Abstract
Application of mechanical stretch to cultured adult rat muscle satellite cells results in release of hepatocyte growth factor (HGF) and accelerated entry into the cell cycle. Stretch activation of cultured rat muscle satellite cells was observed only when medium pH was between 7.1 and 7.5, even though activation of satellite cells was accelerated by exogenous HGF over a pH range from 6.9 to 7.8. Furthermore, HGF was only released in stretched cultures when the pH of the medium was between 7.1 and 7.4. Conditioned medium from stretched satellite cell cultures stimulated activation of unstretched satellite cells, and the addition of anti-HGF neutralizing antibodies to stretch-conditioned medium inhibited the stretch activation response. Conditioned medium from satellite cells that were stretched in the presence of nitric-oxide synthase (NOS) inhibitor Nω-nitro-l-arginine methyl ester hydrochloride did not accelerate activation of unstretched control satellite cells, and HGF was not released into the medium. Conditioned medium from unstretched cells that were treated with a nitric oxide donor, sodium nitroprusside dihydrate, was able to accelerate the activation of satellite cells in vitro, and HGF was found in the conditioned medium. Immunoblot analysis indicated that both neuronal and endothelial NOS isoforms were present in satellite cell cultures. Furthermore, assays of NOS activity in stretched satellite cell cultures demonstrated that NOS is stimulated when satellite cells are stretched in vitro. These experiments indicate that stretch triggers an intracellular cascade of events, including nitric oxide synthesis, which results in HGF release and satellite cell activation.
INTRODUCTION
In adult skeletal muscle, satellite cells are quiescent most of the time, but when muscle is damaged or overused, they are activated to enter the cell cycle, divide, and differentiate. Therefore, a mechanism must exist to translate mechanical changes in muscle tissue into chemical signals that can activate satellite cells. There are two factors that have been demonstrated to activate quiescent satellite cells. The first is hepatocyte growth factor (HGF); HGF can activate quiescent rat muscle satellite cells in vitro and in vivo (Allen et al., 1995; Tatsumi et al., 1998). HGF is a heparin-binding growth factor that has been localized in the extracellular domain of uninjured skeletal muscle fibers, and after injury HGF quickly associates with satellite cells (Tatsumi et al., 1998; Anderson, 2000). Furthermore, quiescent and activated satellite cells have been shown to express the c-met receptor, which mediates the intracellular signaling response of HGF (Cornelison and Wold, 1997; Allen et al., 1998; Tatsumi et al., 1998), which is consistent with the proposed role of HGF in activating quiescent satellite cells.
The second factor involved in satellite cell activation is nitric oxide (NO). Anderson (2000) demonstrated that NO mediated the rapid morphological changes associated with satellite cell activation after crush injury, including cell hypertrophy and detachment from the adjacent fiber. At the cellular level, shear forces generated by contraction or retraction of damaged fibers within the basal lamina are thought to stimulate nitric-oxide synthase (NOS) to produce bursts of NO synthesis. Such bursts of NO were hypothesized by Anderson (2000) to provide the initial signal for activating satellite cells. Consequently, there is a strong hypothetical link between mechanical changes in muscle, NO synthesis, and satellite cell activation.
In previous work, we used a FlexerCell system to apply cyclic stretch to isolated rat satellite cells at 12 h postplating and found that stretched satellite cells were activated sooner than unstretched control satellite cells (Tatsumi et al., 2001). Furthermore, we found that the activation response was due to HGF release from its extracellular association with satellite cells. In the present report, we extend these observation by exploring the relationship between HGF binding to satellite cells and stretch-induced HGF release.
MATERIALS AND METHODS
Materials
Dulbecco's modified Eagle's medium (DMEM; low-glucose type, 31600-034), minimum essential medium-α (12000-022), horse serum (HS) (16050-122), antibiotic-antimycotic (15240-062), and gentamicin (15710-064) were purchased from Invitrogen (Carlsbad, CA). Poly-l-lysine (P-9155), bovine plasma fibronectin (F-1141), protease type XIV (P-5147), and 5-bromo-2′-deoxyuridine (BrdU) were purchased from Sigma-Aldrich (St. Louis, MO).
Human recombinant HGF (294-HG), goat polyclonal anti-human recombinant HGF antibody (AB-294-NA), peroxidase-conjugated polyclonal anti-human recombinant HGF antibody (Quantikine Human HGF Immunoassay kit DHG00), human recombinant HGF receptor (358-MT), and tetramethylbenzidine substrate solution (DY999) were purchased from R & D Systems (Minneapolis, MN). Rabbit polyclonal anti-c-met antibody (m-Met SP260) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and G3G4 mouse monoclonal anti-BrdU antibody and D3 mouse monoclonal antidesmin antibodies were obtained from the Developmental Studies Hybridoma Bank (Iowa City, IA). Affinity-purified horseradish peroxidase-conjugated goat anti-mouse IgG (A-4416) was purchased from Sigma-Aldrich. Affinity-purified biotinylated rabbit anti-goat IgG (BA-5000), affinity-purified biotinylated horse anti-goat IgG (BA-9500), affinity-purified biotinylated goat anti-rabbit IgG (BA-1000), affinity-purified biotinylated horse anti-mouse IgG (BA-2000), and avidin and biotinylated horseradish peroxidase kit (PK-6100) were purchased from Vector Laboratories (Burlingame, CA). Bovine serum albumin (BSA fraction V, 735078) was purchased from Roche Applied Science (Mannheim, Germany), and 3, 3′-diaminobenzidene (D-5637) and goat serum were purchased from Sigma-Aldrich.
