Abstract
Most heavy metal ions are carcinogenic and non-biodegradable, posing threats to ecological balance and human health in trace amount. Therefore, there is a pressing demand for rapid and dependable detection technologies. Electrochemical sensing technology distinguishes itself with its ease of use and swiftness, rendering it perfect for the expeditious detection of heavy metal elements. This review examines various electrochemical detection techniques for on-site real-time monitoring of heavy metal ions. Advanced methods using innovative electrochemical sensor technologies are explored, highlighting the importance of sensing strategies for the quick and easy monitoring of metal levels in different environments. Additionally, the role of nanotechnology and electrochemical techniques in enhancing the sensitivity and selectivity of sensors for better detection of heavy metals is discussed. Finally, the future direction of sensor development is addressed, focusing on integrating new materials and technologies to improve the performance of sensor in environmental monitoring, food safety and public health.
Keywords: Electrochemical sensing detection, Heavy metal ions, Sensing strategy, Nanomaterial
Graphical abstract

Highlights
-
•
Innovative electrochemical sensing strategies are comprehensively summarized.
-
•
The combination of new strategies, techniques and nanomaterials improves electrochemical sensor performance.
-
•
The challenges and perspectives are also discussed in depth.
1. Introduction
Heavy metal pollution is a serious environmental problem characterized by the presence of metals and metalloid elements with a specific gravity exceeding 4.0, posing a threat to human health (Bansod, Kumar, Thakur, Rana, & Singh, 2017). These pollutants are non-biodegradable and can accumulate in the environment, entering the food chain and affecting various physiological processes. Elements such as lead, cadmium, mercury, chromium and arsenic can cause severe health problems even in trace amounts (Ferrari, Carrington, Rowley-Neale, & Banks, 2020). For instance, cadmium and chromium can bind to the sulfhydryl groups of biomolecules like amino acids and enzymes, leading to their inactivation, while lead can interfere with heme synthesis. Although trace amounts of metal ions, such as Cu, Fe and Zn are necessary for maintaining normal biological processes, excessive consumption of these elements can be harmful (Kempahanumakkagari, Deep, Kim, Kumar Kailasa, & Yoon, 2017; Zhang, Wang, Xie, Gao, & Yu, 2024). Thus, it is crucial to develop a technology capable of achieving rapid, highly sensitive, highly selective and trace detection of heavy metal ions, which has become a focal point of current research (Buledi, Amin, Haider, Bhanger, & Solangi, 2021).
Although highly sensitive spectroscopic techniques such as atomic absorption spectrometry (AAS), inductively coupled plasma mass spectrometry (ICP-MS), atomic emission spectroscopy (AES) and X-ray fluorescence spectroscopy (XRF) can detect trace amounts of heavy metals in complex matrices, they are often limited by high equipment costs, complex operating procedures and lack of portability, making them unsuitable for rapid on-site detection (Waheed, Mansha, & Ullah, 2018). In contrast, electrochemical sensors offer several advantages, including low development cost, simple operation, portability and short detection and analysis times, enabling real-time detection and prompt action when heavy metal ions concentrations exceed standard limits (Liu, Yao, Ying, & Ping, 2019). Despite these benefits, the relatively low sensitivity and high detection limits of electrochemical techniques compared to spectroscopic techniques necessitate improvements in their design to enhance performance in detecting heavy metal ions (Li et al., 2024). Research indicates that integrating diverse electrochemical techniques with distinct sensing strategies and implementing modified nanomaterials on the electrode surface can enhance sensor sensitivity and minimize detection limits (Meng et al., 2023).
Several reviews have explored the fundamental principles of electrochemical sensors, including their classification and the latest progress in nanomaterial modification (Gudić & Krišto, 2023; Kempahanumakkagari et al., 2017; Power, Gorey, Chandra, & Chapman, 2018; Yang, Denno, Pyakurel, & Venton, 2015). However, few studies have focused on electrochemical strategies. Therefore, this paper provides a brief overview of electrochemical techniques and nanomaterials, emphasizing the innovative mechanisms and strategies of nanomaterial-based electrochemical sensors for detecting heavy metal ions (Gumpu, Sethuraman, Krishnan, & Rayappan, 2015). Integrating these new strategies, electrochemical techniques and nanomaterials not only addressed the challenges faced by traditional electrochemical analysis, but also supported the development of novel sensors and innovative electrochemical detection technologies, which points out the future directions of electrochemical sensing technologies.
2. Electrochemical techniques for heavy mental detection
Electrochemical techniques based on heavy metal ions offer versatile and sensitive method for detecting and quantifying heavy metal ions in various environments. These methods leverage the unique electrochemical properties of metal ions to achieve high sensitivity, specificity and rapid analysis. Electrochemical sensors are categorized based on their electrochemical detection principles and include voltammetric, potentiometric, conductive and impedance sensors. To achieve sensitive detection, these sensors measure the concentration of heavy metal ions by calculating the corresponding electrochemical signals (potential, current and conductivity). According to the current research progress, voltammetric electrochemical sensors have been the most widely studied at present (Beitollahi et al., 2020).
2.1. Voltammetry
The electrode potential of the voltammetric sensor alters based on a specific program (e.g., linear or cyclic scan), and the current flowing through the working electrode (WE) is measured. As there is a linear relationship between the current signal and analyte concentration within a certain range, the obtained current signal can be transformed into the analyte concentration according to the linear equation, thereby achieving highly sensitive detection of heavy metal ions (Ding, Liu, Weerasooriya, & Chen, 2024). Depending on the change in applied potential over time, there are several types of voltammograms (Gulaboski, 2022).
2.1.1. Linear sweep voltammetry
Linear sweep voltammetry (LSV) involves linearly scanning the potential from its initial value to its final value over an extended period of time (Tukur & Azah Yusof, 2014). During the potential scanning process, a redox reaction occurs on the surface of the electrode, resulting in the generation of a current (Singh, Maurya, & Malviya, 2024). The change of current is recorded, thereby permitting the acquisition of a voltammetric curve (i.e., a current-voltage curve) (Zhao et al., 2023).Tanvi et al. electrodeposited MnO onto ITO electrodes and constructed an electrochemical sensor for As(III) detection using LSV. The limit of detection was 1 ppb (Gupte et al., 2019). Similarly, Tu et al. modified gold nanoparticles (AuNPs) on screen-printed carbon electrodes and constructed an AuNP-SPCE sensor to achieve highly sensitive detection of Cr(VI) using LSV. The linear range of the sensor was 20–200 μg/L, the limit of detection was 5.4 μg/L (Tu, Gan, Liang, Wan, & Wang, 2018).
2.1.2. Cyclic voltammetry
Cyclic voltammetry (CV) provides valuable information regarding the electron transfer rate, electrode interface reaction kinetics and redox properties of the analyte (Puthongkham & Venton, 2020). Although CV is valuable for the optimization of analytical conditions and preliminary identification of analytes, it is not sensitive for trace analysis of heavy metal ions (Hu et al., 2018). However, there have been studies on electrochemical determination using CV (Rajendrachari, Somashekharappa, Mahale, Vasanth, & Chikkegouda, 2022). Liu et al. developed an electrochemical aptasensor based on Ti-Co3O4 nanoparticles for the determination of Cd(II) using CV. The Ti-Co3O4 nanoparticles were used as signal amplifiers, which served as recognition elements for Cd(II), and thionine on the aptamers served as an electrochemical probe. The basic principle of Cd(II) determination involves the formation of a Cd(II)–Apt complex via specific recognition, which increases the thionine current signal. CV was used to characterize each optimization step as well as the binding of the aptamer to Cd(II). Under optimal conditions, the calibration curve of ∆I and Cd(II) concentration was established through CV, with a linear range of 0.20–15 ng/mL, a limit of detection of 0.49 ng/mL, and the recovery of 98.71 % ∼ 109.95 % in tap water (Liu et al., 2020).
2.1.3. Differential pulse voltammetry
Differential pulse voltammetry (DPV) is a technique that involves superimposing periodic equally spaced pulses on a slowly changing potential and recording the changes in the current before and after the pulse for analysis. This method enables determination of the relationship between the peak current and the concentration of the analyte to be measured. Additionally, DPV uses the obtained peak potential to distinguish different compounds with similar potentials, demonstrating a high resolution. The calibration curve obtained by measuring the current and heavy metal ions concentration using DPV can also be used to determine the detection limit and sensitivity of the sensor, thus providing an effective method for quantitative analysis in the field of electrochemical detection (Xu, Dai, & Jin, 2019). Singh et al. developed an electrochemical sensor for detecting Cd(II) by DPV. A novel nanoscale copper MOF (CNT-Cu-MOF) functionalized with multi-walled carbon nanotubes (MWCNTs) was prepared via a hydrothermal reaction and was modified on the surface of the WE. A successful sensing interface was constructed and the limit of detection was extremely low, reaching 0.27 nM (Singh et al., 2021).
2.1.4. Square wave voltammetry
Square wave voltammetry (SWV) is a technique that employs periodic square-wave pulses superimposed on the basic potential. The square-wave pulse consists of alternating positive and negative pulses of the same length. At the end of each cycle of the square-wave pulse, the current is measured and the difference between the positive and negative pulse currents was calculated. The SWV is suitable for analyzing the redox characteristics of trace electroactive substances and can reduce the noise of redox reactions (Ayenimo & Adeloju, 2016). Xiao et al. utilized a one-step hydrothermal technique to synthesize Fe-MOF/MXene in situ, which was then modified onto the WE to prepare a sensitive As(III) electrochemical sensor. The strong bonding effect between As(III) and hydroxyl groups was evident in the Fe-MOF/MXene-modified electrode, resulting in exceptional electrochemical performance. As(III) exhibited a high current response with an LOD of 0.58 ng/L, and has been successfully applied for the detection of As(III) in real water samples (Xiao et al., 2022).
2.1.5. Stripping voltammetry
Stripping voltammetry is a highly sensitive electrochemical analysis technique which includes measuring the concentration of a substance on the electrode surface, followed by the oxidation or reduction of the substance through a change in potential, thereby obtaining a voltammogram and achieving quantitative analysis of the target substance. Common stripping voltammetry methods include anodic stripping voltammetry (ASV) and cathodic stripping voltammetry (CSV). CSV is usually used to detect anionic substances, whereas ASV is more suitable for heavy metal detection because heavy metal ions are easily enriched on the electrode. Although ASV has many advantages, its accuracy and sensitivity can be adversely affected by the presence of interfering ions in the complex samples. To address this issue, it is necessary to implement strategies, such as adding masking agents or modifying the electrode. Rahman et al. developed a highly sensitive sensor for Hg(II) detection using ASV. A sensing interface was successfully constructed by modifying a platinum (Pt) electrode with graphene oxide (GO) and silver nanowires (AgNWs) composite materials. Under optimal conditions, the sensor demonstrated a linear detection in the range of 1–70 nM with a detection limit of 2.0 nM. The sensor has been successfully employed for on-site detection of Hg(II) in water (Rahman et al., 2019).
2.1.6. Amperometry
Amperometry is a highly sensitive electrochemical method that has emerged as a promising alternative to traditional analytical techniques for the detection and quantification of toxic metals. For instance, amperometric inhibition biosensors have been successfully used to detect persistent organic pollutants and heavy metals such as Cd, Pb and Cu. This approach leverages the bioelectrocatalytic activity of immobilized enzymes such as horseradish peroxidase on modified electrodes (Scandurra & Mirabella, 2021). Karthika et al. constructed a g-C3N4/AgM/Nf/GCE electrochemical sensor to detect Cr(VI) in water samples using an amperometric method. The sensor exhibited a satisfactory linear range (0.1–0.7 μM) and an exceptionally low detection limit (1.6 nM). Additionally, the sensor achieved good recovery rates for various water samples (Karthika, Nikhil, Suganthi, & Rajarajan, 2020).
2.2. Potentiometric method
Potentiometric analysis is a technique that measures the potential difference between an indicator and a reference electrode (RE) in close proximity to zero current. This potential difference is linearly proportional to the concentration of heavy metal ions, thereby quantifying heavy metals. This method has several advantages, including nondestructive testing, simple operation, ability to analyze precious samples and ease of automation (Lisak, 2021). An ion-selective electrode (ISE) is the most commonly used sensor in potentiometric analysis and consists of a two-electrode system comprising a WE with an ion-selective membrane (ISM) and a RE. ISM incorporates ionophores that transport specific ions through the membrane, inducing ion activity and generating an electric potential (Ding, Cheong, Zhao, & Lisak, 2021). This provides selectivity for the potentiometer in the presence of other analytes, thereby avoiding the negative effects of interfering ions. Conductive poly (sulfonamide anthraquinone) nanoparticles are suitable ionophores. Based on this carrier, Huang et al. constructed a Pb(II) ISE sensor for measuring Pb(II) ions in aqueous solutions. The detection limit of the potentiometric sensor was 16 μM (Huang, Ding, & Li, 2014).
2.3. Conductivity method
Conductivity sensors determine the signal based on alterations in conductance caused by the oxidation or reduction of the analyte. Modifications in the concentration of charged ions with varying mobilities result in changes in conductivity. Conductivity sensors do not require the use of a RE and are insensitive to light. Nevertheless, conductivity sensors can only measure the aggregate conductance of all ions and cannot distinguish between different ion species, resulting in poor specificity and limited applications (Gulaboski, 2022). Koo et al. designed a simple technique for the fabrication of highly active and conductive porous materials by embedding catalytic metal nanoparticles (NPs) within 2D metal-organic frameworks (MOFs). In particularly, Pd or Pt NPs were placed within the cavities of Cu3(HHTP)2 MOFs. This specific structure confines the size of the metal NPs to approximately 2 nm. The modified MOFs exhibited improved NO2 sensing performance at room temperature owing to the high reactivity of the metal NPs and porosity of the MOFs. The study also demonstrated the enhancement of NO2 sensing in Cu3(HHTP)2 by Pd and Pt NPs (Koo, Kim, Jang, Kim, & Kim, 2019).
2.4. Impedance method
The impedance method is usually represented by electrochemical impedance spectroscopy (EIS), which provides information on the internal resistance of electrode materials. The impedance spectrum often includes both semicircular and linear components. The high-frequency semicircle represents the electron-transfer-limited process. The larger the diameter of the semicircle, the slower the charge transfer process and the poorer the reaction kinetics of the electrode. Conversely, the linear part at lower frequencies corresponds to a diffusion-limited process, with a larger slope indicating a slower diffusion process and a smaller slope indicating faster diffusion. The interaction between the heavy metal ions and nanomaterial-modified electrodes changes the electrochemical impedance characteristics of the electrode interface. By measuring these impedance changes, the heavy metal ions in the solution can be detected both qualitatively and quantitatively. Tan et al. constructed an electrochemical resistance sensor based on reduced graphene oxide (rGO) for the sensitive and specific detection of Hg(II) in water samples. The sensor exhibited a selective response to Hg(II) in the presence of other metal ions, with a minimum detection concentration of 0.5 nM. The detection of Hg(II) showed no significant differences across matrices (Tan et al., 2016).
In general, different types of electrochemical techniques have their own set of advantages and application areas. We need to choose the appropriate method according to the specific detection requirements and conditions.
3. Nanomaterials
Electrochemical detection techniques are widely preferred because of low cost, rapidity, high sensitivity, ease of operation and high accuracy. These methods often cause some problems including poor stability and selectivity (Manikandan, Yoon, & Chang, 2023).The electrode modification effectively enhanced the electrochemical response signal. Current research more focuses on the preparation of high-performance WE through electrode surface modification (Chen, Xie, Zhao, & Han, 2022). Common electrode surface modification materials include carbon nanomaterials, metal nanomaterials, polymers, organic frame materials, MXene materials and other materials. In view of the extensive literature on this part, this chapter briefly introduces common carbon nanomaterials and metal nanomaterials.
3.1. Carbon material
Carbon materials include graphene (GR) and its derivatives, carbon nanotubes and graphitic carbon nitride. GR and its derivatives, including GO and reduced rGO (Petrucci et al., 2019; Yan et al., 2022). Qian et al. developed a hydrophilic GR/GO composite material for the electrochemical detection of Cd(II). The sensor exhibited a low LOD (0.087 μM) and wide linear range (0–10 μM) (Shan, Tian, Ding, & Wu, 2022). Carbon nanotubes (CNTs), including MWCNTs and single-walled carbon nanotubes (SWCNTs) (Fiyadh et al., 2019; Li et al., 2023). Specifically, small SWCNTs facilitate rapid electron transport, whereas the layered structure of MWCNTs provides more surface active sites (Bassyouni et al., 2019). Peng et al. synthesized PEI-functionalized MWCNTs and constructed an MWCNTs-PEI/GCE sensor for the electrochemical detection of trace amounts of Cr(VI) (Peng, Wang, Li, & He, 2016). Graphitic carbon nitride (g-C3N4) is a nanostructured material with strong covalent bonds between carbon and nitrogen (N) in its structure, which can replace the carbon‑carbon (C—C) bonds in graphite layers (Zhao, Zou, Hu, Long, & Jiao, 2021). This material plays an important role in electrochemical sensors because of its multiple catalytically active sites, high conductivity and large surface area (Chen et al., 2020). Wang et al. constructed a simple g-C3N4@ GCE electrochemical sensor for the detection of three heavy metal ions, with detection limits of 0.228, 0.103 and 0.217 μM for Pb(II), Cu(II) and Hg(II), respectively (Wang, Zhao, Sun, & Zhang, 2019).
3.2. Metal nanoparticles
Metal nanoparticles, comprising pure metal and metal oxide nanoparticles, are increasingly being used to enhance electrochemical signals (Sawan, Maalouf, Errachid, & Jaffrezic-Renault, 2020). Metal nanoparticles, including AuNPs, silver nanoparticles (AgNPs) and bismuth nanoparticles (BiNPs), are used in electrochemical sensors to enhance signal response owing to their excellent conductivity properties. Gimenez-Gomez et al. modified AuNPs on a gold thin film electrode. The linear range of the sensor was 1–150 μg/L, and the limit of detection was 0.42 μg/L (Giménez-Gómez, Baldi, Ayora, & Fernandez-Sanchez, 2019a). Sonkoue et al. used simple AgNPs modified gold electrodes for the determination of As(III) in water. The electrical signal was improved owing to the large surface area of AgNPs. The linear range of this sensor was 0.05–0.2 μM, with a detection limit of 13.8 nM (Sonkoue, Tchekwagep, Nanseu-Njiki, & Ngameni, 2018). Li et al. successfully measured Pb(II), Cd(II) and Zn(II) by in situ electrodeposition of a bismuth film on a screen-printed gold electrode (SPAuE). The sensor exhibited a wide linear range (1–120 μg/L). In addition, the limit of Pb(II), Cd(II) and Zn(II) detection were 0.04 μg/L, 0.02 μg/L and 0.05 μg/L, respectively (Li, Zhao, Zhao, & Cui, 2021). Zhao et al. developed an electrochemical sensor based on an electrode modified with Au—Ag bimetallic nanoparticles for the simultaneous determination of two chromium species, Cr(III) and Cr(VI). The linear range and detection limit of Cr (VI) are 0.05–5 ppm and 0.1 ppb respectively, and the linear range and detection limit of Cr (III) are 0.05–1 ppm and 0.1 ppb respectively (Zhao, Ge, Wong, Zhou, & Lisak, 2021). In another study, Yang et al. synthesized Au—Cu bimetallic nanoparticles using a simple hydrothermal method, which exhibited a low detection limit of 2.09 ppb and ultrahigh anti-interference performance for As(III) detection (Yang et al., 2016).
Various metal oxides, primarily transition metals, have been used to modify electrodes, including iron oxide (Fe2O3), ferric oxide (Fe3O4), zinc oxide (ZnO), bismuth oxide (Bi2O3), chromium oxide (Cr2O3), titanium oxide (TiO2) and cobalt oxide (Co3O4), on the electrode surface to construct sensing interfaces. Among these, Fe2O3-based materials have a high affinity for heavy metal ions. Li et al. used Fe2O3 nanorods and Fe2O3 hollow nanocubes-modified glassy carbon electrodes for the electrochemical detection of Pb(II). They found that the Fe2O3 nanorod-modified electrode exhibited a lower detection limit (0.0034 μM) than the Fe2O3 hollow nanocube-modified electrode (Li et al., 2018).
4. Sensing strategies
Sensing strategies are an essential aspect of electrochemical sensors, allowing the detection limit of the sensor to be reduced by several orders of magnitude. Existing literature predominantly discussed the classification of nanomaterials for electrochemical sensors, primarily emphasizing the roles and types of nanomaterials employed. However, there is a lack of emphasis on the key role of sensing strategies in electrochemical sensors. Therefore, this section highlights the innovative strategies for heavy metal ions detection in electrochemical sensors by referring to literature published in the past decade (2014–2024), and demonstrates the pivotal role of electrochemical sensing strategies in detection.
4.1. IIPs-based electrochemical sensor
Ion-imprinted polymers (IIPs) are materials synthesized through free-radical polymerization that exhibit specific recognition of particular metal ions (Abdul Halim, Sulaiman, Nordin, & Bajunaid Hariz, 2022). These materials are similar to molecularly imprinted polymers, which are synthesized using a solvent to remove template ions after the polymerization process, leaving behind cavities that are highly matched to the target metal ions in terms of size, shape and functional groups. Consequently, IIPs can achieve highly selective recognition of specific heavy metal ions (Dahaghin, Kilmartin, & Mousavi, 2018). For instance, Hu et al. constructed a chitosan-IIP-GCE electrochemical sensor for measuring trace levels of Pb(II) in food and water samples. This sensor utilized chitosan to stabilize IIPs on the electrode surface, resulting in enhanced selectivity for Pb(II). Compared to NIP, IIP increased the adsorption capacity of Pb(II) by 2 times. The sensor exhibited a linear range of 0.05–60 μM and a detection limit of 0.01 μM (Hu, Xiong, Huang, & Lai, 2016). Masi et al. initially synthesized IIP for determining Cu(II) levels. The IIP was modified on the surface of the SPCE. The IIP sensor demonstrated a higher affinity for Cu(II) than the NIP, with a sensitivity that was four times higher. The sensor had a linear range of 0.95–244 nM, a detection limit of 2.7 nM, and a recovery rate of 95–105 % in spiked drinking water (Fig. 1a) (Di Masi et al., 2020). Khairnar et al. developed Zn(II)-IIP through free radical polymerization on the surface of vinyl silica particles and detected Zn(II) using DPV, demonstrating a linear range of 6.12–45.9 nM with a detection limit of 13.51 nM (Khairnar et al., 2020). Ma et al. prepared nanoporous gold (NPG) via green electrodeposition using As(III) as the template ion and o-phenylenediamine as the functional monomer. A layer of IIP was synthesized in situ on the NPG surface via electropolymerization, and an electrochemical sensor of IIP/NPG/GE was constructed for the determination of As(III) in water. The linear range of this sensor for As(III) was 9.0–200.0 nM, and the limit of detection was 7.1 pM (Ma, Chang, Zhao, & Ye, 2020).
Fig. 1.
(a) An electrosynthesised ion imprinted polymeric sensor for the detection of Cu(II) (Khairnar, Jirimali, Patil, & Gite, 2020). Copyright 2020, Elsevier. (b) Illustration of the chemical structure of IIP (Coelho et al., 2017). Copyright 2021, Elsevier. (c) Nano-structured Cr(III)-imprinted sensing platform for the detection of Cr(III) (Hu, Sedki, Shen, Mulchandani, & Gao, 2021). Copyright 2017, Elsevier.
Cd(II)-imprinted polymers were synthesized for Cd(II) detection. Coelho et al. constructed a carbon paste electrode/ion-imprinted polymer (CPE/IIP) electrochemical sensor to detect Cd(II) in water samples. The detection limit of IIP was lower than that of NIP for Cd(II) detection. The limit of detection was 4.95 μg/L, the limit of quantification was 16.4 μg/L. This sensor was successfully applied to Cd(II) detection in mineral, tap water and lake water samples (Coelho et al., 2017). Hu et al. synthesized IIP using polyethyleneimine (PEI) and methacrylic acid (MAA) as bifunctional monomers and Cd(II) ions as template agents, and constructed an electrochemical resistive sensor with IIP-functionalized rGO (IIP/rGO) for Cd(II) determination in water. The imprinted membrane (PEI-MAA) exhibited a higher selectivity for Cd(II). The sensor demonstrated a linear range of 2–200 ppb and a detection limit of 0.83 ppb, which was successfully applied for Cd(II) detection in tap water, lake water and river water with a recovery rate of 94.5 % to 113.5 % (Fig. 1b) (Hu et al., 2021). Motlagh et al. prepared nanostructured Cd-IIP for the first time by surface-imprinting technique using the sol-gel method, and employed DPASV to determine Cd(II) at a carbon paste electrode (CPE), where the maximum adsorption amounts of NIP and Cd -IIP showed maximum adsorption of 12.2 and 24.7 mg/g, respectively (Ghanei-Motlagh & Taher, 2017). This suggests that the high ratio of imprinted sites in the Cd-IIP materials provides a favorable high-performance platform for Cd(II) sensing.
In other studies, Cr(III)- imprinted polymers were also synthesized for the Cr(III) detection. Alizadeh et al. synthesized Cr(III)-imprinted polymers using itaconic acid and ethylene glycol dimethacrylate as the complexing monomers and crosslinkers, respectively, and constructed IIP-IMA nanoparticles to construct an electrochemical sensor for IIP-CCE. The oxidized peak current response of Cr(III) at the IIP-modified electrode was significantly higher than that of CCE and NIP-CCE. The peak current of Cr(III) ions in IIP-CCE was approximately 18 times higher than that in NIP-CCE. The IIP not only accumulated Cr(III) on the surface of the electrode, thereby increasing its electrochemical signal, but also enhanced the charge transfer rate at the electrode surface, further increasing the electrochemical signal of Cr(III) on IIP-CCE. The sensor demonstrated a linear range of 0.1–10 μM, a lower limit of detection of 17.6 nM, and was successfully applied to the determination of Cr(III) in water and urine (Fig. 1c) (Alizadeh, Rafiei, Hamidi, & Ganjali, 2017). Aravind et al. also developed a method for the detection of Cr(III), they established an ion imprinting technique for the determination of Cr(III) in water by using MAA as a functional monomer, N,N′ -methylene-bis-acrylamide as a cross-linking agent and potassium persulfate as an initiator. Simultaneously, they produced vinyl-functional MWCNTs, formed a coating of an ion-imprinted polymer (IIP) on their surface, and then applied it to platinum electrodes to form a novel Cr(III) ion-imprinted MWCNTs-IIP electrochemical sensor. This sensor demonstrated a rapid response to Cr(III) ions by integrating highly specific binding sites in the IIP, and was suitable for the selective detection of Cr(III) ions with a detection limit of 0.054 μM (Aravind & Mathew, 2018).
In conclusion, electrochemical sensors based on the imprinting strategy have promising applications in the detection of heavy metal ions.
4.2. Flexible electrochemical sensor
Flexible substrates play a supporting role in electrochemical sensors. In contrast to the brittle substrates used in traditional electrochemical sensors, flexible substrates are bendable, stretchable, wearable, easily miniaturized, portable, easily manufactured, and can make direct contact with the skin and other surfaces for noninvasive detection. Commonly used flexible substrates include polydimethylsiloxane (PDMS), polyvinyl alcohol (PVA), polycarbonate resin (PC), polyimide (PI), polyethylene terephthalate (PET) and polyethylene naphthalate (PEN). The flexible design and cost-effective manufacturing of flexible sensors make them ideal for field inspection (Mathew, Radhakrishnan, Vaidyanathan, Chakraborty, & Rout, 2021).
Flexible electrochemical sensors are becoming increasingly popular because of their ability to be thin, lightweight, stretchable and bendable. These sensors were designed to satisfy the requirements of different application environments. By employing micromachining technology and printed electronics technology, researchers can precisely configure the electrodes and sensing interfaces on flexible substrates to realize high-performance flexible electrochemical sensing platforms (Gao et al., 2023). For example, Neethipathi et al. used screen printing technology to print a three-electrode system onto a flexible polyvinyl chloride (PVC) substrate with a hybrid carbon paste ink to form a thick-film electrode SPCE, and modified the SPCE with two-dimensional MoS2 nanoparticles to successfully construct an electrochemical sensor for detecting Cu(II) in water. Comparing with the electrochemical impedance of the modified SPCE, the results showed that the SPCE flexible sensor had better performance than the traditional glassy carbon electrode, with a linearity of the standard curve of 0.99, an extremely wide detection range of 5–5000 μM, and an LOD of 5.43 μM (Fig. 2a) (Neethipathi et al., 2023). On the basis of the flexible sensor to detect single ions, studies have shown that the self-made flexible sensor can detect multiple metal ions at the same time. Zhao et al. successfully synthesized BiNPs@LIG nanostructures using flexible PI films as substrates and 470 nm laser irradiation. Subsequently, BiNPs@LIG was wrapped with a Nafion film to form a Nafion/BiNPs @LIG composite material, which was modified at the WE and assembled with an Ag/AgCl RE and a Pt wire counter electrode to form a three-electrode system. This flexible electrode was connected to an electrochemical instrument for simultaneous determination of Pb(II) and Cd(II) with a measurement range of 5.0–50.0 μg/L. When applied to standard addition recovery experiments on soil samples, the recovery rates of Cd(II) and Pb(II) reached 99.07 % and 98.95 %, respectively, demonstrating the potential application of this sensor to real-world samples (Fig. 2b) (Zhao, Wang, & Liu, 2022). Zhao et al. employed electrodes using full printing technology by printing a commercial carbon ink onto the surface of PET to form a conductive layer. An EDTA@PANI/MWCNTs composite material was used as the electronic ink to print the WE. The redox current peaks of the Cu(II), Pb(II) and Hg(II) ions did not overlap and showed obvious differences in their positions, which allowed for the simultaneous detection of these ions. The limit of detection for Cu(II), Pb(II) and Hg(II) ions were 55.4 pM, 22 pM and 17.8 pM, respectively (Zhao et al., 2020).
Fig. 2.
(a) Manufacturing procedure of bulk electrodes for MoS2-modified screen printed sensor (Neethipathi, Beniwal, Bass, Scott, & Dahiya, 2023). Copyright 2023, IEEE Xplore. (b) The fabrication process of the disposable and flexible electrochemical sensor for the sensitive detection of Cd(II) and Pb(II) (Li, Zhang, & Li, 2025). Copyright 2022, Elsevier. (c) The electrode fabrication, detection mechanism and sensing process of electrochemical sensor (Jiang et al., 2020). Copyright 2020, American Chemical Society. (d) System design of the fully integrated battery-free and flexible electrochemical tag (Xu et al., 2020). Copyright 2020, Elsevier.
Traditional electrochemical sensors are typically connected to commercial electrochemical workstations, which greatly limits the integration of the system and real-time monitoring of heavy metal ions (Li et al., 2025). Later, some portable integrated systems which combined sensors and microprocessors were developed; however, they still require an external battery or a wired connection to a smartphone for power supply and data transmission, which still has limitations for on-site detection. Therefore, wireless electromagnetic induction technologies based on NFC and Bluetooth have emerged. Jiang et al. modified a bismuth (Bi) film on a textile substrate to prepare a flexible electrode and combined it with a printed circuit board (PCB), Bluetooth technology and electrochemical technology to successfully prepare a flexible electrochemical sensor for real-time detection of human sweat, seawater, Zn(II), Cd(II) and Pb(II) in cosmetics and drinking water. The designed circuit and microcontroller were integrated on a small board, and the printed circuit board (PCB) was fabricated using electronic printing technology, then a flexible wearable sensor was integrated with a PCB and Bluetooth system. The PCB was responsible for data acquisition and processing, the Bluetooth system transmitted the data to the mobile device, and the sensor enabled wireless transmission and real-time detection. The sensor was bendable as well as washable, cheaper and easier to scale up, with a lower LOD of 6.32, 15.04 and 9.08 ppb for Zn(II), Cd(II) and Pb(II), respectively (Fig. 2c) (Jiang et al., 2020). Xu et al. developed a wireless electrochemical sensor that could detect Pb(II) and Cd(II) in a range of containers without batteries. The sensor was composed of a flexible circuit board that replaced the traditional commercial electrochemical workstation and integrated circuits, NFC modules and flexible electrode arrays. The electrode array had two WEs for the detection of two heavy metal ions: one modified with AuNPs for Pb(II) detection and the other with BiNPs for Cd(II) detection. The miniaturized flexible circuit board design allows it to be attached directly to the inner surface of the container, allowing the wireless detection of heavy metal ions from the outside using only an NFC-enabled smartphone. The NFC technology provides a stable voltage power supply to the circuit board so that the test results can be wirelessly transmitted to a mobile phone without any external power supply or equipment, which is convenient for easy access or subsequent analysis. The sensor achieved the low detection limits of 2.20 μg/L and 1.51 μg/L for Pb(II) and Cd(II), respectively (Fig. 2d) (Xu, Li, et al., 2020).
Many flexible electrochemical sensors have been used to detect heavy-metal pollution in the environment, such as soil and water. Furthermore, studies have been conducted to monitor the levels of heavy metal ions in human sweat and urine. Hui et al. successfully developed a flexible electrochemical sensor by ultrasonically combining Ti3C2Tx and MWCNTs nanomaterials, modifying them on Au PET electrodes, and depositing antimony (Sb) on the electrode surface. This sensor was used to simultaneously detect Cu(II) and Zn(II) in human urine and sweat, respectively. Taking Cu(II) as an example, the study showed slight changes in the oxidation electrode potential of the sensor at different bending angles (70°, 45° and 30°). Compared with the original state, the changes in the peak currents were only 1.3 %, 0.6 % and 1.9 %, respectively. This result highlighted the excellent flexibility of the electrodes, allowed them to be directly attached to the skin and toilet bowl for monitoring Cu(II) and Zn(II) levels in sweat and urine with detection limits of 0.1 ppb and 1.5 ppb were achieved, respectively. The application of this technology opens new possibilities for home health monitoring and real-time tracking of heavy metal ions levels in the body (Hui et al., 2020).
Flexible electrochemical sensors are highly conducive to rapid on-site detection owing to their adaptability. As technology continues to advance and optimize, it is likely that additional flexible electrochemical sensors with superior performance will be developed to meet a wider range of detection requirements.
4.3. Paper-based electrochemical sensor
Paper possesses microporous characteristics with adjustable pore size, hydrophilicity, strong liquid absorption capacity, thinness, lightness, customizable functional group modification, and can generate flow by capillary action. Cellulose is the primary component of paper, and various cellulose-based papers containing a large number of hydroxyl and carboxyl groups have been used as electrode substrates, demonstrating their potential for heavy-metal adsorption (Ding, Cheong, Ahamed, & Lisak, 2021). Furthermore, recent investigations have explored the application of paper for the assessment of heavy metals, they highlighted the significance of paper substrates in shaping sensing responses and presented a cost-effective and accessible alternative for heavy metal analysis (Özbek & Berkel, 2022).
Paper-based sensors are commonly used in the development of colorimetric and fluorescent sensors, and have also been studied for the detection of heavy metal ions using electrochemical sensors. In contrast, electrochemical sensing methods provide better response times and sensitivities, with less background noise. Hu et al. modified BiNPs and nitrogen-doped carbon (NC) materials on flexible graphene paper electrodes to enable the simultaneous detection of various heavy metals, with linear ranges of 10–1200 ppb for Zn(II), 0.5–1200 ppb for Cd(II), and a linear range of 0.5 ppb ∼ 2.1 g/L for Pb(II). The detection limits for the BiNPs@NC/GP sensors were 5 ppb for Zn(II), 0.05 ppb for Cd(II), and 0.02 ppb for Pb(II) (Hu, He, Xiao, & Liu, 2023). Kamel et al. developed a paper-based sensor for assessing Cu(II) levels in blood. They used filter paper as a supporting substrate and macrocyclic pyridine-pentapeptide derivatives as novel ionophores for Cu(II) detection. The paper-based sensor had a linear range of 0.5 μM ∼ 1.0 mM and a detection limit of 80.0 nM. The advantage of this sensor is that it can detect blood without pretreatment and it is relatively faster (Kamel, Amr, Almehizia, Elsayed, & Moustafa, 2021). Feng et al. prepared a cost-effective, disposable and paper-based sensor for the electrochemical detection of Pb(II). The sensor featured a disposable WE composed of carbon ribbons and indium tin oxide (ITO) glass, which was coated with a bismuth film, and filter paper was placed at the sensing interface for heavy metal detection. The linear range of this paper-based sensor for Pb(II) was 10–500 μg/L with a detection limit of 2 μg/L. The sensor was simple to prepare and inexpensive, and the electrode preparation price was approximately $ 0.05, which was convenient for practical applications (Fig. 3a) (Feng et al., 2013). Mariana et al. developed a disposable paper-based sensing device that combined filtering and detection capabilities. The linearity of Pb(II) and Cd(II) in the water samples was 10–100 ppb, with detection limits of 7 and 11 ppb, respectively. In addition, the inclusion of filter paper in this sensing platform imparts filtering properties to the sensor and allows sample pretreatment. Pb(II) and Cd(II) can be detected in complex matrices such as mud and seawater without sample pretreatment (Medina-Sanchez et al., 2015). Ruecha et al. used graphene-polyaniline composite (GO/PANI) to modify SPCE and added Bi NPs to the buffer solution to achieve the detection of Zn(II), Cd(II) and Pb(II). The linear range of the sensor was 0.1–300 ppb, and the limit for Zn(II), Cd(II) and Pb(II) detection were 1 ppb, 0.1 ppb and 0.1 ppb, respectively. Compared with the bare electrode, the sensitivity of the modified electrode was increased by 2 times. The recovery rates of Zn(II), Cd(II) and Pb(II) in human serum spiked with these metals were between 93.8 % and 109.7 % (Fig. 3b) (Ruecha et al., 2015).
Fig. 3.
(a) The fabrication process of paper-based analytical devices (Kamel et al., 2021). Copyright 2013, Elsevier. (b) The electrochemical sensor fabrication on plastic film or paper (Medina-Sanchez, Cadevall, Ros, & Merkoci, 2015). Copyright 2015, Elsevier.
4.4. Ratio electrochemical sensor
In recent years, ratiometric techniques originally developed for fluorescence analysis have been applied to electrochemical detection. Ratiometric sensors improve the reliability of electrochemical detection by measuring the variations in the ratio between two electrochemical signals (analytical and reference signals). Compared with single-signal electrochemical sensors, the self-calibration of the ratio of the two signals is less susceptible to unavoidable interference caused by instrumental or environmental factors, exhibits good stability and addresses the issue of poor reproducibility in electrochemical sensors (Wang et al., 2019).
Ratio electrochemical sensors can be divided into two categories according to the different manifestations of the reference signal: the reference signal remains constant and the reference signal changes. In the first type, when the analysis signal changes, the reference signal remains unchanged. In the second type, when the analysis signal changes, the reference signal also changes. Wang et al. constructed a ratio electrochemical sensor using AuNPs as an internal reference signal. Cu(II) were successfully detected, and the reproducibility was greatly improved (Fig. 4a) (Wang, Liu, et al., 2019).
Fig. 4.
(a) Embedded Au nanoparticles-based ratiometric electrochemical sensing strategy for the detection of copper ions (Ruecha et al., 2015). Copyright 2019, American Chemical Society. (b) The ratiometric electrochemical sensor for the detection of Pb(II) and Cd(II) (Zhou et al., 2021). Copyright 2020, American Chemical Society. (c) The DNAzyme-based dual-signal ratiometric electrochemical biosensor for the detection of Pb(II) (Yang et al., 2024). Copyright 2018, Elsevier.
The reference signals of the above sensors were kept constant, whereas some studies used the strategy of changing the reference signal. Rong et al. constructed a ratiometric electrochemical sensors based on MOFs for the detection of Cd(II) and Pb(II) and the Cu(II) signals of the center ions of the MOFs were used as the internal reference signals. The central metal ions in the original MOFs were replaced by ion-exchange reactions in the presence of Cd(II) and Pb(II). The peak current of Cu(II) decreased, the peak currents of Cd(II) and Pb(II) increased, and the final output signals of the ratiometric sensors were used as the I Cd(II)/I Cu(II) and I Pb(II)/I Cu(II) signals. The output signal established a linear relationship with the concentrations of Cd(II) and Pb(II) (Fig. 4b) (Hu, Zhang, Chi, Yang, & Yang, 2020). Ma et al. constructed a DNAzyme-based dual-signal ratiometric electrochemical biosensor for the detection of Pb(II). The biosensor was self-assembled on a gold electrode using a methylene thiocyanate blue-labeled catalytic DNA probe (MB-P1) and a complementary ferrocene-modified substrate DNA probe (Fc-P2). MB-P1 served as the catalytic probe and the “turn-on” element, and Fc-P2 served as the substrate probe and the “turn-on” elements. The presence of Pb(II) activates DNAzyme, cleaving Fc-P2 into two fragments and dissociating it from the sensing interface, and is accompanied by an MB-P1 probe approaching the sensing platform. As a result, the peak current of the MB increases and the peak current of Fc decreases, generating a “turn on” and “turn off” element of a dual signal-ratio electrochemical output. According to the comparison of two peak currents, it is possible to detect Pb(II) in biological fluids, such as serum. DNAzyme has an efficient recognition capability and signal amplification strategy, and the biosensor has a wide detection range (0.1–5 μM) and a low detection limit (45.8 pM) (Fig. 4c) (Ma et al., 2018).
Ratiometric electrochemical analysis is a widely used method for detecting heavy metal ions, because it is ability to eliminate environmental interference. This technique is particularly useful in electrochemical detection.
4.5. Dual-mode electrochemical sensor
The signal of a single-mode sensor is susceptible to interference from the external environment, particularly the sample matrix, which may lead to inaccurate results, such as false positive or false negative. However, dual-mode sensors compensate for the limitations of single-mode sensors by integrating the benefits of both and exhibiting synergistic effects. These two signals can verify each other more reliably after interference from the external environment. Moreover, dual-mode sensors offer greater versatility in dealing with intricate sample-matrix interference (Zhou et al., 2021).
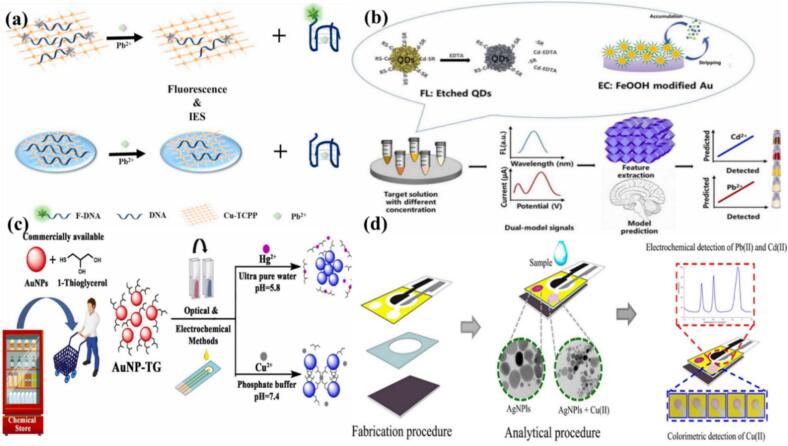
Among the various sensors, fluorescence and colorimetric sensors belong to the optical field category and are more traditional dual-mode sensors. However, electrochemical fluorescence dual-mode sensors belong to two fields and have few applications. These have attracted wide attention in recent years. Fluorescence sensors have low detection limits in trace samples as well as turbid samples, electrochemical sensors have low reproducibility, and bimodal sensors are used considering the limitations of both sensors (Yang et al., 2024). Karakuş et al. designed a sensing platform by combining aggregation-induced emission (AIE) fluorophores and electrochemically active units into a single structure, which could provide an effective sensing platform for detecting the dual-signal response of Cu(II) ions. An easily synthesized AIE fluorescent probe (ANT-Fe) was developed with anthracene dye as the fluorophore and ferrocene formaldehyde as the redox-active unit. The probe has dual-modal sensing potential, namely a fluorescence “turn-off” signal mode and a cathode-shifted electrochemical signal mode, for the detection of Cu(II) in water. This sensor has a low detection limit (2.5 ppb), short response time (<30 s), and good selectivity for Cu(II) ions (Karakuş, Gunduz, Liv, & Ozturk, 2020). Chen et al. constructed a fluorescent electrochemical dual-mode sensor to successfully detect Pb(II) ions in tap water and fertilizer samples. In the optical field, GDNA was labeled with FAM (F-GDNA) at the 3′ end as a recognition probe for Pb(II) ions and 2D-MOF nanosheets were used as quenchers. These two molecules are mixed by π–π stacking and hydrogen bonding, leading to FRET and the quenching of F-GDNA fluorescence. In the presence of Pb(II), the separation of F-GDNA resulted in the recovery of fluorescence in the sensing system, thereby establishing a linear relationship between fluorescence intensity and Pb(II) concentration. The detection limit of the fluorescence sensor was 3.3 nM. In the field of electrochemistry, 2D-MOF nanosheets were modified on an electrode, followed by the modification of single-stranded GDNA on a 2D-MOF/GCE. The addition of Pb(II) ions led to the formation of G-quadruplexes from single-stranded DNA. The difference in the 2D-MOF affinity for single-stranded GDNA and G-quadruplexes resulted in a change in the impedance of the electrode surface, enabling the detection of Pb(II) ions. This electrochemical sensor was highly sensitive, with a low detection limit of 8.7 pM (Fig. 5a) (Chen, Bai, Jin, & Zheng, 2021). Wang et al. used fluorescence electrochemistry dual mode to solve the problem of Cd(II) and Pb(II) detection in practical situations. For the first time, a sea urchin-like FeOOH-modified Au electrode was used to detect Pb(II) in a solution. EDTA quenched the fluorescence of CdTe/CdS quantum dots. Fluorescence was recovered in the presence of Cd(II), and the degree of fluorescence recovery was related to the concentration of Cd(II). In this study, the convolutional neural network (CNN) technique was applied for the first time to an electrochemical-fluorescence (EC-FL) two-mode strategy for the determination of Cd(II) and Pb(II) content in water. In particularly, the prediction errors of the developed CNN model for Cd(II) and Pb(II) were significantly lower than those of the conventional linear regression (0.2176 and 0.6002 μg/L, respectively), confirming its high accuracy and reliability in the analysis of real water samples (Fig. 5b) (Wang et al., 2022).
Fig. 5.
(a) Fluorescent and electrochemical Pb(II) sensor based on 2D-MOF nanosheets (Wang et al., 2022). Copyright 2021, Elsevier. (b) Fluorescence and electrochemistry dual-modal sensor for detection of Cd(II) and Pb(II) (Erdemir et al., 2023). Copyright 2022, Elsevier. (c) Optical–electrochemical method of detecting Hg(II) and Cu(II) (Chaiyo, Apiluk, Siangproh, & Chailapakul, 2016). Copyright 2023, Elsevier. (d) Dual electrochemical and colorimetric detection of Pb(II), Cd(II) and Cu(II) (Cui, Ren, Song, & Ren, 2022). Copyright 2016, Elsevier.
In the field of heavy-metal detection, in addition to electrochemical fluorescence dual-mode sensors, researchers have successfully constructed electrochemical colorimetric dual-mode sensors. Erdemir et al. developed 1-mercapto triglyceride (TG)-modified AuNPs and used optical and electrochemical methods to identify and detect Hg(II) and Cu(II) by varying the solution medium. In ultrapure water, Hg(II) ions changed the original wine red color of AuNP-TG to blue, the initial absorbance value at 520 nm decreased, and a new absorbance peak appeared at 670 nm. In the PB solution, the Cu(II) ions produced a similar reaction. In terms of electrochemical detection, the change in the redox peak of Cu(II) originated from the reduction of its complex with the hydroxyl group adjacent to TG, whereas the electrochemical detection of Hg(II) originated from the disruption of AuNP-S bonds by Hg(II), followed by the aggregation of AuNPs and a significant increase in the electron transfer rate of the electrode. In this study, electrochemical and colorimetric dual-mode sensors were constructed and successfully used for the detection of Hg(II) and Cu(II) in spring, drinking and wastewater samples. Moreover, color-intensity changes in colorimetric sensors can be color-resolved using a smartphone, thus enabling on-site identification (Fig. 5c) (Erdemir et al., 2023). Chaiyo et al. also constructed an electrochemical and colorimetric dual-mode sensor to quantitatively detect Pb(II), Cd(II) and Cu(II). The sensor combined bismuth-modified boron-doped diamond electrodes (Bi-BDDEs) for the electrochemical analysis of Pb(II) and Cd(II), and the catalytic etching of silver nanoplates (AgNPls) using thiosulfate (S2O32−) for the colorimetric analysis of Cu(II). Under Cu(II) catalysis, the AgNPls experienced size reduction and a color change from pink to colorless. This sensing strategy provided an extremely low detection limits of 0.1 ng/mL for Pb(II) and Cd(II), whereas the detection limit for Cu(II) is 5.0 ng/mL (Fig. 5d) (Chaiyo et al., 2016).
4.6. Microfluidics-based electrochemical sensor
Microfluidics were initially used in the medical field, and research on food testing has increased annually in recent years. Combining microfluidic chips with electrochemical sensors can realize real-time, rapid and sensitive on-site detection of analyte. Microfluidics can accurately control fluid flow at the micron and nanoscale, reduce the amount of reagents and analytes, reduce costs, detect multiple analytes simultaneously, reduce analysis time and achieve higher sensitivity at low analyte levels. And the preparation, reaction, concentration, separation, purification and detection of sample can integrated into one device, laying a solid foundation for point-of-care testing (POCT). The microfluidic channel causes turbulence in the electrolyte, resulting in more metal ions contacting the electrodes, enhancing the enrichment of metal ions and increasing the electrochemical signal. Therefore, electrochemical sensing devices based on microfluidic chips exhibit easy miniaturization, suitability for field applications and the ability to provide early warnings (Cui et al., 2022).
In the early stages of microfluidic development, small microfluidic chips and electrodes were developed to efficiently process tiny fluids using simple devices. Studies have shown that CeO2@Au NPs nanomaterials can promote the decomposition of H2O2, generate active oxygen, and oxidize colorless o-PD to form the yellow oxidation state 2,2′-diaminoazobenzene (o-PDox), which served as an electrochemical chemical testing indicator. According to this, Huang et al. proposed a microfluidic electrochemical sensing chip for detecting trace amounts of Pb(II). It was used to hybridize substrate chain S1 labeled with CeO2 @AuNPs with DNAzyme, which was activated by Pb(II)-induced activation of DNAzyme, and S1 was separated into two fragments and detached from the sensing interface. The electrochemical response was reduced because of a reduction in the number of CeO2@Au NPs. In this chip architecture, there was no need for complex chip components, such as pumps or valves. Instead, three-electrode microchambers with water-tank structures were integrated into the three-dimensional chip. When an appropriate amount of fluid was added to the microfluidic device, the solution flowed into three-electrode microchambers, realizing a three-electrode connection. The electrode microchamber was filled with o-PD and H2O2 to ensure that the electrochemical reaction could proceed normally. Moreover, this microfluidic sensor could detect 10 samples simultaneously. Using nanopore gold nanolayer (PAuNL) as the sensing substrate and CeO2@Au NPs as the signal amplification strategy, the detection limit of Pb(II) can reach 3.1 pM (Fig. 6a) (Huang et al., 2021).
Fig. 6.
(a) The three-dimensional model and the analysis process of MESC (Hossain, Karim, Seo, Park, & Shim, 2023). Copyright 2021, Elsevier. (b) A disposable microfluidic channel sensor for the detection of Cu(II), Cd(II), Hg(II) and Pb(II) (Giménez-Gómez, Baldi, Ayora, & Fernandez-Sanchez, 2019b). Copyright 2023, American Chemical Society. (c) Design of the microfluidic system (Dong et al., 2016). Copyright 2019, American Chemical Society. (d) The microfluidic devices with the SPE (Liang et al., 2021). Copyright 2016, American Chemical Society.
Research on electrochemical microfluidic technology in the field of heavy metal ions detection has evolved from initial basic research to current highly integrated and intelligent development (Lace & Cleary, 2021). The microfluidic system was integrated and intelligent, integrating microfluidic components such as microelectrodes, sensor arrays, micropumps and valves, which are used in conjunction with smartphones and cloud computing technology to achieve automated sample processing and efficient and rapid on-site detection. This system does not have to be operated by laboratory professionals; the data will be transmitted to smartphones and computers to achieve intelligent operation of the sample into the system results directly output. Hossain et al. recently constructed a disposable AC electrodynamic microfluidic device consisting of a screen-printed carbon channel and a detection electrode on a polyethylene film substrate, with a WE modified with a composite of a conductive polymer and GO nanoparticles. This study applied a small AC potential symmetrically on the channel wall, and soluble organic and inorganic substances could be precisely separated based on the mass, charge, size and dipole moment difference of the target substance. Different types of hydrophilic plastic caps have been utilized to demonstrate the flow of fluids through microfluidic channels via capillary action and electrodynamic forces. Heavy metal ions have been used as model analytes for precise separation and sensitive detection using simple amperometric methods. The detection limits of the microfluidic sensor for Cu(II), Cd(II), Hg(II) and Pb(II) were 0.04, 0.29, 0.07 and 0.14 ppb respectively. Finally, the electrochemical results were compared with the ICP – MS results with a confidence level of 95 % (Fig. 6b) (Hossain et al., 2023).Giménez-Gómez et al. demonstrated an electrochemical sensor integrated with microfluidic technology for precise monitoring of inorganic arsenic (III) in water. This advanced microfluidic system has a modular design that enables automated sample handling and the highly sensitive detection of As(III). A flexible and transparent microfluidic module made by rapid prototyping technology can easily integrate gold nanoparticle-modified gold film electrodes. The system not only simplified the traditional arsenic detection process, but also significantly improved the accuracy and efficiency of detection. The linear range is 1–150 μg/L, and the detection limit is 0.42 μg/L. This represents an important advancement in microfluidic electrochemical sensors in the field of environmental monitoring, particularly for the rapid detection of arsenic contamination (Fig. 6c) (Giménez-Gómez et al., 2019b).
In recent years, 3D printing technology has been gradually applied to the manufacturing of sensors. As an economical and environmentally friendly production method, 3D printing can achieve mass production while ensuring equipment standards. Hong et al. used 3D printing to fabricate a microfluidic cell adapted to screen-printed electrodes (SPEs), which were integrated with SPEs, USB interfaces, electrochemical workstations, pumps and iPads, to establish a real-time sampling system for the detection of Cd(II) and Pb(II). The sensor had a low detection limit of 0.5 μg/L and 0.2 μg/L for Cd(II) and Pb(II), respectively (Fig. 6d) (Dong et al., 2016).
In the future, as advancements in artificial intelligence (AI) and machine learning continue to evolve, the integration of these technologies with electrochemical microfluidic detection systems will propel their development towards heightened intelligence and automation. This evolution is set to significantly enhance our capabilities in safeguarding public health and environmental integrity, leveraging the enhanced precision and efficiency that these advanced technological frameworks can offer.
4.7. Electrochemical sensor based on hybrid strategy
In the field of electrochemical detection, single-technology strategies have shown advantages for heavy metal ions. These methods are based on their respective characteristics, such as the low cost and personnel friendliness of paper sensors, high efficiency and fine control of microfluidic systems, adaptability of flexible sensors, high accuracy of dual-modal technology, anti-interference ability of ratiometric technology and high selectivity of IIP, which have greatly contributed to the development of this field. Nevertheless, these strategies may still encounter challenges in terms of sensitivity and selectivity when used for the detection of trace heavy metal ions in complex matrices (Liang et al., 2021).
Therefore, integrated methods incorporating multiple detection strategies are gradually becoming a research hotspot, aiming to compensate for the shortcomings of a single technology to achieve the efficient detection of heavy metal ions at extremely low concentrations. Through strategic integration, they complement one another's advantages. For example, combining microfluidic technology and flexible sensors can provide sufficient flexibility to adapt to different detection environments while maintaining precise sample control, and combining IIP with dual-mode detection technology can simultaneously provide both high target-ion selectivity and enhanced detection sensitivity. Such cross-strategy integration not only optimizes the detection performance but also expands the application prospects of electrochemical sensing technology in a number of important fields, such as environmental monitoring, food quality inspection and public health.
A single strategy works well for heavy metal ions, but still has its own drawbacks; therefore, researchers have combined different strategies to detect heavy metal content at lower concentrations. Li et al. integrated flexible sensors, chemical sensor arrays, and modified MoS2 flexible materials to achieve simultaneous detection of Cd(II), Hg(II) and Pb(II). The sensor array had a detection limit of 5 ng/mL for Cd(II) and could detect Hg(II) in tap water and Na(I) in human sweat samples (Fig. 7a) (Li, Zhang, & Wu, 2019). Zhang et al. constructed a smartphone-controlled portable wearable microfluidic electrochemical sensor that does not require external energy input. Under capillary action and a surface tension gradient, it can fill the entire channel of the microfluidic device. The surface-tension gradient-driven fluid flow can accelerate the pre-enrichment and shorten the detection time. An induced graphene electrode (LIG) was prepared using a laser method, and flower-cluster-shaped ZnO nanorods (FC-ZnONRs) were decorated on the LIG. The microfluidic system exhibited a good detection performance for Cu(II) in the range of 1–2100 μg/L, with a low detection limit of 0.0368 μg/L. In addition, portable microfluidic systems have been successfully used for the detection of Cu(II) in human sweat in conjunction with portable workstations and smartphones (Fig. 7b) (Zhang, Ma, et al., 2024). Hu et al. proposed an microfluidic paper-based analysis device (mPAD) based on IIPs for the detection of Cd(II) in water. rGO was modified into a paper-based screen-printed carbon electrode (pSPCE), and the linear range of this sensor was 1–100 ng/mL, with a detection limit of 0.05 ng/mL. When spiked into water samples, the recovery rate was 96.5 % ∼ 114.2 % (Fig. 7c) (Hu et al., 2024). The combination of various electrochemical strategies makes up for the deficiency of a single strategy and makes the performance of the sensor better. This may also be an important direction for future electrochemical sensors.
Fig. 7.
(A) Flexible integrated MoS2 chemical sensor arrays fabrication processes (Zhang et al., 2024). Copyright 2019, Elsevier. (B) The fabrication process of the microfluidic device and the electrochemical sensing Cu(II) (Hu et al., 2024). Copyright 2024, Elsevier. (C) μPAD with IIPs for the detection of Cd(II) (P. Pathak, H.J. Cho, Graphene Oxide-Chitosan Composite-Based Flexible Electrochemical Sensors for Lead ION Detection, 2021). Copyright 2024, Royal Society of Chemistry.
This section is the key part of this review, this section mainly focuses on the latest advancements in the field of heavy metal ions detection using electrochemical sensing strategy, which have been conducted comprehensively over the last decade (Table 1). Through the summary of the table, we can intuitively understand the various nanomaterials and technologies applied in electrochemical sensors.
Table 1.
Summary of heavy metal ions detection using electrochemical sensors.
| Metals | Type | Linear Range | LOD | Samples | Recovery | Refs. |
|---|---|---|---|---|---|---|
| Pb(II) | LSV | 0.05–60 μM | 0.01 μM | Water and rice | 95–103 % | (Hu et al., 2016) |
| Cd(II) | DPV | 10.1–19,620 μg/L | 4.95 μg/L | Water | / | (Coelho et al., 2017) |
| Zn(II) | DPV | 6.12–45.9 nM | 13.51 nM | Water | / | (Khairnar et al., 2020) |
| Cd(II) | CR | 2–200 ppb | 0.83 ppb | Water | 94.5–113.5 % | (Hu et al., 2021) |
| Cr(III) | EIS | 0.1–10 μM | 17.6 nM | Water and urine | / | (Alizadeh et al., 2017) |
| Cr(III) | DPV | 1–5 ppm | 0.051 μM | Metal plating industry | 99.6 % | (Aravind & Mathew, 2018) |
| As(III) | CV | 0.02–9 nM | 7.1 pM | Water | 90.1–109.2 % | (Ma et al., 2020) |
| Cd(II) | DPASV | 0.5–40 μg/L | 0.15 μg/L | Water | 97.0–101.7 % | (Ghanei-Motlagh & Taher, 2017) |
| Cu(II) | DPV | 5 μM–5 mM | 5.43 μM | Water | / | (Neethipathi et al., 2023) |
| Pb(II) | SWASV | 2–14 ppb | 0.81 ppb | Water | / | (P. Pathak, H.J. Cho, Graphene Oxide-Chitosan Composite-Based Flexible Electrochemical Sensors for Lead ION Detection, 2021) |
| Cu(II), Pb(II) and Hg(II) | SWV | 0.2 nM − 1.5 μM, 0.2–100 nM, 0.1–10 nM | 5.4 pM, 22 pM and 17.8 pM | Mineral water and tap water | 90–95 % | (Zhao et al., 2020) |
| Pb(II) and Cd(II) | SWASV | 5.0–50.0 μg/L | 0.8 μg/L and 0.5 μg/L | Soil | 99.07 %, 98.95 % | (Zhao et al., 2022) |
| Zn(II), Cd(II) and Pb(II) | SWV | 0–3500 μg/L, 0–5000 μg/L, 0–2000 μg/L | 6.32 ppb, 15.04 ppb and 9.08 ppb | Human sweat, seawater and cosmetics | / | (Jiang et al., 2020) |
| Pb(II) and Cd(II) | SWASV | 50–300 μg/L and 100–300 μg/L | 2.20 μg/L and 1.51 μg/L | Enameled cup, ceramic Teapot and dark-red enameled pottery |
93.3–119.1 %, 98.7–107.2 % | (Xu, Li, et al., 2020) |
| Cu(II) and Zn(II) | SWASV | 10–500 ppb and 350–850 ppb | 0.1 ppb and 1.5 ppb | Soil, mackerel, crayfish and water | 96.2–100.4 % | (Hui et al., 2020) |
| Zn(II), Cd(II) and Pb(II) | SWASV | 10–1200 ppb, 0.5–1200 ppb and 0.5 ppb–2.1 g/L | 5 ppb, 0.05 ppb and 0.02 ppb | Tap water and lake | 94.9–100.7 %, 105–105.8 %, 105.5–106.1 % | (Hu et al., 2023) |
| Cu(II) | EC | 0.5 μM–1.0 mM | 80.0 nM | Serum and whole blood | 92.0–105.0 % | (Kamel et al., 2021) |
| Pb(II) | SWV | 10–500 μg/L | 2 μg/L | Toys samples | / | (Feng et al., 2013) |
| Pb(II) and Cd(II) | SWV | 10–100 ppb | 7 ppb and 11 ppb | Water | / | (Medina-Sanchez et al., 2015) |
| Zn(II), Cd(II) and Pb(II) | SWASV | 0.1–300 ppb | 1 ppb, 0.1 ppb and 0.1 ppb | Human serum | 93.8–109.7 % | (Ruecha et al., 2015) |
| Cu(II) | DPV | 2.5–500 μg/L | 0.9 μg/L | Seawater | 94.0–102.0 % | (Wang, Liu, et al., 2019) |
| Cu(II) | DPV | 10 nM–10 mM | 0.2 μM | Water | 99.9–102.2 % | (Hu et al., 2020) |
| Pb(II) | ACV | 0.1–5 μM | 45.8 pM | Serum | / | (Ma et al., 2018) |
| Cu(II) | DPV and fluorescence | 0–9 equiv. and 0–10 equiv | 2.5 ppb | / | / | (Karakuş et al., 2020) |
| Pb(II) | EIS and fluorescence | 5 nM–1 μM | 8.7 pM and 3.3 nM | Tap water and soluble | 97.3–103.0 % | (Chen et al., 2021) |
| Cd(II) and Pb(II) | EIS and fluorescence | 3.1–9.8 μg/L and 10.0–49.1 μg/L | 2.82 μg/L | Tap water | 99.18 %, 101.60 % | (Wang et al., 2022) |
| Hg(II) and Cu(II) | Colorimetric and CV | 50–200 nM | 45 nM and 50 nM(Colorimetric), 293 nM and 173 nM(CV) | Drinking water, spring water and wastewater | 98.2–103.6 % | (Erdemir et al., 2023) |
| Pb(II), Cd(II) and Cu(II) |
ASV and colorimetric | 0.5–70 ng/mL, 0.5–70 ng/mL and 10–350 ng/mL | 0.1 ng/mL, 0.1 ng/mL and 4.12 ng/mL | Stream water, groundwater, rice and fish | 82.2–102.6 %, 81.6–102.9 %, 71.7–96.9 % | (Chaiyo et al., 2016) |
| Pb(II) | DPV | 0.01 nM–5 μM | 3.1 pM | Wastewater | 95.12–109.4 % | (Huang et al., 2021) |
| Cu(II), Cd(II), Hg(II) and Pb(II) | SWASV | 0.1–200.0 ppb | 0.04 ppb,0.29 ppb,0.07 ppb and 0.14 ppb | Water | / | (Hossain et al., 2023) |
| As(III) | LSV | 1–150 μg/L | 0.42 μg/L | Water | 90.0–98.2 % | (Giménez-Gómez et al., 2019b) |
| Cd(II) and Pb(II) | DPASV | 0.5–8 μg/L and 10–100 μg/L | 0.5 μg/L, 0.2 μg/L | Water | 94.7–102.2 %, 93.2–101.9 % | (Dong et al., 2016) |
| Cd(II) | EC | 50–500 μg/mL | 5 ng/mL | Tap water | / | (Li et al., 2019) |
| Cu(II) | DPV | 1–2100 μg/L | 0.0368 μg/L | Sweat | 96.5–101.6 % | (Zhang, Ma, et al., 2024) |
| Cd(II) | SWASV | 1–100 ng/mL | 0.05 ng/mL | Water | 96.5–114.2 % | (Hu et al., 2024) |
5. Conclusion and Prospect
The design and construction of heavy metal electrochemical sensing platform has always been a hot field of interest for researchers. In this review, nanomaterials and common electrochemical techniques are briefly described. On this basis, electrochemical sensing strategies are discussed, including IIPs-based, flexible, paper-based, ratio, dual-mode, microfluidic and composite strategies. We summarize, illustrate and highlight the importance of these sensing strategies for the construction of electrochemical sensing platforms, which, through clever design and innovation, can increase sensitivity and reduce detection limits, showing great potential for complex sample matrix detection (Li, Chen, Deng, & Gao, 2022; Manikandan et al., 2024). In summary, the combination of these new strategies, electrochemical techniques and nanomaterials solves the challenges faced by traditional electrochemical analysis and promotes a new trend in food safety sensing (Xu, Liu, Xiao, Jiang, & Yi, 2020).
Electrochemical sensors have made great progress on the basis of new technologies and strategies, but there are still challenges in performance, materials, practical applications and commercialization. In terms of sensor performance, when detecting multiple substances at the same time, the sensitivity and selectivity of the electrochemical sensor may cause interference, affecting the accuracy of the detection (del Campo, 2023). In terms of material preparation, there are problems such as complicated process, not environmental protection, not green, and high cost. In addition, the sensor portability, miniaturization and integration face the challenges of weak electrode signal and difficult manufacturing (Yang, Shen, Duan, Liu, & Wang, 2025). In practical applications, complex real samples can interfere with detection, and field measurements need to adapt to harsh environments and respond quickly (Ghaani, Azimzadeh, Büyüktaş, Carullo, & Farris, 2024). Finally, the process of commercializing electrochemical sensors is difficult. When transforming from the laboratory to the market, it is necessary to solve many problems such as production process and quality control (Harun-Or-Rashid, Aktar, Preda, & Nasiri, 2024).
In the future, these platforms will need deeper requirements: integrating environmental protection concepts into detection operations, strengthening real-time monitoring and preventive measures, and using advanced technology for data processing. Firstly, with the growing awareness of global environmental protection, the development of electrochemical sensors should increasingly emphasize the use of decomposable and recyclable materials, renewable energy sources, and reduction of reagent volumes and waste liquids to achieve more environmentally friendly heavy-metal detection (Jiao et al., 2024; Zhao & Liu, 2018). Then, the future direction of electrochemical sensor technology will focus on portable real-time monitoring and application in the field to ensure that effective measures are taken quickly in the early stages of pollution, ensure full-chain protection, effectively reduce the risk of environmental pollution and protect public health and safety. Lastly, with the latest wireless technologies, including Bluetooth, NFC and 5G, the instant collection and rapid analysis of monitoring data can be achieved. By leveraging AI and machine learning algorithms, the sensing platform can automatically interpret detection data, identify data patterns and predict future trends. In the future, electrochemical sensors will support environmental monitoring, food safety testing and public health more effectively (Gu et al., 2022; Mukherjee et al., 2021; Wang et al., 2024).
CRediT authorship contribution statement
Yu Tian: Writing – review & editing, Writing – original draft, Investigation, Formal analysis. Jinli Liu: Writing – original draft, Visualization, Investigation, Formal analysis. Jiali Qiao: Visualization, Methodology, Investigation. Fuguo Ge: Investigation, Formal analysis. Yukun Yang: Writing – review & editing, Supervision, Formal analysis. Qi Zhang: Resources, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Science and Technology Plan Project of China Tobacco Industry Development Center (No. ZYSYQ-2022-3), National Natural Science Foundation of China (No. 32072301), Excellent Youth Cultivation Project of Shanxi (No. 20210302122004), Key R&D Plan of Shanxi (Agricultural Field, No. 202102140601018), the Open Project Program of Xinghuacun College of Shanxi University (Shanxi Institute of Brewing Technology and Industry) (No. XCSXU-KF-202306).
Contributor Information
Yukun Yang, Email: yangyukun@sxu.edu.cn.
Qi Zhang, Email: 95204601@qq.com.
Data availability
No data was used for the research described in the article.
References
- Abdul Halim A., Sulaiman S.S., Nordin A.N., Bajunaid Hariz H. Systematic review study on application of ion imprinted polymer (IIP) in heavy metals detection. International Journal of Environmental Analytical Chemistry. 2022:1–25. doi: 10.1080/03067319.2022.2106426. [DOI] [Google Scholar]
- Alizadeh T., Rafiei F., Hamidi N., Ganjali M.R. A new electrochemical sensing platform for Cr(III) determination based on nano-structured Cr(III)-imprinted polymer-modified carbon composite electrode. Electrochimica Acta. 2017;247:812–819. doi: 10.1016/j.electacta.2017.07.081. [DOI] [Google Scholar]
- Aravind A., Mathew B. Electrochemical sensor based on nanostructured ion imprinted polymer for the sensing and extraction of Cr(III) ions from industrial wastewater. Polymer International. 2018;67(12):1595–1604. doi: 10.1002/pi.5683. [DOI] [Google Scholar]
- Ayenimo J.G., Adeloju S.B. Rapid amperometric detection of trace metals by inhibition of an ultrathin polypyrrole-based glucose biosensor. Talanta. 2016;148:502–510. doi: 10.1016/j.talanta.2015.11.024. [DOI] [PubMed] [Google Scholar]
- Bansod B., Kumar T., Thakur R., Rana S., Singh I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosensors & Bioelectronics. 2017;94:443–455. doi: 10.1016/j.bios.2017.03.031. [DOI] [PubMed] [Google Scholar]
- Bassyouni M., Mansi A.E., Elgabry A., Ibrahim B.A., Kassem O.A., Alhebeshy R. Utilization of carbon nanotubes in removal of heavy metals from wastewater: A review of the CNTs' potential and current challenges. Applied Physics A. 2019;126(1) doi: 10.1007/s00339-019-3211-7. [DOI] [Google Scholar]
- Beitollahi H., Khalilzadeh M.A., Tajik S., Safaei M., Zhang K., Jang H.W., Shokouhimehr M. Recent advances in applications of Voltammetric sensors modified with ferrocene and its derivatives. ACS Omega. 2020;5(5):2049–2059. doi: 10.1021/acsomega.9b03788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buledi J.A., Amin S., Haider S.I., Bhanger M.I., Solangi A.R. A review on detection of heavy metals from aqueous media using nanomaterial-based sensors. Environmental Science and Pollution Research International. 2021;28(42):58994–59002. doi: 10.1007/s11356-020-07865-7. [DOI] [PubMed] [Google Scholar]
- del Campo F.J. Self-powered electrochemical sensors. Current Opinion in Electrochemistry. 2023;41 doi: 10.1016/j.coelec.2023.101356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaiyo S., Apiluk A., Siangproh W., Chailapakul O. High sensitivity and specificity simultaneous determination of lead, cadmium and copper using μPAD with dual electrochemical and colorimetric detection. Sensors and Actuators B: Chemical. 2016;233:540–549. doi: 10.1016/j.snb.2016.04.109. [DOI] [Google Scholar]
- Chen G., Bai W., Jin Y., Zheng J. Fluorescence and electrochemical assay for bimodal detection of lead ions based on metal-organic framework nanosheets. Talanta. 2021;232 doi: 10.1016/j.talanta.2021.122405. [DOI] [PubMed] [Google Scholar]
- Chen Z., Xie M., Zhao F., Han S. Application of nanomaterial modified aptamer-based electrochemical sensor in detection of heavy metal ions. Foods. 2022;11(10) doi: 10.3390/foods11101404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Zhang S., Liu Y., Alharbi N.S., Rabah S.O., Wang S., Wang X. Synthesis and fabrication of g-C(3)N(4)-based materials and their application in elimination of pollutants. Sci Total Environ. 2020;731 doi: 10.1016/j.scitotenv.2020.139054. [DOI] [PubMed] [Google Scholar]
- Coelho M.K.L., De Oliveira H.L., De Almeida F.G., Borges K.B., Tarley C.R.T., Pereira A.C. 2017. Development of carbon paste electrode modified with cadmium ion-imprinted polymer for selective voltammetric determination of Cd2+ International journal of environmental analytical chemistry 97 (14–15) pp. 1378–1392. [DOI] [Google Scholar]
- Cui W., Ren Z., Song Y., Ren C.L. Development and potential for point-of-care heavy metal sensing using microfluidic systems: A brief review. Sensors and Actuators A: Physical. 2022;344 doi: 10.1016/j.sna.2022.113733. [DOI] [Google Scholar]
- Dahaghin Z., Kilmartin P.A., Mousavi H.Z. Determination of cadmium(II) using a glassy carbon electrode modified with a cd-ion imprinted polymer. Journal of Electroanalytical Chemistry. 2018;810:185–190. doi: 10.1016/j.jelechem.2018.01.014. [DOI] [Google Scholar]
- Di Masi S., Pennetta A., Guerreiro A., Canfarotta F., De Benedetto G.E., Malitesta C. Sensor based on electrosynthesised imprinted polymeric film for rapid and trace detection of copper(II) ions. Sensors and Actuators B: Chemical. 2020;307 doi: 10.1016/j.snb.2019.127648. [DOI] [Google Scholar]
- Ding R., Cheong Y.H., Ahamed A., Lisak G. Heavy metals detection with paper-based electrochemical sensors. Analytical Chemistry. 2021;93(4):1880–1888. doi: 10.1021/acs.analchem.0c04247. [DOI] [PubMed] [Google Scholar]
- Ding R., Cheong Y.H., Zhao K., Lisak G. Acidified paper substrates for microfluidic solution sampling integrated with potentiometric sensors for determination of heavy metals. Sensors and Actuators B: Chemical. 2021;347 doi: 10.1016/j.snb.2021.130567. [DOI] [PubMed] [Google Scholar]
- Ding Z.J., Liu Y., Weerasooriya R., Chen X. Electrochemical determination of chromium (VI) with au/UiO-66 modified glassy carbon and screen-printed electrodes by linear sweep voltammetry (LSV) Analytical Letters. 2024;57(5):753–771. doi: 10.1080/00032719.2023.2222425. [DOI] [Google Scholar]
- Dong X., Hong Y., Wu M., Chen G., Dai Z., Zhang Y., Chen G. 3D printed microfluidic device with microporous Mn(2)O(3)-modified screen printed electrode for real-time determination of heavy metal ions. ACS Applied Materials & Interfaces. 2016;8(48):32940–32947. doi: 10.1021/acsami.6b10464. [DOI] [PubMed] [Google Scholar]
- Erdemir E., Suna G., Liv L., Eğlence-Bakır S., Şahin M., Karakuş E. Smartphone-assisted dual-channel discriminative detection of hg(II) and cu(II) ions with a simple, unique, readily available probe. Sensors and Actuators B: Chemical. 2023;382 doi: 10.1016/j.snb.2023.133487. [DOI] [Google Scholar]
- Feng Q.M., Zhang Q., Shi C.G., Xu J.J., Bao N., Gu H.Y. Using nanostructured conductive carbon tape modified with bismuth as the disposable working electrode for stripping analysis in paper-based analytical devices. Talanta. 2013;115:235–240. doi: 10.1016/j.talanta.2013.04.071. [DOI] [PubMed] [Google Scholar]
- Ferrari A.G.M., Carrington P., Rowley-Neale S.J., Banks C.E. Recent advances in portable heavy metal electrochemical sensing platforms. Environmental Science: Water Research & Technology. 2020;6(10):2676–2690. doi: 10.1039/D0EW00407C. [DOI] [Google Scholar]
- Fiyadh S.S., AlSaadi M.A., Jaafar W.Z., AlOmar M.K., Fayaed S.S., Mohd N.S., Hin L.S., El-Shafie A. Review on heavy metal adsorption processes by carbon nanotubes. Journal of Cleaner Production. 2019;230:783–793. doi: 10.1016/j.jclepro.2019.05.154. [DOI] [Google Scholar]
- Gao F., Liu C., Zhang L., Liu T., Wang Z., Song Z., Xue N. Wearable and flexible electrochemical sensors for sweat analysis: A review. Microsystems & Nanoengineering. 2023;9(1):1–21. doi: 10.1038/s41378-022-00443-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaani M., Azimzadeh M., Büyüktaş D., Carullo D., Farris S. Electrochemical sensors in the food sector: A review. Journal of Agricultural and Food Chemistry. 2024;72(44):24170–24190. doi: 10.1021/acs.jafc.4c09423. [DOI] [PubMed] [Google Scholar]
- Ghanei-Motlagh M., Taher M.A. Novel imprinted polymeric nanoparticles prepared by sol–gel technique for electrochemical detection of toxic cadmium(II) ions. Chemical Engineering Journal. 2017;327:135–141. doi: 10.1016/j.cej.2017.06.091. [DOI] [Google Scholar]
- Giménez-Gómez P., Baldi A., Ayora C., Fernandez-Sanchez C. Automated determination of as (III) in waters with an electrochemical sensor integrated into a modular microfluidic system. ACS Sensors. 2019;4(12):3156–3165. doi: 10.1021/acssensors.9b01286. [DOI] [PubMed] [Google Scholar]
- Giménez-Gómez P., Baldi A., Ayora C., Fernandez-Sanchez C. Automated determination of as (III) in waters with an electrochemical sensor integrated into a modular microfluidic system. ACS Sensors. 2019;4(12):3156–3165. doi: 10.1021/acssensors.9b01286. [DOI] [PubMed] [Google Scholar]
- Gu Y., Li Y., Ren D., Sun L., Zhuang Y., Yi L., Wang S. Recent advances in nanomaterial-assisted electrochemical sensors for food safety analysis. Food Frontiers. 2022;3(3):453–479. doi: 10.1002/fft2.143. [DOI] [Google Scholar]
- Gudić S., Krišto N. Determination of Cu2+ ions concentration by electrochemical methods. Kemija u Industriji. 2023;5-6 doi: 10.15255/kui.2022.061. [DOI] [Google Scholar]
- Gulaboski R. Future of voltammetry. Macedonian Journal of Chemistry and Chemical Engineering. 2022;41(2) doi: 10.20450/mjcce.2022.2555. [DOI] [Google Scholar]
- Gumpu M.B., Sethuraman S., Krishnan U.M., Rayappan J.B.B. A review on detection of heavy metal ions in water – An electrochemical approach. Sensors and Actuators B: Chemical. 2015;213:515–533. doi: 10.1016/j.snb.2015.02.122. [DOI] [Google Scholar]
- Gupte T., Jana S.K., Mohanty J.S., Srikrishnarka P., Mukherjee S., Ahuja T., Sudhakar C., Thomas T., Pradeep T. Highly sensitive as(3+) detection using electrodeposited nanostructured MnO(x) and phase evolution of the active material during sensing. ACS Applied Materials & Interfaces. 2019;11(31):28154–28163. doi: 10.1021/acsami.9b06023. [DOI] [PubMed] [Google Scholar]
- Harun-Or-Rashid M., Aktar M.N., Preda V., Nasiri N. Advances in electrochemical sensors for real-time glucose monitoring. Sensors & Diagnostics. 2024;3(6):893–913. doi: 10.1039/D4SD00086B. [DOI] [Google Scholar]
- Hossain M.M., Karim M.M., Seo K.D., Park D.S., Shim Y.B. Capillary and electrodynamic forces-driven separation detection of metal ions using a disposable microfluidic sensor with a composite electrode. Analytical Chemistry. 2023;95(45):16701–16709. doi: 10.1021/acs.analchem.3c03518. [DOI] [PubMed] [Google Scholar]
- Hu J., Sedki M., Shen Y., Mulchandani A., Gao G. Chemiresistor sensor based on ion-imprinted polymer (IIP)-functionalized rGO for cd(II) ions in water. Sensors and Actuators B: Chemical. 2021;346 doi: 10.1016/j.snb.2021.130474. [DOI] [Google Scholar]
- Hu J., Wang L., Song Y., Li Y., Shen Y., Gao G., Mulchandani A. Ion imprinted polymers integrated into a multi-functional microfluidic paper-based analytical device for trace cadmium detection in water. Analytical Methods. 2024;16(2):179–188. doi: 10.1039/D3AY01787G. [DOI] [PubMed] [Google Scholar]
- Hu L., Zhang L., Zhou Y., Meng G., Yu Y., Yao W., Yan Z. Chitosan-stabilized gold nano composite modified glassy carbon electrode for electrochemical sensing trace Hg2+ in practice. Journal of the Electrochemical Society. 2018;165(16):B900. [Google Scholar]
- Hu M., He H., Xiao F., Liu C. Bi-MOF-derived carbon wrapped bi nanoparticles assembly on flexible graphene paper electrode for electrochemical sensing of multiple heavy metal ions. Nanomaterials (Basel) 2023;13(14) doi: 10.3390/nano13142069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R., Zhang X., Chi K.N., Yang T., Yang Y.H. Bifunctional MOFs-based Ratiometric electrochemical sensor for multiplex heavy metal ions. ACS Applied Materials & Interfaces. 2020;12(27):30770–30778. doi: 10.1021/acsami.0c06291. [DOI] [PubMed] [Google Scholar]
- Hu S., Xiong X., Huang S., Lai X. Preparation of Pb (II) ion imprinted polymer and its application as the interface of an electrochemical sensor for trace lead determination. Analytical Sciences. 2016;32(9):975–980. doi: 10.2116/analsci.32.975. [DOI] [PubMed] [Google Scholar]
- Huang M.R., Ding Y.B., Li X.G. Combinatorial screening of potentiometric Pb(II) sensors from polysulfoaminoanthraquinone solid ionophore. ACS Combinatorial Science. 2014;16(3):128–138. doi: 10.1021/co400140g. [DOI] [PubMed] [Google Scholar]
- Huang Y., Han Y., Gao Y., Gao J., Ji H., He Q., Tu J., Xu G., Zhang Y., Han L. Electrochemical sensor array with nanoporous gold nanolayer and ceria@gold corona-nanocomposites enhancer integrated into microfluidic for simultaneous ultrasensitive lead ion detection. Electrochimica Acta. 2021;373 doi: 10.1016/j.electacta.2021.137921. [DOI] [Google Scholar]
- Hui X., Sharifuzzaman M., Sharma S., Xuan X., Zhang S., Ko S.G., Yoon S.H., Park J.Y. High-performance flexible electrochemical heavy metal sensor based on layer-by-layer assembly of Ti(3)C(2)T(x)/MWNTs nanocomposites for noninvasive detection of copper and zinc ions in human biofluids. ACS Applied Materials & Interfaces. 2020;12(43):48928–48937. doi: 10.1021/acsami.0c12239. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Cui S., Xia T., Sun T., Tan H., Yu F., Su Y., Wu S., Wang D., Zhu N. Real-time monitoring of heavy metals in healthcare via twistable and washable Smartsensors. Analytical Chemistry. 2020;92(21):14536–14541. doi: 10.1021/acs.analchem.0c02723. [DOI] [PubMed] [Google Scholar]
- Jiao B., Wang L., Gui H., Ni Z., Du R., Hu Y. Study on the green extraction of corncob xylan by deep eutectic solvent. Grain & Oil Science and Technology. 2024;7(1):50–59. doi: 10.1016/j.gaost.2024.01.003. [DOI] [Google Scholar]
- Kamel A.H., Amr A.E.E., Almehizia A.A., Elsayed E.A., Moustafa G.O. Low-cost potentiometric paper-based analytical device based on newly synthesized macrocyclic pyrido-pentapeptide derivatives as novel ionophores for point-of-care copper(ii) determination. RSC Advances. 2021;11(44):27174–27182. doi: 10.1039/d1ra04712d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakuş E., Gunduz S., Liv L., Ozturk T. Fluorescent and electrochemical detection of cu (II) ions in aqueous environment by a novel, simple and readily available AIE probe. Journal of Photochemistry and Photobiology A: Chemistry. 2020;400 doi: 10.1016/j.jphotochem.2020.112702. [DOI] [Google Scholar]
- Karthika A., Nikhil S., Suganthi A., Rajarajan M. A facile sonochemical approach based on graphene carbon nitride doped silver molybdate immobilized nafion for selective and sensitive electrochemical detection of chromium (VI) in real sample. Advanced Powder Technology. 2020;31(5):1879–1890. doi: 10.1016/j.apt.2020.02.021. [DOI] [Google Scholar]
- Kempahanumakkagari S., Deep A., Kim K.H., Kumar Kailasa S., Yoon H.O. Nanomaterial-based electrochemical sensors for arsenic - a review. Biosensors & Bioelectronics. 2017;95:106–116. doi: 10.1016/j.bios.2017.04.013. [DOI] [PubMed] [Google Scholar]
- Khairnar N.A., Jirimali H.D., Patil K.P., Gite V.V. Zinc ion-imprinted polymer based on silica particles modified carbon paste electrodes for highly selective electrochemical determination of zinc ions. Polymer-Plastics Technology and Materials. 2020;59(15):1698–1714. doi: 10.1080/25740881.2020.1765381. [DOI] [Google Scholar]
- Koo W.T., Kim S.J., Jang J.S., Kim D.H., Kim I.D. Catalytic Metal Nanoparticles Embedded in Conductive Metal-Organic Frameworks for Chemiresistors: Highly Active and Conductive Porous Materials. Adv Sci (Weinh) 2019;6(21) doi: 10.1002/advs.201900250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lace A., Cleary J. A review of microfluidic detection strategies for heavy metals in water. Chemosensors. 2021;9(4) doi: 10.3390/chemosensors9040060. [DOI] [Google Scholar]
- Li B., Xie X., Meng T., Guo X., Li Q., Yang Y., Jin H., Jin C., Meng X., Pang H. Recent advance of nanomaterials modified electrochemical sensors in the detection of heavy metal ions in food and water. Food Chemistry. 2024;440 doi: 10.1016/j.foodchem.2023.138213. [DOI] [PubMed] [Google Scholar]
- Li H., Zhao J., Zhao S., Cui G. Simultaneous determination of trace Pb(II), cd(II), and Zn(II) using an integrated three-electrode modified with bismuth film. Microchemical Journal. 2021;168 doi: 10.1016/j.microc.2021.106390. [DOI] [Google Scholar]
- Li L., Chen S., Deng M., Gao Z. Optical techniques in non-destructive detection of wheat quality: A review. Grain & Oil Science and Technology. 2022;5(1):44–57. doi: 10.1016/j.gaost.2021.12.001. [DOI] [Google Scholar]
- Li P., Zhang D., Wu Z. Flexible MoS2 sensor arrays for high performance label-free ion sensing. Sensors and Actuators A: Physical. 2019;286:51–58. doi: 10.1016/j.sna.2018.12.026. [DOI] [Google Scholar]
- Li S.-S., Zhou W.-Y., Jiang M., Li L.-N., Sun Y.-F., Guo Z., Liu J.-H., Huang X.-J. Insights into diverse performance for the electroanalysis of Pb(II) on Fe2O3 nanorods and hollow nanocubes: Toward analysis of adsorption sites. Electrochimica Acta. 2018;288:42–51. doi: 10.1016/j.electacta.2018.08.069. [DOI] [Google Scholar]
- Li X., Zhang Z.-Y., Li F. Flexible electrochemical sensors based on nanomaterials: Constructions, applications and prospects. Chemical Engineering Journal. 2025;504 doi: 10.1016/j.cej.2024.158101. [DOI] [Google Scholar]
- Li Y., Chen X., Lin Y., Yang Y., Zhang L., Zhao P., Wang C., Fei J., Xie Y. Detection of catechins in tea beverages using a novel electrochemical sensor based on cyclodextrin nanosponges composite. eFood. 2023;4(2) doi: 10.1002/efd2.64. [DOI] [Google Scholar]
- Liang Y., Ma M., Zhang F., Liu F., Lu T., Liu Z., Li Y. Wireless microfluidic sensor for metal ion detection in water. ACS Omega. 2021;6(13):9302–9309. doi: 10.1021/acsomega.1c00941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisak G. Reliable environmental trace heavy metal analysis with potentiometric ion sensors - reality or a distant dream. Environmental Pollution. 2021;289 doi: 10.1016/j.envpol.2021.117882. [DOI] [PubMed] [Google Scholar]
- Liu X., Yao Y., Ying Y., Ping J. Recent advances in nanomaterial-enabled screen-printed electrochemical sensors for heavy metal detection. TrAC Trends in Analytical Chemistry. 2019;115:187–202. doi: 10.1016/j.trac.2019.03.021. [DOI] [Google Scholar]
- Liu Y., Zhang D., Ding J., Hayat K., Yang X., Zhan X., Zhang D., Lu Y., Zhou P. Label-free and sensitive determination of cadmium ions using a Ti-modified co(3)O(4)-based electrochemical Aptasensor. Biosensors (Basel) 2020;10(12) doi: 10.3390/bios10120195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R.-N., Wang L.-L., Zhang M., Jia L.-P., Zhang W., Shang L., Jia W.-L., Wang H.-S. A novel one-step triggered “signal-on/off” electrochemical sensing platform for lead based on the dual-signal ratiometric output and electrode-bound DNAzyme assembly. Sensors and Actuators B: Chemical. 2018;257:678–684. doi: 10.1016/j.snb.2017.10.158. [DOI] [Google Scholar]
- Ma W., Chang Q., Zhao J., Ye B.C. Novel electrochemical sensing platform based on ion imprinted polymer with nanoporous gold for ultrasensitive and selective determination of as(3) Mikrochimica Acta. 2020;187(10):571. doi: 10.1007/s00604-020-04552-9. [DOI] [PubMed] [Google Scholar]
- Manikandan R., Rajarathinam T., Jayaraman S., Jang H.-G., Yoon J.-H., Lee J., Paik H.-J., Chang S.-C. Recent advances in miniaturized electrochemical analyzers for hazardous heavy metal sensing in environmental samples. Coordination Chemistry Reviews. 2024;499 doi: 10.1016/j.ccr.2023.215487. [DOI] [Google Scholar]
- Manikandan R., Yoon J.-H., Chang S.-C. Emerging trends in nanostructured materials-coated screen printed electrodes for the electrochemical detection of hazardous heavy metals in environmental matrices. Chemosphere. 2023;344 doi: 10.1016/j.chemosphere.2023.140231. [DOI] [PubMed] [Google Scholar]
- Mathew M., Radhakrishnan S., Vaidyanathan A., Chakraborty B., Rout C.S. Flexible and wearable electrochemical biosensors based on two-dimensional materials: Recent developments. Analytical and Bioanalytical Chemistry. 2021;413(3):727–762. doi: 10.1007/s00216-020-03002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Sanchez M., Cadevall M., Ros J., Merkoci A. Eco-friendly electrochemical lab-on-paper for heavy metal detection. Analytical and Bioanalytical Chemistry. 2015;407(28):8445–8449. doi: 10.1007/s00216-015-9022-6. [DOI] [PubMed] [Google Scholar]
- Meng R., Zhu Q., Long T., He X., Luo Z., Gu R., Wang W., Xiang P. The innovative and accurate detection of heavy metals in foods: A critical review on electrochemical sensors. Food Control. 2023;150 doi: 10.1016/j.foodcont.2023.109743. [DOI] [Google Scholar]
- Mukherjee S., Bhattacharyya S., Ghosh K., Pal S., Halder A., Naseri M., Mohammadniaei M., Sarkar S., Ghosh A., Sun Y., Bhattacharyya N. Sensory development for heavy metal detection: A review on translation from conventional analysis to field-portable sensor. Trends in Food Science & Technology. 2021;109:674–689. doi: 10.1016/j.tifs.2021.01.062. [DOI] [Google Scholar]
- Neethipathi D.K., Beniwal A., Bass A.M., Scott M., Dahiya R. MoS₂ modified screen printed carbon electrode based flexible electrochemical sensor for detection of copper ions in water. IEEE Sensors Journal. 2023;23(8):8146–8153. doi: 10.1109/jsen.2023.3257188. [DOI] [Google Scholar]
- Özbek O., Berkel C. Recent advances in potentiometric analysis: Paper–based devices. Sensors International. 2022;3 doi: 10.1016/j.sintl.2022.100189. [DOI] [Google Scholar]
- P. Pathak, H.J. Cho, Graphene Oxide-Chitosan Composite-Based Flexible Electrochemical Sensors for Lead ION Detection 21st international conference on solid-state sensors. Actuators and Microsystems (Transducers) 2021;2021:254–258. [Google Scholar]
- Peng Y., Wang Y., Li Y., He X. Polyethylenimine functionalized multi-walled carbon nanotubes for electrochemical detection of chromium(VI) Electroanalysis. 2016;28(9):2029–2036. doi: 10.1002/elan.201501175. [DOI] [Google Scholar]
- Petrucci R., Chiarotto I., Mattiello L., Passeri D., Rossi M., Zollo G., Feroci A.M. Graphene oxide: A smart (starting) material for natural Methylxanthines adsorption and detection. Molecules. 2019;24(23) doi: 10.3390/molecules24234247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power A.C., Gorey B., Chandra S., Chapman J. Carbon nanomaterials and their application to electrochemical sensors: A review. Nanotechnology Reviews. 2018;7(1):19–41. doi: 10.1515/ntrev-2017-0160. [DOI] [Google Scholar]
- Puthongkham P., Venton B.J. Recent advances in fast-scan cyclic voltammetry. Analyst. 2020;145(4):1087–1102. doi: 10.1039/c9an01925a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M.T., Kabir M.F., Gurung A., Reza K.M., Pathak R., Ghimire N., Baride A., Wang Z., Kumar M., Qiao Q. Graphene Oxide–Silver Nanowire Nanocomposites for Enhanced Sensing of Hg2+ ACS Applied Nano Materials. 2019;2(8):4842–4851. doi: 10.1021/acsanm.9b00789. [DOI] [Google Scholar]
- Rajendrachari S., Somashekharappa K.K., Mahale R.S., Vasanth S., Chikkegouda S.P. 2022. A review on cyclic voltammetric investigation of toxic heavy metals IntechOpen. [DOI] [Google Scholar]
- Ruecha N., Rodthongkum N., Cate D.M., Volckens J., Chailapakul O., Henry C.S. Sensitive electrochemical sensor using a graphene-polyaniline nanocomposite for simultaneous detection of Zn(II), cd(II), and Pb(II) Analytica Chimica Acta. 2015;874:40–48. doi: 10.1016/j.aca.2015.02.064. [DOI] [PubMed] [Google Scholar]
- Sawan S., Maalouf R., Errachid A., Jaffrezic-Renault N. Metal and metal oxide nanoparticles in the voltammetric detection of heavy metals: A review. TrAC Trends in Analytical Chemistry. 2020;131(116014) doi: 10.1016/j.trac.2020.116014. [DOI] [Google Scholar]
- Scandurra A., Mirabella S. Square wave anodic stripping voltammetry applied to a nano-electrode for trace analysis of Pb (II) and cd (II) ions in solution. IEEE Sensors Journal. 2021;21(20):22134–22142. doi: 10.1109/JSEN.2021.3051762. [DOI] [Google Scholar]
- Shan Q., Tian J., Ding Q., Wu W. Electrochemical sensor based on metal-free materials composed of graphene and graphene oxide for sensitive detection of cadmium ions in water. Materials Chemistry and Physics. 2022;284 doi: 10.1016/j.matchemphys.2022.126064. [DOI] [Google Scholar]
- Singh K., Maurya K.K., Malviya M. Review of electrochemical sensors and biosensors based on first-row transition metals. Their Oxides, and Noble Metals Nanoparticles, Journal of Analysis and Testing. 2024;8(2):143–159. doi: 10.1007/s41664-023-00292-w. [DOI] [Google Scholar]
- Singh S., Numan A., Somaily H.H., Dawsari M.M.A., Alqarni M.H.S., Alam A., Kumar P. A novel, eco-friendly multi-walled carbon nanotubes functionalized copper metal-organic framework for ultrasensitive potentiometric detection of cadmium ions, journal of environmental. Chemical Engineering. 2021;9(6) doi: 10.1016/j.jece.2021.106534. [DOI] [Google Scholar]
- Sonkoue B.M., Tchekwagep P.M.S., Nanseu-Njiki C.P., Ngameni E. Electrochemical determination of arsenic using silver nanoparticles. Electroanalysis. 2018;30(11):2738–2743. doi: 10.1002/elan.201800520. [DOI] [Google Scholar]
- Tan F., Cong L., Saucedo N.M., Gao J., Li X., Mulchandani A. An electrochemically reduced graphene oxide chemiresistive sensor for sensitive detection of hg(2+) ion in water samples. Journal of Hazardous Materials. 2016;320:226–233. doi: 10.1016/j.jhazmat.2016.08.029. [DOI] [PubMed] [Google Scholar]
- Tu J., Gan Y., Liang T., Wan H., Wang P. A miniaturized electrochemical system for high sensitive determination of chromium(VI) by screen-printed carbon electrode with gold nanoparticles modification. Sensors and Actuators B: Chemical. 2018;272:582–588. doi: 10.1016/j.snb.2018.06.006. [DOI] [Google Scholar]
- Tukur S., Azah Yusof N., Hajian R. Gold nanoparticles modified screen printed electrode for determination of Pb (II) ion using linear sweep anodic stripping voltammetry. IEEE Sensors Journal. 2014:2780–2784. doi: 10.1109/jsen.2014.2379283. [DOI] [Google Scholar]
- Waheed A., Mansha M., Ullah N. Nanomaterials-based electrochemical detection of heavy metals in water: Current status, challenges and future direction. TrAC Trends in Analytical Chemistry. 2018;105:37–51. doi: 10.1016/j.trac.2018.04.012. [DOI] [Google Scholar]
- Wang D., Liu Y., Qian J., Li Y., Hou L., Liu H. Discrimination of pressed sesame oil: A comparison study of non-targeted UV spectral fingerprints combined with different chemometric methods. Grain & Oil Science and Technology. 2024 doi: 10.1016/j.gaost.2024.08.001. [DOI] [Google Scholar]
- Wang W., Zhao J., Sun Y., Zhang H. Facile synthesis of g-C(3)N(4) with various morphologies for application in electrochemical detection. RSC Advances. 2019;9(14):7737–7746. doi: 10.1039/c8ra10166c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Lin W., Chen C., Kong L., Huang Z., Kirsanov D., Legin A., Wan H., Wang P. Neural networks based fluorescence and electrochemistry dual-modal sensor for sensitive and precise detection of cadmium and lead simultaneously. Sensors and Actuators B: Chemical. 2022;366 doi: 10.1016/j.snb.2022.131922. [DOI] [Google Scholar]
- Wang X., Liu G., Qi Y., Yuan Y., Gao J., Luo X., Yang T. Embedded au nanoparticles-based Ratiometric electrochemical sensing strategy for sensitive and reliable detection of copper ions. Analytical Chemistry. 2019;91(18):12006–12013. doi: 10.1021/acs.analchem.9b02945. [DOI] [PubMed] [Google Scholar]
- Xiao P., Zhu G., Shang X., Hu B., Zhang B., Tang Z., Yang J., Liu J. An Fe-MOF/MXene-based ultra-sensitive electrochemical sensor for arsenic(III) measurement. Journal of Electroanalytical Chemistry. 2022;916 doi: 10.1016/j.jelechem.2022.116382. [DOI] [Google Scholar]
- Xu G., Li X., Cheng C., Yang J., Liu Z., Shi Z., Zhu L., Lu Y., Low S.S., Liu Q. Fully integrated battery-free and flexible electrochemical tag for on-demand wireless in situ monitoring of heavy metals. Sensors and Actuators B: Chemical. 2020;310 doi: 10.1016/j.snb.2020.127809. [DOI] [Google Scholar]
- Xu T., Dai H., Jin Y. Electrochemical sensing of lead(II) by differential pulse voltammetry using conductive polypyrrole nanoparticles. Mikrochimica Acta. 2019;187(1):23. doi: 10.1007/s00604-019-4027-z. [DOI] [PubMed] [Google Scholar]
- Xu Z., Liu Z., Xiao M., Jiang L., Yi C. A smartphone-based quantitative point-of-care testing (POCT) system for simultaneous detection of multiple heavy metal ions. Chemical Engineering Journal. 2020;394 doi: 10.1016/j.cej.2020.124966. [DOI] [Google Scholar]
- Yan X., Chen H., Du G., Guo Q., Yuan Y., Yue T. Recent trends in fluorescent aptasensors for mycotoxin detection in food: Principles, constituted elements, types, and applications. Food Frontiers. 2022;3(3):428–452. doi: 10.1002/fft2.144. [DOI] [Google Scholar]
- Yang C., Denno M.E., Pyakurel P., Venton B.J. Recent trends in carbon nanomaterial-based electrochemical sensors for biomolecules: A review. Analytica Chimica Acta. 2015;887:17–37. doi: 10.1016/j.aca.2015.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Wang X., Zhang F., Yu L., Bai B., Zhang J., Zhang B., Tian Y., Qin S., Yang Y. Two birds with one stone: A universal design and application of signal-on labeled fluorescent/electrochemical dual-signal mode biosensor for the detection of tetracycline residues in tap water, milk and chicken. Food Chemistry. 2024;430 doi: 10.1016/j.foodchem.2023.136904. [DOI] [PubMed] [Google Scholar]
- Yang M., Guo Z., Li L.-N., Huang Y.-Y., Liu J.-H., Zhou Q., Chen X., Huang X.-J. Electrochemical determination of arsenic(III) with ultra-high anti-interference performance using au–cu bimetallic nanoparticles. Sensors and Actuators B: Chemical. 2016;231:70–78. doi: 10.1016/j.snb.2016.03.009. [DOI] [Google Scholar]
- Yang T., Shen T., Duan B., Liu Z., Wang C. In vivo electrochemical biosensing Technologies for Neurochemicals: Recent advances in electrochemical sensors and devices. ACS Sensors. 2025 doi: 10.1021/acssensors.4c03314. [DOI] [PubMed] [Google Scholar]
- Zhang M., Wang C., Xie Z., Gao B., Yu L. Chemical structures, analytical approaches and toxicological effects of oxidative derivatives of triglycerides as potential hazards in lipid thermal processing: A review. Grain & Oil Science and Technology. 2024 doi: 10.1016/j.gaost.2024.07.001. [DOI] [Google Scholar]
- Zhang Q., Ma S., Zhan X., Meng W., Wang H., Liu C., Zhang T., Zhang K., Su S. Smartphone-based wearable microfluidic electrochemical sensor for on-site monitoring of copper ions in sweat without external driving. Talanta. 2024;266(Pt 1) doi: 10.1016/j.talanta.2023.125015. [DOI] [PubMed] [Google Scholar]
- Zhao G., Liu G. A portable electrochemical system for the on-site detection of heavy metals in farmland soil based on electrochemical sensors. IEEE Sensors Journal. 2018;18(14):5645–5655. doi: 10.1109/jsen.2018.2845306. [DOI] [Google Scholar]
- Zhao G., Wang X., Liu G., Thuy N.T.D. A disposable and flexible electrochemical sensor for the sensitive detection of heavy metals based on a one-step laser-induced surface modification: A new strategy for the batch fabrication of sensors. Sensors and Actuators B: Chemical. 2022;350 doi: 10.1016/j.snb.2021.130834. [DOI] [Google Scholar]
- Zhao G.-Q., Zou J., Hu J., Long X., Jiao F.-P. A critical review on graphitic carbon nitride (g-C3N4)-based composites for environmental remediation. Separation and Purification Technology. 2021;279 doi: 10.1016/j.seppur.2021.119769. [DOI] [Google Scholar]
- Zhao J., Shi Y., Zhang M., Zhang L., Cui X., Zhu X., Su J., Jing D., Yang D. Preparation of eco-friendly high-performance manganese dioxide supercapacitors by linear sweep voltammetry. Journal of Renewable Materials. 2023;11(1):79–91. doi: 10.32604/jrm.2023.022030. [DOI] [Google Scholar]
- Zhao K., Ge L., Wong T.I., Zhou X., Lisak G. