This systematic review and meta-analysis analyzes associations of exercise with anxiety, depression, and quality of life from randomized clinical trials of older adults with cancer.
Key Points
Question
Is exercise therapy associated with improved depression, anxiety, and health-related quality of life (HRQOL) in older adults with cancer?
Findings
In this systematic review and meta-analysis of 27 randomized clinical trials with 1929 participants, exercise therapy was associated with reduced depression and anxiety severity and improved HRQOL in older adults with cancer. In particular, mind-body exercises (eg, tai chi, yoga, or qigong) improved outcomes significantly.
Meaning
This systematic review meta-analysis found that exercise therapy was associated with reduced depression and anxiety severity and improved HRQOL in older adults with cancer, suggesting that health care professionals and policymakers should focus more on implementing exercise interventions to improve mental health outcomes in this vulnerable population.
Abstract
Importance
Cancer and its treatment negatively impact the mental health of older adults. The potential of exercise interventions as a complementary treatment to alleviate the psychological impacts of cancer is promising, but there are gaps in the current literature.
Objective
To determine if exercise interventions are associated with improvements in psychological outcomes among older adults with cancer.
Data Sources
PubMed, Embase, PsycINFO, and Cochrane databases were searched from database inception to November 5, 2024. Search terms used were geriatrics, cancer, depression, anxiety, quality of life, and exercise interventions.
Study Selection
English-language randomized clinical trials (RCTs) that analyzed the association of various exercise interventions with at least 1 of 3 psychological outcomes (depression, anxiety, or health-related quality-of-life [HRQOL]) were included. The control groups were given usual care. Studies were included if the mean age of participants was older than 60 years and had participants with a diagnosis of any cancer regardless of comorbidities.
Data Extraction and Synthesis
Studies were screened, and data were extracted by 2 independent authors. Random-effects meta-analyses and meta-regressions were used for analysis. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline was followed.
Main Outcomes and Measures
The primary outcomes were depression, anxiety, and HRQOL. Standardized mean difference (SMD) was used to quantify the association of exercise interventions with outcomes.
Results
A total of 27 RCTs with 1929 participants were included. Meta-analyses observed an association of exercise with a significant reduction in levels of depression (SMD = −0.53; 95% CI, −0.79 to −0.28) and anxiety (SMD = −0.39; 95% CI, −0.66 to −0.12) and improvements in overall HRQOL (SMD = 0.63; 95% CI, 0.10 to 1.17). Subgroup analyses revealed that mind-body exercise interventions were significantly associated with improved depression (SMD = −0.89; 95% CI, −1.51 to −0.27) and anxiety levels (SMD = −0.77; 95% CI, −1.54 to −0.01) compared with conventional exercise interventions.
Conclusion
In this systematic review and meta-analysis of 27 RCTs, exercise interventions were found to be associated with significantly reduced levels of depression and anxiety and significantly improved HRQOL in older adults with cancer. These findings suggest that health care professionals and policymakers should focus more on implementing exercise interventions to improve mental health outcomes in this vulnerable population.
Introduction
Cancer is a global health challenge, exhibiting an estimated 19.96 million new cases worldwide in 2022, with projections indicating a surge to more than 35 million cases in 2050.1 Cancer is associated with age,2 and two-thirds of newly-diagnosed cases are made up of adults aged 60 years and older.3,4 Adverse effects of cancer manifest both physically and psychologically,5 with 30% to 35% of patients having received a psychiatric disorder diagnosis.6 Sequelae of cancer include uncertainty about survival,7 bodily deterioration,8 treatment-induced adverse effects,9 phobias, and psychological distress.10 Furthermore, cancer treatment often results in many long-term negative outcomes.11,12,13 The unfavorable psychiatric milieu attributed to cancer elevates suicide risk by 4.4 times compared with the general population.14 The increased distress not only impairs immune surveillance of tumors, but also persistently activates the hypothalamic-pituitary-adrenal axis that regulates stress response,15 thereby increasing the risk of recurrence and mortality.16 Older adults with comorbidities,17 frailty, and lower physiologic reserve18 are more susceptible to complications of the disease and treatment.19 Thus, it is pertinent to address the psychological impact of cancer to improve quality of life and patient outcomes in this population.
Exercise therapy, defined as activities exceeding routine physical function, positively impacts the well-being of patients with cancer. Andersen et al20 and Assi et al21 found that exercise therapy alleviates cancer symptoms like depression, anxiety, nausea, fatigue, and sleep disorders, as well as chemotherapy adverse effects, thereby improving quality of life.22 Exercise also slows down tumor growth, inflammation, and angiogenesis, while speeding up tumor regression.23 Reducing disease burden and recurrence may mitigate the severity of cancer-associated symptoms, indirectly improving the mental well-being of patients with cancer.
Currently, methods of managing poor mental health include pharmacological treatment and cognitive behavioral therapies. However, conventional pharmacological therapies are hindered by potential adverse effects and drug-drug interactions. Especially in older adults, polypharmacy and compromised organ function18,19 pose added challenges in drug administration and lower the overall efficacy of pharmacotherapies.24 Although studies have proven cognitive behavioral therapy to be effective, patients face pervasive social stigma obtaining mental health support25 and may be reluctant to share their feelings with health care professionals.26 Thus, exercise therapy emerges as both a preventive and complementary treatment for both cancer and mental health concerns, functioning synergistically with conventional therapies.
Randomized clinical trials (RCT) remain the benchmark in providing the strongest scientific evidence.27 In this meta-analysis of RCTs, we assessed the significance of exercise therapy in alleviating depression and anxiety severity and improving quality of life in older patients with cancer. Furthermore, we compared the effects of mind-body and conventional exercises on the aforementioned parameters, a direction not taken before, to our knowledge. By filling this gap in literature, our study aims to provide valuable insight into the potential of exercise therapy as a complementary and synergistic cancer treatment that improves psychological outcomes.
Methods
Protocol and Guidance
This systematic review and meta-analysis is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline. Our protocol is registered on PROSPERO (CRD42023394964).
Data Sources, Search Strategy and Definitions
We conducted a literature search in the PubMed, Embase, PsycINFO, and Cochrane databases from database inception to November 5, 2024. Search terms used were geriatrics, cancer, depression, anxiety, quality of life, and exercise interventions. We included relevant synonyms to expand our search for titles, abstracts, and keywords in those databases. Full strategies for the databases are available in eTable 1 in Supplement 1.
Study Selection: Inclusion and Exclusion Criteria
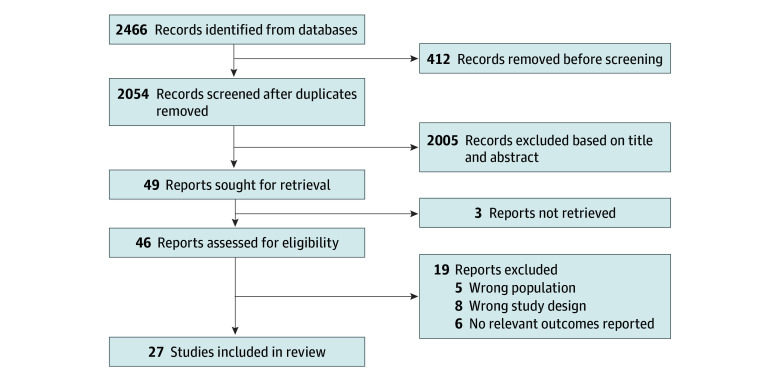
We included English-language peer-reviewed studies that assessed the association of exercise interventions with psychological outcomes in older adults with cancer. We defined exercise interventions as conventional exercises like aerobic-, resistance-, and strength-related physical training or mind-body exercises such as qigong, yoga, and tai chi, which combine physical movement with mental focus. We included studies if the mean age of patients was 60 years or older,28 and participants had a diagnosis of any cancer regardless of comorbidities. We selected only RCTs with control groups (usual care) analyzing at least 1 of these psychological outcomes: depression, anxiety, or health-related quality of life (HRQOL). Studies involving only educational, pharmacological, or surgical interventions were excluded. This selection process is shown in Figure 1.
Figure 1. Flowchart of Studies.