Monoclonal anti-neuronal-NOS (nNOS) antibody (N31020-050) and monoclonal anti-endothelial nitric-oxide synthase (eNOS) antibodies (N30020-050) were purchased from Transduction Laboratories (Lexington, KY). Nω-Nitro-l-arginine methyl ester hydrochloride (l-NAME; 483125), NG-nitro-d-arginine methyl ester hydrochloride (d-NAME; 483124), l-arginine free base (l-Arg; 1820), and sodium nitroprusside dihydrate (SNP; 567538) were obtained from Calbiochem-Novabiochem (La Jolla, CA).
Enhanced chemiluminescence (ECL) detection kit (PRN2106) and nitrocellulose membranes (Hybond ECL; RPN2020D) were purchased from Amersham Biosciences (Piscataway, NJ). X-OMAT-AR x-ray film (166-0760) was obtained from Eastman Kodak (Rochester, NY). Biotinylated molecular weight standards (161-0319) were purchased from Bio-Rad (Hercules, CA).
Satellite Cell Isolation and Culture
Satellite cells were isolated from 9-mo-old male Sprague-Dawley rats according to Allen et al. (1998). Briefly, muscle groups from the hind limb and back were excised, trimmed of fat and connective tissue, hand minced with scissors, and digested for 1 h at 37°C with 1.25 mg/ml pronase. Cells were separated from muscle fiber fragments and tissue debris by differential centrifugation and plated on poly-lysine and fibronectin-coated dishes in DMEM containing 10% HS, 1% antibiotics mixture, and 0.5% gentamicin. For stretch experiments, cells were plated into BioFlex amino-culture plates (BF-AMIN) or Flex-I amino plates (FLX-P1001A) (Flexcell International, McKeesport, PA). For cell culture assays of conditioned medium from stretched cells, 24-well culture plates or 48-well culture plates were used (Costar, Cambridge, MA). Cultures were maintained in a humidified atmosphere of 5% CO2 at 37°C. In addition, companion satellite cell cultures were stained for the presence of desmin at 30 h to determine the percentage of myogenic cells present; cultures with <95% desmin-positive cells were not used. Each experiment was repeated two to five times, and in most cases, one rat was used per experiment.
Cultured satellite cells were subjected to mechanical stretch for variable periods of time from 12 to 36 h in culture in a vacuum-operated cyclic strain-providing instrument (FlexerCell FX-2000 System; Flexcell International, McKeesport, PA). The percentage of stretch and the stretch interval length were optimized for satellite cell activation previously at 25% stretch at 12-s intervals. Also, the viability of cells, as measured by cell density at the end of 36 h of stretch treatment, was shown to be the same for stretched and unstretched cells (Tatsumi et al., 2001).
Adjustment of pH in Medium
The pH of culture media (DMEM-10% HS) was adjusted by varying the NaHCO3 concentration. Figure 1 describes the effect of NaHCO3 addition on culture medium pH after equilibration in a humidified atmosphere of 5% CO2 at 37°C. Subsequent experiments in which medium pH was altered were based on these data.
Figure 1.
Adjustment of culture medium pH. DMEM-10% HS was adjusted by varying the NaHCO3 concentration. (A) Equilibration rate with addition of various concentrations of NaHCO3 in a humidified atmosphere of 5% CO2 at 37°C. (B) Standard curve for pH and NaHCO3.
In Vitro Activation Assay
Cultures were grown for 12 h in DMEM-10% HS, washed with serum-free DMEM, and then cultured for an additional 24 h in treatment medium. During the final 2 h, cultures were pulse labeled with 10 μM BrdU. Cultures were then prepared for immunocytochemistry detection of BrdU by using an anti-BrdU antibody (1:100 dilution in 0.1% BSA-phosphate-buffered saline [PBS]) and a horseradish peroxidase-conjugated secondary antibody (1:500 dilution) according to Tatsumi et al. (1998). The percentage of BrdU-labeled cells was used as an indicator of activation and entry into the cell cycle. Our experience has been that cells attach and progress toward activation more readily in conventional 24-well plates (Costar), and as a result, labeling index is often higher than in satellite cell cultures in the BioFlex plates.
In experiments in which conditioned medium was assayed, cultures were washed with serum-free DMEM at 12 h postplating, and treatments were imposed for 2 h in the presence of serum-free DMEM containing 1.0 mg of NaHCO3/ml. Treatments during the production of conditioned medium included the addition of l-NAME (10 μM), d-NAME (10 μM), l-Arg (2.4 mM), or SNP (30 μM) to stretched or unstretched cultures. Conditioned medium from each treatment was removed, centrifuged for 4 min at 1300 × g, filtered through 0.22-μm filter, and frozen at −80°C. For immunoneutralization experiments, 2 μg of control antibody per milliliter (purified anti-mouse IgG produced in goat; Cappel Research, Durham, NC), anti-HGF antibody (2 μg/ml), and/or HGF (2.5 ng/ml) were added to selected treatment-conditioned media for 2 h before the activation assay. Horse serum (10% final concentration), and antibiotics were added to each treatment medium immediately before assaying for satellite cell activation by using unstretched cultures.
