Abstract
Chemotherapeutic agents such as cisplatin induce persistent activation of N-terminal c-Jun Kinase, which in turn mediates induction of apoptosis. By using a common MAPK Kinase, MEKK1, cisplatin also activates the survival transcription factor NFκB. We have found a cross-talk between c-Jun expression and NFκB transcriptional activation in response to cisplatin. Fibroblast derived from c-jun knock out mice are more resistant to cisplatin-induced cell death, and this survival advantage is mediated by upregulation of NFκB-dependent transcription and expression of MIAP3. This process can be reverted by ectopic expression of c-Jun in c-jun−/− fibroblasts, which decreases p65 transcriptional activity back to normal levels. Negative regulation of NFκB-dependent transcription by c-jun contributes to cisplatin-induced cell death, which suggests that inhibition of NFκB may potentiate the antineoplastic effect of conventional chemotherapeutic agents.
INTRODUCTION
Induction of apoptosis is the primary mechanism of tumor cell killing by radiation and chemotherapeutic agents. Abundant literature has implicated different caspases as the executionery elements at the onset stage of the apoptotic process (Enari et al., 1998). However, the initial events responsible for the later phases of cell death are poorly understood.
Different types of stress such as treatment with DNA-damaging agents used in cancer therapy induce the activation of protein kinase cascades that include the c-jun-N-terminal kinases (JNKs) and the p38 MAP kinase. Each MAP kinase subfamily is activated by a specific upstream MAP kinase kinase (MKKs), which phosphorylates both threonine and tyrosine residues within a conserved T-X-Y motif (Hibi et al., 1993; Dérijard et al., 1994; Kyriakis et al., 1994). These residues are dephosphorylated by dual-specific protein phosphatases, resulting in the inactivation of MAP kinases (Keyse, 2000). Activation of MAP kinase family members leads to phosphorylation of numerous cellular effectors, including protein kinases such as MAPKAPK1, MAPKAP-K2/3, Mnk1/2, and transcription factors such as c-jun, ATF-2, MEF2c, and CHOP, which are responsible for the fate of the cell (Alpert et al., 1999).
cis-Diaminedichloroplatinum (c-DDP, cisplatin) is a DNA-reactive agent commonly used in chemotherapy protocols in the treatment of several kinds of human cancers. Lesions of c-DDP on DNA include intrastrand 1,2-d(GpG), 1,2-d(ApG), 1,3-d(GpNpG), and interstrand cross-link (Fichtinger-Shepman et al., 1985). Like many anticancer agents, c-DDP induces a sustained activation of JNK and p38 (Sánchez-Pérez et al., 2000). Activation of JNK takes place via the MEKK1/SEK1 cascade and is directly related to cell death (Sánchez-Pérez and Perona, 1999). Dephosphorylation and inactivation of JNK by the dual-specific phosphatases CL100 and hVH5 result in protection against cisplatin-induced apoptosis (Sánchez-Pérez et al., 2000). Furthermore, both phosphatases are able to inhibit transcriptional activation of c-Jun, the main physiological substrate of JNK (Kyriakis et al., 1994).
We have previously shown that c-Jun is necessary for the induction of apoptosis in response to cisplatin. A cell line derived from a c-jun knock out mouse is more resistant than normal cells to cisplatin-induced cell death (Sánchez-Pérez and Perona, 1999). This effect is specific to c-Jun because transfection of c-jun into the c-jun−/− cell line, restores its endogenous activity, which results in a phenotype similar to that of parental cells. The role of c-Jun in apoptosis has been described in other cell lines, such as PC12 cells, which undergo apoptosis after NGF withdrawal in a c-jun–dependent mechanism (Ham et al., 1995). In agreement with these results, constitutive expression of c-Jun in NIH3T3 cells induces apoptosis upon serum deprivation (Ham et al., 1995; Bossy-Wetzel et al., 1997).
Little is known about molecules involved in the induction of apoptosis whose expression is regulated by the c-Jun transcription factor. Fas Ligand appears to participate in DNA-damage–induced apoptosis, and its expression seems to depend partially on activation of the JNK pathway (Kasibhatla et al., 1998)
NFκB comprises a family of inducible transcription factors that act as regulators of the host immune and inflammatory response (Collart et al., 1990; Libermann and Baltimore, 1990; Zhang et al., 1990). They also have been involved in protecting cells from apoptosis induced by chemotherapeutic agents (Wang et al., 1999; Baldwin, 2001) or cytokine treatment (Baldwin, 2001). NFκB is a heterodimer composed of p50 and p65/RelA subunits. In unstimulated cells, NFκB is found mainly in the cytoplasm associated with a family of inhibitory molecules known as IκBs (Finco and Baldwin, 1995; Matthews and Hay, 1995; Yin et al., 1998). The activation mechanism of NFκB involves the phosphorylation of IκBs in two critical serine residues (Ser32 and Ser36) via the IκB kinase (IKK) signalosome complex (Brown et al., 1995; Traenckner et al., 1995; Whiteside et al., 1995; DiDonato et al., 1996; O'Connell et al., 1998). Two different kinases that phosphorylate IKKs have been described: NFκB-induced kinase (NIK; Malinin et al., 1997) and MEKK1 (Lee et al., 1998). Once IκBs are phosphorylated, they are targeted for ubiquitination and subsequent degradation by the 26S proteosome. Free p50/p65 heterodimers translocate to the nucleus, where they activate transcription of NFκB responsive genes (Baldwin, 1996; Ghosh et al., 1998). However, there is increasing evidence that an alternative mechanism of NFκB activation that involves phosphorylation of the p65/RelA transactivation subunit takes place (Schmitz et al., 2001). A number of kinases have been shown to phosphorylate p65 NFκB, including the catalytic subunit of protein kinase A (PKAc), whose activity leads to association of p65/RelA with the CREB-binding protein/p300 (CBP/p300) transcriptional activator (Zhong et al., 1998). In addition, AKT/PKB a potent regulator of cell survival, can stimulate the transactivation domain of p65/RelA in a manner that is dependent on IkB kinase and the p38 MAPK activities (Baldwin, 1996; Madrid et al., 2001).
Here we show that treatment of cells with cisplatin induces cell death by modulating both survival and proapoptotic pathways. We have found that activation of MEKK1 by cisplatin upregulates cell death by inducing AP-1–mediated FasL transcription. In parallel, cisplatin also activates NFκB through MEKK1, and this activation is modulated by c-Jun, the main substrate of the JNK pathway. In the absence of c-Jun expression, cells are more resistant to cisplatin, and this correlates with an increase in MEKK1-NFκB–dependent transcription. Accordingly, when NFκB activation was impaired in jun−/− fibroblasts by expression of the IκB—SR (a degradation resistant form of IκBα), resistance to cisplatin was reverted to levels similar to that of wild-type cells. The regulation of NFκB-dependent transcription occurs by the interference of c-jun with p65 transcriptional activation. These findings suggest that chemotherapeutic agents such as cisplatin upregulate proapoptotic pathways, through c-jun–dependent FasL transcription and simultaneous downregulation of survival pathways that are dependent on NFκB transcription.
MATERIALS AND METHODS
Cell Culture, Antibodies, and Reagents
Human embryonic kidney fibroblast cells (293T) were maintained in DMEM supplemented with 10% fetal bovine serum. NIH3T3 cells derived from c-jun knock out mice were cultured in the same medium but supplemented with 5% fetal bovine serum. c-jun−/− and WT cells were obtained from Erwin Wagner (Hilberg et al., 1993; Schreiber et al., 1995). Jun−/+ cells have been previously described (Sánchez-Pérez and Perona, 1999). Phosphorylated forms of JNK were detected with antiphospho JNK (Promega, Madison, WI) antibody. Anti-Fas ligand, anti-IκBα(C-21), antiphospho.-IκBα(B9) and anti-IKKα (77–280) were from Santa Cruz Biotechnology (Santa Cruz, CA). Cisplatin, etoposide, cycloheximide, and thricostatin were purchased from Sigma (St. Louis, MO), and TNF-α was purchased from Upstate Biotechnology (Lake Placid, NY).
Plasmids
(−453/+80) HIVLUC contains the NFκB sites of the HIV promoter, and Δ NFκB HIVLUC contains a three-base pair substitution in each of the NFκB binding sites (Devary et al., 1993). The following plasmids have been already described: GAL4c-Jun (1–223), pCDNAIII derived-MEKK1, pMEKK1 (K-R; Perona et al., 1997), pMEKK6 and p38α (Tamura et al., 2000), RC-CMV-IkBα-Ala32/Ala36 (IkBα-SR; Whiteside et al., 1995), pSG5CL100 (Sánchez-Pérez et al., 2000), and 1xSIE-CAT (Aznar et al., 2001). PCDNAI-c-jun and RCCMV-p65 were obtained from Rodrigo Bravo (Montaner et al., 1998). P-Gal4-p65TAD1 was obtained from Lienhard Schmitz (Schmitz et al., 1995). PCMV-CBP was obtained from Harel-Bellan (Polesskaya et al., 2001). Fas ligand luciferase reporter constructs encoding for a 0.9-kb fragment of the FasL promoter were provided by Douglas Green (Kasibhatla et al., 1998), and p-GST-IkBα was obtained from Mark Hannink (Sachdev and Hannink, 1998).
Transfection and Analysis of Gene Expression
For transient transfection assays, 293T cells were transfected by the calcium phosphate method as described previously (Sánchez-Pérez and Perona, 1999). The total amount of DNA was kept constant at 5 μg per plate with the corresponding empty vector. When indicated, the cells were stimulated for different times and harvested 24 h after transfection. Protein extracts were prepared by lysis with the commercially available reporter lysis buffer (Promega). The total amount of protein was determined with a commercial kit based on the Bradford method (Bio-Rad, Richmond, CA). NIH cells were transfected with Lipofectamine (GIBCO/BRL, Rockville, MD) in six-well plates with 2 × 105 cells per well. For transient transfection assays, the total amount of DNA transfected was brought up to 4 μg per well by addition of empty vector DNA.
For stable transfection, cells were transfected with 3.5 μg of pIκBα-SR expression plasmid and 0.5 μg of p-PUR plasmid (Clontech, Palo Alto, CA). Twenty-four hours after transfection, cells were selected for puromycin resistance. Luciferase assays were performed according to manufacturer's instruction (Promega), and β-galactosidase (β-gal) assays were done as previously described (Perona et al., 1997). Transfection efficiencies were corrected by cotransfection of p-CMVβ-gal and by measuring β-gal activity. Each assay was performed in triplicate, in a single experiment, and repeated in three different experiments with similar results. Chloranphenicol acetyltransferase (CAT) activity was assayed by using a xylene-based method, as described (Aznar et al., 2001).
Cell Extracts and Immunoblotting
Whole-cell lysates and nuclear extracts were prepared as described previously (Perona et al., 1997; Sánchez-Pérez et al., 1998). Western blotting was carried out by standard methods (Sánchez-Pérez et al., 1998).
EMSA
Nuclear extracts (2 μg of protein) from c-DDP (20 μg/ml) or TNF-α (10 ng/ml) treated cells were incubated with a 32P-labeled probe containing the NFκB binding site (Perona et al., 1997). Samples treated with c-DDP or TNF-α were incubated for supershift assays with preimmune serum, or antibodies specific for either p50 or p65. Extracts were incubated 15min with the corresponding antibody before probe binding. The protein-DNA complexes were analyzed by EMSA as previously described (Perona et al., 1997).
RNA Extraction and Reverse Transcriptase (RT)-PCR
Total RNA was isolated from cells using Trizol reagent (Life Technologies, Rockville, MD) following the manufacturer's instructions. For RT-PCR single-strand cDNA was synthesized from total RNA using a primer oligo(dT)12–18 and the Superscript reverse transcriptase (Clontech). Each cDNA was amplified by PCR using Taq DNA polymerase. The sequences of the primers were MIAP3-F (5′AGTGGGGCACCACATGTTAT-3′) and MIAP3-R (5′CGGAAACAGTGCTGTTAGCA-3′) and mouse β-actin-F (5′GGTATGGAATCCTGTGGCATCCATGAAA3′) and β-actin-R (5′GTGTAAAACGCAG-CTCAGTAACAGTCCG 3′). The conditions for reactions were as follows: 1× (95°C, 1 min), 25× (95°C, 30 s; 55°C 1 min; 72°C, 30 s) and 1× (72°C, 3 min) and for β-actin 1× (95°C, 1 min), 25× (95°C, 30 s; 60°C, 1 min; 72°C, 30 s) and 1× (72°C, 3 min). The products were analyzed on a 1% agarose gel and stained with ethidium bromide.
Inmunoprecipitation and Kinase Assays
Cells treated with c-DDP (20 μg/ml) were lysed in buffer A containing 100 mM NaCl, 50 mM Tris (pH 8.0), 1 mM sodium orthovanadate, 1 mM NaF, 0.5 mM B-glycerophosphate, and protease inhibitors. Two hundred micrograms of protein of each cell lysate were incubated with anti-IKKα antibody during 4 h, and 20 μl of protein A-agarose beads were added for 1 h. The inmunoprecipitation complex was extensively washed, and the kinase reaction mixture (10 μCi [32P]ATP, 2 mM MgCl2, 1 mM DTT in buffer A) was added to the agarose beads. All incubations were performed at 4°C. GST-IκBα fusion protein containing amino acids 1–54 was incubated at 30°C for 25 min. The reaction mixtures were then subjected to SDS-PAGE and autoradiography.
Cell Viability Assay
The cell viability was determined using a crystal violet staining method as previously described (Sánchez-Pérez et al., 1998).
RESULTS
Cisplatin Treatment Increases NFκB Activity
We have previously shown that persistent activation of JNK is involved in cisplatin-induced apoptosis (Sánchez-Pérez et al., 1998). In addition, cisplatin has been reported to induce activation of NFκB (Sodhi and Singh, 1998), a transcription factor involved in antiapoptotic processes. Thus, we have investigated the relationship between both pathways in cisplatin-induced cell death. 293T cells were treated with 20 μg/ml cisplatin, nuclear extracts were obtained, and NFκB binding activity was assayed by EMSA. A consensus κB binding sequence and specific antibodies to investigate the composition of the complexes were used for this purpose. We observed that cisplatin induces an increase in NFκB binding activity, with slow kinetics displaying maximal DNA-binding between 3 and 6 h after treatment and decreasing after 9 h (Figure 1). Both p50 and p65/RelA proteins were found in the retarded bands as demonstrated by inhibition in binding of the complexes to DNA by p50 and RelA specific antisera (Figure 1). Similar results were obtained with Pam212 or NIH3T3 cells.
Figure 1.
NFκB activation by c-DDP. 293T cells were treated with c-DDP (20 μg/ml) at the indicated times. Nuclear extracts were prepared, and EMSA was performed as described in MATERIALS AND METHODS. Lanes 10–12 represent competition of nuclear extracts obtained after stimulation with c-DDP for 3 h with p50, p65-specific antibodies, or cold NFκB consensus oligonucleotide (κB), respectively. Arrows show the migration of different NFκB complexes.
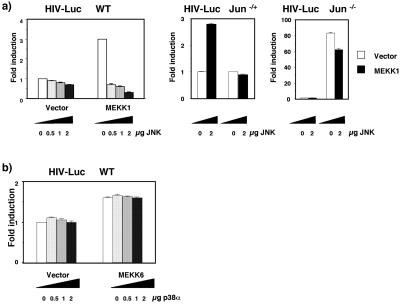
As we have reported before, cisplatin also induces activation of JNK via MEKK1/SEK1, with a delayed kinetics (Sánchez-Pérez and Perona, 1999) very similar to that observed for activation of NFκB complexes. Because MEKK1 also activates NFκB (Lee et al., 1998), we verified if activation of both NFκB and JNK upon cisplatin treatment share a common MEKK1-dependent pathway. 293T cells were transfected with the HIV luciferase (HIVLUC) reporter, and NFκB-dependent transcription was measured (Devary et al., 1993). Cisplatin treatment led to an increase in NFκB-dependent transcription. As well, transfection of an MEKK1 expression plasmid was able by itself to induce activation of NFκB, and a further increase in activity was observed upon treatment with cisplatin of MEKK1-expressing cells. Accordingly, transfection of a dominant negative form of MEKK1 (MEKK1/KR) prevented activation of NFκB, further indicating the role of this kinase in cisplatin-induced activation of the transcription factor. This activity was κB dependent, because no activation was observed when a HIVLUC reporter containing three-base pair substitutions in each NFκB binding site was used (Figure 2a). DNA-binding of NFκB upon cisplatin treatment was also dependent on MEKK1 activity, because ectopic expression of MEKK1 in 293T cells was able to induce translocation of κB binding complexes and expression of MEKK1 (KR) partially blocks the translocation (Figure 2b) in response to cisplatin. The translocation of active complexes (p50/p65) was also inhibited by expression of a plasmid repressor of IκBα containing mutations in the two serines 32/36, phosphorylated by the IκK complexes (IκBα-SR).
Figure 2.
Activation of NFκB by c-DDP requires MEKK1. (a) Activation of the HIV promoter in 293T cells. 293T cells were cotransfected either with 0.5 μg of (−453/−80) HIVLUC or 0.5 μg. of Δ NFκB (−453/−80) HIV-LUC per 60-mm plate, together with the following vectors: pcDNAIII and the derived vectors expressing MEKK1, MEKK1(KR). The total amount of expression vectors was 5 μg per 60-mm plate. Twenty-four hours after transfection, cells were treated with c-DDP (2.5 μg/ml), and luciferase activity was determined 8 h after treatment. Ratios obtained for the empty vectors were considered 1. Data shown in this figure represent the mean of a single experiment performed in triplicate ±SD and are representative of at least three experiments with similar results. (b) EMSA analysis of NFκB complexes induced by c-DDP in transiently transfected 293T cells. 293T cells were cotransfected with 3.5 μg of pcDNAIII empty vector or the derived vector containing IκBα-SR, MEKK1, or MEKK1(KR). After 24 h cells were stimulated, when indicated, with 20g/ml c-DDP during 3 h. Nuclear extracts were assayed for gel retardation assay as indicated in Figure 1. Lanes 8 and 9: competition of nuclear extracts stimulated with c-DDP for 3 h with cold NFκB consensus oligonucleotide (CE) or mutated NFκB consensus oligonucleotide (CI). Arrows show the migration of different NFκB complexes. The experiment was repeated twice with similar results.