Based on the aforementioned criteria, 2 independent reviewers (R.Y.S. and V.O.) screened titles and abstracts of all studies. The full text of studies that were assessed to be either relevant or unclear were then reviewed again. Discrepancies were resolved by a third independent reviewer (C.E.L.).
Data Extraction and Organization
Two reviewers (R.Y.S. and V.O.) independently conducted the extraction process. The data extracted encompassed the study’s objectives, characteristics of the study population, type of exercise intervention and control conducted, alongside primary findings. Standardized mean difference (SMD) was used to quantify the association of exercise interventions. The changes in both mean and SD of depression, anxiety, and HRQOL scores between baseline and postintervention measures were extracted. In the primary analysis, we selected the model from each study that exhibited the highest level of control over confounders. Change in SD was calculated in accordance with Cochrane recommendations; 0.2 represents a small association, 0.5 represents a moderate association, and 0.8 represents a large association.29
Risk of Bias Assessment
We used the Cochrane Risk of Bias Tool 2 checklist to assess risk of bias and methodological quality of the studies. This assessment was done by 2 independent reviewers (R.Y.S. and V.O.).
Statistical Analysis
Data analyses were conducted using the meta and metafor packages in R version 4.1.0 (R Project for Statistical Computing). Statistical significance was determined by a 2-sided P < .05, unless specified otherwise. SMD was used to aggregate the studies involved in this meta-analysis. Sensitivity analyses were conducted, using leave-one-out analysis, common effects, and identifying and excluding potential outliers. Degree of heterogeneity among studies was evaluated through I2 and τ2 statistics. I2 less than 30% indicated minimal heterogeneity, 30% to 60% suggested moderate heterogeneity, and I2 greater than 60% denoted substantial heterogeneity. The potential influence of categorical and hierarchical variables on the results were investigated using subgroup analyses and meta-regression. Quantitative and qualitative assessment of publication bias were done via an Egger test and visual inspection of funnel plot asymmetry, respectively. Because publication bias was suspected, a sensitivity analysis utilizing the trim-and-fill method (R0 estimator, fixed-random effects models) was conducted; this reestimated the overall association size after imputing potentially missing studies, assuming normal distribution of association size around the center of the funnel plot in absence of publication bias.
Results
Of the 2466 articles identified from databases and citation searching, 2439 studies were omitted after removing duplicates and excluding irrelevant studies. The remaining 27 RCTs30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56 met the final inclusion criteria (Figure 1).
Of the 27 RCTs, all but one40 reported on HRQOL, while 12 studies 32,35,38,40,44,46,47,50,51,53,55,56 included data on depression and 9 studies38,40,44,47,50,51,53,55,56 included data on anxiety. In total, 1929 participants were included in our analysis, with sample sizes of each study varying from 17 to 252 participants. The majority of studies included patients with prostate cancer (15 studies),30,32,33,35,36,37,38,40,42,44,45,48,52,54,55 while 4 others recruited participants with various cancer types,43,50,51,53 3 focused on lung cancer,39,41,49 2 focused on breast cancer,34,46 and 1 study each focused on head and neck,31 colorectal47 and bladder56 cancer. A summary of the characteristics of the studies is presented in the Table.
Table. Characteristics of Included Studies.
| Source | Country | Cancer type | Participants, No. (Sex, No. [%]) | Race, No. (%) | Age, mean (SD), y | Study methodology and characteristics | Instrument, scales, and diagnostic criteria for assessing depression, anxiety, and HRQOL |
|---|---|---|---|---|---|---|---|
| Segal et al,30 2003 | Canada | Prostate | 155 (155 male [100%]) | NR | 68.0 (7.7) | RCT with males recruited from Ottawa Regional Cancer Centre in Ontario) and Cross Cancer Institute in Edmonton in Alberta | HRQOL: FACT-P |
| McNeely et al,31 2004 | Canada | Head and neck | 17 (14 Male [82%]; 3 female [18%]) | NR | 61.0 (7.7) | RCT with participants recruited from Cross Cancer Institute and University of Alberta in Edmonton | HRQOL: FACT-G |
| Monga et al,32 2007 | US | Prostate | 21 (21 Male [100%]) | Black (57%); Hispanic (10%); White (33%) (self-reported) | 69.2 (4.8) | RCT with males referred for radiotherapy service at the Houston Veterans Affairs Medical Center in Texas for radiation treatment of localized prostate cancer | Depression: BDI; HRQOL: FACT-P |
| Segal et al,33 2009 | Canada | Prostate | 121 (121 Male [100%]) | NR | 66.3 (7.0) | RCT with participants receiving radiation therapy, recruited from Ottawa Hospital Regional Cancer Centre | HRQOL: FACT-G |
| Banasik et al,34 2010 | US | Breast | 18 (18 Female [100%]) | 18 White (100%) (self-reported) | 62.9 (6.9) | RCT with females recruited via mail from a local cancer center database in Spokane, Washington | HRQOL: FACT-B |
| Culos-Reed et al,35 2010 | Canada | Prostate | 100 (100 Male [100%]) | NR | 67.6 (8.6) | RCT with participants receiving androgen deprivation therapy recruited from hospitals | Depression: CES-D; HRQOL: EORTC QLQ-C30 |
| Galvão et al,36 2010 | Australia | Prostate | 57 (57 Male [100%]) | NR | 69.8 (7.2) | RCT with participants undergoing androgen suppression therapy, recruited from Sir Charles Gairdner Hospital (Perth, Western Australia) | HRQOL: SF-36 |
| Bourke et al,37 2011 | UK | Prostate | 50 (50 Male [100%]) | NR | 71.8 (7.0) | RCT with males recruited from outpatient urology clinics in Sheffield | HRQOL: FACT-G |
| Cormie et al,38 2013 | Australia | Prostate | 20 (20 Male [100%]) | NR | 72.2 (7.1) | RCT with participants recruited via referral by oncologists and urologists in Perth, Western Australia | Depression: BSI-18 DEPR; Anxiety: BSI-18 ANX; HRQOL: SF-36 |
| Arbane et al,39 2014 | UK | Lung | 131 (83 Male [64%]; 48 female [36%]) | NR | 68.0 (11.0) | RCT with participants admitted for curative surgery, recruited from 2 clinical-academic centers in London | HRQOL: SF-36 |
| Campo et al,40 2014 | US | Prostate | 40 (40 Male [100%]) | 3 Other (7%); 37 White (93%) (source of classification NR)a | 73.8 (8.1) | RCT with males recruited via Huntsman Cancer Institute clinics, University of Utah, cancer registries, and community-based strategies | Depression: BSI-18 DEPR; Anxiety: BSI-18 ANX |
| Edvardsen et al,41 2014 | Norway | Lung | 61 (29 Male [47%]; 32 female [53%]) | NR | 65.2 (8.9) | RCT with participants recruited from Oslo University Hospital | HRQOL: SF-36 |
| Galvão et al,42 2014 | Australia and New Zealand | Prostate | 100 (100 Male [100%]) | NR | 71.7 (6.4) | RCT with males enrolled in the RADAR trial (examining effect of adjuvant androgen deprivation therapy duration on recurrence-free survival) from 3 centers, contacted by a letter of invitation from their oncologist | HRQOL: SF-36 |
| Miki et al,43 2014 | Japan | Various | 78 (35 Male [45%]; 43 female [55%]) | NR | 74.2 (5.8) | RCT with participants recruited from the outpatient clinic of Hiroshima University Hospital | HRQOL: FACT-G |
| Cormie et al,44 2015 | Australia | Prostate | 63 (63 Male [100%]) | NR | 68.4 (7.1) | RCT with males referred by oncologists and urologists in Perth, Western Australia | Depression: BSI-18 DEPR; Anxiety: BSI-18 ANX; HRQOL: SF-36 |
| Nilsen et al,45 2015 | Norway | Prostate | 58 (58 Male [100%]) | NR | 66.0 (5.8) | RCT with males recruited from 2 units at Oslo University Hospital | HRQOL: EORTC QLQ-C30 |
| Yagli et al,46 2015 | Turkey | Breast | 20 (20 Female [100%]) | NR | 68.7 (4.7) | RCT with females who were part of the preventive/conservative rehabilitation at the Hacettepe University, Faculty of Health Sciences, Department of Physiotherapy and Rehabilitation, Samanpazari in Ankara | Depression: BDI; HRQOL: NHP |
