Abstract
The family of p21-activated kinases (PAKs) have been implicated in the rearrangement of actin cytoskeleton by acting downstream of the small GTPases Rac and Cdc42. Here we report that even though Cdc42/Rac1 or Akt are not activated, phosphatidylinositol-3 (PI-3) kinase activation induces PAK1 kinase activity. Indeed, we demonstrate that PI-3 kinase associates with the N-terminal regulatory domain of PAK1 (amino acids 67–150) leading to PAK1 activation. The association of the PI-3 kinase with the Cdc42/Rac1 binding-deficient PAK1(H83,86L) confirms that the small GTPases are not involved in the PI-3 kinase-PAK1 interaction. Furthermore, PAK1 was activated in cells expressing the dominant-negative forms of Cdc42 or Rac1. Additionally, we show that PAK1 phosphorylates actin, resulting in the dissolution of stress fibers and redistribution of microfilaments. The phosphorylation of actin was inhibited by the kinase-dead PAK1(K299R) or the PAK1 autoinhibitory domain (PAK1(83–149)), indicating that PAK1 was responsible for actin phosphorylation. We conclude that the association of PI-3 kinase with PAK1 regulates PAK1 kinase activity through a Cdc42/Rac1-independent mechanism leading to actin phosphorylation and cytoskeletal reorganization.
INTRODUCTION
Cytoskeletal elements are central to many cellular functions and their polymerization dynamics are subject to fine regulatory control (Lim et al., 1996a; Tapon and Hall, 1997; Papakonstanti et al., 2000). Early cellular responses induced by a variety of stimuli, including opioid agonists, seem to involve changes in the polymerization state of actin and cell morphology (Isenberg, 1996; Papakonstanti et al., 1996, 1998; Adam et al., 1998; Koukouritaki et al., 1999). Several studies indicate that opioids, which have been shown to influence the proliferation and many other functions of human and animal cells (Hatzoglou et al., 1996; Olson et al., 1998), induce regulatory mechanisms involving phosphoinositide metabolism, phosphorylation of their receptors by G-protein receptor kinases, and recruitment of cellular components to transmit their signals, but the molecular details of opioid receptor signal transduction are not well understood (Mosaddeghi et al., 1995; Law and Loh, 1999, Law et al., 2000). Even less is known about the effects of opioids on the dynamics or the organization of microfilaments, and moreover, the overall signaling pathway by which opioids affect the actin cytoskeleton has not been addressed to date.
Generally, opioids act through membrane receptors, which belong to the seven transmembrane loop receptor superfamily, that are coupled to heterotrimeric G-proteins (Reisine and Bell, 1993). This receptor class can be coupled to the activation of PI-3 kinase that is directly stimulated by the G-protein βγ subunits released from the α-subunit when the latter is activated by binding GTP (Vanhaesebroeck et al., 1997; Hazeki et al., 1998; Maier et al., 1999). The downstream effectors of PI-3 kinase, Cdc42, and Rac1 have been implicated in the formation of peripheral filopodia, membrane ruffles, and loss of stress fibers (Lim et al., 1996b; Hall, 1998). In the active, GTP-bound state these small GTPases interact with a variety of effector proteins to elicit cellular responses, including cytoskeletal reorganization (Machesky and Hall, 1996; Narumiya, 1996; Hall 1998). Recently, a family of serine/threonine kinases known as p21-activated kinases (PAKs) has been identified as downstream target of activated Cdc42 and Rac (Manser et al., 1994, 1995; Bagrodia et al., 1995). PAK activity is regulated by different classes of membrane receptors including G-protein coupled receptors, tyrosine kinase receptors, and cytokine receptors (Knaus et al., 1995; Zhang et al., 1995; Kjøller and Hall, 1999). On binding to Cdc42/Rac1, PAK1 is activated and autophosphorylates, thus increasing its catalytic activity toward exogenous substrates (Manser et al., 1994). Activation of PAK1 has been shown to result in depolymerization of stress fibers and peripheral actin reorganization including cortical actin polymerization and formation of filopodia and membrane ruffles (Dharmawardhane et al., 1997; Daniels and Bokoch, 1999). However, several studies have shown that constitutively active PAK1 or PAK1/PAK3 mutants, which are unable to bind Cdc42 or Rac, mediate morphological changes reminiscent of those induced by Cdc42 and Rac. These findings indicated that p21-binding domains are not involved or that PAK could function independently of Rac or as an upstream activator of Rac (Manser et al., 1997; Sells et al., 1997; Daniels et al., 1998; Obermeier et al., 1998). More recently, it was found that PAK1 is stimulated by activated Akt through a GTPase-independent mechanism (Tang et al., 2000) as well as by PDK1 through a PI-3 kinase–independent mechanism (King et al., 2000). In addition, direct interactions with GTPase exchange factors, adapters such as Nck, receptors, and lipids have been implicated in activation of PAK1 (Bokoch et al., 1996, 1998; Lu et al., 1997; Daniels et al., 1998). Thus, it remains to be elucidated how these and other signaling molecules are involved in the effects of PAK on actin cytoskeleton.
Because opioid receptors are coupled to heterotrimeric G-proteins that are implicated in PAK1 and PI-3 kinase activation (Knaus et al., 1995; Vanhaesebroeck et al., 1997; Wymann and Pirola 1998; Murga et al., 2000), we thought it worthwhile to investigate whether the mechanism(s) by which opioids transmit their signal to promote morphological changes that are characteristic of Cdc42/Rac-PAK activation involves these candidates. We previously showed that exposure of opossum kidney (OK) cells to the opioid agonists, αs1 casomorphin and ethylketocyclazocine (EKC) resulted in substantial alterations of actin polymerization dynamics and microfilament distribution, which occurred within 15 min and persisted for at least 2 h (Papakonstanti et al., 1998). The results of the present study provide evidence that opioids induce a novel signal transduction pathway, which does not involve Cdc42/Rac1 or Akt activation. Instead, PI-3 kinase activation was followed both, by its association with the N terminal regulatory domain of PAK1 (to the region between amino acids 67 and 150) and by PAK1 activation. Subsequently, PAK1 directly phosphorylated actin, resulting in the disassembly of stress fibers and cortical actin reorganization.
MATERIALS AND METHODS
Materials
Rhodamine-phalloidin and Slow Fade Antifade kit were from Molecular Probes Inc. (Eugene, OR). Polyclonal antibodies for PAK1 (rabbit), LIMK1 (goat), actin (goat), αPIX (goat), and Rac1 (rabbit) as well the RhoA and p110β monoclonal antibodies and protein G-Agarose were from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Rabbit polyclonal anti–PI-3 kinase(p85) antibody, mouse monoclonal anti-Nck antibody, protein A-Agarose, and Cdc42 or Rac activation assay kit [including GST-fusion of the PAK1 p21-binding domain (PBD, amino acids 67–150) bound to glutathione-Agarose, lysis/wash buffer, GTPγS, GDP, and monoclonal anti-Cdc42 or anti-Rac antibodies] were purchased from Upstate Biotechnology Inc. (Lake Placid, NY). Phospho-specific (Thr308) Akt and anti-Akt antibodies were from New England Biolabs (Beverly, MA). The anti-Gβγ antibody was obtained from Calbiochem (La Jolla, CA). Phosphatidylinositol-4,5-bisphosphate (from bovine brain) and myelin basic protein (MBP) were obtained from Sigma (St. Louis, MO). Silica gel 60 sheets were from Merck (Poole, Dorset, UK). ECL Western blotting kit, monoclonal antiactin antibody (for immunoblotting), glutathione-Sepharose beads and [γ-32P]ATP were from Amersham Corp (Arlington Heights, IL). The rabbit anticofilin antibody was obtained from Cytoskeleton Inc. (Denver, CO). EKC and αs1-casomorphin were kindly provided by Dr. E. Castanas (University of Crete, Greece). All other chemicals were obtained from usual commercial sources at the highest grade available.
Cell Culture and Transfections
OK cells were from the American Type Culture Collection (Manassas, VA) and were studied between passages 40 and 50. Cells were maintained in a humidified atmosphere of 5% CO2–95% air at 37°C and fed twice weekly with a 1:1 DMEM-Ham's F12 medium, supplemented with 10% fetal calf serum (FCS) and 2 mM glutamine, 20 mM NaHCO3, 22 mM HEPES, 50 IU/ml penicillin, and 50 mg/ml streptomycin. Subcultivation was performed with Ca2+- and Mg2+-free phosphate-buffered saline (PBS), containing 0.25% trypsin and 5 mM EDTA. Initially, cells were cultured for 48 h with complete medium as described above. The medium was changed to serum-free medium, containing 0.1% bovine serum albumin, 15–20 h before the actual experiments. Exposure of cells to opioid agonists was performed as described earlier (Papakonstanti et al., 1998). The PI-3 kinase inhibitor wortmannin was used in a concentration of 100 nM, and it was added for the last 30 min of the 15- to 20-h preincubation period, and then cells were stimulated with EKC or αs1-casomorphin (10−8 M) for the indicated times. All experiments described below were performed while cells were still in their logarithmic growth phase.
Cells grown on 100-mm tissue culture dishes were transiently transfected, using the calcium-phosphate DNA coprecipitation protocol, with 10 μg of pJ3H vector containing either the HA-tagged kinase-dead PAK1(K299R) or p21-binding–deficient PAK1(H83,86L) or with 10 μg of pCMV6 M vector containing either the Myc-tagged autoinhibitory domain of PAK1(PAK1(83–149)), dominant-negative Cdc42(T17N), or dominant-negative Rac1(T17N). Cells were allowed to express the protein for 40 h after transfection and were then washed in PBS, scraped into lysis buffer, and used for immunoprecipitation experiments as described below.
Plasmids
pGEX-2T-TRBD, pCMV6 M expression plasmids encoding the Cdc42(T17N) or PAK1(83–149) and pJ3H expression plasmids encoding the PAK1(K299R) or PAK1(H83,86L) were kindly provided by Dr. A. Moustakas (Ludwing Institute for Cancer Research, Uppsala, Sweden) and Dr. G.M. Bokoch (The Scripps Research Institute, La Jolla, California).
Expression and Purification of Recombinant Proteins
The pGEX-2T plasmids expressing the glutathione S-transferase (GST)-fusion proteins were transformed into Escherichia coli DH5α and induced with isopropyl-β-d-thiogalactopyranoside (IPTG) to express GST-fusion protein. The bacteria were collected by centrifugation and resuspended in E. coli lysis buffer (50 mM Tris-HCl, pH 7.5, 1% Triton X-100, 150 mM NaCl, 5 mM MgCl2, 1 mM DTT, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM PMSF). Vigorous sonication was performed before centrifugation at 14,000 rpm for 30 min, and the resulting supernatants were saved as crude exctracts containing GST-fusion protein, which was purified with glutathione-Sepharose beads. pCMV and pJ3H plasmids were transformed into E. coli DH10B for protein expression. Recombinant proteins from 250-ml culture were purified by the high-purity plasmid purification system (Life Technologies-BRL, Rockville, MD) according to the manufacturer's instructions.