Cisplatin Induces FasL Expression through Activation of the SAPK Pathway
Recent studies have shown that the apoptotic response to chemotherapeutic agents in some cell types may proceed through the expression of FasL (Faris et al., 1998; Kasibhatla et al., 1998). To verify if FasL expression was induced by cisplatin, we treated 293T cells with cisplatin at various times and studied its expression by Western blot (Figure 3a). Expression of FasL was detected 9 h after treatment with cisplatin, at a time when both JNK and NFκB activities were present (Figure 1; Sánchez-Pérez et al., 1998). Regulation of FasL expression by some chemotherapeutic agents occurs at the transcriptional level and might involve the activation of NFκB and the SAPK pathway (Kasibhatla et al., 1998). Therefore, we next investigated whether cisplatin was able to modulate the FasL transcription. 293T cells were transiently transfected with a plasmid containing the FasL promoter joined to the luciferase gene as a reporter, and cells were treated for different times with cisplatin. The results shown in Figure 3b indicate that cisplatin induces activation of the FasL promoter almost up to fourfold, in a similar magnitude to the positive control etoposide (Kasibhatla et al., 1998). Because cisplatin seems to activate both SAPK/JNK and the NFκB pathway through activation of MEKK1, we tested the involvement of this kinase in the activation of FasL promoter by cisplatin. 293T cells were contransfected with the FasL promoter reporter and either the MEKK1 or MEKK1(KR) expression vectors. The results shown in Figure 3c indicate that expression of MEKK1 increases both the basal and cisplatin-induced activity of this promoter. MEKK1 is required for activation of the FasL promoter in response to cisplatin because expression of the MEKK1(KR) construct abolishes transcription of the FasL promoter by this drug. The promoter construct used in our assays contains both the AP-1 and NFκB binding sites that control FasL transcription (Kasibhatla et al., 1998). Thus the relative contribution of these two transcription factors in FasL transcription was verified by specifically inhibiting both pathways. 293T cells were cotransfected with the IκBα-SR and MEKK1 expression vectors together with the FasL promoter construct. Transcriptional activation of FasL promoter was not dependent on the NFκB pathway (Figure 3d, top panel). As a functional control of IkBα-SR inhibition, MEKK1-dependent transcription of HIV-luc is inhibited under similar conditions (Figure 3d, bottom panel). However, inhibition of JNK by transient expression of the CL100 dual phosphatase (Sánchez-Pérez et al., 2000) abolishes MEKK1-dependent activation of the FasL promoter (Figure 3e). Altogether these results indicate that although cisplatin and MEKK1 activate both NFκB and JNK pathways, only JNK plays a positive role in the transcription of the FasL promoter, and therefore, this could represent a proapoptotic mechanism of the JNK pathway in response to cisplatin.
Figure 3.
c-DDP induces Fas ligand expression through the activity of the JNK pathway. (a) Levels of expression of FasL after treatment with c-DDP. 293T cells were exposed to c-DDP at 10 μg/ml. At the indicated times cultured cells were collected, and FasL expression examined by immunoblotting using a polyclonal antiserum against FasL. (b) Regulation of the transcriptional activation of the FasL promoter by c-DDP and etoposide. 293T cells were transiently transfected with 1 μg of FasL-LUC reporter plasmid. Twenty-four hours after transfection, cells were treated with 2.5 μg/ml c-DDP or 5 mM Etoposide for different times. Cells were lysed and analyzed for luciferase activity. The fold increase in luciferase activity was calculated based on the values for untreated cells. These data are representative of three experiments. (c) Luciferase activity showing the effect of MEKK1 and MEKK1 (KR) expression on FasL promoter activation by stress stimuli. 293T cells were transiently cotransfected with 1 μg of FasL-Luc construct and 2 μg of MEKK1 or MEKK1 (KR). Twenty-four hours after transfection cells were treated with c-DDP. The cells were lysed 5 h later and analyzed for luciferase activity. Fold increase in luciferase activity was calculated based on the value for untreated control cells. (d and e) Contribution of AP1 and NFκB transcription factor on FasL promoter activation. 293T cells were transiently cotransfected with 1 μg of FasL-Luc (d, top panel) or HIV-LUC (d, bottom panel) construct and either 2 μg of pMEKK1, 2 μg of IKBα-SR, or 2 μg of CL100. Luciferase activity was analyzed as in c. Data shown in this figure represent the mean of a single experiment performed in triplicate ±SD and are representative of at least three experiments with similar results.
Inhibition of the NFκB Pathway Reverts Cisplatin Resistance of Cells
We have previously demonstrated that fibroblasts derived from jun−/− embryos show a higher ID50 for cisplatin than parental NIH3T3 cells (Sánchez-Pérez and Perona, 1999). Moreover, when c-jun expression is restored in c-jun−/− cells, the resultant cell line c-jun−/+ is more sensitive to cisplatin (Sánchez-Pérez and Perona, 1999). As well, inhibition of JNK activation by expression of the CL100 dual phosphatase specifically inhibits cisplatin-induced apoptosis (Sánchez-Pérez et al., 2000). Work from other laboratories has identified NFκB-regulated genes as mediators of cell survival to proapoptotic stimuli (Baldwin, 2001; Barkett and Gilmore, 1999) Thus, we investigated the physiological role of NFκB activation in the behavior of the parental WT cells and jun−/− cells upon cisplatin treatment. Stable cell lines were generated by transfecting IκBα-SR into WT and c-jun−/− cells (WT/IκBα and c-jun−/−/IkBα, which were tested for sensitivity to cisplatin. As a functional control of the dominant negative activity of IκBα-SR, these cells were treated with TNF-α and HIVLUC reported activity was assayed. As shown in Figure 4a, both cell lines showed impaired NFκB activation upon TNF-α treatment when compared with the parental cell lines.
Figure 4.
(a) Activation of HIV promoter by TNF-α in NIH cell lines of different genotypes for c-jun. Cells were transfected by lipofectamine with 0.5 μg of (−453/−80) HIVLUC per well. Twenty-four hours after transfection cells were exposed to TNF-α 10 ng/ml for 5 h. The luciferase activity was determined as in Figure 2. Data shown in this figure represent the mean of a single experiment performed in triplicate ±SD and are representative of at least three experiments with similar results. (b) Cell viability after incubation with different doses of c-DDP in WT, Jun −/−, WT/IκB, Jun −/−/IκB. Viability was measured by the crystal violet–based staining method 48 h after treatment. (c) jun−/− and jun−/−/IKB cell lines were exposed to 10 μg for the indicated times. Activated JNK was detected in Western blots using a specific antibody for phospho-JNK1/JNK2.
It should be noted that c-jun−/− cells showed a fivefold increase in basal NFκB activity with respect to WT cells (Figure 4a). We then used all four cell lines to test the viability after cisplatin treatment. As expected, c-jun−/− cells are more resistant to cisplatin-induced cell death (Sánchez-Pérez and Perona, 1999) than the parental WT cells. On the other hand c-jun−/−/IκBα showed a similar increase in sensitivity to cisplatin and became similar to that of the parental WT cells. Moreover, expression of IκBα-SR in the WT cells did not result in noticeable changes in viability toward cisplatin. The prolonged kinetics for JNK activation after cisplatin treatment is important for the induction of apoptosis (Sánchez-Pérez et al., 1998). However, we have observed that the differences observed in cisplatin sensitivity between c-jun−/− and c-jun−/−/IκBα cells are not due to differences in the kinetics of JNK activation by cisplatin (Figure 4c). Thus, altogether these results suggest that the lower sensitivity to cisplatin observed in c-jun−/− cells is NFκB dependent.
Activation of c-Jun by the MEKK1/JNK Pathway Downregulates NFκB Transcription
Exposure of 293T cells to cisplatin activates MEKK1, and this pathway contributes, on one hand to c-Jun transcriptional activation and on the other to NFκB activation. Because c-jun−/− cells are less susceptible to cisplatin-induced cell death in a NFκB-dependent manner, we have studied the contribution of MEKK1 to NFκB activation in these cells. Cells of the three genotypes (c-jun+/+, c-jun−/−, and c-jun−/+) as well as the IκB-α–expressing cells (WT/IkBα and jun−/−/IκBα) were cotransfected with the MEKK1 expression vector and the HIVLUC reporter plasmid (Figure 5a). Expression of MEKK1 is able to induce activation of the HIVLUC reporter in all cell lines with the exception of the IkBα degradation resistant–expressing cells. Interestingly, c-jun−/− cell line displays a drastic increase in NFκB activity with respect to WT cells, even if the basal NFκB activity in these cells is already higher than that of the WT cells (fivefold). This effect is specific of c-Jun, because c-jun−/+ cells showed an induction similar to that of WT cells. The differences observed are not due to changes in transfection efficiencies, because all luciferase values were normalized to that of an internal β-gal control. As well, these differences between WT and c-jun−/− cells are specific for NFκB, because transcription dependent of STAT-3 (Figure 5b) and SRF was almost equal in both cell lines. Furthermore, the differences in NFκB activity are not due to changes in the levels of p65 or p50 because no variations in the expression of both transcription factors in cells treated with cisplatin were observed. Altogether, the results suggest that transcriptional activation of c-Jun triggered by MEKK1 in WT cells negatively regulates NFκB activation.
