUPAS48148), we described and compared cohorts of adult patients with CKD and T2D initiating a sodium-glucose cotransporter 2 inhibitor (SGLT2i) before the launch of finerenone in Europe, Japan, and the United States (US).
Methods
This was a multinational, multi-cohort study of patients with T2D in five data sources: the Danish National Health Registers (DNHR) (Denmark), PHARMO Data Network (The Netherlands), Valencia Health System Integrated Database (VID) (Spain), Japan Chronic Kidney Disease Database Extension (J-CKD-DB-Ex) (Japan), and Optum’s de-identified Clinformatics® Data Mart Database (CDM) (US). Eligible patients had CKD (based on either diagnosis codes, eGFR values, and/or urine ACR) and initiated an SGLT2i between 2012 and 2021. Baseline demographic, lifestyle, and clinical characteristics were analyzed, and drug utilization patterns were described.
Results
The final cohorts included 21,739 patients in DNHR, 381 in PHARMO, 31,785 in VID, 1157 in J-CKD-DB-Ex, and 56,219 in CDM. Across data sources, approximately 41–70% had CKD stage 1 or 2 at baseline; severe CKD (stage 4) was uncommon (1.6–6.7%). The median duration of SGLT2i therapy ranged from 7.5 months in PHARMO to 17.0 months in VID. At least 50% of patients were currently receiving SGLT2i treatment at 1 year after initiation.
Conclusions
At a 1-year follow-up, at least half of the patients with CKD and T2D were receiving SGLT2i treatment across the data sources. In patients initiating SGLT2i, treatment options for T2D and CKD were heterogeneous and dynamic within and among data sources.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13300-024-01671-x.
Keywords: Chronic kidney disease, CKD, Type 2 diabetes, T2D, Drug utilization, SGLT2 inhibitor, FOUNTAIN, Danish National Health Registers (DNHR), PHARMO Data Network, Valencia Health System Integrated Database (VID), Japan Chronic Kidney Disease Database Extension (J-CKD-DB-Ex), Optum’s de-identified Clinformatics® Data Mart Database (CDM)
Key Summary Points
| Why carry out this study? |
| As the treatment landscape for the prevention of progression of chronic kidney disease (CKD) in type 2 diabetes (T2D) evolves, we conducted a multinational multi-cohort study in Japan, Europe, and the United States to characterize cohorts with CKD and T2D initiating a sodium-glucose cotransporter 2 inhibitor (2012–2021). |
| What was learned from the study? |
| Across data sources, 41%–70% of patients had CKD stage 1 or 2 at baseline; severe CKD was uncommon. |
| T2D and CKD treatment patterns were heterogeneous and dynamic across five data sources. |
Introduction
Type 2 diabetes (T2D) is a primary cause of chronic kidney disease (CKD) worldwide, and the prevalence and incidence of CKD are higher in people with T2D than in those without [1, 2]. The estimated prevalence of CKD among people with T2D is 17–24% in Denmark [3, 4], 28% in Spain [5], 26% in The Netherlands [6], 38% in the United States (US) [7], and 46% in Japan [8]. People with CKD have an increased risk of kidney failure, cardiovascular disease, and premature death compared with people without CKD [9]. The treatment of patients with CKD involves a multidisciplinary approach, with a primary goal of slowing the progression of kidney disease to avoid dialysis or transplantation and a secondary goal of reducing the significant cardiovascular disease burden that accompanies CKD. For patients with T2D and CKD, cardiovascular disease outcomes are an especially relevant concern [10, 11].
The Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guidelines recommend sodium-glucose cotransporter 2 inhibitor(s) (SGLT2i) as first-line drug therapy for patients with T2D and CKD as part of a comprehensive management approach and strongly recommend SGLT2i as first-line therapy for the prevention of CKD progression and cardiovascular events, regardless of other glucose-lowering treatment [12, 13]. SGLT2i can be added to other glucose-lowering drugs (GLDs), and updated guidelines are currently recommending SGLT2i for patients with an estimated glomerular filtration rate (eGFR) ≥ 20 ml/min/1.73 m2 [13–15]. After SGLT2i first became available in 2012 [16], their beneficial effects on CKD progression and cardiovascular outcomes were recognized, leading to regulatory approval in 2020 (in the US) and 2021 (in Europe and Japan) for use in patients with decreased eGFR. Despite wide use of SGLT2i in these settings, evidence about the characteristics of patients treated and utilization patterns is limited.
As new therapies for the prevention of CKD progression emerge (e.g., finerenone and glucagon-like peptide-1 receptor agonists [GLP-1 RAs]), evaluating the characteristics of populations with CKD and T2D initiating SGLT2i in different health settings and determining patterns of use is an important first step in understanding target populations for these therapies. We aimed to describe patient profiles (e.g., demographic, lifestyle, and clinical characteristics) and treatment patterns in adults with CKD and T2D who initiated SGLT2i between 2012 and 2021, while evidence of the CKD-protective benefits of these therapies was emerging and before they were approved to prevent CKD progression in Europe, Japan, and the US, using real-world data from population-based data sources in these regions. We also sought to explore cross-country demographic, lifestyle, and clinical characteristics; disease severity; and comedications and usage patterns in different data sources. This study (NCT05526157) was performed as part of the FOUNTAIN (FinerenOne mUlti-database NeTwork for evidence generAtIoN) platform [17].
Methods
Study Design
This was a multinational multi-cohort study that analyzed real-world data from five participating data sources in Europe, Japan, and the US. This study used de-identified data from electronic health records. Ethics committee review was waived for the Danish National Health Registers (DNHR) and the PHARMO Data Network of the PHARMO Institute for Drug Outcomes Research (PHARMO). The study protocol was reviewed and approved by the Comité Ético de Investigación con Medicamentos del Hospital Clínico Universitario de Valencia for the Valencia Health System Integrated Database (VID) (2022/164). This study protocol was reviewed and approved by the ethics committee of the Shiga University of Medical Science for the Japan Chronic Kidney Disease Database Extension (J-CKD-DB-Ex) (R2022-143). Optum de-identified Clinformatics® Data Mart Database (CDM) data are de‐identified and are compliant with the Health Insurance Portability and Accountability Act of 1996. This study was deemed to not constitute research involving human subjects according to 45 Code of Federal Regulations 46.102(f) and was deemed exempt from board oversight. The institutional review board of RTI International deemed the study exempt from full review. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Patient consent for participation and patient consent for publication are not applicable.
Data Sources
The five data sources are described in detail in Appendix A in the Supplementary Material, and Appendix Table A1 (Supplementary Material) summarizes the characteristics of participating data sources. Briefly, the DNHR is a large network of population-based health registers covering the entire population of Denmark, with administrative data from the Danish Health Care System [18–21], including inpatient, outpatient, emergency department, and general practice (GP) encounters (only information on number of encounters available); prescription data; and laboratory data. PHARMO is a population-based network of electronic healthcare databases, combining data from different primary and secondary healthcare settings, including data from GPs, inpatient and outpatient pharmacies, clinical laboratories, hospitals, the cancer registry, pathology registry, and perinatal registry in The Netherlands [22]. VID is a set of multiple public population-wide electronic databases, including sociodemographic and administrative data and clinical, pharmaceutical, and healthcare utilization data from hospital, emergency, specialty, and primary care settings, for Valencia, the fourth most populated region in Spain [23]. J-CKD-DB-Ex is a nationwide comprehensive clinical database of patients aged ≥ 18 years with CKD (proteinuria ≥ 1 + [dipstick test] and/or eGFR < 60 ml/min/1.73 m2) that is based on electronic health record (EHR) data—including inpatient and outpatient encounters, prescriptions, diagnosis codes, and laboratory measurements—from five participating university hospitals [24, 25]. CDM is derived from a database of administrative health claims for members of large commercial and Medicare Advantage health plans [26].
Study Population
Focusing on patients with CKD and T2D, the study population in each data source included all adult patients (aged ≥ 18 years) with at least 12 months of continuous enrollment or registration in the data source with recorded evidence of CKD and T2D before SGLT2i use and who initiated an SGLT2i from January 1, 2012, through June 30, 2021. In Japan, the study period began on January 1, 2014. New users of SGLT2i were identified at an outpatient prescription or dispensing (hereafter, prescription), depending on the data source, with no record of a prescription for any SGLT2i during the previous 12 months. The index date was the date of the first SGLT2i prescription that fulfilled the definition of new use during the study period. Appendix Figure B1 (Supplementary Material) depicts the study design features regarding cohort eligibility, cohort entry, baseline assessment periods, and follow-up. Excluded patients were those who on or ever before the index date had type 1 diabetes (T1D), a diagnosis of kidney cancer, or chronic kidney failure.
Variables
Eligibility Criteria
The definition of active registration was specific to each data source, and T1D and T2D were defined by algorithms specific to each data source (Appendix Table A1, Supplementary Material). CKD was assessed through diagnosis codes (a single diagnosis code for stage 2, 3, 3a, 3b, 4, or stage unspecified), eGFR test results (occurrence of two different eGFR test results ≥ 15 ml/min/1.73 m2 and < 60 ml/min/1.73 m2 separated by at least 90 days and ≤ 540 days), or urine albumin-to-creatinine ratio (ACR) test results (2 different results ≥ 30 mg/g separated by at least 90 days and ≤ 540 days). Chronic kidney failure was defined according to diagnosis codes for CKD stage 5, occurrence of 2 eGFR results < 15 ml/min/1.73 m2 separated by at least 90 days and ≤ 540 days, receipt of chronic kidney replacement therapy, or kidney transplantation. Kidney cancer was assessed by diagnosis codes.
Exposures
Exposures to medications of interest were identified from records in the EHR or administrative data for prescription of medications, depending on the data source. SGLT2i were defined by Anatomical Therapeutic Chemical (ATC) codes (Appendix Table C1, Supplementary Material). Analyses were conducted by exposure group defined by SGLT2i class and any concurrent medication class with potential CKD-protective effect, including GLP-1 RAs, angiotensin-converting enzyme inhibitor(s) (ACEi) or angiotensin receptor blockers (ARBs), or steroidal mineralocorticoid receptor antagonists (sMRAs). Information on indication was not available, so it could not be determined whether these classes of drugs were used for renoprotection or other indications. We described prescription of these other drugs in three distinct time periods: in the 90 days before the index date, on the index date, and in the 90 days after the index date.
The study-defined categories were SGLT2i initiated as the only drug of interest (“monotherapy”), simultaneous initiation of SGLT2i together with another drug of interest (“combination therapy”), “add-on therapy” in which SGLT2i is added to an existing drug of interest, “switched-to therapy” in which an existing drug of interest is replaced by SGLT2i, both add-on and switched-to therapy at the same time, and non-evaluable index therapy (Appendix B, Supplementary Material). Current-use periods of SGLT2i were defined from the day after the index date to the end of presumed supply for consecutive prescriptions plus a grace period of 30 days in all data sources except in DNHR, which did not have data on dispensed days’ supply. In DNHR, days’ supply was estimated using a data-driven approach using the upper quartile of the times between prescriptions with a 30-day grace period. All data sources used a common approach to account for gaps off drug and for potential stockpiling of drug supply (Appendix B, Supplementary Material).
Demographic, Lifestyle, and Clinical Characteristics
Demographic and lifestyle variables available in each data source at the index date included age, sex, evidence of obesity using qualitative diagnosis codes or body mass index (if available), smoking status using the latest value recorded before the index date, and alcohol abuse and related conditions, such as alcoholic fatty liver, alcoholic hepatitis, alcoholic hepatic failure, or alcoholic liver disease. Baseline clinical characteristics evaluated included markers of T2D severity and markers of severity of kidney dysfunction (see Appendix Tables C3 and C4, Supplementary Material), medications other than GLDs used in the 180 days before or on the index date, and comorbidities at any time before or on the index date using all available history. Baseline demographic and clinical characteristics were calculated overall for each data source. To examine associations of receiving an ACR test or not in clinical practice with patient characteristics and treatment choices, analyses were further stratified by whether or not an ACR test was performed in the 365 days before or on the index date.
Treatment Utilization
Treatment utilization outcomes of interest during follow-up were treatment discontinuation (end of current use), defined as the date following the last day of current use, treatment switches, and add-on treatments. The duration of the index medication episode and the duration of total exposure to an SGLT2i across all follow-up time (regardless of discontinuations) also were described. Because J-CKD-DB-Ex is a hospital database, only encounters in the inpatient or hospital outpatient clinic setting were recorded. Patients may be referred to a community clinic after care in a hospital clinic; prescriptions received in community settings are not captured in the database.
Statistical Analyses
Statistical analyses were performed on site by each research partner according to a common protocol and a common statistical analysis plan with data source-specific adaptations. Analyses were performed in SAS software version 9.4 or higher (SAS Institute, Inc.), except at VID, where R software version 4.1.0 was used [27]. Aggregated results for each data source were provided to the coordinating center in regional-specific data sets. Descriptive statistics were compiled and, according to country-specific data privacy standards, categorical variables with low frequencies for a specific level were masked. The percentage of missing data for individual variables was reported.
Analyses for treatment changes over time were restricted to 3 years after the index date. The following treatment states were assessed at discrete times (“checkpoints”; 90 days, 180 days, 270 days, 1 year, 2 years, and 3 years) after the index date: (1) treated with index medication (i.e., patient was in any continuous current-use period on the date of the checkpoint); (2) untreated with index medication; (3) death (information on death was not available in J-CKD-DB-Ex); and (4) lost to follow-up, end of study, or censored. Sankey diagrams were constructed to illustrate the movement of patients between different treatment states across all checkpoints [28, 29].
Results
After applying inclusion and exclusion criteria, the final analysis cohorts of SGLT2i new users with T2D and CKD comprised 21,739 patients in DNHR, 381 in PHARMO, 31,785 in VID, 1157 in J-CKD-DB-Ex, and 56,219 in CDM (Appendix Table C2, Supplementary Material).
Baseline Demographic Characteristics
The mean age of SGLT2i initiators in the data sources was comparable, ranging from 66.5 to 70.7 years, and there was a higher percentage of men than women in all data sources (Table 1). The percentage of patients with documented obesity ranged from 8.4% (J-CKD-DB-Ex) to 66.6% (VID). Overall, annual counts of new SGLT2i initiators were generally skewed toward the later years of the study period, and by design, only 6 months of observation were included in 2021.
Table 1.
Selected baseline characteristics of SGLT2i new users, by data source
| Characteristic | DNHR (n = 21,739) | PHARMO (n = 381) | VID (n = 31,785) | J-CKD-DB-Ex (n = 1157) | CDM (n = 56,219) |
|---|---|---|---|---|---|
| Demographic/lifestyle | |||||
| Age at the index date, mean (SD), years | 66.5 (11.4) | 69.4 (9.5) | 70.7 (10.9) | 67.1 (11.7) | 68.6 (10.1) |
| Sex, n (%) | |||||
| Female | 7710 (35.5) | 169 (44.4) | 12,910 (40.6) | 431 (37.3) | 25,633 (45.6) |
| Calendar year of index date, n (%) | |||||
| 2012 | N/A | 0 (0.0) | 0 (0.0) | N/A | 0 (0.0) |
| 2013 | 123 (0.6) | 4 (1.0) | 5 (0.02) | N/A | 730 (1.3) |
| 2014 | 231 (1.1) | 21 (5.5) | 377 (1.2) | N/A | 2142 (3.8) |
| 2015 | 666 (3.1) | 15 (3.9) | 2343 (7.4) | 83 (7.2) | 3503 (6.2) |
| 2016 | 1651 (7.6) | 32 (8.4) | 3941 (12.4) | 186 (16.1) | 3633 (6.5) |
| 2017 | 2663 (12.2) | 78 (20.5) | 4175 (13.1) | 172 (14.9) | 5355 (9.5) |
| 2018 | 3276 (15.1) | 78 (20.5) | 4754 (15.0) | 186 (16.1) | 6219 (11.1) |
| 2019 | 3891 (17.9) | 72 (18.9) | 6346 (20.0) | 184 (15.9) | 9818 (17.5) |
| 2020 | 5044 (23.2) | 81 (21.3) | 5552 (17.5) | 208 (18.0) | 13,704 (24.4) |
| 2021 | 4194 (19.3) | N/A | 4292 (13.5) | 138 (11.9) | 11,115 (19.8) |
| Obesity, yes, n (%)a | 5811 (26.7) | 219 (57.5) | 21,156 (66.6) | 97 (8.4) | 26,443 (47.0) |
| Alcohol abuse, yes, n (%)a | 1054 (4.8) | 6 (1.6) | 1094 (3.4) | 35 (3.0) | 954 (1.7) |
| Comorbidities, n (%)b | |||||
| Complications of diabetesc | |||||
| Retinopathy | 4724 (21.7) | 5 (1.3) | 8333 (26.2) | 231 (20.0) | 12,937 (23.0) |
| Nephropathy | 18,424 (84.8) | 358 (94.0) | 31,785 (100.0) | 390 (33.7) | 33,245 (59.1) |
| Neuropathy | 4803 (22.1) | 24 (6.3) | 6236 (19.6) | 295 (25.5) | 22,737 (40.4) |
| Coronary heart disease | 6774 (31.2) | 129 (33.9) | 8550 (26.9) | 677 (58.5) | 19,663 (35.0) |
| Cerebrovascular disease | 2863 (13.2) | 40 (10.5) | 4140 (13.0) | 484 (41.8) | 6854 (12.2) |
| Peripheral vascular disease | 3352 (15.4) | 52 (13.6) | 6674 (21.0) | 199 (17.2) | 15,737 (28.0) |
| Amputation | 457 (2.1) | 1 (0.3) | 323 (1.0) | 0 (0) | 991 (1.8) |
| Hyperkalemiad | 244 (1.1) | 8 (2.1) | 2917 (9.2) | 73 (6.3) | 3639 (6.5) |
| Associated with risk of CKD or cardiovascular disease risk factore | |||||
| Hypertension | 17,575 (80.8) | 271 (71.1) | 28,846 (90.8) | 957 (82.7) | 52,558 (93.5) |
| Glomerulonephritis (all causes) | 484 (2.2) | 12 (3.1) | 989 (3.1) | 233 (20.1) | 965 (1.7) |
| Renovascular disease | 74 (0.3) | 0 (0) | 371 (1.2) | 36 (3.1) | 532 (0.9) |
| Autoimmune disease | 1077 (5.0) | 7 (1.8) | 2229 (7.0) | 327 (28.3) | 3387 (6.0) |
| Polycystic kidney disease | 104 (0.5) | 0 (0) | 132 (0.4) | 1 (0.1) | 170 (0.3) |
| Gout or hyperuricemiac | 1051 (4.8) | 15 (3.9) | 9397 (29.6) | 381 (32.9) | 6046 (10.8) |
| Hypercholesterolemia | 7369 (33.9) | 134 (35.2) | 25,186 (79.2) | 887 (76.7) | 50,291 (89.5) |
| Congestive heart failure | 3611 (16.6) | 58 (15.2) | 1776 (5.6) | 717 (62.0) | 12,073 (21.5) |
| Comedications other than glucose-lowering drugs, n (%)f | |||||
| Angiotensin-converting enzyme inhibitors | 8252 (38.0) | 152 (39.9) | 6728 (21.2%) | 135 (11.7) | 22,793 (40.5) |
| Angiotensin receptor blockers | 9702 (44.6) | 135 (35.4) | 18,984 (59.7) | 615 (53.2) | 29,637 (52.7) |
| Thiazide-like diuretics | 3099 (14.3) | 76 (19.9) | 1617 (5.1) | 74 (6.4) | 17,919 (31.9) |
| Loop diuretics | 5557 (25.6) | 77 (20.2) | 8360 (26.3) | 159 (13.7) | 12,595 (22.4) |
| Potassium-sparing diuretics | 162 (0.7) | 9 (2.4) | 6728 (21.2) | 0 (0) | 1255 (2.2) |
| Beta-blockers | 9195 (42.3) | 220 (57.7) | 11,552 (36.3) | 309 (26.7) | 28,416 (50.5) |
| Calcium channel blockers | 8607 (39.6) | 128 (33.6) | 8056 (25.4) | 471 (40.7) | 18,711 (33.3) |
| Statins | 16,870 (77.6) | 292 (76.6) | 23,797 (74.9) | 504 (43.6) | 43,609 (77.6) |
| Insulin use | 6657 (30.6) | 82 (5.6) | 10,088 (31.7) | 562 (48.6) | 18,447 (32.8) |
DNHR Danish National Health Registers, J-CKD-DB-Ex Japan Chronic Kidney Disease Database Extension, CDM Optum’s de-identified Clinformatics® Data Mart Database, SGLT2i sodium-glucose cotransporter 2 inhibitors, N/A not available, SD standard deviation, VID Valencia Health System Integrated Database
aThe definition of this variable varied by data source
bComorbidities for PHARMO are based on hospital information, although some comorbidities listed are recorded and managed by general practitioners in The Netherlands. Therefore, some proportions may be underestimates
cEvaluation window for these variables is any time before or on the index date (study days [− ∞, 0]) for all data sources except CDM, in which the evaluation window was 365 days before or on the index date
dEvaluation window for hyperkalemia was 365 days before or on the index date
eEvaluation window for these variables is any time before or on the index date (study days [− ∞, 0])
fComedications; assessed in the 180 days before or on the index date
Markers of Severity of T2D at the Index Date
The estimated median recorded duration of T2D in the data sources ranged from 4.2 years in CDM to 12.4 years in PHARMO (Appendix Table C3, Supplementary Material). Hemoglobin A1c (HbA1c) values recorded in the 180 days before the index date suggested that patients in J-CKD-DB-Ex had better glycemic control of their T2D than patients in the other data sources. GLP-1 RA medications were prescribed to 24.4% of patients in DNHR, 17.8% in CDM, 17.5% in J-CKD-DB-Ex, 10.9% in VID, and 4.7% in PHARMO. Use of alpha-glucosidase inhibitors was < 1% in all data sources except for J-CKD-DB-Ex (25.8%). Use of thiazolidinediones was highest in J-CKD-DB-Ex (17.0%) and CDM (9.4%) but was < 3% in the other data sources. Meglitinide use was < 1.5% in DNHR, PHARMO, and CDM but was 18.1% in VID and 19.3% in J-CKD-DB-Ex. The percentage of patients with no use of any GLD classes other than insulin in the 180 days before or on the index date was < 10% in the European data sources, 12.5% in J-CKD-DB-Ex, and 16.0% in CDM. Most patients in the European data sources (just over 81%) and in CDM (67.8%) had used 1 or 2 GLD classes in this time period, but in J-CKD-DB-Ex, 42.3% had used drugs from 1 or 2 classes and 45.1% had used drugs from 3 or 4 GLD classes. Insulin use was recorded in 48.6% of SGLT2i initiators in J-CKD-DB-Ex; in just over 31% of patients in DNHR, VID, and CDM; but in only 5.6% of patients in PHARMO (Appendix Table C3, Supplementary Material). In addition, the Diabetes Severity Complications Index Score [30, 31] was higher in J-CKD-DB-Ex and VID (median for both: 4) than in CDM (median: 3) and in the DNHR and PHARMO (median for both: 2) (Appendix Table C3, Supplementary Material).
Markers of Severity of Kidney Dysfunction at the Index Date
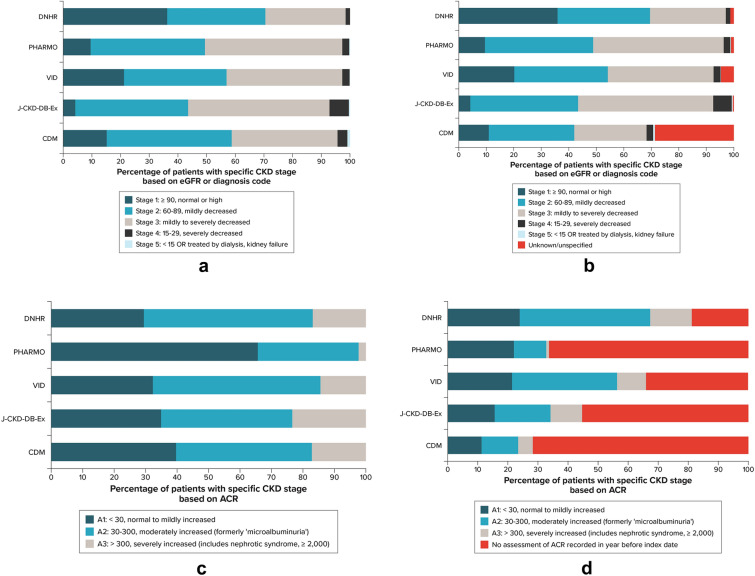
The estimated median recorded duration of CKD at the index date based on all available data (history of CKD diagnosis codes, eGFR test results, or ACR test results) was ~ 2 years in the VID and J-CKD-DB-Ex, ~ 3 years in DNHR and CDM, and 6 years in PHARMO (Appendix Table C4, Supplementary Material). Based on CKD stage (defined by eGFR or diagnosis code only) (Fig. 1a and 1b), the proportion of patients in stage 1 CKD at baseline was highest in the DNHR (35.9%) and lowest in J-CKD-DB-Ex (4.1%), which has a requirement of CKD (proteinuria and/or eGFR of < 60 ml/min/1.73 m2) to enter the database. Across data sources, approximately 31–39% of patients were in stage 2 and 28–49% were in stage 3 CKD at baseline. Severe CKD (stage 4) was uncommon, ranging from 1.6–6.7%, with the highest percentage in J-CKD-DB-Ex.
Fig. 1.
CKD severity at the index date, by data source. a CKD stage at the index date defined by eGFR value or diagnosis code, among those with a test result. b CKD stage at the index date defined by eGFR value or diagnosis code, including those with no assessment of GFR or diagnosis code any time before or on the index date. c ACR categories among those with an ACR recording in the year before the index date. d ACR categories among all patients, including those with no ACR assessment in the year before index date. ACR albumin-to-creatinine ratio, CKD chronic kidney disease, DNHR Danish National Health Registers, eGFR estimated glomerular filtration rate, J CKD-DB-Ex Japan Chronic Kidney Disease Database Extension, PHARMO PHARMO Data Network, VID Valencia Health System Integrated Database. All patients met the inclusion eligibility criteria for CKD, which was assessed through diagnosis codes, eGFR test results, or urine ACR test results. For CDM, 58.2% of patients in the category of no assessment of ACR recorded before the index date had a recorded claim for an ACR test, but no result was available. Patients with stage 1 did not meet eGFR cohort inclusion criteria but could be included if they had two ACR measurements ≥ 30 mg/g
In the DNHR and VID, a majority of patients had an ACR test performed in the year before or on the index date (DNHR: 81.1% ACR, 18.9% no ACR; VID: 66.0% ACR, 34.0% no ACR). For the other data sources, there were fewer patients who had an ACR test performed than there were patients without an ACR test (CDM: 28.3% ACR, 71.7% no ACR; PHARMO: 33.6% ACR, 66.4% no ACR; J-CKD-DB-Ex: 44.6% ACR, 55.4% no ACR; note that in CDM, of the 39,382 patients with a record of a test for ACR, 15,894 had a result recorded for the ACR test). The highest proportion of patients was in ACR category A1 in PHARMO, and similar proportions were categorized as A1 in DNHR and VID (Fig. 1c). In the DNHR, with missing ACR values on only 18.9% of patients, 23.9% were categorized as A1, 43.5% as A2, and 13.8% as A3 at baseline (Fig. 1d). Baseline characteristics stratified by ACR or no ACR assessment are listed in Appendix Table C5 (Supplementary Material).
Baseline Comorbidities and Comedications
Of the clinical conditions known to be associated with an increased risk of CKD and assessed at any time before or on the index date, hypertension was the most common in all data sources (> 90% in CDM and VID, > 80% in DNHR and J-CKD-DB-Ex, and 71.1% in PHARMO) (Table 1). Hypercholesterolemia was the next most common baseline comorbidity in all data sources, recorded in ~ 90% of patients in CDM, and 77–79% in J-CKD-DB-Ex and VID; congestive heart failure was also common in J-CKD-DB-Ex (62.0%) (Appendix Table C6, Supplementary Material). Coronary heart disease was also frequent, ranging from 26.9% in VID to 58.5% in J-CKD-DB-Ex.
Medications other than GLDs recorded in the 180 days before or on the index date are shown in Appendix Table C7 (Supplementary Material). A high proportion of SGLT2i initiators had previous use of medication classes of interest with potential CKD-protective effect (ACEi or ARB, sMRA, GLP-1 RA) before initiating an SGLT2i (Fig. 2). Of the other cardiovascular medications, beta-blockers, calcium channel blockers, statins, anticoagulants, and antiplatelet agents all were commonly used.
Fig. 2.
Previous use of CKD-protective medications in relation to the index SGLT2i medication. ACEi angiotensin-converting enzyme inhibitors, ARB angiotensin receptor blockers, CKD chronic kidney disease, GLP-1 RA glucagon-like peptide-1 receptor agonists, SGLT2i sodium-glucose cotransporter 2 inhibitors, sMRA steroidal mineralocorticoid receptor antagonists. Note that, by design, SGLT2i use could not occur from −365 days to the day before the index date
Characteristics of the Index Medication at Baseline and During Follow-Up
The index SGLT2i was most commonly prescribed as “monotherapy” (defined as no concomitant ACE/ARB, GLP-1 RA, or sMRA treatment) according to the study definition (Appendix B, Supplementary Material) for patients in J-CKD-DB-Ex (78.0%) and as an “add-on therapy” to another drug of interest in the other data sources (60.8%, VID; 59.6%, PHARMO; 57.6%, CDM; 47.2%, DNHR) (Table 2). Empagliflozin was the most common type of SGLT2i medication prescribed at the index date in the DNHR (60.8%), J-CKD-DB-Ex (30.8%), VID (47.5%), and CDM (61.6%), whereas dapagliflozin was the most common index SGLT2i in PHARMO (57.5%) (Appendix Figure C1, Supplementary Material). The median duration of total follow-up was 15.8 months in CDM and ranged from 20.7 to 26.5 months across the other 4 data sources. The median duration of the initial SGLT2i exposure episode was only 2.8 months in PHARMO, but almost a year in VID (11.6 months) and in other data sources ranged from 5.4 months in CDM to 9.7 months in DNHR. The median days’ supply of the index SGLT2i was ~ 3.5 months in DNHR and ~ 1 month in the other 4 data sources.
Table 2.
Characteristics of the index SGLT2i at baseline and during follow-up, by data source
| Characteristic | DNHR (n = 21,739) | PHARMO (n = 381) | VID (n = 31,785) | J-CKD-DB-Ex (n = 1157) | CDM (n = 56,219) |
|---|---|---|---|---|---|
| Classification of the index SGLT2i at the index date, n (%) | |||||
| Monotherapy | 4769 (21.9) | 98 (25.7) | 4247 (13.4) | 902 (78.0) | 10,117 (18.0) |
| Combination therapy | 1645 (7.6) | 10 (2.6) | 2828 (8.9) | 35 (3.0) | 1846 (3.3) |
| Add-on | 10,268 (47.2) | 227 (59.6) | 19,339 (60.8) | 205 (17.7) | 32,392 (57.6) |
| Switch | 2162 (9.9) | 14 (3.7) | 1499 (4.7) | 6 (0.5) | 3972 (7.1) |
| Index SGLT2i was an “add-on” to…, n (%) | |||||
| GLP-1 RA | 3918 (18.0) | 14 (3.7) | 2058 (6.5) | 75 (6.5) | 6801 (12.1) |
| sMRA | 1209 (5.6) | 46 (12.1) | 1990 (6.3) | 136 (11.8) | 2873 (5.1) |
| ACEi/ARB | 10,102 (46.5) | 223 (58.5) | 19,858 (62.5) | 156 (13.5) | 36,511 (64.9) |
| Duration of initial SGLT2i treatment after cohort entry, median (1st, 99th percentiles), months | 9.7 (0, 66) | 2.8 (0,12) | 11.6 (0.3, 71) | 7.7 (0, 67) | 5.4 (1, 55) |
| Days’ supply of index SGLT2i, mean (SD); median, days | 121.8 (96.7); 105 | 28.9 (17.8); 28 | 29.5 (5.8); 30 | 36.0 (24.5); 34 | 47.2 (27.8); 30 |
| No. of prescriptions during follow-up for the SGLT2i drug class, median (1st, 99th percentiles) | 5 (1, 50) | 3 (0, 78) | 11 (1, 76) | 7 (1, 82) | 3 (1, 42) |
| No. of distinct “current-use” periods (treatment episodes) during follow-up for the index SGLT2i drug class, n (%) | |||||
| 1 | 17,801 (81.9) | 316 (82.9) | 23,381 (73.6) | 879 (76.0) | 28,202 (50.2) |
| 2 | 2949 (13.6) | 42 (11.0) | 5482 (17.3) | 199 (17.2) | 13,912 (24.7) |
| 3+ | 989 (4.5) | 23 (6.0) | 2922 (9.2) | 79 (6.8) | 14,105 (25.1) |
| No. of distinct prescriptions during follow-up for the index SGLT2i drug class, median (1st, 99th percentiles) | 6 (1, 54) | 6 (1, 100) | 17 (1, 79) | 10 (1, 102) | 8 (1, 57) |
| No. of discontinuations (interruptions) of current use during follow-up, n (%) | |||||
| 0 | 13,123 (60.4) | 316 (82.9) | 23,381 (73.6) | 535 (46.2) | 28,202 (50.2) |
| 1 | 6942 (31.9) | 42 (64.6) | 5482 (17.3) | 469 (40.5) | 13,912 (24.7) |
| 2 | 1244 (5.7) | 16 (24.6) | 1732 (5.5) | 109 (9.4) | 6748 (12.0) |