Triton X-100 Fractionation
The Triton X-100 soluble and insoluble fractions of cells exposed to opioids, in the absence or in the presence of wortmannin, were prepared as previously described (Golenhofen et al., 1995) with minor modifications. Cells were incubated in 500 μl of Triton-extraction buffer (0.3% Triton X-100, 5 mM Tris, pH 7.4, 2 mM EGTA, 300 mM sucrose, 2 μM phalloidin, 1 mM PMSF, 10 μg/ml leupeptin, 20 μg/ml aprotinin, 1 mM sodium orthovanadate, and 50 mM NaF) for 5 min on ice. After removing the soluble proteins, the Triton-insoluble fraction remaining on the plate was scraped directly into 500 μl of RIPA buffer (50 mM Tris.HCl, pH 7.4, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 0.15 M NaCl, 1 mM EDTA, 1 mM dithiothreitol, and 1 mM sodium orthovanadate), and any remaining insoluble material was removed by centrifugation. Equal volumes of each fraction were subjected to SDS-PAGE and Western blotting.
Immunoprecipitation, Kinase Assays, and Immunoblotting Analysis
Cells were washed three times with ice-cold PBS and suspended in cold lysis buffer containing 1% Nonidet P-40, 50 mM Tris, pH 7.5, and 150 mM NaCl supplemented with protease and phosphatase inhibitors as described (Knaus et al., 1995; Okano et al., 1995). Cleared lysates were preadsorbed with protein A-Agarose (before incubation with rabbit anti-PAK1 and anti–PI-3 kinase (p85) and mouse anti-HA or anti-Myc epitope antibodies) or with protein G-Agarose (before incubation with goat anti-LIMK1 and antiactin antibodies) for 1 h at 4°C and centrifuged, and the supernatants (equal amounts of protein) were subjected to immunoprecipitation using the indicated antibodies and the respective protein A- or G-Agarose beads.
PAK1 kinase assays were performed as described (Knaus et al., 1995) using both autophosphorylation and phosphorylation of the exogenous substrate myelin basic protein (MBP) to assess activity. Protein A-Agarose beads containing immunoprecipitated PAK1 were washed twice with lysis buffer and three times with kinase buffer (50 mM HEPES, pH 7.5, 10 mM MgCl2, 2 mM MnCl2, and 0.2 mM DTT). Kinase activity was measured in 60 μl of kinase buffer containing 10 μCi of [γ-32P]ATP (5000 Ci/mmol) for 20 min at 30°C. Reactions were stopped by addition of SDS sample buffer and loading on a 10% SDS-PAGE. Proteins were transferred onto nitrocellulose membranes and 32P-labeled proteins were visualized by autoradiography.
Protein G-Agarose beads containing immunoprecipitated LIMK1 were washed three times with kinase buffer (50 mM HEPES, pH 7.2, 150 mM NaCl, 5 mM MgCl2, 5 mM MnCl2, 10 mM NaF, 1 mM Na3VO4), and then kinase activity was measured in 40 μl of kinase buffer, containing 15 μM ATP and 5 μCi of [γ-32P]ATP (5000 Ci/mmol), for 20 min at 30°C (Okano et al., 1995). Proteins were resolved by a 12% SDS-PAGE and transferred onto nitrocellulose membranes, and 32P-labeled proteins were visualized by autoradiography.
The lipid kinase activity of PI-3 kinase was measured by the method of Auger et al. (1989) with minor modifications. Protein A-Agarose beads containing immunoprecipitated PI-3 kinase or protein complexes that were coprecipitated with GST-PBD (amino acids 67–150) were washed three times with buffer A (20 mM Tris, pH 7.4, 137 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, 1% Nonidet P-40, 0.1 mM Na3VO4), three times with 5 mM LiCl in 0.1 M Tris (pH 7.4) and twice with TNE (10 mM Tris, pH 7.4, 150 mM NaCl, 5 mM EDTA, 0.1 mM Na3VO4). The immunoprecipitates or protein complexes were then resuspended in TNE, and the PI-3 kinase activity was assayed using 0.2 mg/ml phosphatidylinositol-4,5-bisphosphate (PI-4,5-P2) as a substrate, in the presence of 58 μM ATP, 10 μCi of [γ-32P]ATP (5000 Ci/mmol), and 14 mM MgCl2, for 10 min at 37°C. The reaction was stopped by the addition of 1 M HCl and methanol:chloroform (1:1). After mixing vigorously and centrifuging to separate the phases, the lipids in the organic lower phase were separated by TLC on oxalated silica gel 60 sheets, as described by Singh et al. (1996). Chromatographed lipids were also visualized by iodine staining and compared with the migration of known standards.
For immunoblot analysis, the cell lysates or the immunoprecipitates were suspended in Laemmli's sample buffer and separated by SDS-PAGE. Proteins were transferred onto nitrocellulose membrane, and the membrane was blocked with 5% nonfat dry milk in TBS-T (20 mM Tris, pH 7.6, 137 mM NaCl, 0.05% Tween-20) for 1 h at room temperature. Antibody solutions (in TBS-T containing 5% nonfat dry milk) were added overnight at 4°C (first antibody) and for 1 h (second horseradish peroxidase–coupled antibody). Blots were developed using the ECL system and the band intensities were quantitated by PC-based image analysis (Image Analysis Inc., St. Catherines, Ontario, Canada).
Affinity Precipitation
Affinity precipitation with GST-PBD was performed using an assay based on the method of Benard et al. (1999). Cells were lysed in Mg2+ lysis buffer (MLB), which was provided by the assay kit (UBI, Lake Placid, NY), mixed with 8 μg GST-PBD bound to glutathione-Agarose, and incubated for 1 h at 4°C. For a positive control, cell lysates were incubated for 15 min at 30°C with 100 μM GTPγS in the presence of 1 mM EDTA. The loading reaction was stopped by addition of 60 mM MgCl2. GTPγS loaded lysates were incubated with GST-PBD for 30 min at 4°C. Finally, precipitates were washed three times with MLB and suspended in Laemmli's sample buffer. Proteins were separated by 12% SDS-PAGE, transferred onto nitrocellulose membrane, and blotted with anti-Cdc42 or anti-Rac1 antibody.
Affinity precipitation with GST-RBD was performed as described by Ren et al. (1999). Cells were washed with ice-cold TBS and then lysed in RIPA buffer (50 mM Tris, pH 7.2, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 500 mM NaCl, 10 mM MgCl2 supplemented with protease inhibitors). Cleared cell lysates were incubated with 30 μg GST-RBD attached to glutathione-Sepharose at 4°C for 1 h. GTP-bound RhoA was detected by immunoblotting using an mAb.
Immunofluorescence Microscopy
For morphological observations by immunofluorescence microscopy, cells were cultured onto type IV collagen-covered glass slides (22 × 22 mm), to assure cellular attachment and orientation conditions analogous to those obtaining for renal tubular epithelia in situ. The procedure of cell fixation and direct fluorescence staining of microfilaments by rhodamine-phalloidin included incubation of cells with 3.7% formaldehyde, which was followed by a short incubation with acetone at −20°C. The cells were then incubated for 40 min at room temperature with rhodamine-phalloidin to stain the filamentous actin (Koukouritaki et al., 1996). Double labeling with additional indirect staining for fluorescence of HA-tagged kinase-dead PAK1(K299R) or Myc-tagged dominant-negative Cdc42(T17N) to detect the transfected cells was performed using monoclonal anti-HA or anti-Myc epitope antibody and the fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse antibody. Slides were mounted using the Slow Fade Antifade kit. All specimens were examined with a Olympus BH-2 microscope (Lake Success, NY) equipped with epifluorescence illumination. Micrographs were photographed with a 35-mm (C-35AD-4) camera and Kodak P3200 black and white films (Eastman Kodak, Rochester, NY).
RESULTS
Stimulation of PI-3 Kinase by Opioids
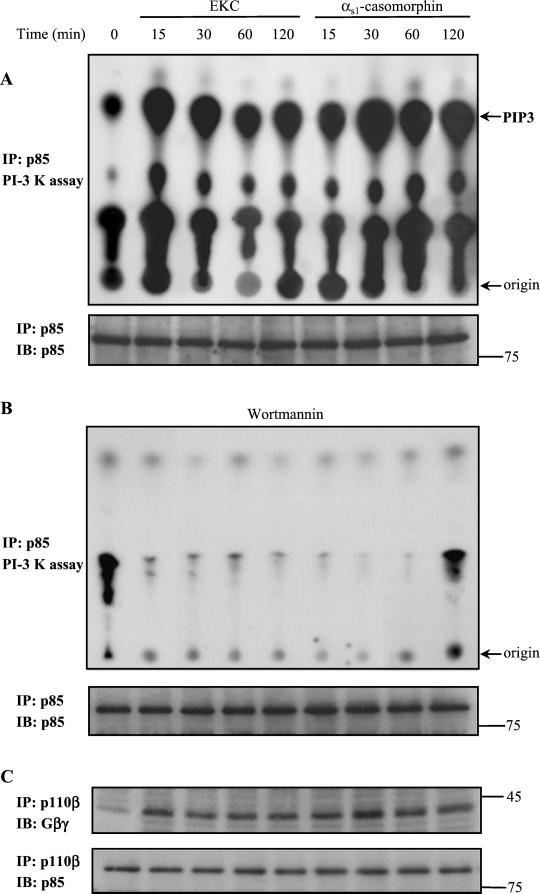
First, we examined the effect of opioid agonists, EKC and αs1-casomorphin, on PI-3 kinase activity. An in vitro kinase assay was performed on anti–PI-3 kinase(p85) immune complexes isolated from equal amounts of protein of untreated and opioid treated cells (Figure 1, A and B). We chose the indicated incubation times because our previous study has shown that alterations in the polymerization state and the distribution of actin were detectable within 15 min and persisted for 120 min (Papakonstanti et al., 1998). We found that PI-3 kinase activity was induced to a maximum within 15 min of EKC treatment and within 30 min of αs1-casomorphin treatment, and it remained above basal levels for 2 h (Figure 1A, top panel). Preincubation of cells with the specific PI-3 kinase inhibitor wortmannin (100 nM) abolished the formation of phosphatidylinositol-3,4,5-trisphosphate (PIP3), confirming the presence of PI-3 kinase in the immunoprecipitates (Figure 1B, top panel). Immunoblotting of PI-3 kinase(p85) immunoprecipitates, obtained from the same cell lysates that were used for the kinase assay, with the anti–PI-3 kinase(p85) antibody confirms that equal amounts of PI-3 kinase protein were immunoprecipitated in the kinase assay (Figure 1, A and B, bottom panels).
Figure 1.
Effect of EKC and αs1-casomorphin on the lipid kinase activity of PI-3 kinase. (A) Cells were incubated for the indicated times with 10−8 M EKC or αs1-casomorphin. Equal amounts of proteins of cell lysates were immunoprecipitated with an anti–PI-3 kinase(p85) antibody and subjected to an in vitro PI-3 kinase assay, as described in MATERIALS AND METHODS, using phosphatidylinositol-4,5-bisphosphate (PIP2) as substrate. The reaction products were separated by TLC and visualized by autoradiography (top panel). The amount of PI-3 kinase protein that was immunoprecipitated in the kinase assay was assessed by immunoblotting (IB) with anti–PI-3 kinase(p85) antibody (bottom panel). (B) Cells were pretreated with the PI-3 kinase inhibitor wortmannin for 30 min and then stimulated with 10−8 M EKC or αs1-casomorphin. Anti–PI-3 kinase(p85) immune complexes were assayed for kinase activity using PIP2 as substrate (top panel). The amount of PI-3 kinase protein that was immunoprecipitated in the kinase assay was assessed by immunoblotting (IB) with anti–PI-3 kinase(p85) antibody (bottom panel). (C) Cells were stimulated with EKC or αs1-casomorphin for the indicated times and lysed, and then equal amounts of protein were immunoprecipitated (IP) with an anti-p110β antibody. Coimmunoprecipitated Gβγ was detected by immunoblot (IB) with a specific anti-Gβγ antibody (top panel). Reprobing of the membrane with the anti–PI-3 kinase(p85) antibody confirms the presence of the regulatory subunit in the immunoprecipitates (bottom panel). Results shown are representative of four similar experiments. PIP3, phosphatidylinositol-3,4,5-trisphosphate.
Because it has been recently shown that Gβγ, liberated by G protein couple receptors (GPCRs), significantly stimulates the lipid kinase activity of PI-3 kinase β (Maier et al., 1999; Murga et al., 2000), we examined whether the same mechanism was involved in the PI-3 kinase activation observed in our system. Thus, the cells were exposed to opioid agonists, and then equal amounts of protein were subjected to immunoprecipitation with the anti–PI-3 kinase(p110β) antibody. Immunoblotting analysis with a specific anti-Gβγ antibody revealed that indeed, Gβγ was coprecipitated with PI-3 kinase β under the experimental conditions used (Figure 1C, top panel). Reprobing of the nitrocellulose membrane with the anti–PI-3 kinase(p85) antibody (Figure 1C, bottom panel), which was used for the lipid kinase activity assay, confirms that the regulatory subunit was coprecipitated with the catalytic subunit of PI-3 kinase, which is the target of Gβγ (Maier et al., 1999).
Opioids Stimulate PAK1 Kinase Activity through a PI-3 Kinase–dependent but Cdc42/Rac1-independent Mechanism
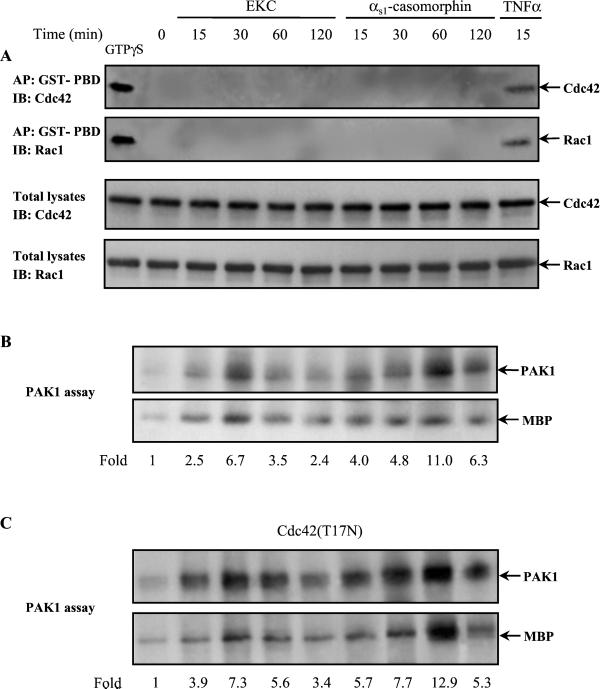
Because the downstream effectors of PI-3 kinase, Cdc42, and Rac1 have been implicated in morphological changes (Hall, 1998), we tested whether these small GTPases are activated by opioids. We performed affinity precipitation experiments with a GST-fusion protein corresponding to the p21-binding domain of PAK1 (GST-PBD) that specifically binds to and precipitates Cdc42-GTP and Rac-GTP from cell lysates (Benard et al., 1999). The presence of each GTPase was assessed with specific antibodies. As shown in Figure 2A (top two panels), GST-PBD effectively interacted with the active GTPγS-bound form of Cdc42 and Rac1 as well as with the GTP-Cdc42 and GTP-Rac1 in cell lysates obtained from TNF-α–treated cells that were used as positive controls. However, no interaction was observed between GST-PBD and GTP-Cdc42 or GTP-Rac1 in cells exposed to opioids, indicating that these small GTPases were not activated under the experimental conditions used. In addition, immunoblotting analysis of total lysates revealed no changes in the expression levels of both proteins (Figure 2A, bottom two panels). Surprisingly, opioids induced a strong activation of PAK1 kinase activity, as was determined by its autophosphorylation and its activity toward myelin basic protein (MBP; Figure 2B), indicating that the GTPase's activation was not required for PAK1 activation. To confirm further that PAK1 was activated independently of the small GTPases and to exclude the possibility that it was due to undetectable amounts of the GTP-Cdc42 or -Rac, the cells were transfected with inactive Cdc42(T17N) and exposed to opioid agonists, and then equal amounts of protein were subjected to immunoprecipitation for PAK1 kinase assay. As shown in Figure 2C, instead of being attenuated, PAK1 was activated by opioids, following the same kinetics as in untransfected cells (Figure 2B). Similar results were obtained when cells were transfected with inactive Rac1(T17N) (our unpublished results). Because the transfection efficiency was >40%, as determined by immunofluorescence microscopy from three independent experiments (our unpublished results), we consider the above result meaningful. Comparison of Figure 2B with Figure 1A revealed that opioid-induced activation of PAK1 followed the kinetics of PI-3 kinase activation, with a maximum 6.7-fold activation by 30 min of treatment with EKC and 11-fold activation by 60 min of αs1-casomorphin treatment. This may suggest that PAK1 activation is a relatively proximal consequence of PI-3 kinase activation.
Figure 2.
Stimulation of PAK1 kinase activity through a Cdc42/Rac1-independent mechanism. (A) GTPγS loaded lysates and lysates from untreated, EKC-, αs1-casomorphin-, or TNF-α (100 ng/ml, positive control)-treated cells were affinity precipitated (AP) with GTP-PBD bound to glutathione-Agarose beads. Precipitated GTP-Cdc42 or GTP-Rac1 was detected by immunoblot (IB) with anti-Cdc42 or anti-Rac1 antibody respectively (top two panels). Equal amount of proteins (100 μg) of untreated and opioid-treated or TNF-α–treated cells were subjected to SDS-PAGE, transferred to nitrocellulose membrane and immunoblotted (IB) with monoclonal anti-Cdc42 or anti-Rac1 antibody, respectively (bottom two panels). (B) Cells were stimulated with EKC or αs1-casomorphin for the indicated times and lysed, and anti-PAK1 immune complexes were assayed for kinase activity, including autophosphorylation and phosphorylation of the exogenous substrate MBP, by an in vitro kinase assay as described in MATERIALS AND METHODS. The reaction products were separated by SDS-PAGE and transferred to nitrocellulose membrane, and phosphorylation was visualized by autoradiography. (C) Cells were transfected with the inactive Cdc42(T17N) and then stimulated with opioid agonists for the indicated times. Anti-PAK1 immune complexes were assayed for kinase activity, including autophosphorylation and phosphorylation of the exogenous substrate MBP, by an in vitro kinase assay as described in MATERIALS AND METHODS. The number below each lane indi-cates the -fold autophosphorylation of PAK1, with that of untreated cells taken as 1. Results shown are representative of three independent experiments with similar results. MBP, myelin basic protein.
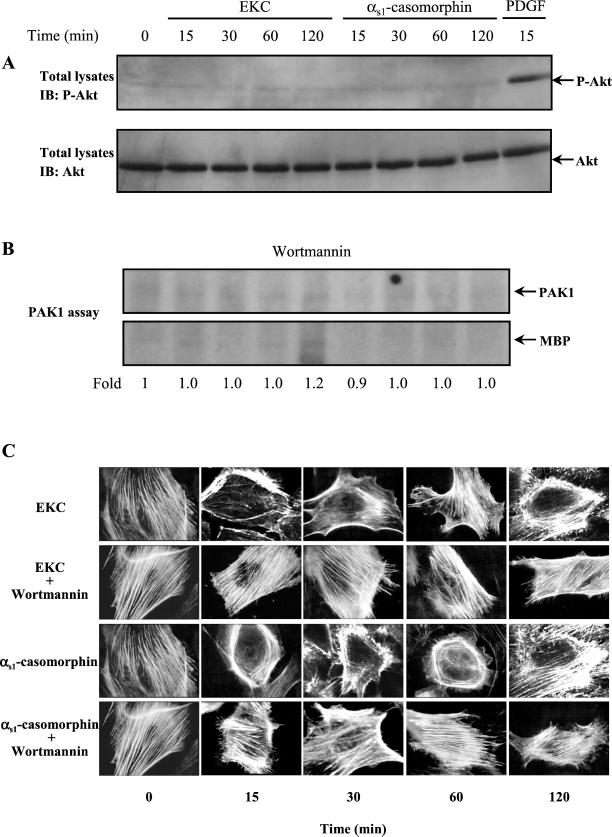
Recently, it has been shown that activated Akt stimulates PAK1 through a GTPase-independent mechanism (Tang et al., 2000). Accordingly, we examined whether Akt was activated in our system, thus mediating the signal from opioid-induced PI-3 kinase activation to PAK1. Cell extracts, from untreated and opioid-treated cells as well as from PDGF-treated cells, which were used as positive control, were analyzed by immunoblotting using a phospho-specific anti-Akt antibody. As shown in Figure 3A (top panel), Akt phosphorylation was strongly induced by PDGF but not by opioids. Reprobing of the nitrocellulose membrane with an anti-Akt antibody confirms that the amounts of Akt protein were equal in each sample (Figure 3A, bottom panel). These data suggest that Akt is not involved in PAK1 activation observed in our system.
Figure 3.
PAK1 activation and the redistribution of actin filaments induced by opioids depend on PI-3 kinase activity. (A) Equal amount of proteins (50 μg) of untreated and opioid-treated or PDGF(40 ng/ml)-treated cells were subjected to SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted (IB) with an anti–phospho-Akt (top panel) or anti-Akt (bottom panel) antibody. (B) Cells were pretreated with wortmannin for 30 min and then stimulated with 10−8 M EKC or αs1-casomorphin for the indicated times. PAK1 activity was determined in immunoprecipitates by an in vitro kinase assay. The reaction products were separated by SDS-PAGE and transferred to nitrocellulose membrane, and phosphorylation was visualized by autoradiography. (C) Cells were grown on type IV collagen-coated glass coverslips, pretreated with Wormannin or with its vehicle (DMSO), and then incubated for the indicated times with 10−8 M EKC or αs1-casomorphin. The redistribution of filamentous actin was determined with rhodamine-phalloidin staining by immunofluorescence microscopy. Magnification, ×1000. Similar results were obtained in three independent experiments.
Recently, it was also reported that PAK1 is activated by PDK1 and that this activation is not blocked by pretreatment with wortmannin (King et al., 2000). Thus, we examined whether PAK1 activation observed here is dependent on PI-3 kinase activity. It was noteworthy, that the PI-3 kinase inhibitor wortmannin abolished PAK1 kinase activity (Figure 3B), suggesting a close correlation between PI-3 kinase and PAK1 activity, independently of guanine nucleotide exchange factors (GNEFs) and Akt. In addition, preincubation of opioid treated cells with wortmannin was accompanied by suppression of opioid-induced actin reorganization. Indeed, as shown in Figure 3C, in cells exposed to EKC or αs1-casomorphin a clear redistribution of actin filaments was observed, including the formation of peripheral filopodia and membrane ruffles and loss of stress fibers. However, when cells were preincubated with wortmannin, opioid agonists failed to induce reorganization or disassembly of the actin microfilaments (Figure 3C). These findings were further corroborated by quantitative immunoblot analysis of the Triton X-100 soluble (TS) and insoluble (TI) actin cytoskeleton fractions of cells exposed to opioids (Table 1). Because the Triton X-100 insoluble fraction has been used to isolate cytoskeletal-associated proteins, it is widely accepted that Triton X-100 insoluble actin is primarily filamentous (F-) actin and Triton soluble actin is composed primarily of monomeric (G-) actin (Golenhofen et al., 1995). As calculated from the relative band intensities, the TS/TI actin ratio increased significantly in cells incubated with opioids for 15 min to 2 h, consistent with a decrease in the proportion of filamentous actin. Instead, no quantitative difference was observed between opioid treated and control cells that preincubated with wortmannin (Table 1).
Table 1.
Effect of EKC and αs1-casomorphin on the polymerization state of actin, in the absence or in the presence of the PI-3 kinase inhibitor wortmannin
| Incubation time (min) | TS/TI actin ratio(fold)
|
|||
|---|---|---|---|---|
| EKC | EKC + Wortm. | αs1-casomorphin | αs1-casomorphin + Wortm. | |
| 0 | 1 | 1 | 1 | 1 |
| 15 | 1.64 | 0.97 | 2.28 | 0.98 |
| 30 | 1.94 | 0.97 | 2.54 | 1.03 |
| 60 | 1.58 | 1.02 | 2.76 | 1.04 |
| 120 | 1.53 | 1.06 | 1.82 | 1.02 |
| 240 | 1.25 | — | 1.36 | — |
| 360 | 1.14 | — | 1.12 | — |
Cells were pretreated with wortmannin for 30 min and then stimulated with 10−8 M of either of the two opioids for the indicated times. Triton-soluble (TS) and Triton-insoluble (TI) actin cytoskeleton fractions were prepared as described in MATERIALS AND METHODS and analyzed for their content of actin by immunoblotting. Data presented correspond to the -fold TS/TI actin ratio with that of untreated cells, in each condition, taken as 1. These data are representative of two independent experiments.
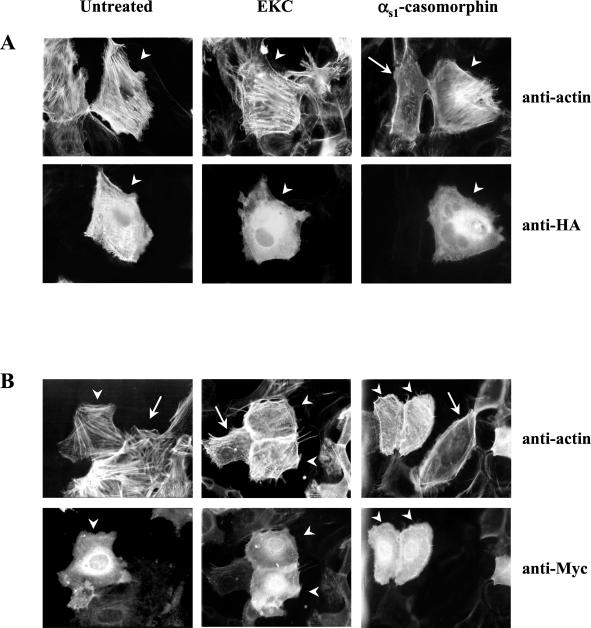
To determine whether the opioid-induced actin reorganization was dependent on PAK1 activation, cells were transfected with the kinase-dead PAK1(K299R), and opioid agonists were added for 30 min. As shown in Figure 4A, in cells expressing the kinase-dead PAK1(K299R) the dissolution of stress fibers as well as the reorganization of actin, including peripheral actin accumulation and the formation of membrane ruffles or filopodia, were completely blocked (arrowheads). On the contrary, these cytoskeletal changes were still observed in untransfected cells (arrows). On the other hand, Figure 4B shows that actin redistribution was induced by opioids in untransfected cells (arrows) as well as in cells expressing the dominant-negative Cdc42(T17N) (arrowheads). These results further support our findings that opioid-induced actin remodeling occurs through a PAK1-dependent but GTPase-independent mechanism.
Figure 4.
The opioid-induced actin remodeling occurs through a PAK1-dependent but GTPase-independent mechanism. (A) Cells were transfected with HA-tagged kinase-dead PAK1(K299R) and then incubated with either opioid for 30 min. Transfected cells were identified by double immunofluorescence as described in MATERIALS AND METHODS. Cells expressing HA-tagged PAK1(K299R) are indicated by an arrowhead; arrows show untransfected cells. (B) Cells were transfected with Myc-tagged dominant-negative Cdc42(T17N) and then incubated with either opioid for 30 min. Transfected cells were identified by double immunofluorescence as described in MATERIALS AND METHODS. Cells expressing Myc-tagged Cdc42(T17N) are indicated by an arrowhead; arrows show untransfected cells. Magnification, ×400. Similar results were obtained in three independent experiments.
PAK1 Associates with PI-3 Kinase
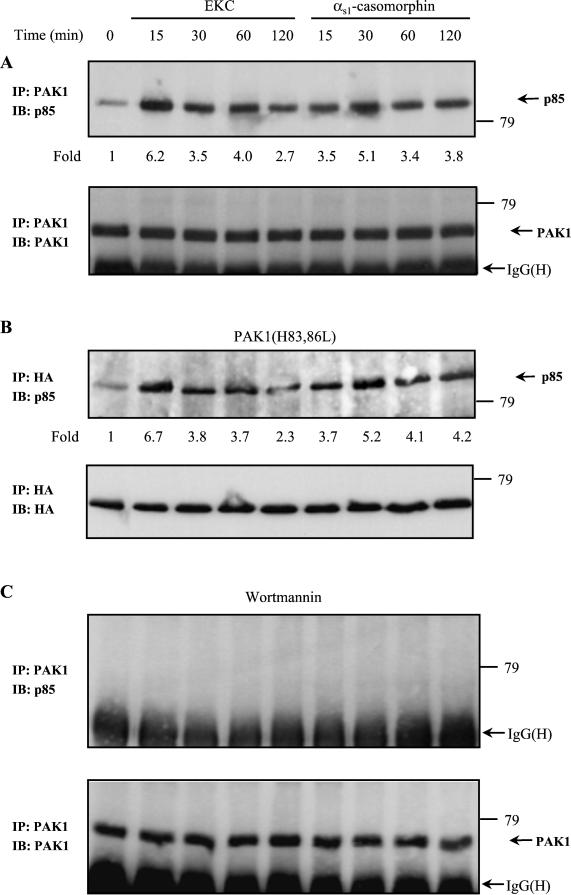
The above results revealed that, even though Cdc42/Rac1 and Akt were not activated, PAK1 kinase activity as well as actin alterations were directly dependent on PI-3 kinase activation. Thus, we explored the potential interaction between PI-3 kinase and PAK1 by immunoblotting PAK1 immunoprecipitates with an anti–PI-3 kinase(p85) antibody (Figure 5). We found that PI-3 kinase weakly associates with PAK1 under control conditions, but this association increased strongly in opioid-stimulated cells (Figure 5A, top panel). Furthermore, the kinetics of PI-3 kinase activation, which reached a maximum within 15 min of EKC treatment and within 30 min of αs1-casomorphin treatment (Figure 1A), was shown to correlate with the increase in the amount of PI-3 kinase that coimmunoprecipitated with PAK1. Indeed, the interaction between PI-3 kinase and PAK1 was induced to a maximum 6.2-fold within 15 min of EKC treatment and 5.1-fold within 30 min of αs1-casomorphin treatment, compared with control cells (Figure 5A, top panel). Reprobing of the membrane with the anti-PAK1 antibody confirmed that equal amounts of PAK1 protein were immunoprecipitated (Figure 5A, bottom panel). To exclude the possibility that the PI-3 kinase-PAK1 interaction occurred secondary to GTPase association, cells were transfected with an hemagglutinin (HA)-tagged PAK1(H83,86L) mutant that is unable to bind to Cdc42 or Rac1 (Sells et al., 1997). Immunoblotting of anti-HA immunoprecipitates with an anti–PI-3 kinase(p85) antibody revealed that the PI-3 kinase was associated with PAK1(H83,86L) (Figure 5B, top panel), indicating that the small GTPases are not involved in the PAK1-PI-3 kinase interaction. Immunoblotting of anti-HA immunoprecipitates with an anti-HA antibody confirmed the presence of PAK1(H83,86L) in the immunoprecipitates (Figure 5B, top panel). The observed interaction between PI-3 kinase and PAK1, both under control conditions and in opioid-treated cells, was dependent on the PI-3 kinase activity. Figure 5C (top panel) shows that preincubation of cells with the PI-3 kinase inhibitor wortmannin blocked the association of PI-3 kinase with PAK1. Immunoblotting of PAK1 immunoprecipitates with an anti-PAK1 antibody confirmed the presence of PAK1 in the immunoprecipitates (Figure 5C, top panel). These results indicate that PAK1 associates with PI-3 kinase only when the latter is activated and that this association could be an essential step for the stimulation of PAK1 kinase activity. To control this hypothesis, we examined whether αPIX may be involved in this interaction. First, we investigated whether opioids induce an interaction between PAK1 and αPIX. For this, equal amounts of protein from untreated, opioid-treated, and PDGF-treated (positive control) cells were subjected to immunoprecipitation with the anti-PAK1 antibody. Immunoblotting of PAK1-immunoprecipitates with anti-αPIX antibody revealed that PAK1–αPIX interaction was induced by PDGF. Any interaction between these proteins was not detected in opioid-treated cells (Figure 6A, top panel). Reprobing of the membrane with anti-PAK1 antibody confirms the presence of PAK1 in the immunoprecipitates (Figure 6A, middle panel). Furthermore, αPIX was immunodepleted, and PAK1 was immunoprecipitated from the remaining cell lysates. Immunoblot analysis with the anti–PI-3 kinase(p85) antibody revealed that PAK1 associates with the PI-3 kinase in the absence of αPIX (Figure 6A, bottom panel), implying that the PAK1-PI-3 kinase association is not mediated by αPIX.
Figure 5.
Association of endogenous PAK1 with PI-3 kinase. (A) Cells were stimulated with EKC or αs1-casomorphin for the indicated times and lysed, and PAK1 was immunoprecipitated (IP) with rabbit polyclonal anti-PAK1 antibody. Coimmunoprecipitated PI-3 kinase was detected by immunoblot (IB) with rabbit polyclonal anti–PI-3 kinase(p85) antibody (top panel). Stripping and reprobing of the nitrocellulose membrane confirmed that equal amounts of PAK1 protein were immunoprecipitated in each sample (bottom panel). (B) Cells were transfected with the Cdc42/Rac1 binding–deficient PAK1(H83,86L) and then stimulated with EKC or αs1-casomorphin for the indicated times. HA-tagged PAK1(H83,86L) was immunoprecipitated (IP) with mouse monoclonal anti-HA epitope antibody and Western blot of immunoprecipitates was probed (IB) with rabbit polyclonal anti–PI-3 kinase(p85) antibody (top panel). The blot was stripped and reprobed with anti-HA antibody to confirm the presence of PAK1(H83,86L) in the immunoprecipitates (bottom panel). (C) Requirement of PI-3 kinase activity for PI-3 kinase–PAK1 interaction. Cells were pretreated with Wormannin for 30 min and then stimulated with 10−8 M EKC or αs1-casomorphin for the indicated times. Cell lysates were immunoprecipitated (IP) with anti-PAK1 antibody, and precipitates were separated by SDS-PAGE, followed by transfer to nitrocellulose membrane and immunoblot (IB) with anti–PI-3 kinase(p85) antibody (top panel). The blot was stripped and reprobed with anti-PAK1 antibody to confirm the presence of PAK1 in the immunoprecipitates (bottom panel). The number below each lane indicates the -fold amount of PI-3 kinase coimmunoprecipitated with PAK1, with that of untreated cells taken as 1. The experiments were repeated five times with similar results. IgG(H), immunoglobulin G (heavy chain).
