Abstract
Introduction
Functional constipation is common in childhood, with chronicity leading to a significant impact on patients and their families. There is a significant range of therapies available to healthcare professionals for this condition, with many novel or recently studied. There is a need for an update to the joint European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN)/North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) guidelines last released in 2014. We present the prospectively agreed operating procedure and technical review protocol in this manuscript.
Methods
‘Grading of Recommendations Assessment, Development and Evaluation’ (GRADE) will be used for all phases of this guideline development. The Guideline Development Group is formed by paediatric gastroenterologists from both the ESPGHAN as well as the NASPGHAN. A prospective exercise will agree on key outcomes, thresholds of magnitude that are significant at small, moderate and large levels. Systematic evidence searches, selection, extraction, appraisal and analysis will be performed following Cochrane guidance and GRADE guidance for objectively agreeing the certainty of findings. Additional use of network meta-analysis will identify areas of broad triangulation in the evidence. Summary of findings tables will be produced and inform evidence to decision frameworks. These will guide GRADE recommendations with voting to reach a consensus.
Keywords: Gastroenterology, Child Health
WHAT IS ALREADY KNOWN ON THIS TOPIC
A significant growth in published research regarding functional constipation (FC) in childhood has occurred since the last European Society for Paediatric Gastroenterology, Hepatology and Nutrition/North American Society for Pediatric Gastroenterology, Hepatology and Nutrition guidance published in 2014.
An update to this guidance is indicated using the most appropriate up-to-date methods of guideline production.
WHAT THIS STUDY ADDS
High-quality guideline production is built on technical reviews of the evidence and transparent prospectively agreed methods. This manuscript being published represents a key step in this transparent high-quality method.
These operating procedures describe the organisation of the Guideline Development Group (GDG), as well as key steps in agreeing on questions, outcomes and thresholds of outcome magnitudes. It also describes the methods of evidence synthesis and finally, the methods that will be used to summarise, present and vote on recommendations in line with Grading of Recommendations Assessment, Development and Evaluation methodology.
This manuscript and the methods contained within have been approved by all members of the GDG. Several methodological elements are novel within the field.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
We aim for this guideline to provide a tool for the treatment of children aged 0–18 years with FC worldwide for all treatment settings.
This could lead to more uniformity in treatment, as well as yield more capacity for collaboration in a scientific setting worldwide.
Introduction
Functional constipation (FC) is an extremely common problem in children of all ages worldwide, with a pooled prevalence of 9.5%.1 Constipation is often associated with sporadic or occasional and/or infrequent painful defaecation, faecal incontinence and abdominal pain and therefore causes significant distress to children, young people and their families. In addition, FC has a significant impact on healthcare services.2
In 2014, the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) published a joint evidence-based guideline on the treatment of childhood constipation.3 Since then, many new studies have been published. Additionally, the diagnostic criteria have been updated with the publication of the latest Rome-IV criteria for paediatric FC.4 5 The final area of significant development relates to the methodological advances within guideline development, most notably within the procedures of the ‘Grading of Recommendations Assessment, Development and Evaluation’ (GRADE) approach to both appraising evidence and producing guidelines.
This protocol describes the prospectively designed and agreed standard operating procedures that will be followed to produce a GRADE international treatment guideline, seeking to include recommendations for all approaches, used in clinical practice today. The final guideline will additionally consider faecal impaction (FI) and refractory constipation (RC) as well as the surgical management of constipation. The final guideline will contain the official recommendations of the Guideline Development Group (GDG) on all treatment aspects.
The guideline will support health professionals, patients and their families. The methods used for technical review, GRADE analysis and decision-making will allow its dissemination. The prospective publishing of this document is part of that process of systematic guideline production.
Methods
The production of this guideline will be aligned with the procedures of GRADE as described in the GRADE handbook, supported by the WHO handbook for guideline development.6 The team will use the Guideline international network (GIN)-McMaster guideline development checklist (McMaster 2021), an 18-point process map to support the steps in a GRADE-compliant guideline development process.7
The scope of the guideline will include studies on the treatment of FC, FI and RC. Studies on FI will be analysed separately. Studies on RC will be included with the main cohort, as the patient populations are very similar to most included studies. The definitions of FI and RC will be included in the guideline. Definitions will be established based on current literature and by consensus of the guideline committee.