Immunoblotting and Enhanced Chemiluminescence (ECL)
Conditioned media from 2-h stretched cells were subjected to SDS-PAGE on 10% polyacrylamide gels under reducing conditions (Tatsumi et al., 2001). Separated proteins were transferred to nitrocellulose membranes, which were then blocked with 10% powdered milk in 0.1% Tween 20/Tris-buffered saline (TTBS) before incubation with 1:500 dilution of anti-HGF antibody overnight at room temperature. Membranes were subsequently treated with biotinylated rabbit anti-goat secondary antibody at a 1:1000 dilution in 1% powdered milk in TTBS for 1 h at room temperature then with horseradish peroxidase-labeled avidin at a 1:500 dilution in TTBS for 30 min at room temperature, followed by ECL detection onto X-OMAT-AR x-ray films according to manufacturer's recommendation (Eastman Kodak).
Immunoblot analysis of NOS in lysates of 12-h cultured satellite cells was conducted in the same manner by using a monoclonal anti-nNOS or anti-eNOS antibodies (1:500 dilution) and a biotinylated rabbit anti-mouse secondary antibody (1:1000 dilution).
Enzyme-linked Immunosorbent Assay (ELISA) of HGF Binding to c-Met
ELISA plates (EIA/RIA Strip Plate-8; Costar, 2587) were coated overnight at 25°C with c-met/Fc chimera by adding 100 μl of 1 μg of protein/ml in PBS to each well, which was then blocked with 1% BSA, 5% sucrose, and 0.05% sodium azide in PBS for 2 h at 25°C. Plates were washed with DMEM and incubated in CO2 incubator for 2 h with 100 μl of 2.5 ng of HGF/ml in DMEM. Plates were subsequently washed once with DMEM, immediately fixed with cold 3.7% paraformaldehyde in PBS for 5 min at room temperature, and retreated with the blocking solution overnight at 4°C. The binding of HGF to c-met was detected with peroxidase-conjugated anti-HGF polyclonal antibody and tetramethylbenzidine substrate solution, followed by optical density measurements at wavelengths of 450 and 540 nm.
NOS Assay
NOS activity was assessed by subjecting cultured satellite cells to mechanical stretch for variable periods between 12 and 32 h postplating in minimum essential medium-α–10% HS (containing 1.11 mg of NaHCO3/ml) in the presence or absence of 10 μM l-NAME, 10 μM d-NAME, and 2.4 mM l-arginine. Conditioned media were collected at each time point (100 μl/vial × 3) in polypropylene vials (Alltech Associates, Deerfield, IL) with Teflon liners (98094; Alltech Associates) and screw caps (73044; Alltech Associates). Samples (20 μl/run) were applied to an automated NO detector–high-performance liquid chromatography system (Eicom, Kyoto, Japan), which is composed of Autosampler model 33, column oven (ATC-10), NOx Analyzer (ENO-10), NOx Detector (NOD-10), and Chromatocorder model 21, to determine NO2− and NO3− concentrations. Assays were standardized with 10 μM sodium nitrite and 10 μM sodium nitrate. Briefly, NO2− and NO3− in the medium were separated by a reverse-phase separation column (NO-PAK; Eicom) packed with polystyrene polymers. NO3− was reduced to NO2− in a reduction column (NO-RED; Eicom) packed with copper-plated cadmium fillings. NO2− was mixed with Griess reagent (NORE-A/B; Eicom), and the absorbance was read at 540 nm by using a flow-through spectrophotometer. The apparent amount of NO produced in the cultured satellite cells was defined as the total value of NO2− and NO3− measured (NOx).
Statistical Analysis
Analysis of variance procedures were used to analyze experimental results using general linear model procedures of SRISTAT2 for Windows software (Social Survey Research Information, Tokyo, Japan). Least-squares means for each treatment were separated based on least significant differences.
RESULTS
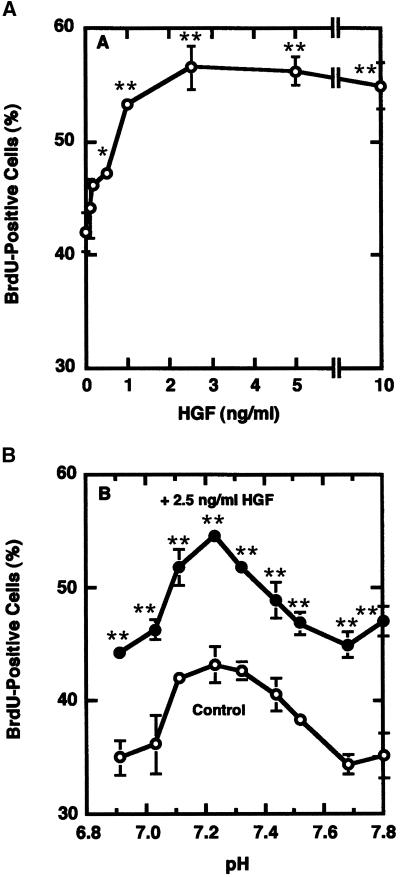
The role of extracellular association of HGF with satellite cells and stretch activation was examined by assaying satellite cell activation in response to stretch as a function of pH. The pH range used in these experiments encompassed the normal range of pH encountered physiologically. By adjusting the pH of medium (Figure 1), 25% stretch was applied at 12-s intervals from 12 to 36 h postplating. As illustrated in Figure 2, activation in control unstretched cultures was pH dependent with a peak at pH 7.23. Activation in stretch-treated cultures exhibited the same pH optimum. Activation above control in stretch treatment only occurred between pH 7.1 and 7.5 with the maximum stretch effect at pH 7.23.