Figure 6.
PI-3 kinase preferentially associates with the PAK1-PBD (amino acids 67–150). (A) Cells were incubated for the indicated times with EKC, αs1-casomorphin, or PDGF and lysed, and PAK1 was immunoprecipitated (IP) with the anti-PAK1 antibody. Coimmunoprecipitated αPIX was detected by immunoblotting (IB) with an anti-αPIX antibody (top panel). The blot was stripped and reprobed with anti-PAK1 antibody to confirm the presence of PAK1 in the immunoprecipitates (middle panel). αPIX was immunodepleted, and PAK1 was immunoprecipitated from the remaining cell lysates. The presence of PI-3 kinase in PAK1 immunoprecipitates was assessed by immunoblotting with the anti–PI-3 kinase(p85) antibody (bottom panel). (B) Cells were stimulated with EKC or αs1-casomorphin for the indicated times. Lysates from untreated and opioid-treated cells were incubated with GST-PBD (amino acids 67–150), whereas control lysates were incubated with GST only bound to glutathione-Agarose beads. Precipitates were assayed for PI-3 kinase activity, as described in MATERIALS AND METHODS, using PIP2 as substrate. The reaction products were separated by TLC and visualized by autoradiography. (C) The remaining cell exctracts were reprecipitated (IP) with anti-PAK1 antibody, and immunoprecipitates were analyzed by immunoblotting (IB) with anti–PI-3 kinase(p85) antibody (top panel). The blot was stripped and reprobed with anti-PAK1 antibody to confirm the presence of PAK1 in the immunoprecipitates (bottom panel). The experiments were repeated four times with similar results. PIP3, phosphatidylinositol-3,4,5-trisphosphate; IgG(H), immunoglobulin G (heavy chain).
From the above presented results it was obvious that PAK1-PI-3 kinase interaction is not mediated by the small GTPases or by αPIX. Accordingly, we explored the potential direct association of PI-3 kinase with the N terminal regulatory domain of PAK1. We performed an in vitro PI-3 kinase assay on protein(s) that coprecipitated with the PAK1 fragment (amino acids 67–150) expressed as a GST fusion protein bound to glutathione agarose. Figure 6B shows that PI-3 kinase activity associated with GST-PBD (amino acids 67–150), as was indicated by the synthesis of PIP3 in the phosphoinositide kinase assay. Negligible activity was found in the GST control. Additionally, the pattern of PIP3 synthesis was similar to that observed when the in vitro kinase assay was performed on anti–PI-3 kinase immune complex (Figure 1A, top panel). The same pattern of PIP3 synthesis was obtained by the in vitro kinase assay that was performed on anti-PAK1 immune complex (our unpublished results). To examine further the specificity of the PI-3 kinase-PBD (amino acids 67–150) interaction, PAK1 was immunoprecipitated from the remaining cell lysates, obtained after removal of the GST-PBD, and the nitrocellulose membrane was probed with an anti–PI-3 kinase(p85) antibody. As shown in Figure 6C (top panel), no association of PI-3 kinase with PAK1 was observed, indicating that the PI-3 kinase preferentially associates with the N terminal regulatory domain of PAK1 to the region between amino acids 67 and 150. Stripping and reprobing of the membrane with an anti-PAK1 antibody confirmed the presence of PAK1 in the immunoprecipitates (Figure 6C, bottom panel). The association of PI-3 kinase with the PAK1 fragment (amino acids 67–150) confirms that this association is not mediated by αPIX, because PIX binds to the fourth PH domain of PAK1 (Manser et al., 1998).
PAK1 Phosphorylates Actin
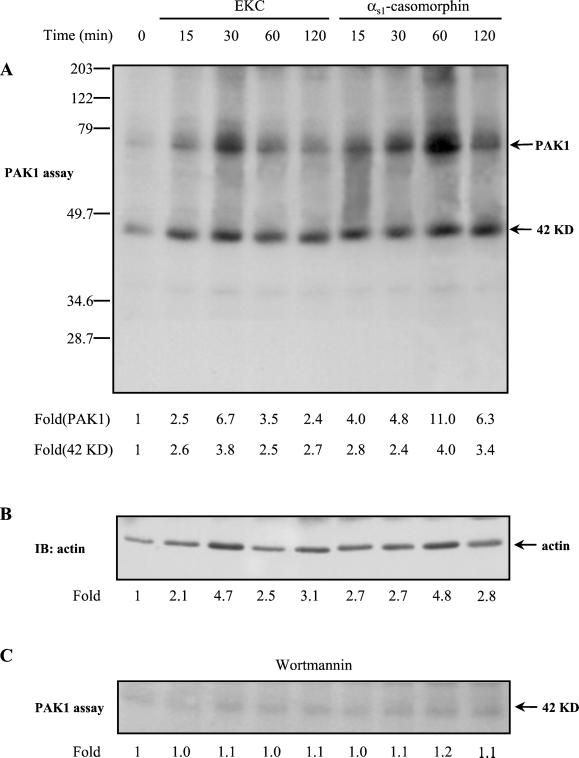
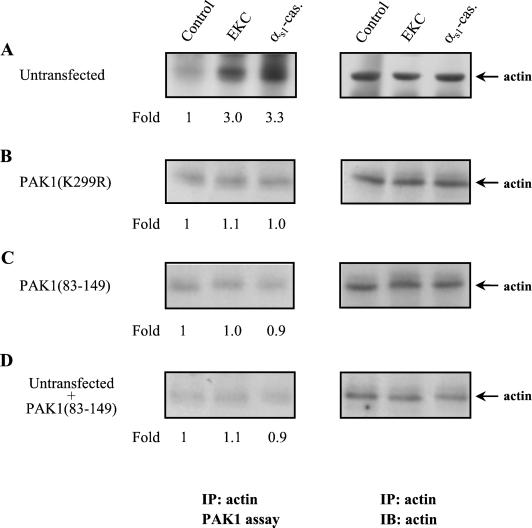
When PAK1 was immunoprecipitated from cell lysates (equal amounts of protein) and subjected to immune complex kinase assay, visualization of phosphorylated proteins by autoradiography revealed that an endogenous phosphorylated protein of 42 kDa coprecipitated with PAK1. Notably, the phosphorylation of this protein, in response to stimulation with opioids, followed similar kinetics to PAK1 autophosphorylation (Figure 7A). The PAK1-associated protein Nck was excluded to be the observed phosphorylated protein because it was recognized by a monoclonal anti-Nck antibody but in a different position on the nitrocellulose membrane (our unpublished results). Moreover, Nck was not phosphorylated under the experimental conditions used. The 42-kDa protein was identified as actin by immunoblotting analysis of the same nitrocellulose membrane in which the phosphorylated proteins, obtained from PAK1 kinase assay, had been transferred (Figure 7B). It is also noteworthy that the increase in phosphorylation of actin is associated with the increase in the amount of actin that associates with PAK1 (Figure 7, compare A and B). Figure 7C shows that preincubation of cells with the PI-3 kinase inhibitor wortmannin resulted in blockage of the phosphorylation of actin and supports the hypothesis that the later could serve as a substrate for phosphorylation by PAK1. To confirm this suggestion, actin was immunoprecipitated from untransfected cells (Figure 8A) and cells that were transfected either with kinase-dead PAK1(K299R) (Figure 8B) or with the autoinhibitory domain of PAK1 (PAK1(83–149)) (Figure 8C) and subjected to PAK1 kinase assay. Actin was also immunoprecipitated from untransfected cells ,and the autoinhibitory domain of PAK1 (PAK1(83–149)) was added to the in vitro kinase assay (Figure 8D). In each of the above conditions cells were incubated for 15 min with either opioid. As shown in Figure 8A (left panel) the increase in the phosphorylation of actin in untransfected cells was almost equal to that observed when actin coprecipitated with PAK1 (Figure 7A). Interestingly, opioid agonists failed to enhance actin phosphorylation in cells expressing the PAK1(K299R) or PAK1(83–149) as well as when PAK(83–149) was added to the in vitro kinase assay. These results strongly suggest that actin served as a substrate for phosphorylation by PAK1 and not coprecipitated with other kinases.
Figure 7.
The phosphorylated protein that was coprecipitated with PAK1 identified as actin. (A) Cells were stimulated with EKC or αs1-casomorphin for the indicated times and lysed, and anti-PAK1 immune complexes were assayed for kinase activity by an in vitro kinase assay as described in MATERIALS AND METHODS. The reaction products were separated by SDS-PAGE and transferred to nitrocellulose membrane, and phosphorylated proteins were visualized by autoradiography. The number below each lane indicates the -fold autophosphorylation of PAK1 (fold (PAK1)) or the -fold phosphorylation of the protein of 42 kDa (fold (42KD)), with that of untreated cells taken as 1. (B) The nitrocellulose membrane described in the previous panel was immunoblotted (IB) with antiactin mAb. The number below each lane indicates the -fold amount of actin coimmunoprecipitated with PAK1, with that of untreated cells taken as 1. (C) The phosphorylation of actin by PAK1 was examined in cells that were pretreated with wortmannin and then stimulated with 10−8 M EKC or αs1-casomorphin. The number below each lane indicates the -fold phosphorylation of actin, with that of untreated cells taken as 1. The experiments were repeated three to four times with similar results.
Figure 8.
In vivo phosphorylation of actin by PAK1. Cells were incubated for 15 min with EKC or αs1-casomorphin and lysed, and actin was immunoprecipitated (IP) with an anti-actin antibody. The anti-actin immune-complexes were subjected to PAK1 kinase assay, resolved on SDS-PAGE and transferred to nitrocellulose membrane, and the phosphorylated actin was visualized by autoradiography (left panels). The membrane was then immunoblotted (IB) with anti-actin mAb (right panels). The phosphorylation of actin was examined in untransfected cells (A), in cells that were transfected with kinase-dead PAK1(K299R) (B), or with the autoinhibitory domain of PAK1 (PAK1(83–149)) (C) as well as in untransfected cells in which the autoinhibitory domain of PAK1 was added directly to the in vitro kinase assay (D). The number below each lane indicates the -fold phosphorylation of actin with that of untreated cells taken as 1. The experiments were repeated three to four times with similar results.