Organisation, planning and training
In July 2023, members of both ESPGHAN and NASPGHAN discussed a potential collaboration on an FC treatment guideline update with methodological support from the ‘Biomedical Evidence Synthesis and translations to practice’ (BEST) evidence synthesis and guideline production unit at the University of Central Lancashire (which houses the editorial centre for the Cochrane Gut group).
The protocol was developed by the BEST methods team (MGor) and then reviewed and edited by the ESPGHAN and NASPGHAN teams. An ESPGHAN core team (MAB, AdG, MT) in collaboration with BEST (MGor, VS) will be responsible for the technical review, including searches, the tables and synthesis of the result section. Subsequently, a meeting with members of both societies (NASPGHAN and ESPGHAN) will be organised face-to-face in October 2024 in person to discuss the results in depth and to formulate recommendations.
The joint guideline chairs will be appointed as content and field experts from both societies and will be joined with a lead and non-voting GRADE methodologist as co-chair (MGor) in line with GRADE procedures.8 Administrative support will be offered from both host higher education institutions of the co-chairs and access to a Cochrane and National Institute for Health and Care Excellence expert information specialist arranged through these institutions.
Guideline Development Group
The GDG is formed by members of ESPGHAN and NASPGHAN. Included are paediatricians and paediatric gastroenterologists with expertise in FC and its treatment. Also, one clinical psychologist, physical therapist and dietician with extensive experience in the treatment of children with FC is a voting member of the GDG. The methodological chair remains non-voting.
The lead and senior authors for the guidelines were approved by the member societies prior to appointment. All members agreed to maintain the confidentiality of the discussions within the guideline process as well as the confidentiality of the content of the guideline prior to publication and to be coauthors of the full guideline. Members had to declare all conflicts of interest prior to recruitment.
GDG priority setting and identifying target audience
Patient and family stakeholders were consulted through a Delphi process in a previous study to contribute to the formation of a core outcome set for assessing treatment success in FC.9 This core outcome set forms the basis for this treatment guideline.9
Stages of production
The following sequential steps will be followed in the guideline
The standard operating procedure and technical summary protocol will be agreed, peer-reviewed and published in an open-access journal (this manuscript).
A Delphi exercise will be performed to prospectively agree on the outcomes of focus for the guideline and the thresholds that will be considered for the magnitude of health benefits or harm categories to support GRADE analysis and stakeholder utility.10
The completion of technical reviews of randomised controlled trial (RCT) evidence using methodologically rigorous methods and production of a GRADE summary of findings for all outcomes to allow preparation of evidence to decision frameworks and GDG decision-making.11
Sharing of all evidence, supporting data, extra relevant studies and the draft evidence to decision frameworks with GDG members.
A face-to-face GDG meeting to discuss the evidence within the evidence to decision frameworks. This will be followed by a voting process to agree on recommendations.
The publishing of a main guideline that summarises key recommendations, the certainty of underpinning evidence and the strength of the recommendations all within the main published journal output.
Approach to technical review
The technical reviews will be completed in line with methods guidance from Cochrane, the GRADE handbook, JCE (Journal of Clinical Epidemiology) GRADE guidelines12 and our previously developed approaches to such guidelines:13 14
Studies against placebo, no treatment and all active comparators will be considered. Network Meta-analysis will be deployed to triangulate findings and where certainty is high, moderate or low and clinical homogeneity exists, be presented as additional data with the use of appropriate Graphics On Recommendations Diagram Of Network Meta-analysis Plots.15 Subgroup analyses will be performed for outcome measures in the case of different comparator groups, given that heterogeneity and a sufficient volume of studies exist.
Outcome measures were based on the previous publication of a core outcome set for defining the treatment effects of FC in children.16 Following the GRADE handbook, outcomes were defined as critical, important or not important through a Delphi process and a face-to-face meeting held to agree on the final set of outcomes in May 2024 (stated below).