Figure 5.
(a) Cells from different genotypes for c-Jun, (WT, c-jun−/− c-jun−/+, WT/IκBα, jun−/−/IκB-α) were transfected by lipofectamine with 0.5 μg of (−453/−80) HIVLUC per well and the expression vector encoding MEKK1 (0.5 μg). Luciferase activity was determined as in Figure 4. (b) WT and c-jun−/− cells were transfected with lipofectamine with 0.5 μg of SIE-CAT per well, and when indicated the cells were stimulated with 20% fetal bovine serum. CAT activity was determined as indicated in MATERIALS AND METHODS. Relative fold induction for each cell line has been included under the corresponding figure. Data shown in this figure represent the mean of a single experiment performed in triplicate ±SD and are representative of at least three experiments with similar results
To further prove if the inhibitory effect of c-jun over NFκB on MEKK1 expression is specific, we transfected a MEKK1/JNK or MEKK6/p38 combination of expression vectors in the different cell lines. As observed in Figure 6a, expression of increasing amounts of JNK1 in WT cells was able to repress NFκB activation by MEKK1 in a dose-dependent manner. However, whereas coexpression of MEKK1 and a high dose of the JNK expression plasmid in c-jun−/− cells have a mild effect on NFκB transcription, the constitutively c-jun–expressing cells, c-jun−/+, behave like the control WT cells. Furthermore, activation of p38 in cell lines of the three genotypes in response to cisplatin does not show any difference in the kinetics of activation, indicating that p38 is not responsible for the high levels of NFκB activation observed in c-jun−/− cells transfected with MEKK1. Accordingly expression of MEKK6 alone in WT cells induces only a minor increase in NFκB activation (Figure 6b), and no inhibition was observed with increasing doses of the p38α expression vector in contrast with the results obtained with MEKK1/JNK.
Figure 6.
(a) Cells from different genotypes for c-Jun, (WT, c-jun−/−, c-jun−/+) were transfected by lipofectamine with 0.5 μg of (−453/−80) HIVLUC and the indicated amounts of the JNK expression vector. Luciferase activity was determined as in Figure 4. (b) WT cells were transfected with lipofectamine with 0.5 μg of (−453/−80) HIVLUC per well and expression vectors encoding MEKK6 (0.5 μg). And the indicated amounts of a p38α expression vector. Luciferase activity was determined as in Figure 4. Data shown in this figure represent the mean of a single experiment performed in triplicate ±SD and are representative of at least three experiments with similar results.
Increased NFκB Activation in c-jun−/− Cells Is Mediated by Transcriptional Activation of p65/RelA Subunit
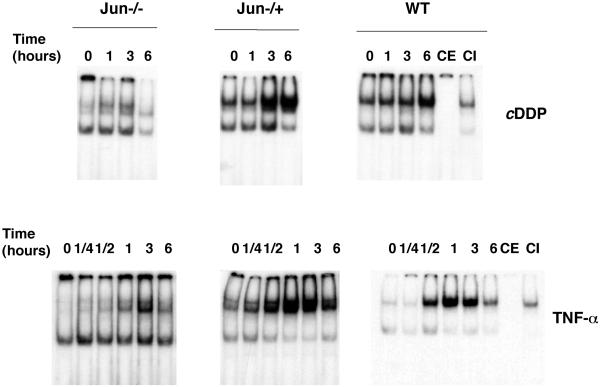
NFκB is regulated in part by a cellular process that involves phosphorylation and degradation of its inhibitory subunit IkBα that allows active NFκB complexes to translocate to the nucleus and activate transcription (Schmitz et al., 2001). Thus, we have studied whether cisplatin stimulation of c-jun−/− cells results in nuclear translocation of NFκB complexes more efficiently than in cells expressing c-jun, accounting for the high NFκB activity observed. To this purpose, WT, c-jun−/−, and c-jun−/+ cells were treated with cisplatin in a time range of 0–6 h, and nuclear extracts were isolated to perform EMSA with a radiolabeled κB element. As shown in Figure 7, all three cell lines display an increase in NFκB DNA-binding activity between 3 and 6 h of cisplatin treatment. Interestingly, nuclear extracts from c-jun−/− cells failed to show a significant increase in NFκB binding activity that would account for the differences observed in NFκB-dependent transcription (Figure 5a). On the contrary, cells constitutively expressing c-jun show a faster and stronger induction in κB binding than WT cells. Furthermore, all three cell lines respond to TNF-α in a similar manner and show a similar pattern of NFκB binding (Figure 7). Moreover, although jun−/− cells show an attenuated translocation of NFκB complexes, both c-jun−/+ and WT cells show the same profile of NFκB activation. These results suggest that although expression of c-Jun is required for optimal induction of NFκB translocation and binding to DNA after activation with cisplatin or TNF-α, a second mechanism of NFκB modulation takes place that accounts for the high NFκB activity present in the jun−/− cells.
Figure 7.
The amount of NFκB DNA-binding complexes is not higher in c-jun−/− cells than in WT cells. Nuclear extracts prepared from cells of the three c-Jun genotypes were treated with c-DDP or TNF-α and were analyzed by EMSA for NFκB binding activity as in Figure 1. The data are representative of three independent experiments.
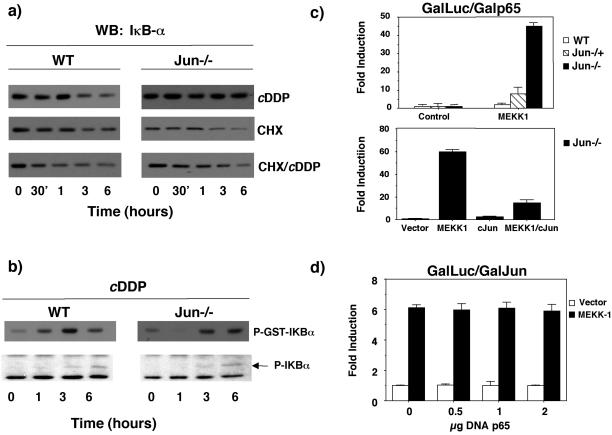
Even though we could not detect important differences in nuclear translocation of NFκB active complexes between WT and c-jun−/− cells, we measured the kinetics of IkBα degradation upon cisplatin exposure. As shown in Figure 8a, the half-life of IκBα in c-jun−/− cells after treatment with cisplatin is longer than 6 h in contrast with WT cells. We therefore analyzed the rate of IκBα turnover in both cell lines. Exponentially growing WT or c-jun−/− cells were treated with the protein synthesis inhibitor cyclohexymide for periods of 30 min, 1, 3, and 6 h (Figure 8a), and the same experiment was carried out after stimulation with cisplatin. Cytoplasmic extracts were then isolated and subjected to immunoblot analysis of IκBα expression. In WT and c-jun−/− cells, the half-life of the IkBα protein upon cisplatin treatment is similar (3–6 h), and after incubation with cyclohexymide decreases to <3 h in WT cells and is slightly lower in jun−/− cells. These results suggest that the levels of IκBα in c-jun−/− cells are maintained by new protein synthesis, and this could be responsible for the results obtained in the gel retardation assays described in Figure 7, because c-jun−/− cells have lower levels of NFκB complexes than WT cells treated.
Figure 8.
c-jun inhibits p65 transcriptional activation. (a) Sustained levels of IκBα in jun−/− require de novo protein synthesis. WT and jun−/− fibroblast were preincubated during 1 h with CHX (2 μM), then treated with c-DDP, and collected after different times, as indicated. Western blots were performed using anti-IκBα antibody. (b) WT and c-jun−/− cells were stimulated with cisplatin at different times, and IKK activity was determined in an immunocomplex assay using IkBα as a substrate. Bottom: panel: the levels of phosphorylated IkBα were determined by Western blot with a specific antiphospho-IkBα antibody. (c) Top panel: WT, jun−/−, and jun−/− cells were cotransfected with a 5X-Gal-luc reporter plasmid (0.25 μg) and expression vectors encoding GAL4-p65 TAD1 (0.25 μg) and MEKK1 (0.5 μg), as indicated. After 24 h cells were collected, and relative luciferase activity was determined. Bottom panel: jun−/− cells were- cotransfected with a 5X-Gal-luc reporter plasmid (0.25 μg) and expression vectors encoding GAL4-p65 TAD1 (0.25 μg), MEKK1 (0.5 μg), and c-Jun (2 μg), as indicated. After 24 h cells were collected, and relative luciferase activity was determined. (d) Expression of p65/RelA does not inhibit c-Jun transcriptional activity. WT fibroblasts were cotransfected with a 5X-Gal-luc reporter plasmid (1 μg) and expression vectors encoding GAL4-c-Jun (1–223) (0.5 μg), MEKK1 (0.5 μg), and RC-CMVp65 (0.5–2 μg), as indicated. Luciferase activity was determined as in c. Data shown in this figure represent the mean of a single experiment performed in triplicate ±SD and are representative of at least three experiments with similar results.