Figure 2.
Effect of medium pH on stretch activation of satellite cells. (A) Stretch pattern applied to culture. (B) Effect of pH on BrdU incorporation in stretched (●) and control (○) satellite cell cultures at 36 h postplating. Points represent means and SE for four cultures per treatment.
The effect of pH on stretch-induced activation could be due to the effect on HGF release from the extracellular matrix or to the effect of pH on HGF binding to its signaling receptor, c-met. To investigate whether the effect was due to changes in HGF activity as a function of pH, unstretched control cultures were subjected to exogenous HGF from 12 to 36 h postplating. Figure 3 illustrates the effect of pH on activation of unstretched satellite cells by HGF. In contrast to results with stretched and unstretched cells in Figure 2, HGF stimulated activation at all pH treatments examined, between 6.9 and 7.8. The HGF and control curves peaked at pH 7.23 but were parallel over the entire range. This suggests that the pH effect on stretch activation is not due to changes in the ability of HGF to interact with c-met on the target cell to generate the appropriate intracellular signals.
Figure 3.
Effect of pH on activation of unstretched satellite cells by HGF. (A) Dose-response curve for HGF and satellite cell activation, as assessed by BrdU labeling index at 36 h postplating. (B) pH dependence of HGF action on satellite cell activation at 36 h postplating: 2.5 ng HGF/ml (●) and control (○). Points represent means and SE for four cultures per treatment.
The issue was further examined by assessing the effect of pH on HGF binding to a c-met-Fc chimera in an ELISA assay. Figure 4 shows a standard curve for HGF binding in the assay (A) and the effect of pH on HGF binding to c-met (B). Over the pH range used in the previously described cell culture assays, there was no difference in HGF binding to c-met, which agrees with the biological effect of HGF on satellite cell activation in Figure 3.
Figure 4.
Effect of pH on HGF binding to c-met. An ELISA binding assay was conducted that used a c-met-Fc chimera bound to plate and soluble HGF ligand; bound HGF was detected by anti-HGF antibody conjugated to horseradish peroxidase. (A) Standard curve for HGF binding. (B) Effect of pH on HGF Binding. Points represent means and SE for four wells per treatment.
The second potential explanation for the affect of pH on stretch activation of satellite cells was a pH-dependent alteration in release of HGF from the extracellular domain. Release of HGF into medium in stretched cultures as a function of pH was investigated by immunoblot analysis (Figure 5). Very little HGF was present in stretch-conditioned medium at pH 7.0 or 7.1 (Figure 5, top). Maximum release occurred at pH 7.2, with diminishing amounts at pH 7.4 and 7.7. No HGF was detected in unstretched control cultures (Figure 5, bottom) at any pH. Maximum release occurred at the same pH as maximum stretch-induced satellite cell activation in Figure 2.
Figure 5.
Effect of pH on release of HGF from stretched satellite cells. Medium was analyzed from stretched and unstretched control satellite cell cultures after 2-h treatment in DMEM beginning at 12 h postplating. Each lane represents proteins from a constant number of cultured satellite cells (1500 cells). Top, immunoblots show the effect of pH on HGF release into medium in stretched cultures. Bottom, immunoblots from unstretched control culture conditioned medium. The first lane in the top panel shows molecular weight standards, and the first lane in the bottom panel shows a control with pH 7.2 stretched medium and no first antibody.
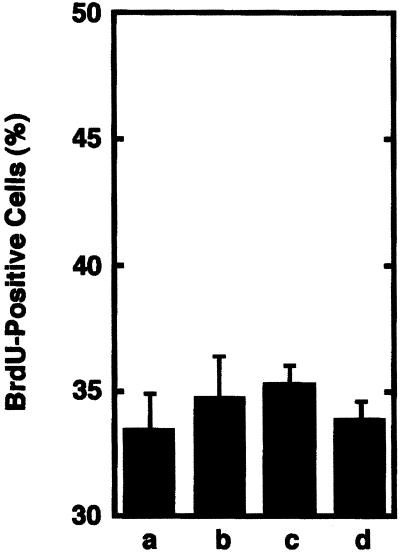
The release of biologically active HGF was further examined by stretching cells at pH 7.7 for variable periods of time from 2 to 20 h (Figure 6). Conditioned medium generated from satellite cells that were stretched for 2, 10, or 20 h did not stimulate satellite activation above the levels produced in cultures that received conditioned medium from unstretched cultures. These results demonstrated that even very long periods of stretch could not generate an activation response if pH was unfavorable for stretch-induced release of HGF.