| Cramer et al,47 2016 | Germany | Colorectal | 54 (33 Male [61%]; 21 female [39%]) | NR | 68.3 (9.7) | RCT with participants recruited from the Department of Surgery and Centre for Minimal Invasive Surgery, Kliniken Essen-Mitte in Essen and the Tempelhof Colon Centre, St. Joseph’s Hospital in Berlin | Depression: HADS; Anxiety: HADS; HRQOL: FACT-C |
| Winters-Stone et al,48 2016 | US | Prostate | 64 (64 Male [100%]) | 59 White (92%); 5 other (8%) (self-reported)a | 71.8 (7.2) | RCT with participants recruited through the Oregon State Cancer Registry program run by the Oregon Department of Human Service | HRQOL: SF-36 |
| Lai et al,49 2017 | China | Lung | 60 (34 Male [57%]; 26 female [43%]) | NR | 72.1 (2.8) | RCT with participants recruited from Department of Thoracic Surgery, West China Hospital, Sichuan University, Chengdu | HRQOL: EORTC-QLQ-C30 |
| Loh et al,50 2019 | US | Various | 252 (20 Male [8%]; 232 female [92%]) | 229 White (91%); 23 other (9%) (source of classification NR)a | 66.7 (5.4) | RCT with participants recruited from 19 community oncology practices across the US | Depression: POMS; Anxiety: STAI; HRQOL: FACT-G emotional well-being scale |
| Cheng et al,51 2021 | China | Various | 60 (34 Male [57%]; 26 female [43%]) | NR | 66.3 (7.6) | RCT with participants recruited from Nanjing Jiangning Hospital and Jiangsu Cancer Hospital | Depression: PHQ-9; Anxiety: GAD-7; HRQOL: QLQ-CCC |
| Mardani et al,52 2021 | Iran | Prostate | 80 (80 Male [100%]) | NR | 69.9 (5.6) | RCT with participants admitted to the radiotherapy department of a large referral teaching hospital in an urban area | HRQOL: EORTC-QLQ-C30 |
| Mikkelsen et al,53 2022 | Denmark | Various | 84 (36 Male [43%]; 48 female [57%]) | NR | 71.7 (5.3) | RCT with participants recruited from the Department of Oncology at Copenhagen University Hospital, Herlev, and Gentofte Hospital | Depression: HADS; Anxiety: HADS; HRQOL: EORTC-QLQ-C30 |
| Capela et al,54 2023 | Portugal | Prostate | 50 (50 Male [100%]) | NR | 71.8 (5.9) | RCT with participants recruited at the Oncology and Urology departments of the Vila Nova de Gaia-Espinho Hospital Centre | HRQOL: EORTC QLQ-C30 |
| Langlais et al,55 2023 | US | Prostate | 25 (25 Male [100%]) | 19 White (76%); 6 other (24%) (source of classification NR)a | 69.3 (8.4) | RCT with participants living within a 3-hour drive of the University of California, San Francisco, recruited through physician referral and patient lists | Depression: CES-D; Anxiety: STAI; HRQOL: FACT-G |
| Porserud et al,56 2024 | Sweden | Bladder | 90 (59 Male [66%]; 31 female [34%]) | NR | 71.5 (8.5) | RCT with participants recruited from Karolinska University Hospital | Depression: HADS; Anxiety: HADS; HRQOL: EORTC QLQ-C30 |
Abbreviations: BDI, Beck Depression Inventory; BSI-18-ANX, The Brief Symptom Inventory for Anxiety; BSI-18-DEPR, The Brief Symptom Inventory for Depression; CES-D, Center for Epidemiologic Studies Depression Scale; EORTC-QLQ-C30, European Organisation for the Research and Treatment of Cancer Quality-of-life Questionnaire; FACT-B, The Functional Assessment of Cancer Therapy-Breast; FACT-C, The Functional Assessment of Cancer Therapy-Colorectal; FACT-G, The Functional Assessment of Cancer Therapy-General; FACT-P, The Functional Assessment of Cancer Therapy-Prostate; GAD-7, General Anxiety Disorder-7; HADS, Hospital Anxiety and Depression Scale; HRQOL, health-related quality of life; NHP, Nottingham Health Profile; NR, not reported; PHQ-9, Patient Health Questionnaire; POMS, Profile of Mood States; QLQ-CCC, Quality of Life Questionnaire for Chinese Cancer Patients Receiving Chemobiotherapy; RADAR, Risk-Adapted therapy Directed According to Response; RCT, randomized clinical trial; SF-36, Medical Outcomes Study 36-Item Short Form Health Survey; STAI, State Trait Anxiety Inventory.
No definition provided for other race.
Various scales were adopted to measure depression, anxiety, and HRQOL. Notably, the Functional Assessment of Cancer Therapy was the most used to measure HRQOL, while the Hospital Anxiety and Depression Scale and Brief Symptom Inventory were most frequently employed to evaluate both depression and anxiety. Exercise interventions varied in frequency, duration, setting, and format. Exercise intensity also varied; some interventions progressively increased the difficulty of exercises at various intervals,30,31,33,36,38,40,41,42,44,45,47,48,50,52,53,55 while others kept intensity constant throughout the intervention.32,34,35,37,39,43,46,49,51,54,56 Additionally, different interventions were adopted; the majority30,31,32,33,35,36,37,38,39,41,42,43,44,45,48,49,50,52,54,55,56 involved conventional exercises, which include structured physical activities like resistance training, aerobic exercise, and strength training and focus primarily on improving physical fitness. The remaining studies34,40,46,47,51,53 employed mind-body exercises, such as qigong, yoga, and tai chi, which combine physical movement with mental focus to promote physical, emotional, and mental well-being. All except 4 interventions39,49,50,55 took place under the guidance of physiotherapists, instructors, or researchers. Six interventions were conducted in a group setting,34,36,46,48,51,54 12 were conducted individually,30,31,32,33,37,39,43,45,49,50,55,56 and the remaining 9 were multimodal, combining both group and individual components.35,38,40,41,42,44,47,52,53 All the studies included components conducted in either a hospital or community setting, except for 1 study50 that was entirely home-based. Regarding control type, 11 studies30,32,33,35,41,44,45,50,51,53,55 assigned their control group to receive only medical therapy, 6 studies34,36,38,43,47,48 instructed their control participants to maintain their daily activities, 3 studies37,52,54 provided routine check-ups, 2 studies39,49 prescribed respiratory care management, 2 studies 31,40 prescribed light stretches, 2 studies46,56 prescribed other types of exercise, and 1 study42 provided an educational booklet. Detailed descriptions of all exercise and control interventions are found in eTable 2 in Supplement 1.
Significant Improvement in Mean Depression Levels
Pooled results based on 12 studies32,35,38,40,44,46,47,50,51,53,55,56 and 826 participants in a meta-analysis (Figure 2) revealed significant reduction in depression levels after patients underwent exercise programs (SMD = −0.53; 95% CI, −0.79 to −0.28). Results of subgroup analyses of depression severity are presented in eTable 3 in Supplement 1. When the Hospital Anxiety and Depression Scale was used to measure depression, it was associated with a significant decrease in severity of depression (SMD = −0.69; 95% CI, −1.23 to −0.15) compared with other scales. Additionally, mind-body exercises were associated with a more significant reduction in depression severity (SMD = −0.89; 95%CI, −1.51 to −0.27) compared with conventional forms of exercises like aerobic and resistance exercises (SMD = −0.39; 95% CI, −0.64 to −0.13) (eFigure 1 in Supplement 1). However, there was no association of depression levels with other categorical variables such as age, region of study, year of study, type of cancer, control type, or frequency, intensity, and duration of exercise interventions. Meta-regression revealed a significant association of longer exercise interventions (12 weeks or more) with greater reductions in depression severity, compared with shorter interventions. There were no significant associations for age or the effects of exercise intervention over time (eTable 4 in Supplement 1).
Figure 2. Meta-Analysis of Exercise and Depression Levels Among Older Adults With Cancer.

The size of the boxes indicates the weight of each study to the overall pooled estimate. SMD indicates standardized mean difference.