We have also analyzed IKK activation upon cisplatin treatment in both cell lines either by directly measuring IKK activity or by determining the phosphorylation state of IκBα (Figure 8b). We could not observe important differences that would justify the increased basal or MEKK1-induced NFκB transcriptional activity in c-jun−/− cells. These results are in agreement with work from other laboratories that indicated that MEKK1 is not a physiological IKK kinase (Xia et al., 2000; Yujiri et al., 2000).
There are different cellular stimuli that can activate NFκB transcription by a mechanism independent of its nuclear translocation (Schmitz et al., 2001). These alternative mechanisms involve stimulation of the transactivation domain of both the basal and induced levels of the p65 subunit of NFκB. Therefore, we studied if the differences observed were dependent on the transcriptional activation of p65. To address this question, we used a plasmid encoding the Gal4-p65 fusion protein, where the sequences encoding the DNA binding domain of Gal4 have been joined with sequences encoding the TAD1 of p65 (Schmitz et al., 1995). This construction once transfected with the Gal4-Luc reporter allowed us to determine if cellular signals triggered by MEKK1 regulate gene expression by specifically targeting TAD 1 of the p65/relA protein. WT, c-jun−/−, and c-jun−/+ cells were cotransfected with 4x-Gal4-Luc reporter and the Gal4-p65 expression construct and when indicated with an expression vector of MEKK1. As shown in Figure 8c, basal activation of the p65 TAD 1 was very similar in cell lines with the three genotypes. However, in c-jun−/− cells transfected with MEKK1, the activation of p65 TAD1 was almost 50-fold higher than in the other two cell lines. These results indicate that MEKK1 stimulates NFκB transcriptional activity in c-jun−/− cells mainly by increasing p65 transactivation potential. Therefore, because the increase in NFκB-dependent transcription measured by HIVLUC reporter activity and p65 activation in c-jun−/− cells are very similar in magnitude, c-Jun might act as an attenuating factor at some point in the pathway in c-Jun–expressing cells. Accordingly, the high transcriptional activation observed in c-jun−/− cells is reverted back to normal levels when c-jun is transiently expressed. The inhibitory effect of c-Jun on p65 transcriptional activation is not reciprocal. We analyzed the effect of p65 on transactivation of c-Jun with a hybrid Gal4-c-jun protein that contains the Gal4 DNA binding domain fused to the transcriptional activation domain of c-Jun. As shown in Figure 8d, transactivation of the Gal-4–dependent reporter plasmid is induced by expression of Gal4-c-jun and MEKK1, but expression of p65 is not able to inhibit c-Jun transcriptional activation. Altogether these results are compatible with two hypotheses: either MEKK1 activates a signaling pathway that inhibits p65 transcriptional activation or alternatively c-Jun by itself interferes with p65 transcriptional efficiency.
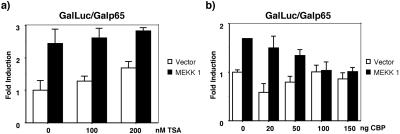
Coregulatory activator or repressor proteins have been shown to be required for the regulation of gene expression by various transcription factors. We have explored the possible involvement of some of the coregulatory proteins in the regulation of NFκB activity by c-Jun. Recently, it has been reported that p65/relA interacts with the histone deacetylase repressors HDAC1 and HDAC2, which downregulate NFκB-dependent transcription (Ashburner et al., 2001). Because in c-Jun–expressing cells there is an attenuation of p65-dependent transcription, we examined if treatment of WT cells with the HDAC inhibitor, thricostatin A (TSA), had any effect on MEKK1-NFκB–dependent transcription. As described previously, cells treated with different concentrations of TSA show an increase in the basal transcription of NFκB (Figure 9a; Ashburner et al., 2001). Under similar conditions, transcriptional activation by MEKK1 was not significantly modified, indicating that HDAC activities are not involved in control of NFκB transcription by MEKK1. Previous works have indicated that p65 can inhibit c-jun–dependent transcription by competing for the coactivator protein p300 (Maggirwar et al., 2000). Because both c-Jun and NFκB interact with the same domain of p300 (Bannister et al., 1995; Gerristen et al., 1997), we designed experiments to investigate if expression of p300 modified the transcriptional activation of NFκB in WT cell that expressed MEKK1. The results indicate that expression of increasing amounts of p300 (20–150 ng) was not able to increase the transcriptional activity of NFκB (Figure 9b).
Figure 9.
(a) Inhibition of HDAC activity does not affect the p65-dependent reporter gene expression. WT fibroblasts were cotransfected with a 5X-Gal-luc reporter plasmid (0.25 μg) and expression vectors encoding GAL4-p65 TAD1 (0.25 μg) and MEKK1 (0.5 μg). Sixteen hours after transfection cells were treated with TSA. Luciferase activity was measured 8 h later as in Figure 7. (b) Expression of p300/CBP does not increase MEKK1-induced transcriptional activation of p65. WT fibroblasts were cotransfected with a 5X-Gal-luc reporter plasmid (0.25 μg) and expression vectors encoding GAL4-p65 TAD1 (0.25 μg), MEKK1 (0.5 μg), and CBP (0.5–2 μg), as indicated. Luciferase activity was determined as in Figure 7. Data shown in this figure represent the mean of a single experiment performed in triplicate ±SD and are representative of at least three experiments with similar results.
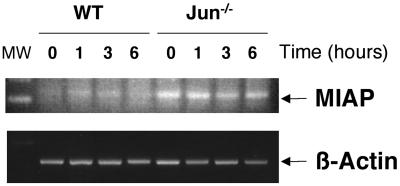
MIAP-3 Is Highly Expressed in c-jun−/− Cells
It is known that NFκB is able to promote transcription of different target genes that block apoptosis induced by different proapoptotic signals (Karin et al., 2002). These antiapoptotic genes include members of BCl2 family such as Bcl-xL and A1/Bfl-1 as well as cellular inhibitors (cIAPs) of apoptosis among others. We studied by RT-PCR the kinetics of expression upon cisplatin treatment of three genes: A1/Bfl-1, Bcl-xL, and the mouse homologue of XIAP, MIAP-3 (Figure 10). WT and c-jun−/− cells were treated with cisplatin and the RNA level estimated by using β-actin as an internal control. Neither A1/Bfl-1 nor Bcl-xL showed different patterns of expression between both cell lines treated with cisplatin. Interestingly MIAP-3 mRNA was present in unstimulated c-jun−/− cells, whereas it was almost undetectable in WT cells. More interestingly although the levels of MIAP-3 mRNA were sustained after several hours of cisplatin treatment, WT cells showed a small and transient increase in MIAP-3 mRNA during the first hours of treatment.
Figure 10.
MIAP-3 mRNA is constitutively transcribed in c-jun−/− cells. WT and c-jun−/− cells were treated with 10 μg/ml cisplatin and harvested at the indicated times. Total RNA was extracted and cDNA was synthesized. The specific cDNA for MIAP3 and β-actin were amplified by PCR.
DISCUSSION
c-Jun plays an important role in different cellular responses such as mitogenesis or DNA damage agents that induced apoptosis. The mechanism involved in c-Jun–mediated mitogenesis is better understood, whereas recently evidence is emerging on the cell death mechanism. The regulation of c-Jun activation by agents that damage DNA takes place through activation on JNK that phosphorylates c-Jun and increases its transactivation potential (Xia et al., 1995; Chen et al., 1996b; Sánchez-Pérez et al., 1998). Activation of JNK occurs as a consequence of activation of MEKK1 and the downstream kinase MEKK4/SEK1, the final JNK activator (Sánchez-Pérez et al., 1998; Chen et al., 1996b).
On the other hand, several signaling pathways have been involved in activation of NFκB in response to different stimuli (Malinin et al., 1997; Lee et al., 1998). A component of the JNK pathway, MEKK1 mediates NFκB-dependent transcription, mainly after treatment with chemotherapeutic agents or TNF-α receptor activation (Minden et al., 1994; Dérijard et al., 1995; Lin et al., 1995; Meyer et al., 1996).
We here show that cisplatin, a commonly used chemotherapeutic agent activates both NFκB and c-Jun by a MEKK1-dependent cascade. Activation of the JNK/SAPK pathway has been extensively shown to mediate the induction of apoptosis upon several types of stress (Xia et al., 1995; Chen et al., 1996a; Sánchez-Pérez et al., 1998). By contrast, the role of NFκB in chemotherapy-induced apoptosis seems to be dependent on the cell system (Kasibhatla et al., 1998; Baldwin, 2001). Activation of NFκB by cisplatin requires MEKK1 activity for both transcriptional activation and nuclear translocation.
Induction of FasL-dependent apoptosis has been shown to take place after exposure to several chemotherapeutic agents, including cisplatin (Kasibhatla et al., 1998; Razzaque et al., 1999). Accordingly, in our cell system c-DDP also induced FasL expression. We here investigated the relative role of JNK and NFκB in the regulation of expression of the proapoptotic protein FasL. We have found that induction of transcription of FasL by cisplatin requires also the activity of MEKK1. Although MEKK1 activates both NFκB and JNK pathways, only activation of JNK pathway seems to be relevant for the induction of FasL transcription in this cell system. We have previously published that CL100 was able to modulate cisplatin-induced apoptosis, both in human and mouse cells mainly due to inhibition of JNK (Sánchez-Pérez et al., 2000). In agreement with these results, expression of CL100 also impairs activation of FasL transcription, further supporting a role of FasL in cisplatin-induced cell death in 293T cells. Accordingly, induction of apoptosis in renal epithelial cells has been shown to be partially dependent on the FasL activation of its receptor (Razzaque et al., 1999). However, activation of NFκB is not required for FasL transcription. On the contrary, evidence in the literature indicates that activity of the NFκB transcription factor is involved in protection to apoptosis induced by different agents (Burow et al., 2000; Chen et al., 2000; Cheng et al., 2000; Baldwin, 2001; Javelaud and Besaçon 2001; Karin et al., 2002).