Figure 6.
Biological activity of stretch-conditioned medium generated from cultures maintained at pH 7.7. Conditioned medium was prepared by subjecting cultures to various periods of stretch in pH 7.7 medium. Conditioned medium was fed to cultures of unstretched satellite cells at 12 h postplating, and BrdU incorporation from 34 to 36 h postplating was assessed at 36 h postplating. Treatment conditioned media were prepared from 2-h unstretched cultures (a), 2-h stretched cultures (b), 10-h stretched cultures (c), and 20-h stretched cultures (d). Bars show means and SEs for four cultures per treatment.
The second objective of this study was to investigate the potential involvement of NO in HGF release and satellite cell activation in response to mechanical stretch. In experiments described in Figure 7, satellite cells were cultured for 12 h and then fed serum-free DMEM containing 1.0 mg of NaHCO3/ml in the presence or absence of stretch and with one of the following: l-NAME, d-NAME, l-Arg, or SNP. Conditioned media were subsequently assayed for satellite cell activation as described previously. As seen in Figure 7A, stretch-conditioned medium (b) and stretch-conditioned medium plus control antibody (c) stimulated significant increases in BrdU incorporation (p < 0.01) relative to unstretched cell conditioned medium (a). Stretch-conditioned medium plus anti-HGF antibody (d) did not stimulate activation relative to the control, and the addition of HGF negated the neutralizing effect of anti-HGF (e). These results are consistent with results from Tatsumi et al. (2001) and serve as important controls for the other treatments within this experiment.
Figure 7.
Stretch activation is affected by altering HGF availability and NO metabolism. Serum-free DMEM, pH 7.2, was used to prepare conditioned medium from satellite cell cultures that were stretched or unstretched for 2 h beginning at 12 h postplating. Medium was analyzed in satellite cell activation assays at 36 h postplating or by immunoblots. (A) Effects on satellite cell activation by the following conditioned medium treatments: control medium from unstretched cultures (a), medium from stretched cells (b), stretch medium plus 2 μg/ml control antibody (c), stretch medium plus 2 μg/ml anti-HGF neutralizing antibody (d), stretch medium plus 2 μg/ml anti-HGF neutralizing antibody plus 20 ng/ml HGF (e), stretch in the presence of 10 μM d-NAME (f), stretch in the presence of 2.4 mM l-Arg (g), stretch in the presence of 10 μM l-NAME (h), stretch in the presence of 10 μM l-NAME followed by addition of 2.5 ng/ml HGF (i), and no stretch in the presence of 30 μM SNP (j). Bars represent the means and SEs from three cultures per treatment, and significant differences from control unstretched means are indicated (∗∗, p < 0.01). (B) Corresponding immunoblots of conditioned medium from the treatments in A: no stretch (a), stretch conditioned medium (b), stretch in the presence of d-NAME (f), stretch in the presence of l-Arg (g), stretch in the presence of l-NAME (h), no stretch in the presence of SNP (k), and control blot of stretch medium without primary antibody (l). Each lane represents proteins from a constant number of cultured satellite cells at 12 h postplating (2700 cells). STD, biotinylated molecular weight standards.
Stretch-conditioned medium was also produced in the presence of d-NAME (f), l-Arg (g), or l-NAME (h) and subsequently tested for activation activity in cultures of unstretched cells. Conditioned medium from stretched cells treated with d-NAME (f) or l-Arg (g) stimulated activation, but conditioned medium from cells stretched in the presence of l-NAME (h), an inhibitor of NOS, did not generate satellite cell-activating activity. When HGF was added to stretch-conditioned medium that was generated in the presence of l-NAME (i) and subsequently assayed for activation activity in cultures of unstretched cells, activation activity was restored, indicating that l-NAME did not directly inhibit satellite cell activation or inhibit HGF action. The final treatment was the addition of an NO-generating compound, SNP, to unstretched cultures (j). Conditioned medium from these cultures stimulated activation comparable to stretch alone.
Figure 7B displays results of immunoblots analyzing the presence of HGF in medium from the experiment. These immunoblots indicate that the α subunit of HGF (bands in the 60-kDa range) was not found in medium from unstretched cultures (lane a), but was present in medium from stretched cultures (lane b), as seen previously (Tatsumi et al., 2001). Cultures stretched in the presence of d-NAME (lane f) or l-Arg (lane g) also released HGF into medium, but cells stretched in the presence of l-NAME (lane h) had diminished amounts of HGF in medium. The addition of SNP, a NO donor, to unstretched cultures stimulated the release of HGF into medium (lane k), similar to the effect of mechanical stretch.