The GDG will complete a Delphi process to prospectively agree on the critical (primary) and important (secondary) outcomes of focus for the guideline and the decision thresholds for the outcome measures before proceeding to data analysis. The threshold ranges will be trivial, small, moderate and large treatment effects.11 These ranges will be identified for each of the included outcome measures separately. The limit of the small treatment effect will represent the minimally important clinical difference for GDG decision-making.17 18
Key to the refinement of the specific questions will be to prioritise outcomes for use that were a reflection of the most clinically relevant and meaningful, as well as always balancing efficacy with safety.
Therapy delivered in all settings and by all professionals, as well as self-administered therapies will be considered but detailed extraction will gather such data to consider as a source of heterogeneity and to aid clinical interpretation.
Study selection
Types of studies
All published, unpublished and ongoing RCTs that compared interventions for the management of FC RC with other active interventions or standard therapy, placebo or no therapy will be considered for inclusion. Studies that described ‘faecal impaction’ will be considered in separate searches.
Observational studies could be considered for inclusion and GRADE only if they met the following criteria: large sample size, clear control of confounding factors and very large differences in effect between groups. Observational studies are not included in formal searches and the GDG will include these based on their knowledge of the field and reference searching of included studies.
Types of participants
Trials enrolling children from the age of 0 to 18 years, with a clinical FC diagnosis, with or without FI, or with an intractable constipation diagnosis as defined by the authors, will be considered for inclusion. If studies do not define FC, FI or intractable constipation, studies will not be included. If studies include a mix of adults and children and the data are not separated, authors will be contacted, and the study will only be included if separate data on children can be provided on request. The diagnostic criteria for FC in children are included in box 1. As FI is not currently defined with international consensus, a working definition will be developed through a systematic review of all published definitions. Similarly, RC has not been included in the scope of the previous Rome IV criteria, but recent work has reviewed published definitions of RC and the following working definition has been proposed ‘Constipation that persists despite administration of two laxatives of different classes (eg, an osmotic and stimulant laxative) with good compliance, over a period of at least three months as assessed during the clinical evaluation in a secondary or tertiary care facility’.19
Box 1. ROME diagnostic criteria for Functional Constipation.
A. Rome IV criteria functional constipation (FC) in infants and toddlers up to 4 years old.5
Must include two or more of the following present for at least 1 month:
Two or fewer defaecations per week
History of excessive stool retention
History of painful or hard bowel movements
Presence of a large-diameter stools
History of large faecal mass in the rectum
In toilet-trained children, the following additional criteria may be used:
At least one episode/week of incontinence after the acquisition of toileting skills
History of large-diameter stools that may obstruct the toilet
B. Rome IV criteria FC in children and adolescents (developmental age ≥4 years).4
Must include two or more of the following occurring at least once per week for a minimum of 1 month with insufficient criteria for a diagnosis of irritable bowel syndrome:
Two or fewer defaecations in the toilet per week
At least one episode of faecal incontinence per week
History of retentive posturing or excessive volitional stool retention
History of painful or hard bowel movements
Presence of a large faecal mass in the rectum
History of large-diameter stools that can obstruct the toilet
After appropriate evaluation, the symptoms cannot be fully explained by another medical condition.
Types of interventions
Pharmacological, non-pharmacological and surgical treatments will be included. Trials studying the pharmacological and non-pharmacological interventions outlined in tables1 2 can be included. Pharmacological treatment will be divided into disimpaction for FI and maintenance treatment, both will be discussed separately in the guideline.
Table 1. Pharmacological interventions for disimpaction and maintenance therapy.
| Oral or rectal treatment | Type | Intervention |
| Oral treatment | Osmotic laxatives | Polyethylene glycol |
| Lactulose | ||
| Lactitol | ||
| Magnesium hydroxide | ||
| Stimulant laxatives | Bisacodyl | |
| Senna | ||
| Sodium picosulfate | ||
| Lubricants | Mineral oil (liquid paraffin) | |
| Novel therapies | Prucalopride | |
| Lubiprostone | ||
| Linaclotide | ||
| Plecanatide | ||
| Bile acid modulators | ||
| Rectal treatment | Enemas | Sodium docusate |
| Sodium lauryl sulfoacetate | ||
| Sodium phosphate | ||
| Soap suds | ||
| Microenema (eg, Promelaxin) | ||
| Fleet bisacodyl enema | ||
| 0.9% NaCl enema | ||
| Suppositories | Glycerin | |
| Effervescent suppositories | ||
| Bisacodyl | ||
| Other | Transanal irrigation | |
| Botox |
Table 2. Non-pharmacological interventions.
| Type | Intervention |
| Lifestyle | Physical activity |
| Dietary interventions | Cow’s milk free diet |
| Fibre supplements and fibres as well-balanced diet | |
| Fluid | |
| Prebiotics, probiotics and synbiotics | |
| Behavioural therapies | Toilet training |
| Behavioural therapy | |
| Biofeedback | |
| Physiotherapy | Pelvic floor muscle exercises |
| Neurostimulation | Transcutaneous electrical stimulation |
| Posterior tibial nerve stimulation | |
| Sacral nerve stimulation | |
| Multidisciplinary treatment | |
| Complementary medicine | Herbal medicine |
| Acupuncture | |
| Homeopathy | |
| Musculoskeletal manipulations (osteopathic and chiropractic) | |
| Yoga | |
| Abdominal massage | |
Trials studying the following surgical interventions can included:
Antegrade continence enema (also consider which agents to be used)
Ileostomy
Colostomy
Sigmoidectomy
Subtotal colectomy
Types of outcome measures
Both dichotomous and continuous outcomes will be valid for inclusion. Ranking of the outcome measures was based on the core outcome set, with the core research team (MG, VS, AG, MT, MAB) proposing a final ranking that received the consent of all GDG members. The set of outcomes includes a mix of outcomes pertaining to the efficacy of treatment (ie, the success of a treatment in reducing symptoms and any consequent beneficial sequelae) and to the safety of treatment (ie, any outcome related to adverse events or their sequelae).
Primary (critical) outcomes
Treatment success as defined by the authors (dichotomous)
Defaecation frequency (dichotomous/continuous)
Withdrawals due to adverse events (dichotomous)
Secondary (important) outcomes
Painful defaecation (dichotomous/continuous)
Stool consistency (dichotomous/continuous)
Quality of life or change in quality of life measured using any validated measurement tool (dichotomous/continuous)
Faecal incontinence (if age appropriate) (dichotomous/continuous)
Abdominal pain (if age appropriate) (dichotomous/continuous)
School attendance (if age appropriate) (dichotomous/continuous)
Serious adverse events (dichotomous)
Total adverse events (dichotomous)
Tolerability or defined as acceptability or compliance (dichotomous/continuous)
Thresholds for outcomes
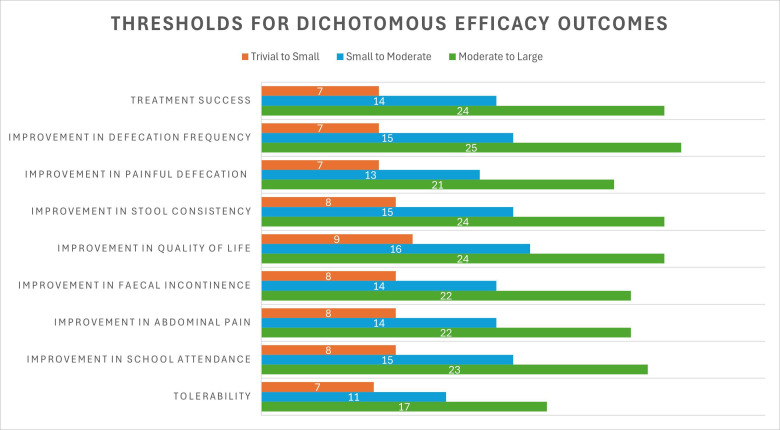
The GDG completed a two-stage modified Delphi process in April and May 2024. This produced the priority outcomes for the guidelines and most importantly the thresholds for outcome measures the magnitude of effect. In round 1, there was good agreement for continuous outcomes which included absolute measures. There was a wider spread for dichotomous outcomes and so these were fed back and a second stage was performed. A face-to-face meeting at DDW (Digestive Disease Week) 2024 was held and a final agreement on the thresholds was reached. This is a unique process. See figures1 2 for the thresholds established by the GDG group for the dichotomous outcomes and table 3 for the continuous outcomes. Thresholds for dichotomous outcomes are expressed as absolute risk differences (%).
Figure 1. Thresholds for FC treatment outcome measures: dichotomous efficacy outcomes. Thresholds are expressed as absolute risk differences (%). FC, functional constipation.
Figure 2. Thresholds for FC treatment outcome measures: safety outcomes. Thresholds are expressed as absolute risk differences (%). FC, functional constipation.
Table 3. Thresholds for FC treatment outcome measures: continuous efficacy outcomes.
| Outcome | Trivial-to-small | Small-to-moderate | Moderate-to-large |
| Increase in defaecation frequency per week | 1.2 | 2.3 | 3.7 |
| Decrease in painful defaecations per week | 1.1 | 2.2 | 3.5 |
| Decrease of pain during defaecation on VAS-score (0–100) | 13 | 26 | 41 |
| Change in stool consistency on the Bristol Stool Form Scale (1–7, 1=very hard stools, 7=very soft stools) | 0.8 | 1.5 | 2.3 |
| Improvement in quality of life on PedsQL score (0–100) | 13 | 23 | 38 |
| Decrease in faecal incontinence frequency per week | 1.0 | 2.4 | 4.0 |
| Decrease of abdominal pain measured on a 0–4-point scale (0=no pain, 4=a lot of pain) | 0.7 | 1.2 | 2.0 |
| Number of school days missed per month | 3 | 6 | 9 |
| Tolerability on 4-point Likert scale (0=poor tolerability, 4=excellent tolerability) | 0.6 | 1.1 | 1.9 |
FCfunctional constipationPedsQLPediatric quality of life inventoryVASVisual Analogue Scale
Extraction and analysis
Key items extracted from all papers will include:
Study details: author, publication year, setting
Patient demographics: age, sociodemographics, disease definition, gender and total number of participants
Definition of the condition covered, including specific international criteria: FC, FC with incontinence, RC
Eligibility criteria: inclusion and exclusion criteria
Trial location: country and number of trial centres
Methods used: study design, total study duration and date
Study flow: randomised numbers to each intervention group and numbers reaching trial end
Intervention and comparator description: type of interventions, treatment duration, dose of pharmacological treatment, details on placebo
Outcomes: outcome definition, unit of measurement, and time of collection, length of follow-up, adverse events
Funding source
All treatment arms are described in the ‘Characteristics of included studies’ tables
Appraisal of included studies
Two authors will independently assess the risk of bias in the included studies based on the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions.20
We will assess the following ‘risk of bias’ domains:
Sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Other biases such as imbalance in participants’ baseline characteristics
The studies will be judged to be at low, high or unclear risk of bias for each domain assessed, based on the guidance in the Cochrane Handbook for Systematic Reviews of Interventions.20 Disagreements will be resolved by reaching consensus.
Measures of treatment effect
We will express dichotomous treatment effects as risk ratios with corresponding 95% CI and mean difference with 95% CI for continuous outcomes. Where end-of-study absolute data and change data are reported, we will use the final data for analysis. However, when combining studies that used different approaches, the standardised mean difference will be used.21
Dealing with missing data
We will contact all study authors when data is missing or information to judge the risk of bias is needed. Studies that failed to report measures of variance will be judged as at high risk of reporting bias.
Unit of analysis issues
The unit of analysis in the technical review will be the individual participant. For studies that involve more than two intervention groups, we intend to conduct multiple pairwise comparisons between all potential pairs of intervention groups. To prevent double counting, we will allocate shared intervention groups proportionally among the comparisons. For dichotomous outcomes, both the number of events and the total number of participants will be divided accordingly. For continuous outcomes, we will only divide the total number of participants, keeping the means and SDs unchanged. Cross-over studies will be included in the quantitative analysis only if data are separately reported for the periods before and after the cross-over, using only the pre-cross-over data.
Assessment of heterogeneity
As concerns are expected with sources of clinical heterogeneity within treatment options (eg, different strains of probiotics, different types of fibre supplements, different age categories), if meta-analyses exhibit visual or statistical tests of concern, we will perform subgroup analyses, given adequate numbers.
A detailed qualitative analysis of the population and study variables across studies will be presented, including chronicity, prior therapy, when and if disimpaction occurred in relation to baseline outcomes measurements, and if rescue therapy was allowed during the treatment period.
When unexplained heterogeneity exists at more than 50% to above 90% with no clear clinical or methodological reasons, the GDG has agreed on an approach: authors will be contacted three times for all details and primary data if possible and if no response is received, the journal editors will be contacted. If no response is received, the study will be removed in a sensitivity analysis. All analyses with heterogeneity of more than 90% will be considered at risk and not used.
To test for statistical heterogeneity, we will employ a χ² test using a p value of less than 0.1 to give an indication of the presence of heterogeneity. Inconsistency was quantified and represented by the I² statistic. We will interpret the thresholds as follows:20
0–40%: might not be important.
30–60%: may represent moderate heterogeneity.
50–90%; may represent substantial heterogeneity.
75–100%: considerable heterogeneity.
Assessment of reporting biases
We plan to investigate publication bias using a funnel plot if there are 10 or more studies in a single analysis in line with established methods.22
Development of recommendations
In line with our operating procedure steps, the full technical reviews, summary of findings tables and evidence to decision frameworks will be given to the GDG members for review. The data and GRADE summary of findings tables will be added to the evidence to decision frameworks.
A face-to-face meeting will be held to discuss any key features of note in the evidence, areas of convergence in individual studies, direct and network meta-analysis.
Where there are clear signals in the evidence base, recommendations will be prepared, followed by voting. Where there is a more disordered or diverging evidence base, discussion and if an appropriate recommendation can be developed, voting will be held.
All recommendations will follow the GRADE approach and nomenclature with the strength of a recommendation based on the evidence to decision frameworks, aligned with the language of the aforementioned level of strength.
The non-voting team will refine this into a final list of recommendations and ensure the strength of the recommendations to be made is consistent with the evidence presented and views of the GDG, as per the GRADE recommendation guidance. They will also facilitate the face-to-face meeting.
Voting will be anonymous and all members with conflicts will abstain. An online voting system will count YES, NO and ABSTAIN votes. If≥75% of voting members agree, a recommendation will be passed. If there is no such an agreement, a further discussion will be held for a maximum of 30 min with either the same or revised recommendation voted. If no consensus can be reached, the recommendation will be left pending further exploration and revisiting.
If the evidence does not support a GRADE level recommendation but there is a sufficiently clear experiential and broader evidence base as well as a significant agreement on a statement that has clear actionable benefit, a Good Practice Statement will be made.23
The final proposals will be agreed by consensus, with the strength of agreement, certainty of evidence and strength of recommendations all presented.
The final synthesised recommendations will be prepared in a guideline to meet the ESPGHAN/NASPGHAN and journal publication standards. The evidence for decision frameworks will be made available as supplementary material and the technical evidence published in full as concomitant outputs to support the main guidance.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. A small fee was made available through the North American society for Paediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) for travel expenses to facilitate physical meetings. The European society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) covered travel expenses to facilitate the face-to-face summit for its delegates. Grant number N/A.
Patient consent for publication: Not applicable.
Ethics approval: Not applicable.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Data availability statement
No data are available.
References
- 1.Koppen IJN, Vriesman MH, Saps M, et al. Prevalence of Functional Defecation Disorders in Children: A Systematic Review and Meta-Analysis. J Pediatr. 2018;198:121–30. doi: 10.1016/j.jpeds.2018.02.029. [DOI] [PubMed] [Google Scholar]
- 2.The Lancet Gastroenterology Hepatology The cost of constipation. Lancet Gastroenterol Hepatol. 2019;4:811. doi: 10.1016/S2468-1253(19)30297-3. [DOI] [PubMed] [Google Scholar]
- 3.Tabbers MM, DiLorenzo C, Berger MY, et al. Evaluation and treatment of functional constipation in infants and children: evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr. 2014;58:258–74. doi: 10.1097/MPG.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 4.Hyams JS, Di Lorenzo C, Saps M, et al. Functional Disorders: Children and Adolescents. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Benninga MA, Faure C, Hyman PE, et al. Childhood Functional Gastrointestinal Disorders: Neonate/Toddler. Gastroenterology. 2016:S0016-5085(16)00182-7. doi: 10.1053/j.gastro.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 6.Organization WH . WHO handbook for guideline development. 2nd. Geneva: World Health Organization; 2014. edn. [Google Scholar]
- 7.Schünemann HJ, Wiercioch W, Etxeandia I, et al. Guidelines 2.0: systematic development of a comprehensive checklist for a successful guideline enterprise. CMAJ. 2014;186:E123–42. doi: 10.1503/cmaj.131237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fretheim A, Schünemann HJ, Oxman AD. Improving the use of research evidence in guideline development: 3. Group composition and consultation process. Health Res Policy Syst. 2006;4:15. doi: 10.1186/1478-4505-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuizenga-Wessel S, Steutel NF, Benninga MA, et al. Development of a core outcome set for clinical trials in childhood constipation: a study using a Delphi technique. BMJ Paediatr Open. 2017;1:e000017. doi: 10.1136/bmjpo-2017-000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alonso-Coello P, Oxman AD, Moberg J, et al. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016;353:i2089. doi: 10.1136/bmj.i2089. [DOI] [PubMed] [Google Scholar]
- 11.Alonso-Coello P, Schünemann HJ, Moberg J, et al. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016;353:i2016. doi: 10.1136/bmj.i2016. [DOI] [PubMed] [Google Scholar]
- 12.Guyatt GH, Oxman AD, Schünemann HJ, et al. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64:380–2. doi: 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Gordon M, Benninga MA, Borlack R, et al. ESPGHAN and NASPGHAN 2023 protocol for paediatric FAPD treatment guidelines (standard operating procedure) BMJ Paediatr Open. 2023;7:e002166. doi: 10.1136/bmjpo-2023-002166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darie A-M, Sinopoulou V, Ajay V, et al. BSG 2024 IBD guidelines protocol (standard operating procedures) BMJ Open Gastroenterol. 2023;10:e001067. doi: 10.1136/bmjgast-2022-001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon M. Maintaining remission in Crohn’s disease post surgery: what can we learn from Cochrane? Frontline Gastroenterol. 2024;15:241–6. doi: 10.1136/flgastro-2023-102559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeevenhooven J, Rexwinkel R, Van Berge Henegouwen VWA, et al. A Core Outcome Set for Clinical Trials in Pediatric Functional Abdominal Pain Disorders. J Pediatr. 2020;221:115–22. doi: 10.1016/j.jpeds.2020.02.032. [DOI] [PubMed] [Google Scholar]
- 17.Iheozor-Ejiofor Z, Lakunina S, Gordon M, et al. Sample-size estimation is not reported in 24% of randomised controlled trials of inflammatory bowel disease: A systematic review. United European Gastroenterol J. 2021;9:47–53. doi: 10.1177/2050640620967899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon M, Lakunina S, Sinopoulou V, et al. Minimum sample size estimates for trials in inflammatory bowel disease: A systematic review of a support resource. World J Gastroenterol. 2021;27:7572–81. doi: 10.3748/wjg.v27.i43.7572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinopoulou V, Gordon M, Rajindrajith S, et al. How do we define therapy-resistant constipation in children aged 4-18 years old? A systematic review with meta-narrative synthesis. BMJ Paediatr Open. 2024;8:e002380. doi: 10.1136/bmjpo-2023-002380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen J. Statistical power analysis for the behavioral sciences . Academic press; 2013. [Google Scholar]
- 22.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dewidar O, Lotfi T, Langendam MW, et al. Good or best practice statements: proposal for the operationalisation and implementation of GRADE guidance. BMJ Evid Based Med. 2023;28:189–96. doi: 10.1136/bmjebm-2022-111962. [DOI] [PMC free article] [PubMed] [Google Scholar]