Work from different laboratories has demonstrated the possibility of a cross-talk between the JNK and NFκB pathway (Maggirwar et al., 2000; Smaele et al., 2001; Tang et al., 2001). To address this point, we used mouse cells defective in either c-Jun and NFκB-dependent transcription or both. We have found that inhibition of NFκB modifies the response of the cells to cisplatin. The survival advantage of jun−/− cells, resulting from the inhibition of Jun-dependent transcription, relies on the activity of NFκB. Expression of the IκBα-SR protein in c-jun−/− cells does not interfere with the activation of JNK; therefore, the sensitization of these cells to cisplatin is not due to the influence of NFκB on JNK activity as described for TNF-α. Two different authors have recently reported that activation of NFκB-dependent transcription by TNF-α inhibits JNK activation, therefore protecting cells from TNF-α–induced apoptosis (Smaele et al., 2001; Tang et al., 2001). Here we observe the contrary effect, because activation of c-jun–dependent transcription seems to negatively modulate the survival effect of NFκB expression. Therefore, if c-Jun is not expressed, cells are able to better tolerate cisplatin treatment. Indeed expression of MEKK1 in cells that lack c-jun induces NFκB-dependent transcription much more efficiently than in WT cells.
Activation of p38 has also been involved in regulation of NFκB signaling by cytokines such as TNF-α (Carter et al., 1999). In our cell system activation of p38 takes place with similar kinetics in cells that lack or express c-Jun. Moreover, modulation of MEKK6/p38 has little influence in NFκB-dependent transcription in contrast with the results reported in TNF-α–treated cells (Alpert et al., 1999). In this system, stimuli such as certain types of stress that produce a sustained activation of p38 are able to induce inhibition of NFκB activation by TNF-α. These results suggest that sustained activation of either JNK or p38 by different types of stress may contribute to apoptosis by inhibiting NFκB activated survival pathways.
NFκB-dependent transcription can be regulated at different levels (Schmitz et al., 2001). Lack of c-Jun expression seems to have different effects in translocation of active NFκB complexes and DNA binding to κB sequences. The amount of NFκB DNA-bound complexes detected in cells treated either with TNF-α or cisplatin is higher in WT cells and jun−/− cells constitutively expressing c-Jun. These results correlate with a lower efficiency in activation of NFκB-dependent transcription in jun−/− cells when treated with TNF-α. Indeed, the kinetics of IκK activation by cisplatin is slower in c-Jun–deficient cells, indicating the importance of c-Jun for optimal induction of the IκKs. Alternatively, because IκBα is a transcriptional target of NFκB, IκBα increased expression could explain the differences observed in the gel retardation assays, between c-jun−/−, c-jun−/+, and WT cells.
Here we demonstrate that the increase in NFκB-dependent transcription, observed in jun−/− cells is due to stimulation of p65 transcriptional activation. Both transient and stable expression of the c-Jun protein in c-jun−/− cells inhibits transcriptional activation of the TAD of p65. p65 and c-Jun do not physically interact (Maggirwar et al., 2000), indicating that the repressive effect observed of c-Jun on p65 TAD is not direct. In agreement with this, we have observed that the effect is not reciprocal, because expression of p65 is not able to inhibit c-Jun transcriptional activation induced by expression of MEKK1.
P65/RelA interacts with the histone deacetylase corepressors HDAC1 and HDAC2, inhibiting the transactivation function of NFκB in both basal and stimulated scenarios (Ashburner et al., 2001). Although treatment of cells with TSA results in an increase of the basal activity of NFκB, MEKK1-induced expression is not modified, suggesting that the mechanism of inhibition of NFκB by c-Jun is not due to activation of histone deacetylase activities, at least HDAC1 or HDAC2.
MEKK1 has been described to stimulate p300-mediated transcription (See, et al., 2001). On the other hand, in PC12 cells, NFκB seems to inhibit c-Jun–dependent transcription by competition for limited amounts of the coactivator protein p300 (Maggirwar et al., 2000). We have not found in our system any variation in p65 transcriptional activation in WT cells transfected with MEKK1 and increased amounts of p300 expression vector. Therefore, the inhibition that c-jun imposes over p65-dependent transcription might be due to competition with other coactivators or by association with repressors other than HDAC1 and 2.
The results presented here indicate that chemotherapeutic agents such as cisplatin are able to induce cell death by modulating concomitantly different signal transduction pathways. Sustained activation of JNK1 and activation of c-Jun upregulates transcription of proapoptotic genes such as FasL (Kasibhatla et al., 1998). On the other hand, c-Jun negatively regulates survival signals triggered by NFκB, such as XIAP, and in the absence of c-Jun, cells survive more to cisplatin (Figure 11). When c-jun is not present in the cells, the activation of the NFκB pathway upregulates expression of MIAP3, protecting cells from apoptosis induced by cisplatin. Works from other laboratories have shown that cisplatin inhibits XIAP expression in cisplatin-sensitive cells (Li et al., 2001; Matsumiya et al., 2001) but not in cisplatin-resistant cells. These observations are consistent with the results that we present. The persistent expression of MIAP-3 in c-jun−/− cells is in agreement with the lower sensitivity of these cells to cisplatin. Thus, both the basal and cisplatin-induced activation of NFκB-dependent transcription could account for the sustained MIAP3 expression and high survival of c-jun−/− cells exposed to cisplatin. c-Jun represses the expression of genes whose transcription is controlled by p53 (Shaulian et al., 2000) after treatment with UV light. This work pointed out the important role of c-jun in controlling cell cycle reentry after UV damage, by repressing p21WAF transcription. c-jun−/− cells are also resistant to apoptosis induced by UV light. It would be interesting to determine if a similar cross-talk between NFκB and JNK pathway in controlling survival in UV irradiated cells as occurs in cisplatin-treated cells
Figure 11.
Regulation of NFκB activity by c-jun. Cisplatin activates both JNK and NFκB-dependent transcription by a common kinase, MEKK1. In normal cells when JNK is activated, c-jun is phosphorylated, and translocation of p50/p65 active complexes takes place. C-jun, on one hand, activates FasL transcription and inhibits NFκB-dependent transcription, inhibiting the expression of genes involved in cell survival such as MIAP3. In c-jun−/− cells, the activation of p65-dependent transcription is no longer inhibited, and even if FasL expression can take place because of the NFκB site in the promoter, the transcription triggered by NFκB leads cells to survival by maintaining expression of MIAP3.
The results presented here may have important implications in cancer therapy protocols that include cisplatin. Different oncogenes involved in the generation of human cancers activate signaling pathways that upregulate NFκB-dependent transcription. Examples of these are activated mutations of ras genes (Madrid et al., 2000), amplification of Her-2/neu (Zhou et al., 2000), or overexpression of EGFr (Hu et al., 1992). All three oncogenes induce survival signals by upregulation of the PI3K and AKT/PKB pathways that leads to an increase of NFκB-dependent transcription. These pathways may compensate for the c-Jun–dependent inhibition of p65-induced transcription when tumors are treated with cisplatin. On the other hand cells derived from several types of carcinomas contain high levels of cIAPs, as a means to escape from drug-induced apoptosis. Furthermore, cell lines derived from ovarian and oral squamous cell carcinoma that constitutively express XIAP are sensitive to cisplatin-induced apoptosis, only when XIAP expression is downregulated. Thus, this implies that resistance to cisplatin involves two complementary pathways that promote (i.e., NFκB) or repress (i.e., c-Jun) XIAP expression. Consequently inhibition of NFκB signaling may represent a good strategy to induce sensitivity to cisplatin in these tumors.
ACKNOWLEDGMENTS
We thank L. Sastre for critical reading of the manuscript and useful comments and Ana Aranda for useful suggestions. I.S.-P. and S.A. are fellows from Comunidad Autónoma de Madrid and Fondo de Investigación Sanitaria, respectively. This study was supported by grants from Fondo de Investigación Sanitaria 00/0862 and 01/1094, and grants 2FD1997-1569 and SAF2001-2042.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–01–0022. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–01–0022.
REFERENCES
- Alpert D, Schwenger P, Han J, Vilcek J. Cell stress and MKK6β-mediated p38 MAP kinase activation inhibit tumor necrosis factor-induced IkB phosphorylation and NF-kB activation. J Biol Chem. 1999;274:22176–22183. doi: 10.1074/jbc.274.32.22176. [DOI] [PubMed] [Google Scholar]
- Ashburner BP, Westerheide SD, Baldwin AS. The p65(RelA) subunit of NF.kB interacts with the histone deacetylase (HDAC) corepressors HDAC1 and HDAC2 to negatively regulated gene expression. Mol Cell Biol. 2001;21:7065–7077. doi: 10.1128/MCB.21.20.7065-7077.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aznar S, Valerón PF, Victoria del Rincón S, Fernández-Pérez F, Perona R, Lacal JC. Simultaneous tyrosine, and serine phosphorylation of STAT 3 transcription factor is involved in RhoA GTPase oncogenic transformation. Mol Biol Cell, 2001;12:3282–3294. doi: 10.1091/mbc.12.10.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin AS., Jr The NFkappa B and Ikappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;19:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Baldwin AS., Jr Control of oncogenesis, and cancer therapy resistance by the transcription factor NF-kB. J Clin Invest. 2001;107:241–264. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister AJ, Oebler T, Wilhelm P, Anjer P, Kouzarides T. Stimulation of c-Jun activity by CBP: c-Jun residues Ser63/73 are required for CBP induced stimulation in vivo and CBP binding in vitro. Oncogene. 1995;11:2504–2514. [PubMed] [Google Scholar]
- Barkett M, Gilmore T. Control of apoptosis by Rel/NFkB transcription factors. Oncogene. 1999;18:6910–6924. doi: 10.1038/sj.onc.1203238. [DOI] [PubMed] [Google Scholar]
- Bossy-Wetzel E, Bakiri L, Yaniv M. Induction of apoptosis by the transcription factor c-Jun. EMBO J. 1997;16:1695–1709. doi: 10.1093/emboj/16.7.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- Burow ME, Weldon CB, Melnik LI, Duong BN, CollinsBurow BM, Beckman BS, McLachlan JA. PI3-K/AKT regulation of NF-kB signaling events in the suppression of TNF-induced apoptosis. Biochem Biophys Res Commun. 2000;271:342–345. doi: 10.1006/bbrc.2000.2626. [DOI] [PubMed] [Google Scholar]
- Carter AB, Knudtson KL, Monick MM, Hunninghake GW. The p38 mitogen-activated protein kinase is required for NF-kB-dependent gene expression. J Biol Chem. 1999;274:30858–30863. doi: 10.1074/jbc.274.43.30858. [DOI] [PubMed] [Google Scholar]
- Chen C, Edelstein LC, Gélinas C. The Rel/NF-kB family directly activates expression of the apoptosis inhibitor Bcl-xL. Mol Cell Biol. 2000;20:2687–2695. doi: 10.1128/mcb.20.8.2687-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-R, Meyer CF, Tan T-H. Persistent activation of c-Jun N-terminal kinase 1 (JNK1) in g radiation-induced apoptosis. J Biol Chem. 1996a;271:631–634. doi: 10.1074/jbc.271.2.631. [DOI] [PubMed] [Google Scholar]
- Chen Y-R, Wang X, Templeton D, Davis RJ, Tan T-H. The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and γ-radiation. J Biol Chem. 1996b;271:31929–31936. doi: 10.1074/jbc.271.50.31929. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Lee HH, Li Y, Cheng G. Upregulation of Bcl-x, and Bfl-1 as a potential mechanism of chemoresistance, which can be overcome by NF-kB inhibition. Oncogene. 2000;19:4936–4940. doi: 10.1038/sj.onc.1203861. [DOI] [PubMed] [Google Scholar]
- Collart MA, Baeuerle P, Vassalli P. Regulation of tumor necrosis factor alpha transcription in macrophages: involvement of for kappa B-like motifs and of constitutive and inducible forms of NF-kappa B. Mol Cell Biol. 1990;4:1498–1506. doi: 10.1128/mcb.10.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dérijard B, Hibi M, Wu I-H, Barret T, Su B, Deng T, Karin M, Davis RJ. JNK1: a protein kinase stimulated by UV light and Ha-ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- Dérijard B, Raingeaud J, Barret T, Wu I-H, Han J, Ulevitch RJ, Davis RJ. Independent human MAP kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- Devary Y, Rosette C, Donato D, Karin M. NF-kB activation by ultraviolet light not dependent on a nuclear signaling. Science. 1993;261:1442–1445. doi: 10.1126/science.8367725. [DOI] [PubMed] [Google Scholar]
- DiDonato J, Mercurio F, Rosette C, Wu-Li J, Suyang H, Ghosh S, Karin M. Mapping of the inducible IkappaB phosphorylation sites that signal its ubiquitination and degradation. Mol Cell Biol. 1996;4:1295–1304. doi: 10.1128/mcb.16.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsy A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;39:143–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- Faris M, Kokot N, Latinis K, Kasibhatla S, Green DR, Koretzky GA, Nel A. The c-Jun N-terminal kinase cascade plays a role in stress-induced apoptosis in Jurkat cells by up-regulating Fas ligand expression. J Immunol. 1998;160:134–144. [PubMed] [Google Scholar]
- Fichtinger-Shepman AM, van der Verr JL, der Hartog JH, Lohman PH, Reedijk J. Adducts of antitumor drug cis-diamminedichloroplatinum (II) with DNA: formation, identification, and quantitation. Biochemistry. 1985;24:707–712. doi: 10.1021/bi00324a025. [DOI] [PubMed] [Google Scholar]
- Finco TS, Baldwin AS. Mechanistic aspects of NF-kappa B regulation: the emerging role of phophorylation and proteolisis. Immunity. 1995;3:263–272. doi: 10.1016/1074-7613(95)90112-4. [DOI] [PubMed] [Google Scholar]
- Gerristen ME, Williams AJ, Neish AS, Moore S, Shi Y, Collins T. CREB-binding proteins/p300 are transcriptional coactivators of p65. Proc Natl Acad Sci USA. 1997;94:2927–2932. doi: 10.1073/pnas.94.7.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, May MJ, Kopp E. NF-kappa B and rel proteins: evolutionan conserved mediators of immune response. Annu Rev Immunol. 1998;16:225–226. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- Ham J, Babij C, Whitfield J, Pfarr CM, Lallemand D, Yaniv M, Rubin LL. A c-Jun dominant negative mutant protects sympathetic neurons against programmed cell death. Neuron. 1995;14:927–939. doi: 10.1016/0896-6273(95)90331-3. [DOI] [PubMed] [Google Scholar]
- Hibi M, Lin A, Minden A, Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- Hilberg F, Aguzzi A, Howells N, Wagner EF. c-Jun is essential for normal mouse development and hepatogenesis. Nature. 1993;365:179–181. doi: 10.1038/365179a0. [DOI] [PubMed] [Google Scholar]
- Hu P, Margolis B, Skolnik EY, Lammers R, Ullrich A, Schlessinger J. Interaction of phosphatidylinositol 3-kinase-associated p85 with epidermal growth facto and platelet-derived growth factor receptor. Mol Cell Biol. 1992;3:981–990. doi: 10.1128/mcb.12.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javelaud D, Besaçon F. NF-kB activation result in a rapid inactivation of JNK in TNFa-treated Ewing sarcoma cells. A mechanism for the anti-apoptotic effect of NF-kB. Oncogene. 2001;20:4365–4372. doi: 10.1038/sj.onc.1204570. [DOI] [PubMed] [Google Scholar]
- Karin M, Cao Y, Greten FR, Li Z. NFkB in cancer from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:310–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- Kasibhatla S, Brunner T, Genestier L, Echevarri F, Mahaboubi A, Green D. DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-kB and AP-1. Mol Cell. 1998;1:543–551. doi: 10.1016/s1097-2765(00)80054-4. [DOI] [PubMed] [Google Scholar]
- Keyse SM. Protein phosphatases, and the regulation of mitogen-activated protein kinase signaling. Curr Opin Cell Biol. 2000;12:186–192. doi: 10.1016/s0955-0674(99)00075-7. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Banerjee P, Nikolakaki E, Dai T, Rubie EA, Ahmad MF, Avruch J, Woodgett JR. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- Lee FS, Peters RT, Dang LC, Maniafis T. MEKK1 activates both IkB Kinase alpha and IkB Kinase beta. Proc Natl Acad Sci USA. 1998;95:9319–9324. doi: 10.1073/pnas.95.16.9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, et al. Human ovarian cancer, and cisplatin resistance. possible role of inhibitor of apoptosis protein. Endocrinology. 2001;142:370–380. doi: 10.1210/endo.142.1.7897. [DOI] [PubMed] [Google Scholar]
- Libermann TA, Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol. 1990;5:2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A, Minden A, Martinetto H, Claret F-X, Lange-Carter C, Mercurio F, Johnson GL, Karin M. Identification of a dual specificity kinase that activates the Jun kinases and p38-Mpk2. Science. 1995;268:286–290. doi: 10.1126/science.7716521. [DOI] [PubMed] [Google Scholar]
- Madrid LV, Mayo MW, Reuther JY, Baldwin AS. Akt stimulates the transactivation potential of the RelA/p65 subunit of NF-kB through utilization of the IkB kinase, and activation of the mitogen activated protein kinase p38. J Biol Chem. 2001;276:18934–18940. doi: 10.1074/jbc.M101103200. [DOI] [PubMed] [Google Scholar]
- Madrid LV, Wang C, Guttridge DC, Schottelius AJG, Baldwin AS, Mayo MW. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-kB. Mol Cell Biol. 2000;20:1626–1638. doi: 10.1128/mcb.20.5.1626-1638.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggirwar SB, Ramirez S, Tong N, Gelbard HA, Dewhurst S. Functional interplay between nuclear factor-kB, and c-Jun integrated by coactivator p300 determines the survival of nerve growth factor-dependent PC12 cell. J Neurochem. 2000;74:527–539. doi: 10.1046/j.1471-4159.2000.740527.x. [DOI] [PubMed] [Google Scholar]
- Malinin NL, Boldin MP, Kovalenkoo AV, Wallach D. MAP3K-related kinase involved in NF-kB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- Matthews JR, Hay RT. Regulation of the DNA binding activity of NF-kappa B. Int J Biochem Cell Biol. 1995;27:865–879. doi: 10.1016/1357-2725(95)00071-v. [DOI] [PubMed] [Google Scholar]
- Matsumiya T, Imaizumi T, Yoshida H, Kimura H, Satoh K. Cisplatin inhibits the expression of X-chromosome-linked inhibitor of apoptosis protein in an oral carcinoma cell line. Oral Oncol. 2001;37:296–300. doi: 10.1016/s1368-8375(00)00102-0. [DOI] [PubMed] [Google Scholar]
- Meyer CF, Wang X, Chang C, Templenton D, Tan T. Interaction between c-Rel and the mitogen-activated protein kinase kinase 1 signaling cascade in the mediating kB enhancer. J Biol Chem. 1996;271:8971–8976. doi: 10.1074/jbc.271.15.8971. [DOI] [PubMed] [Google Scholar]
- Minden A, Lin A, Smeal T, Derijard B, Cobb M, Davis R, Karin M. c-Jun N-terminal phosphorylation correlates with activation of the JNK subgroup but not the ERK subgroup of mitogen-activated protein kinases. Mol Cell Biol. 1994;14:6683–6688. doi: 10.1128/mcb.14.10.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaner S, Perona R, Saniger L, Lacal JC. Multiple signaling pathways lead to the activation of the nuclear factor kappaB by the Rho family of GTPases. J Biol Chem. 1998;273:12779–12785. doi: 10.1074/jbc.273.21.12779. [DOI] [PubMed] [Google Scholar]
- O'Connell MA, Bennett BL, Mercurio F, Manning AM, Mackman N. Role of IKK1 and IKK2 in lipopolysaccharide signaling in human monocytic cell. J Biol Chem. 1998;46:30410–30414. doi: 10.1074/jbc.273.46.30410. [DOI] [PubMed] [Google Scholar]
- Perona R, Montaner S, Saniger L, Sánchez-Pérez I, Bravo R, Lacal JC. Activation of the nuclear factor kB by Rho, Cdc42 and Rac1 proteins. Genes Dev. 1997;11:463–475. doi: 10.1101/gad.11.4.463. [DOI] [PubMed] [Google Scholar]
- Polesskaya A, et al. CBP/p300, and muscle differentiation. no HAT, no muscle. EMBO J. 2001;20:6816–6825. doi: 10.1093/emboj/20.23.6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaque MS, Koji T, Kumatori A, Taguchi T. Cisplatin-induced apoptosis in human proximal tubular epithelial cells is associated with the activation of Fas/Fas ligand system. Histochem Cell Biol. 1999;111:359–365. doi: 10.1007/s004180050368. [DOI] [PubMed] [Google Scholar]
- Sachdev S, Hannink M. Loss of IkappaB alpha-mediated control over nuclear import and DNA binding enables oncogenic activation of c-Rel. Mol Cell Biol. 1998;18:5445–5456. doi: 10.1128/mcb.18.9.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Pérez I, Martínez-Gomariz M, Willians D, Keyse SM, Perona R. CL100/MKP1 modulates JNK activation, and apoptosis in response to cisplatin. Oncogene. 2000;19:5142–5152. doi: 10.1038/sj.onc.1203887. [DOI] [PubMed] [Google Scholar]
- Sánchez-Pérez I, Murguia JR, Perona R. Cisplatin induces a persistent activation of JNK that is related to cell death. Oncogene. 1998;16:533–540. doi: 10.1038/sj.onc.1201578. [DOI] [PubMed] [Google Scholar]
- Sánchez-Pérez I, Perona R. Lack of c-Jun activity increases survival to cisplatin. FEBS Lett. 1999;453:151–158. doi: 10.1016/s0014-5793(99)00690-0. [DOI] [PubMed] [Google Scholar]
- Schmitz ML, Bacher S, Kracht M. IkB-independent control of NF-kB activity by modulatory phosphorylation. Trends Biochem Sci. 2001;26:186–190. doi: 10.1016/s0968-0004(00)01753-9. [DOI] [PubMed] [Google Scholar]
- Schmitz ML, dos Santos-Silva MA, Baeuerle PA. Transactivations domain 2 (TA2) of p65 NFkB similar to TA1 and phorbol ester-stimulated activity and phosphorylation in intact cells. J Biol Chem. 1995;270:15576–15584. doi: 10.1074/jbc.270.26.15576. [DOI] [PubMed] [Google Scholar]
- Schreiber M, Baumann B, Cotten M, Angel P, Wagner EF. Fos is an essential component of the mammalian UV response. EMBO J. 1995;14:5338–5349. doi: 10.1002/j.1460-2075.1995.tb00218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RH, Calvo D, Shi Y, Kawa H, Luke MP, Yuan Z, Shi Y. Stimulation of p300-mediated transcription by the kinase MEKK. J Biol Chem. 2001;276:16310–16317. doi: 10.1074/jbc.M008113200. [DOI] [PubMed] [Google Scholar]
- Shaulian E, Schreiber M, Piu F, Beeche M, Wagner EF, Karin M. The mammalian UV response. c-Jun induction is required for exit from p53-imposed growth arrest. Cell. 2000;103:897–907. doi: 10.1016/s0092-8674(00)00193-8. [DOI] [PubMed] [Google Scholar]
- Smaele E, Zazzeroni P, Nguyen DU, Jin R, Jones J, Cong R, Franzoso G. Induction of gadd45b by NF-kB downregulates pro-apoptotic JNK signaling. Nature. 2001;414:308–313. doi: 10.1038/35104560. [DOI] [PubMed] [Google Scholar]
- Sodhi A, Singh R. Mechanism of NF-kB translocation in macrophages treated in vitro with cisplatin. Inmunol Lett. 1998;63:9–17. doi: 10.1016/s0165-2478(98)00043-1. [DOI] [PubMed] [Google Scholar]
- Tamura K, Sudo T, Senftleben U, Dadak AM, Johnson R, Karin M. Requirement for p38alpha in erythropoietin expression. a role for stress kinases in erythropoiesis. Cell. 2000;102:221–231. doi: 10.1016/s0092-8674(00)00027-1. [DOI] [PubMed] [Google Scholar]
- Tang G, Minemoto Y, Dibling B, Purcell NH, Li Z, Karin M, Lin A. Inhibition of JNK activation through NF-kB target genes. Nature. 2001;414:313–317. doi: 10.1038/35104568. ,. [DOI] [PubMed] [Google Scholar]
- Traenckner EB, Pahl HL, Henkel T, Schmidt KM, Wilk S, Baeuerle PA. Phosphorylation of human I kappa B-alpha on Serines 32 and 3 controls I kappa B-alpha proteolysis and NF-kappa B activation in response to diverse stimuli. EMBO J. 1995;12:2876–2883. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C-Y, Guttridge DC, Mayo MW, Baldwin AS. NF-kB induces expression of the Bcl-2 homologue A1/Bfl-1 to preferentially suppress chemotherapy-induced apoptosis. Mol Cell Biol. 1999;19:5923–5929. doi: 10.1128/mcb.19.9.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside ST, Ernst MK, LeBail O, Laurent-Winter C, Rice N, Israel A. N- and C-terminal sequence control degradation of MAD3/I kappa B-alpha in response to inducers of NF-kappa B activity. Mol Cell Biol. 1995;10:5339–5345. doi: 10.1128/mcb.15.10.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Xia Y, Makris C, Su B, Li E, Yang J, Nemerow GR, Karin M. MEK kinase 1 is critically required for c-Jun N-terminal kinase activation by proinflammatory stimuli, and growth factor-induced cell migration. Proc Natl Acad Sci USA. 2000;97:5243–5248. doi: 10.1073/pnas.97.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature. 1998;39:677–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- Yujiri T, et al. MEK kinase 1 gene disruption alters cell migration, and c-Jun NH2-terminal kinase regulation but does not cause a measurable defect in NF-kappa B activation. Proc Natl Acad Sci USA. 2000;97:7272–7277. doi: 10.1073/pnas.130176697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Lin JX, Vilcek J. Interleukin-6 induction by tumor necrosis factor and interleukin-1 in human fibroblasts involves activation of a nuclear factor binding to a kappa B-like sequence. Mol Cell Biol. 1990;7:3818–3823. doi: 10.1128/mcb.10.7.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong IH, Voll RE, Ghosh S. Phosphorylation of NFkB p65 by PICA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell. 1998;1:661–671. doi: 10.1016/s1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]
- Zhou BP, Hu MCT, Miller SA, Yu Z, Xia W, Lin SY, Hung MC. HER-2/neu blocks tumor necrosis factor-induced apoptosis via the Akt/NF-kB pathway. J Biol Chem. 2000;275:8027–8031. doi: 10.1074/jbc.275.11.8027. [DOI] [PubMed] [Google Scholar]