The involvement of NO production was further examined by determining the presence of different NOS isoforms in satellite cells and by directly demonstrating that NOS activity is stimulated when satellite cells are subjected to mechanical stretch. Figure 8 presents an immunoblot of 12-h cultured satellite cells and positive controls for eNOS and nNOS (provided with antibodies by Transduction Laboratories); three lanes containing crude lysates of 22,000 cells each were blotted for each form of NOS. Bands corresponding to eNOS and nNOS were present, and there were some additional bands of lower molecular weight immunoreactive proteins in lysates. A very faint band with an apparent molecular mass of 140 kDa that comigrated with the endothelial cell lysate positive control was seen in each of the three lysates blotted with anti-eNOS. Blots for nNOS in the same three lysates also contained bands that comigrated with the 155-kDa band of pituitary nNOS in the positive control. Immunostaining of cultures within the same experiment showed positive staining for nNOS in 96.3 ± 0.52% of the cells, compared with positive eNOS staining of 8.24 ± 0.73% of cells; these cultures were 96.7 ± 0.49% positive for c-met, 95.4 ± 1.0% positive for desmin, and 96.8 ± 0.63% positive for HGF. Although a significant amount of immunoreactive protein was present in blots for eNOS, only a minor percentage of cells were immunopositive for eNOS. Resolution of this apparent discrepancy awaits further experimentation. Nonetheless, these data suggest that both forms of NOS are present in cultures of satellite cells, but on a cell-by-cell basis, immunostaining indicated that nNOS expression was more prevalent in the cell population.
Figure 8.
Immunoblot detection of eNOS and nNOS in 12 h satellite cell cultures. Molecular weight standards are shown in the first lane. Bands corresponding to eNOS (*) and nNOS (**) are indicated next to lanes of respective positive controls (PC1, human endothelial cell lysate; PC2 rat pituitary lysate). Three separate 12 h satellite cell culture lysates were analyzed for each form of NOS, cell lysates 1, 2, and 3 are designated under each lane. CNT represents blot of lysate 1 without primary antibody.
Figure 9 describes experiments in which NOS activity was assayed in stretched and control cells as measured by the production of NOx. Figure 9A presents data showing the time course of NOS activity in stretched and control cells and demonstrates that a significant (p < 0.01) increase in NOx was detectable as early as 1 h after initiation of stretch. The difference between NOS activity in stretched and unstretched cells continued to increase for as long as 20 h after the start of stretch. In Figure 9B, the effects of l-NAME (b), d-NAME (c), l-Arg (d), and control medium (a) on NOx production by unstretched cells (open bars) and by stretched cells (solid bars) are compared. NOS activity was significantly increased by stretch in cultures fed control medium, medium containing d-NAME, or medium containing l-Arg. In stretched cultures treated with l-NAME, however, NOS activity was inhibited.
Figure 9.
NOS activity in stretched and control cells. (A) Time course of NOS activity in stretched (●) and control (○) cells and in control medium without cells (⋄). (B) NOS activity in cultures subjected to the following treatments: control medium (a), 10 μM l-NAME (b), 10 μM d-NAME (c), 2.4 mM l-Arg (d), and control medium with no cells (e). Each treatment was applied to unstretched (open bars) and stretched (solid bars) cultures for 20 h from 12 to 32 h postplating. Each point or bar represents the mean and SE for three cultures per treatment, and significant differences from control unstretched means are indicated (∗∗, p < 0.01).
DISCUSSION
Satellite cells from adult, uninjured skeletal muscle are found in a quiescent state and as such, are refractory to many growth factors that stimulate proliferation and differentiation of satellite cells in injured or rapidly growing muscle. The one growth factor that has been demonstrated to have the ability to activate quiescent satellite cells in vitro and in vivo is HGF (Allen et al., 1995; Tatsumi et al., 1998). The results of experiments using the stretch of isolated quiescent skeletal muscle satellite cells in the FlexerCell system demonstrated that satellite cell activation occurred earlier in stretched cells than in unstretched control cells and that HGF mediated this process. In this model system, HGF is apparently released from its tethering in the extracellular domain by stretching and becomes available for interaction with the c-met receptor on the satellite cell (Tatsumi et al., 2001). Our observations to date are consistent with those of Ruwhof et al. (2000) in which cyclic stretch of cardiomyocytes and fibroblasts released growth factors into the medium.
Experiments reported herein provide additional insight into this process by showing that the release of HGF is the critical factor in stretch-induced activation. The release of HGF and the stretch activation response were shown to be pH dependent and only occurred in a pH range between 7.1 and 7.5. HGF release was also shown to be dependent on NO production, and it is interesting in this regard that nNOS activity was previously demonstrated to be optimal between pH 7.0 and 7.5 (Gorren et al., 1998). In contrast, exogenous HGF was shown to stimulate satellite cell activation over a broader pH range, from 6.9 to 7.8, whereas the binding of HGF to the c-met receptor was constant over this pH range. Therefore, the effect of pH on stretch activation of quiescent satellite cells does not seem to be a function of HGF interaction with its signaling receptor. Rather, the pH dependence of stretch activation depends on release of HGF from its extracellular binding to heparan sulfate proteoglycans.
Anderson (2000) demonstrated a second signaling component involved in satellite cell activation in an in vivo crush injury model. Her experiments pointed to production of NO as a signal responsible for the immediate response of satellite cells to mechanical insult. The responses observed in vivo were satellite cell hypertrophy and a rapid release of satellite cells from fibers. Our present experiments with in vitro satellite cell stretch integrate the NO signaling of activation with HGF action. We demonstrated that neither stretch activation of satellite cells nor HGF release from satellite cells occurred if NO synthesis was blocked by l-NAME. Furthermore, the NO donor SNP caused release of HGF into culture medium in cultures of unstretched cells and stimulated precocious satellite cell activation. We also know that the neuronal form and possibly the endothelial form of NOS are present in satellite cells, and an assay for NOS activity revealed that activity is stimulated in satellite cells in response to stretch.