Significant Improvement in Mean Anxiety Levels
Meta-analysis of 9 studies38,40,44,47,50,51,53,55,56 (Figure 3) was conducted, involving 685 participants. Overall, anxiety levels of participants were significantly reduced after exercise (SMD = −0.39; 95%CI, −0.66 to −0.12). Subgroup analyses of anxiety severity among other categorical variables are listed in eTable 5 in Supplement 1. Mind-body exercises were associated with more significant reductions in anxiety severity (SMD = −0.77; 95% CI, −1.54 to −0.01) compared with conventional exercises (SMD = −0.26; 95% CI, −0.47 to −0.06) (eFigure 1 in Supplement 1). However, there was no association of anxiety levels with other categorical variables such as age, anxiety scale used, region of study, year of study, type of cancer, control type, or frequency and duration of exercise interventions. Meta-regression showed that there was no significant association of exercise interventions with reducing anxiety severity, longer intervention period, and age, or effects of intervention over time (eTable 6 in Supplement 1).
Figure 3. Meta-Analysis of Exercise and Anxiety Levels Among Older Adults With Cancer.
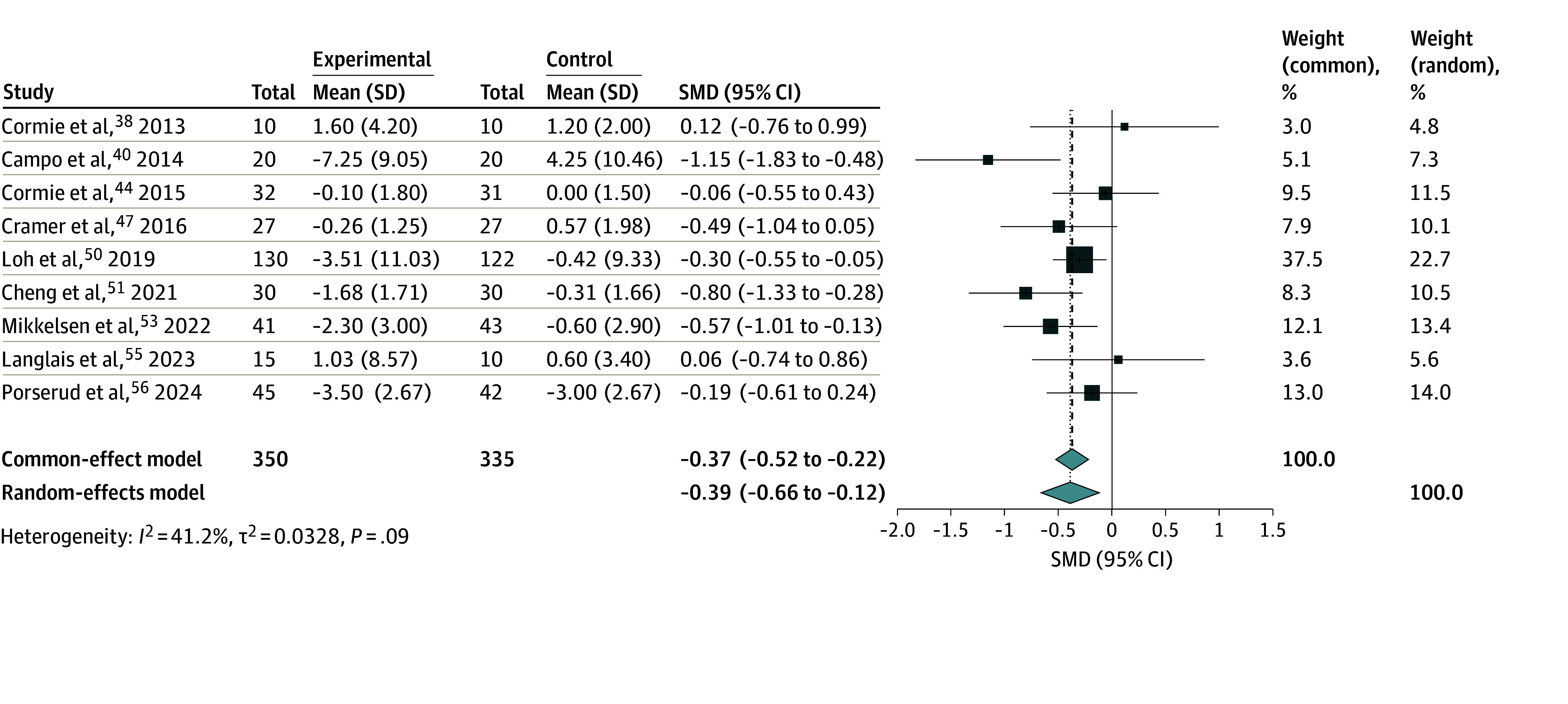
The size of the boxes indicates the weight of each study to the overall pooled estimate. SMD indicates standardized mean difference.
Significant Improvement in Mean HRQOL Scores
Across 26 studies,31,32,33,34,35,36,37,38,39,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56 1866 participants were included in a meta-analysis to investigate the association of exercise with HRQOL (Figure 4). Overall, HRQOL significantly improved upon implementation of the exercise programs (SMD = 0.63; 95% CI, 0.10-1.17). Subgroup analyses on HRQOL based on other variables are presented in eTable 7 in Supplement 1. When results were stratified based on mean age of participants, the subgroup of participants younger than 70 years demonstrated significantly improved HRQOL levels compared with those with a mean age older than 70 years (SMD = 0.91; 95% CI, 0.11-1.71). However, other categorical variables (type of cancer, type of exercise, control type, region of study, year of study, scale used to measure HRQOL, or frequency, duration, and intensity of intervention) were not associated with a significant improvement in HRQOL scores. Meta-regression showed that there was no significant association of exercise interventions with improving HRQOL, longer intervention period, age, or effects of intervention over time (eTable 8 in Supplement 1).
Figure 4. Meta-Analysis of Exercise and Health-Related Quality of Life Levels Among Older Adults With Cancer.

The size of the boxes indicates the weight of each study to the overall pooled estimate. SMD indicates standardized mean difference.
Education, Income, Marital Status, Race, and Social Status
Eighteen studies30,31,32,36,37,38,39,40,42,43,44,46,47,48,49,51,54,56 examined the association of age with enhancement in psychological well-being of participants after exercising (eTable 9 in Supplement 1). Among these, 17 did not find any significant association of social status with improvement of psychological outcomes. However, 1 study51 notably reported that younger males experienced reduced psychological distress compared with older males.
Three studies40,48,49 explored the association of race with improvements in psychological outcomes among participants who took part in exercise interventions (eTable 10 in Supplement 1). Eight studies36,38,40,42,44,47,50,52 evaluated the association of marital status with psychological outcomes after exercise interventions (eTable 11 in the Supplement). Nine studies31,36,40,42,43,47,48,52,56 investigated the association of income level or employment status with improved psychological outcomes after exercise (eTable 12 in Supplement 1). Nine studies32,36,38,43,44,47,48,50,52 examined the association of education level with psychological outcomes after exercise (eTable 13 in the Supplement). Nine studies36,38,39,42,44,49,52,54,56 investigated the association of smoking status with psychological outcomes after exercising (eTable 14 in the Supplement). Overall, none found a significant association of psychological well-being with race, marital status, income level, employment status, education attainment, or smoking status.
Risk of Bias, Publication Bias, and Sensitivity Analyses
This meta-analysis assessed the methodological quality of the 27 studies using the Cochrane Risk of Bias Tool 2 (eTable 15 in Supplement 1). Overall, low risk of bias was noted in 10 studies,31,36,37,39,41,43,44,48,52,56 potential risk of bias in 15 studies,30,32,33,34,38,40,42,45,46,47,49,50,51,53,54 and high risk of bias in 2 studies.35,55
In 11 studies,31,32,34,40,45,46,49,50,51,53,54 allocation sequence of assignment was not properly concealed, while in 15 studies,30,33,34,35,37,38,39,42,45,47,49,51,53,55,56 potential biases arose due to missing outcome data; of those, 2 studies35,55 were severely missing data, resulting in a high risk of bias. Overall, 9 studies32,34,35,36,38,42,46,47,50 did not implement assessor blinding, possibly giving rise to detection bias. One study40 deviated from intended interventions by adopting a per-protocol analysis. As such, the results of the treatment-control analysis should be interpreted after taking the risk of bias analysis into careful consideration. Funnel plots, sensitivity analyses, Egger test, and trim-and-fill procedures indicated the presence of some publication bias (eFigures 2-16 in Supplement 1).