| 3+ | 430 (2.0) | 7 (1.8) | 1190 (3.7) | 44 (3.8) | 7357 (13.1) |
| No. of patients with an interruption of current use lasting 90 days or more, n (%) | 2001 (9.2) | 25 (6.6) | 3769 (11.9) | 494 (42.7) | 18,100 (32.2) |
| Duration of total exposure to index therapy, median (1st, 99th percentile), months | 12.8 (0, 69) | 7.5 (1, 43) | 17.0 (0.3, 73) | 11.9 (0, 70) | 14.5 (2, 79) |
| Other drug classes started during follow-up, n (%) | |||||
| GLP-1 RA | 9233 (42.5) | 53 (13.9) | 6761 (21.3) | 164 (14.2) | 7102 (12.6) |
| sMRA | 3110 (14.3) | 65 (17.1) | 3939 (12.4) | 206 (17.8) | 1519 (2.7) |
| ACEi/ARB | 17,477 (80.4) | 281 (73.8) | 25,060 (78.8) | 709 (61.3) | 1943 (3.5) |
| Duration of total follow-up, median (1st, 99th percentiles), months | 20.7 (0, 80) | 21.6 (0, 82) | 26.5 (0.3, 77) | 24.4 (0, 72) | 15.8 (0, 83) |
ACEi angiotensin-converting enzyme inhibitors, ARB angiotensin receptor blockers, DNHR Danish National Health Registers, GLP-1 RA glucagon-like peptide-1 receptor agonists, J-CKD-DB-Ex Japan Chronic Kidney Disease Database Extension, CDM Optum’s de-identified Clinformatics® Data Mart Database, SD standard deviation, SGLT2i sodium-glucose cotransporter 2 inhibitors, sMRA steroidal mineralocorticoid receptor antagonists, VID Valencia Health System Integrated Database
The proportion of patients with only one distinct current-use episode during follow-up ranged from 50% (CDM) to 83% (PHARMO). A smaller proportion of patients in DNHR (9.2%), VID (11.9%), and PHARMO (6.6%) reported interruption of current use lasting ≥ 90 days, a proxy for discontinuation, than in CDM (32.2%) or J-CKD-DB-Ex (42.7%). The median total duration of SGLT2i therapy ranged from 7.5 months (PHARMO) to 17 months (VID). Of the other drug classes started during follow-up, ACEi or ARB were the most common across data sources (80.4%, DNHR; 61.3%, J-CKD-DB-Ex; 78.8%, VID; 73.8%, PHARMO) except CDM (3.5%), although 73.7% of patients in CDM were already using an ACEi or ARB in the 90 days before the index date (Fig. 2).
Treatment Changes over Time for SGLT2i During Follow-Up
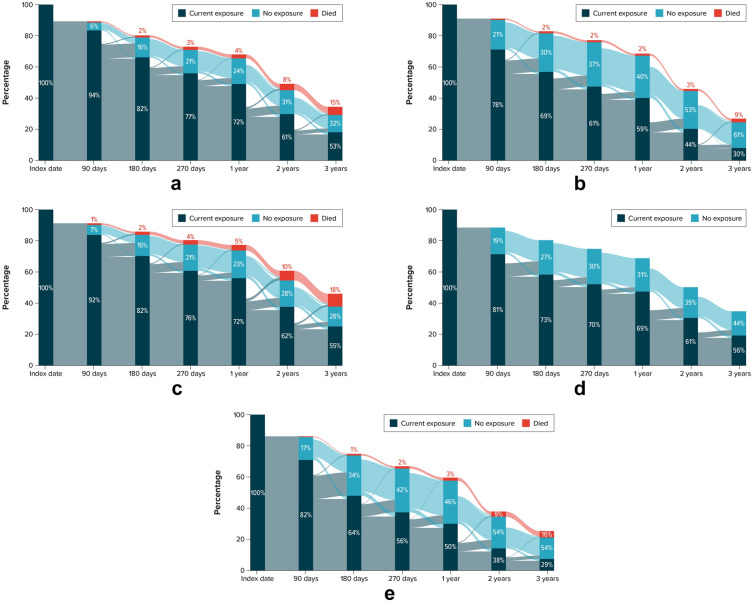
During the study period, the proportions of patients observed to be receiving SGLT2i treatment at each assessment timepoint (90 days, 180 days, 270 days, 1 year, 2 years, 3 years) were similar among the DNHR, VID, and J-CKD-DB-Ex (Fig. 3). The lowest proportions of patients currently receiving SGLT2i treatment of those under observation at yearly timepoints during the study period were observed in CDM (1 year, 50%; 2 years, 38%; 3 years, 29%) and PHARMO (1 year, 59%; 2 years, 44%; 3 years, 30%). Among the other data sources (J-CKD-DB-Ex, VID, DNHR), yearly proportions of patients observed to be receiving current treatment were similar at each timepoint. These percentages represent a combination of both patients who remained continuously on treatment up to the timepoint and other patients who had discontinued and restarted the medications. A common pattern in all data sources was decreasing use over time, with the largest proportional decrease in use occurring between the 90-day and 180-day timepoints. After this, the proportion of patients with no treatment remained fairly stable in each data source except for CDM and PHARMO, in which the proportions not treated increased at 2 and 3 years. At each timepoint, a small proportion of nonusers who remained under observation was found to change and become current users.
Fig. 3.
Treatment states at specific timepoints for SGLT2 initiators for each data sourcea. a DNHR (median follow-up time, 20.7 months). b PHARMO (median follow-up time, 21.6 months). c VID (median follow-up time, 26.5 months). d J-CKD-DB-Exb (median follow-up time, 24.4 months). e CDM (median follow-up time, 15.8 months). DNHR Danish National Health Registers, J-CKD-DB-Ex Japan Chronic Kidney Disease Database Extension, CDM Optum’s de-identified Clinformatics® Data Mart Database, PHARMO PHARMO Data Network, SGLT2i sodium-glucose cotransporter 2 inhibitors, VID Valencia Health System Integrated Database. aAs SGLT2i treatment discontinuation and subsequent re-initiation has been shown to be common [43], it is important to note that estimates presented are cross-sectional estimates of the entire cohort at each timepoint, as opposed to an analysis of individual patient trajectories. Percentages reported in Sankey diagrams are of the number of patients remaining under observation in the study at that time. Individual patients can move between treatment states throughout study follow-up. Note that losses to follow-up and censoring are represented in the white space at the top of each diagram. bInformation on death was not available in J-CKD-DB-Ex
Discussion
In this multinational, multi-cohort study, we analyzed real-world data from five data sources to describe cohorts of individuals with T2D and CKD who initiated an SGLT2i in Europe, Japan, and the US. The cohort sizes ranged from 381 in PHARMO to 56,219 in CDM; the comparatively smaller size of the PHARMO cohort may reflect that SGLT2i were reimbursed in The Netherlands only for those not using insulin during the study period. Depending on database characteristics, the approved indications for SGLT2i in the regions of interest at the time of the study, the availability and completeness of laboratory measurements in the respective data sources, and adherence to recommended laboratory testing routines in this patient population, we observed differences in CKD risk categories across the study cohorts. In particular, heterogeneity in ACR monitoring and capture across the data sources was a major driver of differences in CKD risk profiles: a large percentage of patients in all data sources except the DNHR had no ACR test result recorded in the year before the index date. In DNHR, with near complete availability of laboratory measurements and seemingly high adherence to ACR testing routines and, thus, greater odds of inclusion via ACR criteria and not necessarily by eGFR criteria, nearly 70% of patients had CKD stage 1 and 2 (based on eGFR measurements alone). However, very few patients in DNHR had a diagnosis code for CKD because the database includes only hospital codes (including outpatient specialist visits and hospital admissions) but not diagnosis codes from GP visits. In CDM, with less complete laboratory measurements in the database, just under 37% of patients were classified as having CKD stage 1 or 2 based on eGFR alone, and 42% were classified as stage 1 or 2 based on eGFR or diagnosis code. J-CKD-DB-Ex, in which patients were required to have evidence of proteinuria and/or an eGFR < 60 ml/min/1.73 m2 upon entry into the data source, had the lowest proportion of patients with stage 1 CKD at baseline. The proportions with stage 3 or stage 4 disease, as defined by eGFR or diagnostic code, were highest in J-CKD-DB-Ex and PHARMO. Although ACR test utilization differed across data sources, albuminuria burden was largely consistent across most data sources, with more than 60% of patients with test results having albuminuria category A2 or A3, indicating a clear unmet need in this population to reduce ACR.
Across the study cohorts, median age was similar, and a higher proportion of men than women was observed. Differences were observed in baseline characteristics, including markers of severity of T2D and of kidney dysfunction, baseline comorbidities and medication use, and patterns of SGLT2i utilization at baseline and over time. For example, the proportion of patients with obesity ranged from 8.4% in J-CKD-DB-Ex to 66.6% in VID. This difference could reflect known differences in obesity prevalence across countries, but likely reflects to a higher degree differences in ascertainment in the data sources, with a lower prevalence seen in data sources without body mass index information and a higher prevalence in data sources in which more granular obesity information was recorded. In addition, across the data sources, the median duration of T2D in SGLT2i new users ranged from 4 years (in CDM, derived from a database of administrative health claims for members of large commercial and Medicare Advantage health plans) to 12 years (in PHARMO, a database composed of EHRs from national healthcare systems). Duration of chronic conditions, such as T2D and CKD, before initiating the index treatment is dependent on the length and completeness of pre-index data. Enrollee turnover is high in US commercial health plans [32], resulting in shorter pre-index time than in countries in which most residents have national insurance for a lifetime. Most patients had used GLDs in the 180 days before or on the index date, but the type of GLDs used varied by data source and may reflect differences in diabetes treatment guidelines and practices in the different countries, as well as differences in formulary policies, data characteristics, and secular trends. HbA1c values recorded before or on the index date suggested that patients in J-CKD-DB-Ex had better glycemic control of their T2D than did patients in the other data sources—possibly as a product of more frequent surveillance in an inpatient setting—and were also more likely to use insulin during this period. Insulin use was lowest in PHARMO, potentially because SGLT2i were reimbursed for only those not using insulin at the time of the study [33].
Differences in the populations represented in this analysis and in the performance of the CKD definition reflect inherent heterogeneity in the data sources, as well as differences in healthcare systems, treatment guidelines, country-specific clinical practices, formulary policies, application of diagnostic coding systems in the participating countries, and market introduction of CKD-protective medications. Describing and understanding utilization of current treatments for prevention of CKD progression in T2D and heterogeneity among data sources is an important first step to guide future research on new treatments in this same therapeutic area. For instance, the number of identified new users of SGLT2i in PHARMO was much lower than in the other data sources and cannot be explained by the populations covered in the different data sources. In The Netherlands, SGLT2i were not indicated for patients with CKD during the study period, and limited uptake of SGLT2i is reflected in the relatively short median treatment duration of the index SGLT2i for the PHARMO population. T2D-related treatment guidelines were revised in 2021 to incorporate SGLT2i as either monotherapy or as an add-on to metformin in patients at risk for cardiovascular disease due to their medical history or CKD [34]. However, more recent data from PHARMO were not included in this study.
The treatment landscape for the prevention of progression of CKD in T2D is evolving rapidly [35]. The study period of 2012–2021 was largely before the approval of new CKD indications for existing treatments (SGLT2i and GLP-1 RA) and new treatments (e.g., finerenone, an oral, selective nonsteroidal mineralocorticoid receptor antagonist, approved in the US in 2021 and in Europe and Japan in 2022 [36, 37]). The proportions of patients initiating SGLT2i generally increased during the later years of the study period, as clinical evidence of the CKD-protective therapies emerged (e.g., in the CREDENCE [NCT02065791] and DAPA-CKD [NCT03036150] trials [38, 39]). Increases over time in SGLT2i use in The Netherlands and Japan may have been mitigated by the reimbursement environment in those countries, leading to less pronounced increases in later study years than were observed in the other data sources. In addition, we observed the utilization of many treatment options and therapeutic approaches with available medications that were heterogeneous and dynamic both within and among data sources. This heterogeneity coincides with the multitude of guideline-recommended treatment options that are available for the prevention of CKD progression. Further, we observed frequent treatment discontinuations, restarting, and switching of the index therapy over time. The analysis of SGLT2i treatment patterns over time revealed decreasing use over time, with the largest proportional decrease in SGLT2i use occurring between the 90-day and 180-day timepoints. At the 1-year timepoint, at least 50% of patients were currently receiving SGLT2i treatment across the data sources, with the yearly proportions of patients receiving current treatment lower in CDM and PHARMO and the highest in J-CKD-DB-Ex, VID, and DNHR. As uptake of SGLT2i continues following indications for the prevention of CKD progression in the study countries, and as new treatments emerge, persistence patterns and factors affecting adherence for CKD-preventive therapies should be monitored in future studies. A common finding across all data sources was that ACEi or ARB medications (one of the four classes of medications used to potentially slow the CKD progression) were by far the most frequent type of medication used before initiation of the index SGLT2i. Another consistent finding in all data sources was that hypertension was the most frequently recorded medical condition associated with increased risk of CKD.
The strengths and limitations of this study are acknowledged. The study used existing healthcare data from multiple countries and data sources, which allowed evaluation of study parameters in diverse settings, populations, and healthcare systems. A common protocol and statistical analysis plan were followed, and efforts were made to harmonize approaches as much as possible across all data sources while adjusting criteria to align with the data sources [40]. Nonetheless, as with all studies based on existing healthcare data sources, the data were generated for healthcare delivery or billing rather than research; thus, missing data (due to missing assessments or because results were not recorded in the data sources) or misclassification of study variables is present. The study cohorts reflect selected populations of SGLT2i initiators who have T2D and CKD, and the results may not be generalizable to all people with T2D and CKD in the study countries. We could not examine information on why the index drug was prescribed, as indication information was available in only one data source (VID); thus, we could not capture secular trends within a given country with respect to timing of information on benefits of the SGLT2i for CKD, cardiovascular disease and heart failure outcomes, or label expansions. However, most of the period studied preceded the approval of CKD and cardiovascular indications for SGLT2i, although evidence of the renal protective effects of the class was observed earlier [41, 42]. Further research describing the evolving treatment landscape for people with CKD and T2D is needed to describe the impact of new treatment options on treatment patterns and clinical outcomes.
In conclusion, in this study population with CKD and T2D in 2012–2021, largely before the approval of new CKD indications for existing treatments (SGLT2i and GLP-1 RA) and new CKD treatments (e.g., finerenone), treatment options and therapeutic approaches were heterogeneous and dynamic both within and among data sources. At 1 year of follow-up, half or more of patients who initiated an SGLT2i were currently receiving SGLT2i treatment across the data sources. The treatment landscape for the prevention of progression of CKD in T2D is evolving rapidly. Understanding the characteristics and patterns of use of existing treatments and characterizing the differences in populations and treatment patterns across data sources is a first step in designing future studies to evaluate kidney and cardiovascular outcomes with treatment to prevent CKD progression.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Medical Writing/Editorial Assistance
The authors gratefully acknowledge Daniel Mines, MD, MSCE, of RTI Health Solutions for his coauthorship of the statistical analysis plan, review of study documentation, and scientific contributions to this study. Kate Lothman of RTI Health Solutions provided medical writing support and John Forbes of RTI Health Solutions provided editorial support, with funding from Bayer AG, during manuscript development.
Author Contributions
Conceptualization: Catherine B. Johannes, Ryan Ziemiecki, Manel Pladevall-Vila, J. Bradley Layton, Alfredo E. Farjat, David Vizcaya, Nikolaus G. Oberprieler; Data curation: Ron M.C. Herings, Craig I. Coleman, Hiroshi Kanegae, Yuichiro Yano, Naoki Kashihara, Reimar W. Thomsen, Ina Trolle Andersen, Christian Fynbo Christiansen, Frederik Pagh Bredahl Kristensen, Isabel Hurtado, Celia Robles Cabaniñas, Clara Rodríguez Bernal, Aníbal García-Sempere; Formal analysis: Brenda N. Baak, Jetty A. Overbeek, Fernie J.A. Penning-van Beest, Craig I. Coleman, Hiroshi Kanegae, Yuichiro Yano, Naoki Kashihara, Reimar W. Thomsen, Ina Trolle Andersen, Christian Fynbo Christiansen, Frederik Pagh Bredahl Kristensen, Isabel Hurtado, Celia Robles Cabaniñas, Clara Rodríguez Bernal, Aníbal García-Sempere; Funding acquisition: Nikolaus G. Oberprieler; Investigation: J. Bradley Layton, Catherine B. Johannes, Ryan Ziemiecki, Manel Pladevall-Vila, David Vizcaya, Nikolaus G. Oberprieler, Reimar W. Thomsen, Ina Trolle Andersen, Christian Fynbo Christiansen, Frederik Pagh Bredahl Kristensen, Isabel Hurtado, Celia Robles Cabaniñas, Aníbal García-Sempere, Clara Rodríguez Bernal; Methodology: J. Bradley Layton, Catherine B. Johannes, Ryan Ziemiecki, Manel Pladevall-Vila, Alfredo E. Farjat, David Vizcaya, Nikolaus G. Oberprieler, Hiroshi Kanegae, Yuichiro Yano, Naoki Kashihara, Suguru Okami, Satoshi Yamashita, Reimar W. Thomsen, Ina Trolle Andersen, Christian Fynbo Christiansen, Frederik Pagh Bredahl Kristensen, Isabel Hurtado, Celia Robles Cabaniñas, Aníbal García-Sempere, Clara Rodríguez Bernal, Brenda N. Baak, Jetty A. Overbeek, Fernie J.A. Penning-van Beest; Project administration: Catherine B. Johannes, Ryan Ziemiecki, Manel Pladevall-Vila, J. Bradley Layton, David Vizcaya, Nikolaus G. Oberprieler, Fangfang Liu, Brenda N. Baak, Craig I. Coleman, Suguru Okami; Supervision: Craig I. Coleman, Catherine B. Johannes, Ryan Ziemiecki, Manel Pladevall-Vila, J. Bradley Layton, Alfredo E. Farjat, David Vizcaya, Fangfang Liu, Nikolaus G. Oberprieler; Validation: Ryan Ziemiecki; Writing—review & editing: Catherine B. Johannes, Ryan Ziemiecki, Manel Pladevall-Vila, Natalie Ebert, Csaba P. Kovesdy, Reimar W. Thomsen, Brenda N. Baak, Aníbal García-Sempere, Hiroshi Kanegae, Craig I. Coleman, Michael Walsh, Ina Trolle Andersen, Clara Rodríguez Bernal, Celia Robles Cabaniñas, Christian Fynbo Christiansen, Alfredo E. Farjat, Alain Gay, Patrick Gee, Ron M.C. Herings, Isabel Hurtado, Naoki Kashihara, Frederik Pagh Bredahl Kristensen, Fangfang Liu, Suguru Okami, Jetty A. Overbeek, Fernie J.A. Penning-van Beest, Satoshi Yamashita, Yuichiro Yano, J. Bradley Layton, David Vizcaya, and Nikolaus G. Oberprieler.
Funding
Bayer AG funded this research, development of this article, and the Rapid Service Fee for publication. Authors affiliated with Bayer were involved in the study design, analyses, and development of this publication.
Declarations
Conflict of Interest
Alfredo E. Farjat, Fangfang Liu, Suguru Okami, Satoshi Yamashita, and Nikolaus G. Oberprieler are employees of Bayer, which funded this study. Alain Gay was an employee of Bayer when this research was conducted and is now an employee of Clario, Philadelphia, PA, USA. David Vizcaya was an employee of Bayer when this research was conducted and is now an employee of Alexion Pharma S.L., Barcelona, Spain. Catherine B. Johannes, Ryan Ziemiecki, Manel Pladevall-Vila, and J. Bradley Layton are employees of RTI Health Solutions, which received research funding for this study from Bayer. Brenda N. Baak, Jetty A. Overbeek, Fernie J.A. Penning-van Beest, and Ron M.C. Herings are employees of the PHARMO Institute for Drug Outcomes Research. This independent research institute performs financially supported studies for government and related healthcare authorities and several pharmaceutical companies. Craig I. Coleman has received grant funding and consulting fees from Bayer AG and AstraZeneca Pharmaceuticals. Csaba P. Kovesdy received consulting fees from Abbott, Akebia, Astra Zeneca, Bayer, Boehringer Ingelheim, Cara Therapeutics, CSL Behring, CSL Vifor, GSK, Pharmacosmos, ProKidney, Renibus and Takeda. Yuichiro Yano reports consultancy for Bayer. Naoki Kashihara reports research grants from Daiichi Sankyo, AstraZeneca, and Bayer. Natalie Ebert receives honoraria from Bayer AG. Reimar W. Thomsen, Ina Trolle Andersen, Christian Fynbo Christiansen, and Frederik Pagh Bredahl Kristensen are employees of Aarhus University, which receives institutional research funding from public and private entities, including regulators, pharmaceutical companies, and contract research organizations. This includes the present study. Reimar W. Thomsen has given presentations and lectures on medical research (both with and without financial compensation) for pharmaceutical companies, including AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and Sanofi. Aníbal García-Sempere, Clara Rodríguez Bernal, Celia Robles Cabaniñas, and Isabel Hurtado are employed by FISABIO, a research body in Spain affiliated with the Health Department of the Valencia Government, which receives public and private funding to conduct biomedical research, including the present study.
Ethical Approval
This study used de-identified data from electronic health records. Ethics committee review was waived for DNHR and PHARMO. The study protocol was reviewed and approved by the Comité Ético de Investigación con Medicamentos del Hospital Clínico Universitario de Valencia for VID (2022/164). This study protocol was reviewed and approved by the ethics committee of the Shiga University of Medical Science for J-CKD-DB-Ex (R2022-143). CDM data are de‐identified and are compliant with the Health Insurance Portability and Accountability Act of 1996. This study was deemed to not constitute research involving human subjects according to 45 Code of Federal Regulations 46.102(f) and was deemed exempt from board oversight. The institutional review board of RTI International deemed the study exempt from full review. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Patient consent for participation and patient consent for publication are not applicable.
Footnotes
Alain Gay and David Vizcaya: Affiliation at the time of the study.
Prior presentation: This research was presented in preliminary form as follows: Johannes CB, Ziemiecki R, Pladevall-Vila M, Kovesdy CP, Ebert E, Thomsen RW, et al. Use of SGLT2 inhibitors in patients with type 2 diabetes and chronic kidney disease: a multicountry report from the FOUNTAIN platform. Poster presented at the 2024 Annual Meeting of the International Society for Pharmacoepidemiology; August 24–28, 2024; Berlin, Germany.
References
- 1.Thomas MC, Cooper ME, Zimmet P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat Rev Nephrol. 2016;12(2):73–81. 10.1038/nrneph.2015.173. [DOI] [PubMed] [Google Scholar]
- 2.Li H, Lu W, Wang A, Jiang H, Lyu J. Changing epidemiology of chronic kidney disease as a result of type 2 diabetes mellitus from 1990 to 2017: estimates from Global Burden of Disease 2017. J Diabetes Investig. 2021;12(3):346–56. 10.1111/jdi.13355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandbaek A, Griffin SJ, Sharp SJ, Simmons RK, Borch-Johnsen K, Rutten GE, et al. Effect of early multifactorial therapy compared with routine care on microvascular outcomes at 5 years in people with screen-detected diabetes: a randomized controlled trial: the ADDITION-Europe Study. Diabetes Care. 2014;37(7):2015–23. 10.2337/dc13-1544. [DOI] [PubMed] [Google Scholar]
- 4.Thomsen RW, Nicolaisen SK, Adelborg K, Svensson E, Hasvold P, Palaka E, et al. Hyperkalaemia in people with diabetes: occurrence, risk factors and outcomes in a Danish population-based cohort study. Diabet Med. 2018;35(8):1051–60. 10.1111/dme.13687. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Poncelas A, Garre-Olmo J, Franch-Nadal J, Diez-Espino J, Mundet-Tuduri X, Barrot-De la Puente J, et al. Prevalence of chronic kidney disease in patients with type 2 diabetes in Spain: PERCEDIME2 study. BMC Nephrol. 2013;14:46. 10.1186/1471-2369-14-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serné EH, Overbeek JA, Palmen J, de Jong HJI, Stehouwer CDA. [Prevalence and drug treatment of people with type 2 diabetes mellitus and a very high risk of cardiovascular disease]. Ned Tijdschr Geneeskd. 2023 Sep 19;167. Cited by: Diabetes type 2 én een hoog risico op hart- en vaatziekten. [PubMed]
- 7.Wu B, Bell K, Stanford A, Kern DM, Tunceli O, Vupputuri S, et al. Understanding CKD among patients with T2DM: prevalence, temporal trends, and treatment patterns-NHANES 2007–2012. BMJ Open Diabetes Res Care. 2016;4(1): e000154. 10.1136/bmjdrc-2015-000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohta M, Babazono T, Uchigata Y, Iwamoto Y. Comparison of the prevalence of chronic kidney disease in Japanese patients with type 1 and type 2 diabetes. Diabet Med. 2010;27(9):1017–23. 10.1111/j.1464-5491.2010.03049.x. [DOI] [PubMed] [Google Scholar]
- 9.Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389(10075):1238–52. 10.1016/S0140-6736(16)32064-5. [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, et al. Chronic kidney disease as a global public health problem: approaches and initiatives—a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72(3):247–59. 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Schoolwerth AC, Burrows NR, Williams DE, Stith KR, McClellan W, et al. Comprehensive public health strategies for preventing the development, progression, and complications of CKD: report of an expert panel convened by the Centers for Disease Control and Prevention. Am J Kidney Dis. 2009;53(3):522–35. 10.1053/j.ajkd.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 12.KDIGO. Kidney Disease: Improving Global Outcomes Work Group. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2024;105(Suppl 4):S117-314. 10.1016/j.kint.2023.10.018. [DOI] [PubMed] [Google Scholar]
- 13.KDIGO. Kidney Disease: Improving Global Outcomes Diabetes Work Group. KDIGO 2022 Clinical practice guideline for diabetes management in chronic kidney disease. November 2022. https://www.ncbi.nlm.nih.gov/pubmed/36272764. Accessed 6 Nov 2023.
- 14.ADA. American Diabetes Association. Chronic kidney disease and risk management: standards of care in diabetes—2024. Jan 1, 2024. Report No.: 0149-5992. https://diabetesjournals.org/care/article/47/Supplement_1/S219/153938/11-Chronic-Kidney-Disease-and-Risk-Management.
- 15.Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, et al. Management of hyperglycemia in type 2 diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45(11):2753–86. 10.2337/dci22-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forxiga SmPC. AstraZeneca AB. Forxiga (dapagliflozin) 10 mg film-coated tablets. 2024. https://www.medicines.org.uk/emc/product/7607/smpc. Accessed 7 Mar 2024.
- 17.Oberprieler NG, Pladevall-Vila M, Johannes C, Layton JB, Golozar A, Lavallee M, et al. FOUNTAIN: a modular research platform for integrated real-world evidence generation. BMC Med Res Methodol. 2024;24(1):224. 10.1186/s12874-024-02344-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt M, Schmidt SAJ, Adelborg K, Sundboll J, Laugesen K, Ehrenstein V, Sorensen HT. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol. 2019;11:563–91. 10.2147/CLEP.S179083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pottegård A, Schmidt SAJ, Wallach-Kildemoes H, Sørensen HT, Hallas J, Schmidt M. Data resource profile: the Danish National Prescription Registry. Int J Epidemiol. 2017;46(3):798-f. 10.1093/ije/dyw213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorensen HT, Pedersen L, Jorgensen J, Ehrenstein V. Danish clinical quality databases—an important and untapped resource for clinical research. Clin Epidemiol. 2016;8:425–7. 10.2147/CLEP.S113265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sorensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–90. 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuiper JG, Bakker M, Penning-van Beest FJA, Herings RMC. Existing data sources for clinical epidemiology: the PHARMO Database Network. Clin Epidemiol. 2020;12:415–22. 10.2147/CLEP.S247575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Sempere A, Orrico-Sanchez A, Munoz-Quiles C, Hurtado I, Peiro S, Sanfelix-Gimeno G, Diez-Domingo J. Data resource profile: the Valencia Health System Integrated Database (VID). Int J Epidemiol. 2020;49(3):740–1. 10.1093/ije/dyz266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakagawa N, Sofue T, Kanda E, Nagasu H, Matsushita K, Nangaku M, et al. J-CKD-DB: a nationwide multicentre electronic health record-based chronic kidney disease database in Japan. Sci Rep. 2020;10(1):7351. 10.1038/s41598-020-64123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagasu H, Yano Y, Kanegae H, Heerspink HJL, Nangaku M, Hirakawa Y, et al. Kidney outcomes associated with SGLT2 inhibitors versus other glucose-lowering drugs in real-world clinical practice: the Japan Chronic Kidney Disease Database. Diabetes Care. 2021;44(11):2542–51. 10.2337/dc21-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lukowiak TM, Aizman L, Perz A, Miller CJ, Sobanko JF, Shin TM, et al. Association of age, sex, race, and geographic region with variation of the ratio of basal cell to cutaneous squamous cell carcinomas in the United States. JAMA Dermatol. 2020;156(11):1192–8. 10.1001/jamadermatol.2020.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.R Core Team. R Foundation for Statistical Computing. R: a language and environment for statistical computing. 2023. https://www.R-project.org/. Accessed 7 Jan 2024.
- 28.Thomas S, Chirila C, Ritchey ME. Visualization of patient electronic records to support exploratory analysis and variable derivation of categorical data. 5 November 2017. https://analytics.ncsu.edu/sesug/2017/SESUG2017_Paper-66_Final_PDF.pdf. Accessed 14 Dec 2021.
- 29.Gatto NM, Wang SV, Murk W, Mattox P, Brookhart MA, Bate A, et al. Visualizations throughout pharmacoepidemiology study planning, implementation, and reporting. Pharmacoepidemiol Drug Saf. 2022;31(11):1140–52. 10.1002/pds.5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young BA, Lin E, Von Korff M, Simon G, Ciechanowski P, Ludman EJ, et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care. 2008;14(1):15–23. [PMC free article] [PubMed] [Google Scholar]
- 31.Glasheen WP, Renda A, Dong Y. Diabetes complications severity index (DCSI)—update and ICD-10 translation. J Diabetes Complic. 2017;31(6):1007–13. 10.1016/j.jdiacomp.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 32.Fang H, Frean M, Sylwestrzak G, Ukert B. Trends in disenrollment and reenrollment within US commercial health insurance plans, 2006–2018. JAMA Netw Open. 2022;5(2): e220320. 10.1001/jamanetworkopen.2022.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dutch Healthcare Institute. Advice: expand conditions for medications SGLT-2 inhibitors for diabetes mellitus type 2 with a very high risk of cardiovascular disease. 2021. https://www.zorginstituutnederland.nl/publicaties/adviezen/2021/06/22/gvs-advies-sglt-2-remmers-uitbreiding-bijlage-2#. Accessed 10 June 2024.
- 34.van Schoonhoven AV, Schottler MH, Serne EH, Schrombges PPG, Postma MJ, Boersma C. The health and budget impact of sodium-glucose co-transporter-2 inhibitors (SGLT2is) in The Netherlands. J Med Econ. 2023;26(1):547–53. 10.1080/13696998.2023.2194802. [DOI] [PubMed] [Google Scholar]
- 35.Yau K, Dharia A, Alrowiyti I, Cherney DZI. Prescribing SGLT2 inhibitors in patients with CKD: expanding indications and practical considerations. Kidney Int Rep. 2022;7(7):1463–76. 10.1016/j.ekir.2022.04.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.European Medicines Agency. Kerendia [product information website]. 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/kerendia. Accessed 24 Oct 2023.
- 37.Kerendia PI. Bayer Healthcare. Kerendia (finerenone) tablets. September 2022. https://labeling.bayerhealthcare.com/html/products/pi/Kerendia_PI.pdf. Accessed 6 Nov 2023.
- 38.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–306. 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 39.Heerspink HJL, Stefansson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–46. 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 40.Oberprieler N, Pladevall-Vila M, Johannes C, Layton JB, Golozar A, Lavallee M, et al. FOUNTAIN: a modular research platform for integrated real-world evidence generation. Presented at the 39th International Conference on Pharmacoepidemiology & Therapeutic Risk Management (ICPE); 23–27 August 2023. Halifax, Canada.
- 41.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–34. 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 42.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57. 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 43.Malik ME, Falkentoft AC, Jensen J, Zahir D, Parveen S, Alhakak A, et al. Discontinuation and reinitiation of SGLT-2 inhibitors and GLP-1R agonists in patients with type 2 diabetes: a nationwide study from 2013 to 2021. Lancet Reg Health Eur. 2023;29: 100617. 10.1016/j.lanepe.2023.100617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.