To summarize, our experiments have demonstrated the following points: 1) nNOS is present in quiescent satellite cells, which is consistent with in vivo in situ hybridization and immunolocalization studies (Anderson, unpublished observations); 2) NOS activity is increased when satellite cells are stretched in vitro; 3) NO production mediates the release of HGF; and 4) HGF release is responsible for stretch-induced satellite cell activation in this in vitro system. Our data strongly indicate that NO is intimately involved in the stretch-induced activation of satellite cells in this in vitro system and that it acts upstream from HGF release. The precise details of the mechanism by which NOS stimulation results in HGF release from its extracellular binding are yet to be determined.
The presence of nNOS in satellite cells has only recently been observed (Anderson, unpublished data), even though NOS inhibition was shown by Lee et al. (1994) to inhibit myoblast differentiation into myotubes. The neuronal form of NOS, however, was found in skeletal muscle fibers in association with α1-syntrophin that binds to dystrophin (reviewed in Grozdanovic and Baumgarten, 1999). In this location it is sensitive to mechanical changes in the muscle fiber sarcolemma. In myotube cultures, cyclical stretch for 2 d was reported to increase NO production and nNOS concentration in a calcium-dependent manner (Tidball et al., 1998), and the same study showed that resumption of weight bearing after muscle unloading restored normal nNOS expression and NO release, as did electric stimulation and passive stretch of excised muscle. It is interesting in this regard that in dystrophic muscle without dystrophin, nNOS was absent from the subsarcolemmal region (Brenman et al., 1995, 1996) and that dysregulation of satellite cell activation in vivo was reportedly very similar to that occurring as a result of absent nNOS expression or NOS inhibition (Anderson, 2000).
Normal contraction produces pulses of NO synthesis (Tidball et al., 1998), and during injury nNOS is thought to respond by synthesizing NO. NO in turn is involved in modulating skeletal muscle contraction (Reid, 1998). NO release is also responsive to stretch in cardiac muscle cells where it acts a second messenger to alter ryanodine receptor activity (Petroff et al., 2001). Therefore, the synthesis of NO by nNOS in skeletal muscle fibers and satellite cells and complex regulation of NO activity are consistent with the apparent role of NO in stretch-induced activation of satellite cells in culture.
We have shown in previous studies that HGF is present around the periphery of muscle fibers and not in association with satellite cells. Shortly after a mechanical insult, however, HGF associates with satellite cells and colocalizes with the c-met receptor (Tatsumi et al., 1998; Anderson, 2000). This association is responsible for the HGF that is found on satellite cells after the culture procedure, although activated satellite cells synthesize HGF in vitro and in vivo. Consequently, the in vitro model using single cell cultures may only permit the study of the second stage of satellite cell activation. The importance of our studies with this system of stretched cell cultures is that it has revealed a mechanism that may be the primary event in activation of quiescent satellite cells in their normal association with muscle fibers. In vivo, the HGF that is bound in the extracellular domain of muscle fibers may be the HGF that is initially released when subsarcolemmal nNOS is stimulated in response to muscle injury. HGF released from this compartment may subsequently bind to the extracellular matrix of satellite cells or directly to the c-met receptor on satellite cells. The mechanical stress imposed on satellite cells may then lead to further NO-dependent release of HGF and subsequent binding to c-met signaling receptors. This model builds on the previous model presented by Anderson (2000), but critical mechanistic steps are still not known.
The importance of specific intracellular signaling pathways that include NOS has not been established in stretched satellite cells. It is not known, for example, how NOS is activated in these cells in response to stretch. In skeletal muscle fibers nNOS is linked in the dystrophin glycoprotein complex (reviewed in Bredt, 1999), but the location of NOS in satellite cells has not been established. Recent evidence showed that the spatial distribution of particular NOS isoforms is important in regulating cardiomyocyte responses to NO (Barouch et al., 2002). Therefore, the localization of NOS isoforms in a fiber-satellite cell complex may influence how a mechanical stimulus to satellite cells or fibers may result in NOS stimulation.
The manner in which NOS activity mediates the release of extracellular HGF is also unknown. Evidence suggests that there must be a NO regulated pathway that results in release of HGF from its association with heparan sulfate proteoglycan or release of the entire HGF-heparan sulfate proteoglycan complex. These unanswered questions point out the potential value of studying signal cascades in different models of stretch-induced activation. Recent experiments have used a system of cultured single muscle fibers to model satellite cell quiescence (Anderson and Pilipowicz, 2002). In fiber cultures, satellite cell activation (DNA synthesis) also responded to HGF and to NO in a dose-dependent manner. Ultimately, new experiments to determine the stretch responses of satellite cells on single fibers may provide additional insight into how mechanical stretch results in NO-dependent, pH-dependent HGF release and satellite cell activation.
In summary, the experiments described in this study add to our understanding of how satellite cells are activated in adult skeletal muscle by providing a critical link between mechanical changes in muscle and biochemical signals that result in entry of satellite cells into the cell cycle.
ACKNOWLEDGMENTS
We are grateful to Dr. Takashi Kikuiri and Tetsuo Shirakawa (School of Dentistry, Hokkaido University) for the technical assistance concerning the NO assay. The helpful discussions with Dr. Shannon Sheehan were invaluable. This work was supported by Grants-in-Aid for Encouragement of Young Scientists (No. 10760186 to R.T.) and for Scientific Research (C) (No. 12660299 to R.T.) and (B) (No. 13460115 to A.H.) from the Japan Society for the Promotion of Science, and by research grants from Ito Foundation (to R.T.). The research was also supported by the Arizona Agriculture Experiment Station (R.E.A.) and grants from the USDA National Research Initiative Competitive Grant Program (9504074; to R.E.A.) and the Muscular Dystrophy Association (to R.E.A. and J.E.A.).
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–01–0062. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–01–0062.
Present address: Department of Bioscience and Biotechnology, Graduate School of Agriculture, Kyushu University, Hakozaki, Fukuoka 812-8581, Japan. E-mail address: rtatsumi@agr.kyushu-u.ac.jp.
REFERENCES
- Allen RE, Sheehan SM, Tayler RG, Kendall TL, Rice GM. Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J Cell Physiol. 1995;165:307–312. doi: 10.1002/jcp.1041650211. [DOI] [PubMed] [Google Scholar]
- Allen RE, Temm-Grove CJ, Sheehan SM, Rice G. Skeletal muscle satellite cell cultures. Methods Cell Biol. 1998;52:155–176. doi: 10.1016/s0091-679x(08)60378-7. [DOI] [PubMed] [Google Scholar]
- Anderson JE. A role for nitric oxide in muscle repair: nitric oxide-mediated activation of muscle satellite cells. Mol Biol Cell. 2000;11:1859–1874. doi: 10.1091/mbc.11.5.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, J.E., and Pilipowicz, O. (2002). Activation of muscle satellite cells in single fiber cultures. Nitric Oxide (in press). [DOI] [PubMed]
- Barouch LA, et al. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature. 2002;416:337–340. doi: 10.1038/416337a. [DOI] [PubMed] [Google Scholar]
- Bredt DS. Endogenous nitric oxide synthesis: biological functions and pathophysiology. Free Radical Res. 1999;31:577–596. doi: 10.1080/10715769900301161. [DOI] [PubMed] [Google Scholar]
- Brenman JE, et al. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- Brenman JE, Chao DS, Xia H, Aldape K, Bredt DS. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell. 1995;82:743–752. doi: 10.1016/0092-8674(95)90471-9. [DOI] [PubMed] [Google Scholar]
- Cornelison DDW, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol. 1997;191:270–283. doi: 10.1006/dbio.1997.8721. [DOI] [PubMed] [Google Scholar]
- Gorren ACF, Schrammel A, Schmidt K, Mayer B. Effect of pH on the structure and function of neuronal nitric oxide synthase. Biochem J. 1998;331:801–807. doi: 10.1042/bj3310801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozdanovic Z, Baumgarten HG. Nitric oxide synthase in skeletal muscle fibers: a signaling component of the dystrophin-glycoprotein complex. Histol Histopathol. 1999;14:243–256. doi: 10.14670/HH-14.243. [DOI] [PubMed] [Google Scholar]
- Lee KH, Baek MY, Moon KY, Song WK, Chung CH, Ha DB, Kang MS. Nitric oxide as a messenger molecule for myoblast fusion. J Biol Chem. 1994;269:14371–14374. [PubMed] [Google Scholar]
- Petroff MGV, Kim SH, Pepe S, Dessy C, Marban E, Balligand JL, Sollott SJ. Endogenous nitric oxide mechanisms mediate the stretch dependence of Ca2+ release in cardiomyocytes. Nat Cell Biol. 2001;3:867–873. doi: 10.1038/ncb1001-867. [DOI] [PubMed] [Google Scholar]
- Reid MB. Role of nitric oxide in skeletal muscle: synthesis, distribution and functional importance. Acta Physiol Scand. 1998;162:401–409. doi: 10.1046/j.1365-201X.1998.0303f.x. [DOI] [PubMed] [Google Scholar]
- Ruwhof C, van Wamel AE, Egas JM, van der Laarse A. Cyclic stretch induces the release of growth promoting factors from cultured neonatal cardiomyocytes and cardiac fibroblasts. Mol Cell Biochem. 2000;208:89–98. doi: 10.1023/a:1007046105745. [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Anderson JE, Nevoret CJ, Halevy O, Allen RE. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol. 1998;194:114–128. doi: 10.1006/dbio.1997.8803. [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Sheehan SM, Iwasaki H, Hattori A, Allen RE. Mechanical stretch induces activation of skeletal muscle satellite cells in vitro. Exp Cell Res. 2001;267:107–114. doi: 10.1006/excr.2001.5252. [DOI] [PubMed] [Google Scholar]
- Tidball JG, Lavergne E, Lau KS, Spencer MJ, Stull JT, Wehling M. Mechanical loading regulates NOS expression and activity in developing and adult skeletal muscle. Am J Physiol. 1998;275:C260–C266. doi: 10.1152/ajpcell.1998.275.1.C260. [DOI] [PubMed] [Google Scholar]