Discussion
This systematic review and meta-analysis of RCTs demonstrated that exercise was significantly associated with reduction in depression and anxiety severity and enhanced HRQOL among older adults with cancer. Subgroup analyses revealed that mind-body exercises were significantly associated with reduced anxiety and depression levels more than conventional exercises like aerobic or resistance training. To our knowledge, this is the first systematic review and meta-analysis investigating the association of exercise with improving anxiety, depression, and HRQOL in older adults with cancer.
The 2018 American College of Sports Medicine Roundtable found that exercise can improve anxiety, depression, and HRQOL in all cancer survivors.57 In our study, exercise interventions were also associated with significant improvement in all psychological outcomes. Reduced severity of depression and anxiety may be due to the following mechanisms. First, exercise exerts antidepressant and anxiolytic effects via the release of neurotransmitters that cross the blood brain barrier to mediate brain-derived neurotrophic factor expression, stimulating hippocampal neurogenesis and improving mood control.58 Additionally, exercise stimulates production of monoamines,59,60 generating a natural crescendo that stimulates pleasure, satisfaction, and motivation while regulating fatigue.61 Furthermore, exercise improves sleep quality,62 which has a bidirectional association with depression and anxiety.63,64
Mind-body exercises reduce depression and anxiety levels to a significantly greater extent compared with conventional exercises because they focus heavily on relaxation and mindfulness,65 potentially stimulating additional mechanisms compared with conventional exercises. Miller et al66 found that a walking meditation program resulted in a significantly larger decrease in depression than aerobic walking alone, corroborating our findings and suggesting that additional factors are at play. Conversely, a different study67 showed that conventional exercises reduced depression more significantly than mind-body exercises in younger participants; this may be due to differing exercise preferences because older individuals are predisposed to physical limitations and may prefer low-intensity, slower-paced exercises,68 while younger individuals may prefer fast-paced, high-intensity exercises.69 Additionally, response-shift bias may also affect outcomes because older adults might experience greater mental health benefits from mind-body exercises that align with their preference for mindfulness and relaxation, enhancing perceived effectiveness.70 Likewise, younger individuals may perceive greater improvement from exercises that align with their values and lifestyle.70
In our analysis, longer interventions, specifically those carried out for at least 12 weeks, were more likely to improve depression scores than those of shorter duration. This finding was expected, as shown by Li et al,71 who assessed the effects of different exercise intervention times on depressive symptoms in older adults and found that longer intervention times play a role in ensuring the positive effect of physical exercise. Furthermore, the 2018 American College of Sports Medicine Roundtable found that in particular, thrice-weekly moderate-intensity aerobic training performed for at least 12 weeks significantly reduced anxiety and depression, while an aerobic-resistance program lasting for at least 12 weeks for 2 to 3 times per week improved HRQOL.57 Therefore, it is recommended that future exercise interventions be designed for at least 12 weeks.
Limitations
There are several limitations to this study. We expected heterogenicity in methods of measuring and defining possible important confounding variables like education, income, marital status, and social support, which are well-studied globally4,19,72 across different age groups12,13,73,74 and cancer types.10 We were also unable to control for heterogeneity based on the duration, frequency, and whether the interventions were supervised. Given the variations existing across different study designs and populations, it was unfeasible to statistically pool these variables. As such, we adopted a synthesis without a meta-analysis approach instead. Moreover, because controls did not receive equal amounts of attention as exercise groups, it was not possible to determine how much of the observed effect was due to exercise or simply due to attention.
Next, our study population was intrinsically diverse, with participants undergoing different treatments and varying in their levels of overall health; this introduced variability across the selected studies, which might impact the generalizability of our findings. Moreover, as highlighted by Goel et al,75 stressors from factors like disadvantaged neighborhood environments activate the sympathetic nervous system and hypothalamic-pituitary-adrenal axis, which may contribute to more aggressive tumor biology, increasing cancer recurrence and mortality risk. Thus, in addition to the heterogeneity of health and treatment variables in our study, potential effects of geographic or environmental stressors have also not been accounted for. This limitation further underscores the need for future studies to consider the influence of social and environmental contexts on cancer outcomes.
Conclusions
With advancements in cancer treatment and an aging population, the prevalence of cancer-related decline in psychological well-being can be expected to increase in the following years. Hence, finding effective interventions to mitigate the psychosocial impacts of cancer is essential. Overall, this systematic review and meta-analysis of 27 RCTs found that exercise interventions, especially mind-body exercises, were associated with significant improvements in depression, anxiety, and HRQOL in older adults with cancer. Health care professionals and policymakers should focus more on implementing exercise interventions to improve mental health outcomes in this vulnerable population.
eTable 1. Search Strategy
eTable 2. Specific Details of All Exercise Interventions Used to Improve Psychological Outcomes
eTable 3. Meta-Analyses of Exercise on Depression Severity in OAC Stratified by Categorical Study-Level Characteristics Using the Random Effect Model
eFigure 1. Subgroup Meta-Analyses of Exercise on Depression (A) and Anxiety (B) Levels Among Older Adults With Cancer Stratified by Nature of Exercise
eTable 4. Mixed Effects Meta-Regression of Standardised Mean Differences Against Potential Effect Moderators (Continuous and Categorical Study-Level Characteristics) for Depression Severity After Exercise Interventions in OAC
eTable 5. Meta-Analyses of Exercise on Anxiety Severity in OAC Stratified by Categorical Study-Level Characteristics Using the Random Effect Model
eTable 6. Mixed Effects Meta-Regression of Standardised Mean Differences Against Potential Effect Moderators (Continuous and Categorical Study-Level Characteristics) for Anxiety Severity After Exercise Interventions in OAC
eTable 7. Meta-Analyses of Exercise on HRQOL Improvement in OAC Stratified by Categorical Study-Level Characteristics Using the Random Effect Model
eTable 8. Mixed Effects Meta-Regression of Standardised Mean Differences Against Potential Effect Moderators (Continuous and Categorical Study-Level Characteristics) for HRQOL Improvement After Exercise Interventions in OAC
eTable 9. Evaluation of the Mediating or Confounding Effect of Age of Participants on Psychological Outcomes
eTable 10. Evaluation of the Mediating or Confounding Effect of Race of Participants on Psychological Outcomes
eTable 11. Evaluation of the Mediating or Confounding Effect of Marital Status of Participants on Psychological Outcomes
eTable 12. Evaluation of the Mediating or Confounding Effect of Income Level and Employment Status of Participants on Psychological Outcomes
eTable 13. Evaluation of the Mediating or Confounding Effect of Education Level of Participants on Psychological Outcomes