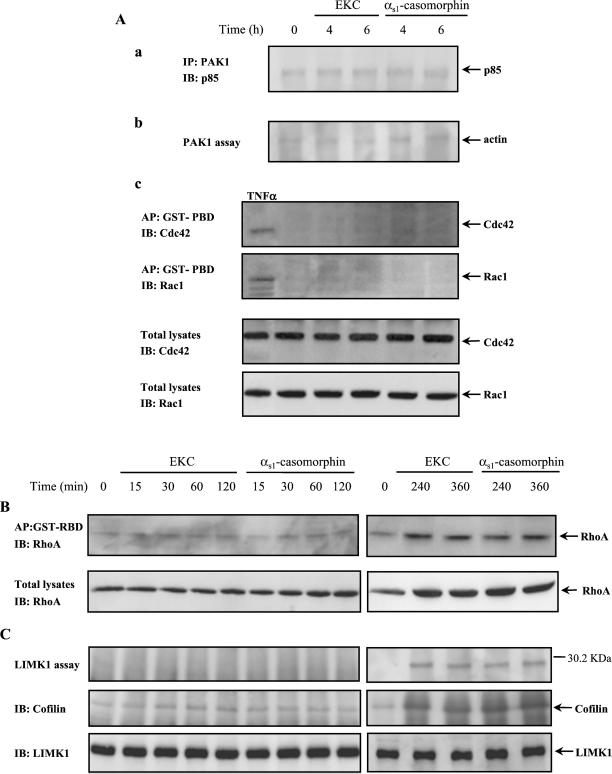
Long-term Exposure of Cells to Opioids Resulted in RhoA and LIMK1 Activation
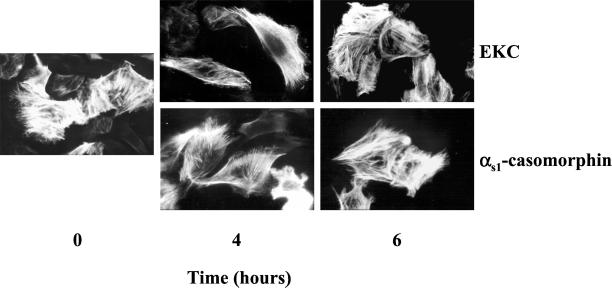
To examine whether the observed microfilament redistribution was a consequence of the PI-3 kinase-PAK1 interaction and actin phosphorylation, we further analyzed the actin repolymerization during the late-phase response of cells to opioids. As shown in Figure 9, neither membrane ruffles nor filopodia was observed 4 h after treatment of cells with opioid agonists. In contrast, the repolymerization of stress fibers was obvious, and it was almost complete 6 h later. These morphological observations were further supported by quantitative immunoblot experiments summarized in Table 1. Interestingly, opioids failed to enhance the association of PI-3 kinase with PAK1 (Figure 10Aa) as well as the phosphorylation of actin by PAK1 (Figure 10Ab) during the same time period. In addition, the long-term exposure of cells to opioids revealed neither Cdc42 or Rac1 activation nor induction of their expression levels (Figure 10Ac). These results suggest that the activation of PAK1 via its association with PI-3 kinase and the consequent actin phosphorylation may be essential for actin depolymerization and microfilament redistribution.
Figure 9.
Repolymerization of actin during the late-phase response of cells to opioids. Cells grown on type IV collagen-coated glass coverslips were incubated for the indicated times with 10−8 M EKC or αs1-casomorphin. The reorganization of filamentous actin was determined with rhodamine-phalloidin staining by immunofluorescence microscopy. Magnification, ×400. Similar results were obtained in three independent experiments.
Figure 10.
Long-term exposure of cells to opioids resulted in RhoA and LIMK1 activation. (A) Discontinuance of PAK1-PI-3 kinase interaction and actin phosphorylation during the late-phase response of cells to opioids. (a) Cells were incubated for the indicated times with EKC or αs1-casomorphin and lysed, and PAK1 was immunoprecipitated (IP) with rabbit polyclonal anti-PAK1 antibody. Coimmunoprecipitated PI-3 kinase was detected by immunoblotting (IB) with rabbit polyclonal anti–PI-3 kinase(p85) antibody. (b) Anti-PAK1 immune complexes were subjected to an in vitro kinase assay, resolved on SDS-PAGE and transferred to nitrocellulose membrane, and the phosphorylated actin was visualized by autoradiography. (c) Lysates from TNF-α (100 ng/ml, 10 min)-treated cells, and lysates from untreated, EKC-, or αs1-casomorphin–treated cells were affinity precipitated (AP) with GTP-PBD bound to glutathione-Agarose beads. Precipitated GTP-Cdc42 or GTP-Rac1 was detected by immunoblotting (IB) with anti-Cdc42 or anti-Rac1 antibody, respectively (top two panels). Equal amount of proteins (100 μg) of TNF-α–treated or untreated and opioid-treated cells were subjected to SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted (IB) with monoclonal anti-Cdc42 or anti-Rac1 antibody, respectively (bottom two panels). (B) Lysates from EKC- or αs1-casomorphin–treated cells were affinity precipitated (AP) with GTP-RBD bound to glutathione-Sepharose beads. Precipitated GTP-RhoA was detected by immunoblotting (IB) with anti-RhoA antibody (top panel). Equal amount of proteins (100 μg) of untreated and opioid-treated cells were subjected to SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted (IB) with monoclonal anti-RhoA antibody (bottom panel). (C) Phosphorylation of endogenous cofilin by LIMK1. Cells were stimulated with EKC or αs1-casomorphin for the indicated times and lysed, and anti-LIMK1 immune complexes were assayed for kinase activity by an in vitro kinase assay as described in MATERIALS AND METHODS. The reaction products were separated by SDS-PAGE and transferred to nitrocellulose membrane, and a phosphorylated protein that coimmunoprecipitated with LIMK1 was visualized by autoradiography (top panel). The membrane was then immunoblotted (IB) with a rabbit polyclonal anticofilin antibody (second panel) or stripped and reprobed with a goat polyclonal anti-LIMK1 antibody (bottom panel). The experiments were repeated three times with similar results. IgG(H), immunoglobulin G (heavy chain).
Because RhoA is a well-characterized Rho GTPase that triggers the formation of stress fibers (Aspenstrom, 1999), we performed affinity precipitation experiments with a GST-fusion protein corresponding to the Rho-binding domain (RBD) of Rhotekin that specifically binds to and precipitates the GTP-RhoA from cell lysates (Ren et al., 1999). The presence of RhoA was assessed with a specific antibody. As shown in Figure 10B (top panel) no increase of RhoA activity was observed in cells exposed to opioids for 15 min to 2 h compared with the control cells. In contrast, RhoA activation was triggered by long-term (4 or 6 h) exposure of cells to opioids. Additionally, RhoA expression was induced during the late-phase response, whereas at early phase response no change of RhoA protein expression levels was observed. (Figure 10B, bottom panel).
To examine whether LIMK1 is activated, we performed an in vitro kinase assay on anti-LIMK1 immune complexes, isolated from cells exposed to opioids for the indicated times, and then the phosphoproteins were visualized by autoradiography. We observed that, only in cells exposed to opioids for 4 or 6 h, an endogenous phosphorylated protein of molecular mass close to that corresponding to cofilin coprecipitated with LIMK1 (Figure 10C, top panel). Indeed, this protein was identified as cofilin by immunoblotting analysis of the same nitrocellulose membrane in which the phosphorylated proteins, obtained from LIMK1 assay, had been transferred (Figure 10C, second panel). The presence of LIMK1 in the immunoprecipitates was confirmed by stripping and reprobing of the nitrocellulose membrane with an anti-LIMK1 antibody (Figure 10C, bottom panel). It is noteworthy that we were unable to document an in vivo interaction between LIMK1 and PAK1 by coimmunoprecipitation experiments (our unpublished results). These results confirm that the cytoskeletal changes observed during the early phase response of cells to opioids depend on actin phosphorylation by PAK1 because the discontinuance of actin phosphorylation occurred concomitantly with the beginning of its repolymerization through a distinct signaling cascade involving RhoA and LIMK1 activation.
DISCUSSION
Previous work has shown that exposure of cells to opioids, EKC, and αs1-casomorphin results in important modifications of the actin cytoskeleton (Papakonstanti et al., 1998). However, it is not known how opioids transmit their signal leading to alterations in actin cytoskeleton dynamics and cell morphology. The results of the present study provide an outline of novel signal transduction events, causing the depolymerization of stress fibers and peripheral actin reorganization.
We found that treatment of cells, for 15 min to 2 h, with two opioid agonists resulted in PI-3 kinase activation, but it was not followed by Cdc42/Rac or Akt activation. Instead, the activation of opioid receptors appeared to trigger a strong kinase activity to exogenous substrates and autophosphorylation of PAK1. This was unexpected because, at least in the case of G-protein–coupled receptors activation, PAKs have been shown to be activated by the GTP-bound forms of Cdc42 or Rac (Daniels and Bokoch, 1999). The Cdc42/Rac1-independent activation of PAK1 was confirmed by the fact that, instead of being attenuated or abolished, PAK1 kinase activity was increased by opioids in cells expressing the dominant-negative Cdc42(T17N) or Rac1(T17N) to the same extend as in untransfected cells. Additionally, our results obtained by immunofluorescence experiments using the kinase-dead PAK1(K299R) or the dominant-negative Cdc42(T17N) support the notion that PAK1 solely was responsible for actin cytoskeleton redistribution induced by opioids. Further support was provided by the inhibition of PI-3 kinase activity with the specific inhibitor wortmannin, which blocked both the activation of PAK1 kinase and the actin cytoskeleton alterations, including depolymerization of stress fibers and cortical actin reorganization. It is well documented that PI-3 kinase can stimulate the small GTPases, which have been implicated in actin redistribution and PAK1 activation (Hall, 1998) as well as the serine-threonine kinase Akt, which also promotes the activation of PAK1 (Tang et al., 2000). Because we found that neither Cdc42/Rac1 nor Akt were activated under the experimental conditions used, our data indicate that, actin alterations should be dependent on PAK1 activation directly via PI-3 kinase. This suggests a close correlation between PI-3 kinase and PAK1 activity and provides evidence for an alternative pathway for PAK1 activation that bypasses Cdc42/Rac1 and Akt. Indeed, we showed a PI-3 kinase-PAK1 interaction that is localized in the PAK1 N terminus to the region between amino acids 67 and 150, which contains the p21-binding domain (amino acids 67–86) and the autoinhibitory regulatory domain (amino acids 83–149). The association of PI-3 kinase with the PAK1(H83,86L) mutant, which is defective in Cdc42 and Rac1 binding (Sells et al., 1997), excludes the possibility that this association occurs indirectly by the concomitant association of undetectable amounts of the small GTPases. The increase in PI-3 kinase activity was found to associate with an increase in the amount of PI-3 kinase that coprecipitates with PAK1. Moreover, the observed interaction between PI-3 kinase and PAK1 was dependent on the PI-3 kinase activity, because pretreatment of cells with the PI-3 kinase inhibitor wortmannin disrupted the interaction between PAK1 and PI-3 kinase as well as abolished the activation of PAK1. Thus, the results described above could mean one of the following: first, PAK1 kinase activity is directly dependent on the PAK1-PI-3 kinase interaction, although the mechanism by which this could occur is not yet understood. Second, PI-3 kinase through its association with PAK1 regulatory domain (amino acids 67–150) regulates PAK1 activity and the consequent effects of the latter on microfilament distribution. Experimental evidence consistent with this hypothesis is provided by our results obtained during the long-term exposure of cells to opioids. The association of PI-3 kinase with PAK1 as well as PAK1 activity was abolished after 4- and 6-h incubation of cells with opioids remaining at basal levels. Additionally, at this time frame the actin microfilaments and cell morphology had been almost restored to control levels, indicating that the interaction of PI-3 kinase with PAK1 (amino acids 67–150) could account for the regulation of PAK1 activity and its effects on actin. This means that the association of PI-3 kinase with PAK1 may be the signal for PAK1 activation, and consequently, the dissociation of PI-3 kinase from PAK1 may be the signal for inactivation of PAK1. A third hypothesis is that the association of PI-3 kinase with PAK1 could facilitate the interaction of PI-3 kinase or PAK1 with other molecules that subsequently promote PAK1 activity. Recently, it was demonstrated that PAK-interacting exchange factor (PIX), which binds to the fourth proline-rich domain in the N-terminus of PAK (Manser et al., 1998), can regulate PAK activity both, by catalyzing GTP exchange on Cdc42/Rac and by direct binding to PAK independently of GTPase activity (Daniels et al., 1999). alphaPIX (αPIX) has been also shown to be activated upon binding of the lipid product (PI-3,4,5-P3) of PI-3 kinase to the PH domain of αPIX, leading to Cdc42/Rac1 activation, which then activate PAK (Yoshii et al., 1999). In the present study we found that PAK1 does not associate with αPIX, under the experimental conditions used, indicating that αPIX is not involved in PAK1 activation. However, the association of PI-3 kinase with PAK1 could facilitate the interaction of PAK1 with other signaling molecules or with lipid species in the cell membrane that has been shown to increase PAK1 activity (Daniels and Bokoch, 1999).
Our findings also provide evidence that actin is a novel downstream target of PAK1. Treatment of cells, for 15 min to 2 h, with either opioid agonists resulted in phosphorylation of actin that was coprecipitated with PAK1. Actin phosphorylation was not observed when actin was immunoprecipitated from cells that had been transfected with the kinase-dead PAK1(K299R) or with the autoinhibitory domain of PAK1 (PAK1(83–149)), providing strong evidence that other kinases than PAK1 in actin phosphorylation could not be expected. Moreover, the phosphorylation of actin was completely blocked when actin was immunoprecipitated from untransfected cells and the autoinhibitory domain of PAK1 (PAK1(83–149)) was added to the kinase assay. Finally, the phosphorylation of actin by kinases that were activated downstream of PAK1 cannot be supported by our experimental findings because such kinases were not detected in autoradiography of PAK1 immunoprecipitates. The observation that expression of kinase-dead PAK1(K299R) resulted in the inhibition of both actin phosphorylation and microfilament redistribution support a physiological role of PAK1-induced actin phosphorylation to actin fiber disassembly. Moreover, exposure of cells for 4–6 h to opioids resulted in the reformation of stress fibers and restoration of actin microfilaments to control levels while concomitantly, at these time intervals PAK1 activation as well as actin phosphorylation were discontinued. These findings suggest that the phosphorylation of actin by PAK1 is a physiologically relevant event and support the notion that PAK1 could directly induce the disassembly of stress fibers and reorganization of peripheral actin by phosphorylation of actin itself. Although the serine residue(s) that is phosphorylated in actin molecule remains to be localized in future studies, the above findings suggest that the association of PAK1 with actin is a dynamic process. Because actin phosphorylation is involved in the actin filament dynamics and actin polymerizability and reorganization (Ohta et al., 1987; Carlier, 1993; Jungbluth et al., 1995), our findings concerning actin phosphorylation by PAK1 may be of functional significance. Indeed, phosphorylated actin could function as a specific binding site for other proteins that are present in membrane ruffles and could also become phosphorylated on both serine/threonine and tyrosine residues. On the other hand, there is evidence that PAK1 can regulate the actin-myosin cytoskeleton by phosphorylating specific substrates. Thus, it can modulate cell contractility through phosphorylation and inhibition of myosin-light-chain kinase (Sanders et al., 1999). Our findings could also explain the effect of EGF on actin cytoskeleton. EGF-receptor tyrosine kinase activation has been shown to induce actin phosphorylation on serine residues, suggesting that actin is not a direct substrate of the EGF-receptor but more likely of a downstream kinase (Van Delft et al., 1995), because it is well known that EGF stimulation leads to activation of PAK1 kinase and redistribution of actin (Tanaka et al., 1995).
Exposure of cells for 4–6 h to opioid agonists resulted in RhoA activation and LIM-kinase 1 (LIMK1) stimulation, because it was documented by phosphorylation of cofilin. Instead, we were unable to document a direct interaction of LIMK1 with PAK1, which indicates that LIMK1 phosphorylates cofilin under the control of Rho. It is well known that LIMKs specifically phosphorylate cofilin, an actin-binding protein that promotes the disassembly of actin filaments, but there are contradictory data with respect to upstream signaling events. According to a proposed mechanism the activity of LIMK1 is regulated by Rac, whereas that of LIMK2 is regulated by Rho and Cdc42 (Arber et al., 1998; Yang et al., 1998; Sumi et al., 1999). According to another mechanism PAK1 binds to and phosphorylates LIMK1, and this association is enhanced by activated Rac or Cdc42 (Edwards et al., 1999). Our data seem to agree with a third proposed mechanism according to which LIMK1 and LIMK2 are phosphorylated and activated by the Rho-dependent protein kinase (Maekawa et al., 1999). However, the reformation of actin stress fibers and microfilament reorganization could be the combined result of the inhibition of actin phosphorylation by PAK1; the activation of RhoA, which mediates the formation of cytoskeletal stress fibers; and the cofilin phosphorylation, which thus becomes inactivated and inhibits its actin-depolymerizing and severing activity.
To conclude, our results define a signal transduction pathway for transmission of extracellular signals to the actin cytoskeleton through PAK1 activation by a Cdc42/Rac1-independent mechanism: PAK1 associates with the activated PI-3 kinase and subsequently autophosphorylates and increases its kinase activity to exogenous substrates. PAK1 might be activated, directly or indirectly, via its binding to PI-3 kinase, and in turn it phosphorylates actin, causing dissolution of stress fibers and redistribution of microfilaments. In a second phase, which represents an essential control point, the association of PAK1 with PI-3 kinase as well as the phosphorylation of actin are discontinued while concomitantly, RhoA is activated and LIMK1 catalyzes the phosphorylation and consequent inactivation of cofilin, resulting in reformation of stress fibers and decreased depolymerization of F-actin.
ACKNOWLEDGMENTS
We are grateful to Dr. A. Moustakas and Dr. G. M. Bokoch for providing the expression plasmids pGEX-2T-TRBD, pCMV-Cdc42(T17N), pCMV6 M-PAK1(83–149), pJ3H-PAK1(K299R), and pJ3H-PAK1(H83,86L). We also thank Dr. E. Castanas for EKC and αs1-casomorphin. We are indebted to Dr. A. Moustakas for a critical reading of the manuscript and his helpful comments. This work was partially supported by grants from Hellenic Pasteur Institute and Biochemical Research Council (KESY). E.A.P. was supported by IKY fellowship grant (AS 304).
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.02–01–0599. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.02–01–0599.
REFERENCES
- Adam L, Vadlamudi R, Kondapaka SB, Chernoff J, Mendelsohn J, Kumar R. Heregulin regulates cytoskeletal reorganization and cell migration through the p21-activated kinase-1 via phosphatidylinositol-3 kinase. J Biol Chem. 1998;273:28238–28246. doi: 10.1074/jbc.273.43.28238. [DOI] [PubMed] [Google Scholar]
- Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–809. doi: 10.1038/31729. [DOI] [PubMed] [Google Scholar]
- Aspenstrom P. Effectors for the Rho GTPases. Curr Opin Cell Biol. 1999;11:95–102. doi: 10.1016/s0955-0674(99)80011-8. [DOI] [PubMed] [Google Scholar]
- Auger KR, Serunian LA, Soltoff SP, Libby P, Cantley C. PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell. 1989;57:167–175. doi: 10.1016/0092-8674(89)90182-7. [DOI] [PubMed] [Google Scholar]
- Bagrodia S, Taylor SJ, Creasy CL, Chernoff J, Cerione RA. Indentification of a mouse p21Cdc42/Rac-activated kinase. J Biol Chem. 1995;270:22731–22737. doi: 10.1074/jbc.270.39.22731. [DOI] [PubMed] [Google Scholar]
- Benard V, Bohl BP, Bokoch GM. Characterization of Rac and Cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J Biol Chem. 1999;274:13198–13204. doi: 10.1074/jbc.274.19.13198. [DOI] [PubMed] [Google Scholar]
- Bokoch GM, Wang Y, Bohl BP, Sells MA, Quilliam LA, Knaus UG. Interaction of the Nck adapter protein with p21-activated kinase (PAK1) J Biol Chem. 1996;271:25746–25749. doi: 10.1074/jbc.271.42.25746. [DOI] [PubMed] [Google Scholar]
- Bokoch GM, Reilly AM, Daniels RH, King CC, Olivera A, Spiegel S, Knaus UG. A GTPase-independent mechanism of Pak activation: regulation by sphingosine and other biologically active lipids. J Biol Chem. 1998;273:8137–8144. doi: 10.1074/jbc.273.14.8137. [DOI] [PubMed] [Google Scholar]
- Carlier M-F. Dynamic actin. Curr Biol. 1993;3:321–323. doi: 10.1016/0960-9822(93)90192-q. [DOI] [PubMed] [Google Scholar]
- Daniels RH, Bokoch GM. p21-activated protein kinase: a crucial component of morphological signaling? Trends Biochem Sci. 1999;24:350–355. doi: 10.1016/s0968-0004(99)01442-5. [DOI] [PubMed] [Google Scholar]
- Daniels RH, Zenke FT, Bokoch GM. αPIX stimulates p21-activated kinase activity through exchange factor-dependent and -independent mechanisms. J Biol Chem. 1999;274:6047–6050. doi: 10.1074/jbc.274.10.6047. [DOI] [PubMed] [Google Scholar]
- Daniels RH, Hall PS, Bokoch GM. Membrane targeting of p21-activated kinase 1 (PAK1) induces neurite outgrowth from PC12 cells. EMBO J. 1998;17:754–764. doi: 10.1093/emboj/17.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmawardhane S, Sanders LC, Martin SS, Daniels RH, Bokoch GM. Localization of p21-activated kinase 1 (PAK1) to pinocytic vesicles and cortical actin structures in stimulated cells. J Cell Biol. 1997;138:1265–1278. doi: 10.1083/jcb.138.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DC, Sanders LC, Bokoch GM, Gill GN. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signaling to actin cytoskeletal dynamics. Nat Cell Biol. 1999;1:253–259. doi: 10.1038/12963. [DOI] [PubMed] [Google Scholar]
- Golenhofen N, Doctor RB, Bacallao R, Mandel LJ. Actin and villin compartmentation during ATP depletion and recovery in renal cultured cells. Kidney Int. 1995;48:1837–1845. doi: 10.1038/ki.1995.482. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hatzoglou A, Bakogeorgou E, Papakonstanti EA, Stournaras C, Emmanouel DS, Castanas E. Identification and characterization of opioid and somatostatin binding sites in the opossum kidney (OK) cell line and their effect on growth. J Cell Biochem. 1996;63:410–421. doi: 10.1002/(SICI)1097-4644(19961215)63:4%3C410::AID-JCB3%3E3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Hazeki O, Okada T, Kurosu S, Takasuga T, Suzuki T, Katada T. Activation of PI-3 kinase by G protein βγ subunits. Life Sci. 1998;62:1555–1559. doi: 10.1016/s0024-3205(98)00106-4. [DOI] [PubMed] [Google Scholar]
- Isenberg G. New concepts for signal perception and transduction by the actin skeleton at cell boundaries. Cell Dev Biol. 1996;7:707–715. [Google Scholar]
- Jungbluth A, Eckerskorn C, Gerisch G, Lottspeich F, Stocker S, Schweiger A. Stress-induced tyrosine phosphorylation of actin in Dictostelium cells and localization of the phosphorylation site to tyrosine-53 adjacent to the DNase I binding loop. FEBS Lett. 1995;375:87–90. doi: 10.1016/0014-5793(95)01165-b. [DOI] [PubMed] [Google Scholar]
- King CC, Gardiner EMM, Zenke FT, Bohl BP, Newton AC, Hemming BA, Bokoch GM. p21-activated kinase (PAK1) is phosphorylated, and activated by 3-phosphoinisitide-dependent kinase-1 (PDK1) J Biol Chem. 2000;275:41201–41209. doi: 10.1074/jbc.M006553200. [DOI] [PubMed] [Google Scholar]
- Kjøller L, Hall A. Signaling to Rho GTPases. Exp Cell Res. 1999;253:166–179. doi: 10.1006/excr.1999.4674. [DOI] [PubMed] [Google Scholar]
- Knaus UG, Morris S, Dong H, Chernoff J, Bokoch GM. Regulation of human leukocyte p21-activated kinases through G protein-coupled receptors. Science (Wash DC) 1995;269:221–223. doi: 10.1126/science.7618083. [DOI] [PubMed] [Google Scholar]
- Koukouritaki SB, Theodoropoulos PA, Margioris AN, Gravanis A, Stournaras C. Dexamethasone alters rapidly actin polymerization dynamics in human endometrial cells: evidence for nongenomic actions involving cAMP turnover. J Cell Biochem. 1996;62:251–261. doi: 10.1002/(SICI)1097-4644(199608)62:2%3C251::AID-JCB13%3E3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Koukouritaki SB, Vardaki EA, Papakonstanti EA, Lianos E, Stournaras C, Emmanouel DS. TNF-α induces actin cytoskeleton reorganization in glomerular epithelial cells involving tyrosine phosphorylation of paxillin and focal adhesion kinase. Mol Med. 1999;5:382–392. [PMC free article] [PubMed] [Google Scholar]
- Law P-Y, Loh HH. Regulation of opioid receptor activities. J Pharmacol Exp Ther. 1999;289:607–624. [PubMed] [Google Scholar]
- Law P, Wong Y, Loh HH. Molecular mechanisms, and regulation of opioid receptor signaling. Annu Rev Pharmacol Toxicol. 2000;40:389–430. doi: 10.1146/annurev.pharmtox.40.1.389. [DOI] [PubMed] [Google Scholar]
- Lim L, Manser E, Leung T, Hall C. Regulation of phosphorylation pathways by p21 GTPases. The p21 Ras-related Rho subfamily and its role in phosphorylation signaling pathways. Eur J Biochem. 1996b;242:171–185. doi: 10.1111/j.1432-1033.1996.0171r.x. [DOI] [PubMed] [Google Scholar]
- Lim L, Hall C, Monfries C. Regulation of actin cytoskeleton by Rho-family GTPases and their associated proteins. Cell Dev Biol. 1996a;7:699–706. [Google Scholar]
- Lu W, Katz S, Gupta R, Mayer BJ. Activation of Pak by membrane localization mediated by an SH3 domain from the adaptor protein Nck. Curr Biol. 1997;7:85–94. doi: 10.1016/s0960-9822(06)00052-2. [DOI] [PubMed] [Google Scholar]
- Machesky LM, Hall A. Rho: a connection between membrane receptor signaling and the cytoskeleton. Trends Cell Biol. 1996;6:304–310. doi: 10.1016/0962-8924(96)10026-x. [DOI] [PubMed] [Google Scholar]
- Maekawa M, et al. Signaling from Rho to the actin cytoskeleton through protein kinase ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- Maier U, Babich A, Nürnberg B. Roles of non-catalytic subunits in Gβγ-induced activation of class I phosphoinositide 3-kinase isoforms β and γ. J Biol Chem. 1999;274:29311–29317. doi: 10.1074/jbc.274.41.29311. [DOI] [PubMed] [Google Scholar]
- Manser E, Chong C, Zhoa Z, Leung T, Michael G, Hall C, Lim L. Molecular cloning of a new member of the p21Cdc42/Rac-activated kinase (PAK) family. J Biol Chem. 1995;270:25070–25078. doi: 10.1074/jbc.270.42.25070. [DOI] [PubMed] [Google Scholar]
- Manser E, Huang H-Y, Loo T-H, Chen X-Q, Dong J-M, Leung T, Lim L. Expression of a constitutively active α-PAK reveals effects of the kinase on actin and focal complexes. Mol Cell Biol. 1997;17:1129–1143. doi: 10.1128/mcb.17.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser E, Leung T, Salihuddin H, Zhoa Z, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- Manser E, Loo TH, Koh CG, Zhao ZS, Chen XQ, Tan L, Tan I, Leung T, Lim L. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol Cell. 1998;1:183–192. doi: 10.1016/s1097-2765(00)80019-2. [DOI] [PubMed] [Google Scholar]
- Mosaddeghi M, Kapusta DR, Minor LD, Duan N, Paul D. Effects of k-opioid receptor agonists on stimulated phosphoinositide hydrolysis in rat kidney. Eur J Pharmacol. 1995;289:411–417. doi: 10.1016/0922-4106(95)90149-3. [DOI] [PubMed] [Google Scholar]
- Murga C, Fukuhara S, Gutkind JS. A novel role for phosphatidylinositol 3-kinase β in signaling from G protein-coupled receptors to Akt. J Biol Chem. 2000;275:12069–12073. doi: 10.1074/jbc.275.16.12069. [DOI] [PubMed] [Google Scholar]
- Narumiya S. The small GTPase Rho: cellular functions and signal transduction. J Biochem. 1996;120:215–228. doi: 10.1093/oxfordjournals.jbchem.a021401. [DOI] [PubMed] [Google Scholar]
- Obermeier A, Ahmed S, Manser E, Yen SC, Hall C, Lim L. PAK promotes morphological changes by acting upstream of Rac. EMBO J. 1998;17:4328–4339. doi: 10.1093/emboj/17.15.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta Y, Akiyama T, Nishida E, Sakai H. Protein kinase C and cAMP-dependent protein kinase induce opposite effects on actin polymerizability. FEBS Lett. 1987;222:305–310. doi: 10.1016/0014-5793(87)80391-5. [DOI] [PubMed] [Google Scholar]
- Okano I, Hiraoka J, Otera H, Nunouel K, Ohashi K, Iwashita S, Hirai M, Mizuno K. Identification and characterization of a novel family of serine/threonine kinases containing two N-terminal LIM motifs. J Biol Chem. 1995;270:31321–31330. doi: 10.1074/jbc.270.52.31321. [DOI] [PubMed] [Google Scholar]
- Olson GA, Olson RD, Vaccarino AL, Kastin AJ. Endogenous opiates: 1997. Peptides. 1998;19:1791–1843. doi: 10.1016/s0196-9781(98)00137-5. [DOI] [PubMed] [Google Scholar]
- Papakonstanti EA, Bakogeorgou E, Castanas E, Emmanouel DS, Hartig R, Stournaras C. Early alterations of actin cytoskeleton in OK cells by opioids. J Cell Biochem. 1998;70:60–69. doi: 10.1002/(sici)1097-4644(19980701)70:1<60::aid-jcb7>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Papakonstanti EA, Emmanouel DS, Gravanis A, Stournaras C. Na+/Pi co-transport alters rapidly cytoskeletal protein polymerization dynamics in opossum kidney cells. Biochem J. 1996;315:241–247. doi: 10.1042/bj3150241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papakonstanti EA, Emmanouel DS, Gravanis A, Stournaras C. PLC-γ1 signaling pathway, and villin activation are involved in actin cytoskeleton reorganization induced by Na+/Pi cotransport up-regulation. Mol Med. 2000;6:303–318. [PMC free article] [PubMed] [Google Scholar]
- Reisine T, Bell GI. Molecular biology of opioid receptors. Trends Neurosci. 1993;16:506–510. doi: 10.1016/0166-2236(93)90194-q. [DOI] [PubMed] [Google Scholar]
- Ren X-D, Kiosses WB, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders LC, Matsumura F, Bokoch GM, de Lanerolle P. Inhibition of myosin light chain kinase by p21-activated kinase. Science. 1999;283:2083–2085. doi: 10.1126/science.283.5410.2083. [DOI] [PubMed] [Google Scholar]
- Sells MA, Knaus UG, Bagrodia S, Ambrose DM, Bokoch GM, Chernoff J. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr Biol. 1997;7:202–210. doi: 10.1016/s0960-9822(97)70091-5. [DOI] [PubMed] [Google Scholar]
- Singh SS, Chauhan AC, Murakami N, Chauhan VPS. Profilin and gelsolin stimulate phosphatidylinositol 3-kinase activity. Biochemistry. 1996;35:16544–16549. doi: 10.1021/bi9609634. [DOI] [PubMed] [Google Scholar]
- Sumi T, Matsumoto K, Yoshimi T, Nakamura T. Cofilin phosphorylation and actin cytoskeletal dynamics regulated by Rho- and Cdc42-activated LIM-kinase 2. J Cell Biol. 1999;147:1519–1532. doi: 10.1083/jcb.147.7.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Gupta R, Mayer BJ. Differential inhibition of signaling pathways by dominant-negative SH2/SH3 adaptor proteins. Mol Cell Biol. 1995;15:6829–6837. doi: 10.1128/mcb.15.12.6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Zhou H, Chen A, Pittman RN, Field J. The Akt Proto-oncogene links Ras to Pak, and cell survival signals. J Biol Chem. 2000;275:9106–9109. doi: 10.1074/jbc.275.13.9106. [DOI] [PubMed] [Google Scholar]
- Tapon N, Hall A. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr Opin Cell Biol. 1997;9:86–92. doi: 10.1016/s0955-0674(97)80156-1. [DOI] [PubMed] [Google Scholar]
- Van Delft S, Verkleij AJ, Boonstra J, Van Bergen en Henegouwen PMP. Epidermal growth factor induces serine phosphorylation of actin. FEBS Lett. 1995;357:251–254. doi: 10.1016/0014-5793(94)01359-9. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Leevers SJ, Panayotou G, Waterfield MD. Phosphoinositide 3 kinases: a conserved family of signal transducers. Trends Biochem Sci. 1997;22:267–72. doi: 10.1016/s0968-0004(97)01061-x. [DOI] [PubMed] [Google Scholar]
- Wymann MP, Pirola L. Structure and function of phosphoinositide 3-kinases. Biochim Biophys Acta. 1998;1436:127–150. doi: 10.1016/s0005-2760(98)00139-8. [DOI] [PubMed] [Google Scholar]
- Yang N, Higuchi O, Ohashi K, Nagata A, Wada A, Kangawa K, Nishida E, Mizuno K. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393:809–812. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- Yoshii S, et al. αPIX nucleotide exchange factor is activated by interaction with phosphatidylinositol 3-kinase. Oncogene. 1999;18:5680–5690. doi: 10.1038/sj.onc.1202936. [DOI] [PubMed] [Google Scholar]
- Zhang S, Han J, Sells MA, Chernoff J, Knaus UG, Ulevitch RJ, Bokoch GM. Rho family GTPases regulate p38 MAP kinase through the downstream mediator Pak1. J Biol Chem. 1995;270:23934–23936. doi: 10.1074/jbc.270.41.23934. [DOI] [PubMed] [Google Scholar]