eTable 14. Evaluation of the Mediating or Confounding Effect of Smoking Status of Participants on Psychological Outcomes
eTable 15. Quality Assessment of Included Studies Using the Cochrane Risk-of-Bias Tool 2
eFigure 2. Funnel Plot for Visual Inspection of Publication Bias in Studies Assessing Depression Severity in OAC
eFigure 3. Trim-and-Fill Analysis for Publication Bias in Studies Assessing Depression Severity in OAC
eFigure 4. Quantitative Assessment Publication Bias in Studies Assessing Depression Severity in OAC
eFigure 5. Leave-One-Out Analysis of Studies Assessing Depression Severity in OAC, Using the Random Effects Model
eFigure 6. Outlier Assessment of Studies Assessing Depression Severity in OAC, Using the Random Effects Model
eFigure 7. Funnel Plot for Visual Inspection of Publication Bias in Studies Assessing Anxiety Severity in OAC
eFigure 8. Trim-and-Fill Analysis for Publication Bias in Studies Assessing Anxiety Severity in OAC
eFigure 9. Quantitative Assessment Publication Bias in Studies Assessing Anxiety Severity in OAC
eFigure 10. Leave-One-Out Analysis of Studies Assessing Anxiety Severity in OAC, Using the Random Effects Model
eFigure 11. Outlier Assessment of Studies Assessing Anxiety Severity in OAC, Using the Random Effects Model
eFigure 12. Funnel Plot for Visual Inspection of Publication Bias in Studies Assessing QoL Levels in OAC
eFigure 13. Trim-and-Fill Analysis for Publication Bias in Studies Assessing QoL Levels in OAC
eFigure 14. Quantitative Assessment Publication Bias in Studies Assessing QoL Levels in OAC
eFigure 15. Leave-One-Out Analysis of Studies Assessing QoL Levels in OAC, Using the Random Effects Model
eFigure 16. Outlier Assessment of Studies Assessing QoL Levels in OAC, Using the Random Effects Model
Data Sharing Statement
References
- 1.Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229-263. doi: 10.3322/caac.21834 [DOI] [PubMed] [Google Scholar]
- 2.White MC, Holman DM, Boehm JE, Peipins LA, Grossman M, Henley SJ. Age and cancer risk: a potentially modifiable relationship. Am J Prev Med. 2014;46(3)(suppl 1):S7-S15. doi: 10.1016/j.amepre.2013.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.KPMG . When cancer grows old: assessing the socio-economic burden of cancer and ageing and the policies required. Published February 2022. Accessed December 23, 2024. https://assets.kpmg.com/content/dam/kpmg/sg/pdf/2022/02/socio-economic-burden-of-cancer-and-ageing.pdf
- 4.Low CE, Yau CE, Tan RY, et al. Association of depression with all-cause and cancer-specific mortality in older adults with cancer: systematic review, meta-analysis, and meta-regression. J Geriatr Oncol. 2024;15(4):101700. doi: 10.1016/j.jgo.2023.101700 [DOI] [PubMed] [Google Scholar]
- 5.Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer. 2008;112(11)(suppl):2577-2592. doi: 10.1002/cncr.23448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caruso R, Breitbart W. Mental health care in oncology. Contemporary perspective on the psychosocial burden of cancer and evidence-based interventions. Epidemiol Psychiatr Sci. 2020;29:e86. doi: 10.1017/S2045796019000866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alfano CM, Lichstein KL, Vander Wal GS, et al. Sleep duration change across breast cancer survivorship: associations with symptoms and health-related quality of life. Breast Cancer Res Treat. 2011;130(1):243-254. doi: 10.1007/s10549-011-1530-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shapiro CL. Cancer Survivorship. N Engl J Med. 2018;379(25):2438-2450. doi: 10.1056/NEJMra1712502 [DOI] [PubMed] [Google Scholar]
- 9.Smith HR. Depression in cancer patients: pathogenesis, implications and treatment (review). Oncol Lett. 2015;9(4):1509-1514. doi: 10.3892/ol.2015.2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Low CE, Loke S, Pang GE, Sim B, Yang VS. Psychological outcomes in patients with rare cancers: a systematic review and meta-analysis. EClinicalMedicine. 2024;72:102631. doi: 10.1016/j.eclinm.2024.102631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shahrokni A, Wu AJ, Carter J, Lichtman SM. Long-term toxicity of cancer treatment in older patients. Clin Geriatr Med. 2016;32(1):63-80. doi: 10.1016/j.cger.2015.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee ARYB, Yau CE, Low CE, Li J, Ho RCM, Ho CSH. Severity and longitudinal course of depression, anxiety and post-traumatic stress in paediatric and young adult cancer patients: a systematic review and meta-analysis. J Clin Med. 2023;12(5):1784. doi: 10.3390/jcm12051784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee ARYB, Low CE, Yau CE, Li J, Ho R, Ho CSH. Lifetime burden of psychological symptoms, disorders, and suicide due to cancer in childhood, adolescent, and young adult years: a systematic review and meta-analysis. JAMA Pediatr. 2023;177(8):790-799. doi: 10.1001/jamapediatrics.2023.2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaorsky NG, Zhang Y, Tuanquin L, Bluethmann SM, Park HS, Chinchilli VM. Suicide among cancer patients. Nat Commun. 2019;10(1):207. doi: 10.1038/s41467-018-08170-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5(10):617-625. doi: 10.1016/S1470-2045(04)01597-9 [DOI] [PubMed] [Google Scholar]
- 16.Zhu J, Fang F, Sjölander A, Fall K, Adami HO, Valdimarsdóttir U. First-onset mental disorders after cancer diagnosis and cancer-specific mortality: a nationwide cohort study. Ann Oncol. 2017;28(8):1964-1969. doi: 10.1093/annonc/mdx265 [DOI] [PubMed] [Google Scholar]
- 17.Salive ME. Multimorbidity in older adults. Epidemiol Rev. 2013;35:75-83. doi: 10.1093/epirev/mxs009 [DOI] [PubMed] [Google Scholar]
- 18.Knapowski J, Wieczorowska-Tobis K, Witowski J. Pathophysiology of ageing. J Physiol Pharmacol. 2002;53(2):135-146. [PubMed] [Google Scholar]
- 19.Low CE, Pillay RM, Teo FJJ, et al. Educational interventions to reduce depression and anxiety in older adults with cancer in the community: a systematic review, meta-analysis and meta-regression of randomised controlled trials. Age Ageing. 2024;53(6):afae111. doi: 10.1093/ageing/afae111 [DOI] [PubMed] [Google Scholar]
- 20.Andersen C, Adamsen L, Moeller T, et al. The effect of a multidimensional exercise programme on symptoms and side-effects in cancer patients undergoing chemotherapy–the use of semi-structured diaries. Eur J Oncol Nurs. 2006;10(4):247-262. doi: 10.1016/j.ejon.2005.12.007 [DOI] [PubMed] [Google Scholar]
- 21.Assi M, Dufresne S, Rébillard A. Exercise shapes redox signaling in cancer. Redox Biol. 2020;35:101439. doi: 10.1016/j.redox.2020.101439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liska TM, Kolen AM. The role of physical activity in cancer survivors’ quality of life. Health Qual Life Outcomes. 2020;18(1):197. doi: 10.1186/s12955-020-01448-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hojman P, Gehl J, Christensen JF, Pedersen BK. Molecular mechanisms linking exercise to cancer prevention and treatment. Cell Metab. 2018;27(1):10-21. doi: 10.1016/j.cmet.2017.09.015 [DOI] [PubMed] [Google Scholar]
- 24.Ostuzzi G, Benda L, Costa E, Barbui C. Efficacy and acceptability of antidepressants on the continuum of depressive experiences in patients with cancer: systematic review and meta-analysis. Cancer Treat Rev. 2015;41(8):714-724. doi: 10.1016/j.ctrv.2015.06.003 [DOI] [PubMed] [Google Scholar]
- 25.Niedzwiedz CL, Knifton L, Robb KA, Katikireddi SV, Smith DJ. Depression and anxiety among people living with and beyond cancer: a growing clinical and research priority. BMC Cancer. 2019;19(1):943. doi: 10.1186/s12885-019-6181-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kvåle K. Do cancer patients always want to talk about difficult emotions? A qualitative study of cancer inpatients communication needs. Eur J Oncol Nurs. 2007;11(4):320-327. doi: 10.1016/j.ejon.2007.01.002 [DOI] [PubMed] [Google Scholar]
- 27.Hariton E, Locascio JJ. Randomised controlled trials - the gold standard for effectiveness research: study design: randomised controlled trials. BJOG. 2018;125(13):1716. doi: 10.1111/1471-0528.15199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization. Ageing and health. Updated October 1, 2024. Accessed December 23, 2024. https://www.who.int/news-room/fact-sheets/detail/ageing-and-health
- 29.Cohen S. Psychosocial models of the role of social support in the etiology of physical disease. Health Psychol. 1988;7(3):269-297. doi: 10.1037/0278-6133.7.3.269 [DOI] [PubMed] [Google Scholar]
- 30.Segal RJ, Reid RD, Courneya KS, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 2003;21(9):1653-1659. doi: 10.1200/JCO.2003.09.534 [DOI] [PubMed] [Google Scholar]
- 31.McNeely ML, Parliament M, Courneya KS, et al. A pilot study of a randomized controlled trial to evaluate the effects of progressive resistance exercise training on shoulder dysfunction caused by spinal accessory neurapraxia/neurectomy in head and neck cancer survivors. Head Neck. 2004;26(6):518-530. doi: 10.1002/hed.20010 [DOI] [PubMed] [Google Scholar]
- 32.Monga U, Garber SL, Thornby J, et al. Exercise prevents fatigue and improves quality of life in prostate cancer patients undergoing radiotherapy. Arch Phys Med Rehabil. 2007;88(11):1416-1422. doi: 10.1016/j.apmr.2007.08.110 [DOI] [PubMed] [Google Scholar]
- 33.Segal RJ, Reid RD, Courneya KS, et al. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol. 2009;27(3):344-351. doi: 10.1200/JCO.2007.15.4963 [DOI] [PubMed] [Google Scholar]
- 34.Banasik J, Williams H, Haberman M, Blank SE, Bendel R. Effect of Iyengar yoga practice on fatigue and diurnal salivary cortisol concentration in breast cancer survivors. J Am Acad Nurse Pract. 2011;23(3):135-142. doi: 10.1111/j.1745-7599.2010.00573.x [DOI] [PubMed] [Google Scholar]
- 35.Culos-Reed SN, Robinson JW, Lau H, et al. Physical activity for men receiving androgen deprivation therapy for prostate cancer: benefits from a 16-week intervention. Support Care Cancer. 2010;18(5):591-599. doi: 10.1007/s00520-009-0694-3 [DOI] [PubMed] [Google Scholar]
- 36.Galvão DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol. 2010;28(2):340-347. doi: 10.1200/JCO.2009.23.2488 [DOI] [PubMed] [Google Scholar]
- 37.Bourke L, Doll H, Crank H, Daley A, Rosario D, Saxton JM. Lifestyle intervention in men with advanced prostate cancer receiving androgen suppression therapy: a feasibility study. Cancer Epidemiol Biomarkers Prev. 2011;20(4):647-657. doi: 10.1158/1055-9965.EPI-10-1143 [DOI] [PubMed] [Google Scholar]
- 38.Cormie P, Newton RU, Spry N, Joseph D, Taaffe DR, Galvão DA. Safety and efficacy of resistance exercise in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 2013;16(4):328-335. doi: 10.1038/pcan.2013.22 [DOI] [PubMed] [Google Scholar]
- 39.Arbane G, Douiri A, Hart N, et al. Effect of postoperative physical training on activity after curative surgery for non-small cell lung cancer: a multicentre randomised controlled trial. Physiotherapy. 2014;100(2):100-107. doi: 10.1016/j.physio.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 40.Campo RA, Agarwal N, LaStayo PC, et al. Levels of fatigue and distress in senior prostate cancer survivors enrolled in a 12-week randomized controlled trial of Qigong. J Cancer Surviv. 2014;8(1):60-69. doi: 10.1007/s11764-013-0315-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edvardsen E, Skjønsberg OH, Holme I, Nordsletten L, Borchsenius F, Anderssen SA. High-intensity training following lung cancer surgery: a randomised controlled trial. Thorax. 2015;70(3):244-250. doi: 10.1136/thoraxjnl-2014-205944 [DOI] [PubMed] [Google Scholar]
- 42.Galvão DA, Spry N, Denham J, et al. A multicentre year-long randomised controlled trial of exercise training targeting physical functioning in men with prostate cancer previously treated with androgen suppression and radiation from TROG 03.04 RADAR. Eur Urol. 2014;65(5):856-864. doi: 10.1016/j.eururo.2013.09.041 [DOI] [PubMed] [Google Scholar]
- 43.Miki E, Kataoka T, Okamura H. Feasibility and efficacy of speed-feedback therapy with a bicycle ergometer on cognitive function in elderly cancer patients in Japan. Psychooncology. 2014;23(8):906-913. doi: 10.1002/pon.3501 [DOI] [PubMed] [Google Scholar]
- 44.Cormie P, Galvão DA, Spry N, et al. Can supervised exercise prevent treatment toxicity in patients with prostate cancer initiating androgen-deprivation therapy: a randomised controlled trial. BJU Int. 2015;115(2):256-266. doi: 10.1111/bju.12646 [DOI] [PubMed] [Google Scholar]
- 45.Nilsen TS, Raastad T, Skovlund E, et al. Effects of strength training on body composition, physical functioning, and quality of life in prostate cancer patients during androgen deprivation therapy. Acta Oncol. 2015;54(10):1805-1813. doi: 10.3109/0284186X.2015.1037008 [DOI] [PubMed] [Google Scholar]
- 46.Yagli NV, Ulger O. The effects of yoga on the quality of life and depression in elderly breast cancer patients. Complement Ther Clin Pract. 2015;21(1):7-10. doi: 10.1016/j.ctcp.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 47.Cramer H, Pokhrel B, Fester C, et al. A randomized controlled bicenter trial of yoga for patients with colorectal cancer. Psychooncology. 2016;25(4):412-420. doi: 10.1002/pon.3927 [DOI] [PubMed] [Google Scholar]
- 48.Winters-Stone KM, Lyons KS, Dobek J, et al. Benefits of partnered strength training for prostate cancer survivors and spouses: results from a randomized controlled trial of the exercising together project. J Cancer Surviv. 2016;10(4):633-644. doi: 10.1007/s11764-015-0509-0 [DOI] [PubMed] [Google Scholar]
- 49.Lai Y, Huang J, Yang M, Su J, Liu J, Che G. Seven-day intensive preoperative rehabilitation for elderly patients with lung cancer: a randomized controlled trial. J Surg Res. 2017;209:30-36. doi: 10.1016/j.jss.2016.09.033 [DOI] [PubMed] [Google Scholar]
- 50.Loh KP, Kleckner IR, Lin PJ, et al. Effects of a home-based exercise program on anxiety and mood disturbances in older adults with cancer receiving chemotherapy. J Am Geriatr Soc. 2019;67(5):1005-1011. doi: 10.1111/jgs.15951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng D, Wang X, Hu J, et al. Effect of tai chi and resistance training on cancer-related fatigue and quality of life in middle-aged and elderly cancer patients. Chin J Integr Med. 2021;27(4):265-272. doi: 10.1007/s11655-021-3278-9 [DOI] [PubMed] [Google Scholar]
- 52.Mardani A, Pedram Razi S, Mazaheri R, Haghani S, Vaismoradi M. Effect of the exercise programme on the quality of life of prostate cancer survivors: a randomized controlled trial. Int J Nurs Pract. 2021;27(2):e12883. doi: 10.1111/ijn.12883 [DOI] [PubMed] [Google Scholar]
- 53.Mikkelsen MK, Lund CM, Vinther A, et al. Effects of a 12-week multimodal exercise intervention among older patients with advanced cancer: results from a randomized controlled trial. Oncologist. 2022;27(1):67-78. doi: 10.1002/onco.13970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Capela A, Antunes P, Coelho CA, et al. Effects of walking football on adherence, safety, quality of life and physical fitness in patients with prostate cancer: findings from the PROSTATA_MOVE randomized controlled trial. Front Oncol. 2023;13:1129028. doi: 10.3389/fonc.2023.1129028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Langlais CS, Chen YH, Van Blarigan EL, et al. Quality of life for men with metastatic castrate-resistant prostate cancer participating in an aerobic and resistance exercise pilot intervention. Urol Oncol. 2023;41(3):146.e1-146.e11. doi: 10.1016/j.urolonc.2022.11.016 [DOI] [PubMed] [Google Scholar]
- 56.Porserud A, Karlsson P, Aly M, et al. Effects of an exercise intervention in primary care after robot-assisted radical cystectomy for urinary bladder cancer: a randomised controlled trial. BMC Cancer. 2024;24(1):891. doi: 10.1186/s12885-024-12647-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51(11):2375-2390. doi: 10.1249/MSS.0000000000002116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wrann CD, White JP, Salogiannnis J, et al. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013;18(5):649-659. doi: 10.1016/j.cmet.2013.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin TW, Kuo YM. Exercise benefits brain function: the monoamine connection. Brain Sci. 2013;3(1):39-53. doi: 10.3390/brainsci3010039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anderson E, Shivakumar G. Effects of exercise and physical activity on anxiety. Front Psychiatry. 2013;4:27. doi: 10.3389/fpsyt.2013.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jaromin E, Sadowska ET, Koteja P. The effect of monoamines reuptake inhibitors on aerobic exercise performance in bank voles from a selection experiment. Curr Zool. 2019;65(4):409-419. doi: 10.1093/cz/zoy063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alley JR, Mazzochi JW, Smith CJ, Morris DM, Collier SR. Effects of resistance exercise timing on sleep architecture and nocturnal blood pressure. J Strength Cond Res. 2015;29(5):1378-1385. doi: 10.1519/JSC.0000000000000750 [DOI] [PubMed] [Google Scholar]
- 63.Dinis J, Bragança M. Quality of sleep and depression in college students: a systematic review. Sleep Sci. 2018;11(4):290-301. doi: 10.5935/1984-0063.20180045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ji C, Yang J, Lin L, Chen S. Physical exercise ameliorates anxiety, depression and sleep quality in college students: experimental evidence from exercise intensity and frequency. Behav Sci (Basel). 2022;12(3):61. doi: 10.3390/bs12030061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bo A, Mao W, Lindsey MA. Effects of mind-body interventions on depressive symptoms among older Chinese adults: a systematic review and meta-analysis. Int J Geriatr Psychiatry. 2017;32(5):509-521. doi: 10.1002/gps.4688 [DOI] [PubMed] [Google Scholar]
- 66.Miller KJ, Gonçalves-Bradley DC, Areerob P, Hennessy D, Mesagno C, Grace F. Comparative effectiveness of three exercise types to treat clinical depression in older adults: a systematic review and network meta-analysis of randomised controlled trials. Ageing Res Rev. 2020;58:100999. doi: 10.1016/j.arr.2019.100999 [DOI] [PubMed] [Google Scholar]
- 67.Zhang Y, Li G, Liu C, Guan J, Zhang Y, Shi Z. Comparing the efficacy of different types of exercise for the treatment and prevention of depression in youths: a systematic review and network meta-analysis. Front Psychiatry. 2023;14:1199510. doi: 10.3389/fpsyt.2023.1199510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Amireault S, Baier JM, Spencer JR. Physical activity preferences among older adults: a systematic review. J Aging Phys Act. 2019;27(1):128-139. doi: 10.1123/japa.2017-0234 [DOI] [PubMed] [Google Scholar]
- 69.Alley SJ, Schoeppe S, Rebar AL, Hayman M, Vandelanotte C. Age differences in physical activity intentions and implementation intention preferences. J Behav Med. 2018;41(3):406-415. doi: 10.1007/s10865-017-9899-y [DOI] [PubMed] [Google Scholar]
- 70.Blome C, Augustin M. Measuring change in quality of life: bias in prospective and retrospective evaluation. Value Health. 2015;18(1):110-115. doi: 10.1016/j.jval.2014.10.007 [DOI] [PubMed] [Google Scholar]
- 71.Li X, He S, Liu T, et al. Impact of exercise type, duration, and intensity on depressive symptoms in older adults: a systematic review and meta-analysis. Front Psychol. 2024;15:1484172. doi: 10.3389/fpsyg.2024.1484172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Low CE, Loke S, Rana S, Sim B, Ho CSH. Prevalence and incidence of suicide, suicidal ideation and self-harm in caregivers of cancer patients: a systematic review and meta-analysis. Gen Hosp Psychiatry. 2024;90:35-43. doi: 10.1016/j.genhosppsych.2024.06.011 [DOI] [PubMed] [Google Scholar]
- 73.Lee ARYB, Leong I, Lau G, et al. Depression and anxiety in older adults with cancer: systematic review and meta-summary of risk, protective and exacerbating factors. Gen Hosp Psychiatry. 2023;81:32-42. doi: 10.1016/j.genhosppsych.2023.01.008 [DOI] [PubMed] [Google Scholar]
- 74.Low CE, Tan SYP, Loh A, et al. Post-traumatic stress disorder and symptoms in paediatric cancer survivors and their family nucleus: systematic review, meta-analysis and meta-regression. BJPsych Open. 2024;10(6):e207. doi: 10.1192/bjo.2024.805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goel N, Hernandez A, Cole SW. Social genomic determinants of health: understanding the molecular pathways by which neighborhood disadvantage affects cancer outcomes. J Clin Oncol. 2024;42(30):3618-3627. doi: 10.1200/JCO.23.02780 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Search Strategy
eTable 2. Specific Details of All Exercise Interventions Used to Improve Psychological Outcomes
eTable 3. Meta-Analyses of Exercise on Depression Severity in OAC Stratified by Categorical Study-Level Characteristics Using the Random Effect Model
eFigure 1. Subgroup Meta-Analyses of Exercise on Depression (A) and Anxiety (B) Levels Among Older Adults With Cancer Stratified by Nature of Exercise
eTable 4. Mixed Effects Meta-Regression of Standardised Mean Differences Against Potential Effect Moderators (Continuous and Categorical Study-Level Characteristics) for Depression Severity After Exercise Interventions in OAC
eTable 5. Meta-Analyses of Exercise on Anxiety Severity in OAC Stratified by Categorical Study-Level Characteristics Using the Random Effect Model
eTable 6. Mixed Effects Meta-Regression of Standardised Mean Differences Against Potential Effect Moderators (Continuous and Categorical Study-Level Characteristics) for Anxiety Severity After Exercise Interventions in OAC
eTable 7. Meta-Analyses of Exercise on HRQOL Improvement in OAC Stratified by Categorical Study-Level Characteristics Using the Random Effect Model
eTable 8. Mixed Effects Meta-Regression of Standardised Mean Differences Against Potential Effect Moderators (Continuous and Categorical Study-Level Characteristics) for HRQOL Improvement After Exercise Interventions in OAC
eTable 9. Evaluation of the Mediating or Confounding Effect of Age of Participants on Psychological Outcomes
eTable 10. Evaluation of the Mediating or Confounding Effect of Race of Participants on Psychological Outcomes
eTable 11. Evaluation of the Mediating or Confounding Effect of Marital Status of Participants on Psychological Outcomes
eTable 12. Evaluation of the Mediating or Confounding Effect of Income Level and Employment Status of Participants on Psychological Outcomes
eTable 13. Evaluation of the Mediating or Confounding Effect of Education Level of Participants on Psychological Outcomes
eTable 14. Evaluation of the Mediating or Confounding Effect of Smoking Status of Participants on Psychological Outcomes
eTable 15. Quality Assessment of Included Studies Using the Cochrane Risk-of-Bias Tool 2
eFigure 2. Funnel Plot for Visual Inspection of Publication Bias in Studies Assessing Depression Severity in OAC
eFigure 3. Trim-and-Fill Analysis for Publication Bias in Studies Assessing Depression Severity in OAC
eFigure 4. Quantitative Assessment Publication Bias in Studies Assessing Depression Severity in OAC
eFigure 5. Leave-One-Out Analysis of Studies Assessing Depression Severity in OAC, Using the Random Effects Model
eFigure 6. Outlier Assessment of Studies Assessing Depression Severity in OAC, Using the Random Effects Model
eFigure 7. Funnel Plot for Visual Inspection of Publication Bias in Studies Assessing Anxiety Severity in OAC
eFigure 8. Trim-and-Fill Analysis for Publication Bias in Studies Assessing Anxiety Severity in OAC
eFigure 9. Quantitative Assessment Publication Bias in Studies Assessing Anxiety Severity in OAC
eFigure 10. Leave-One-Out Analysis of Studies Assessing Anxiety Severity in OAC, Using the Random Effects Model
eFigure 11. Outlier Assessment of Studies Assessing Anxiety Severity in OAC, Using the Random Effects Model
eFigure 12. Funnel Plot for Visual Inspection of Publication Bias in Studies Assessing QoL Levels in OAC
eFigure 13. Trim-and-Fill Analysis for Publication Bias in Studies Assessing QoL Levels in OAC
eFigure 14. Quantitative Assessment Publication Bias in Studies Assessing QoL Levels in OAC
eFigure 15. Leave-One-Out Analysis of Studies Assessing QoL Levels in OAC, Using the Random Effects Model
eFigure 16. Outlier Assessment of Studies Assessing QoL Levels in OAC, Using the Random Effects Model
Data Sharing Statement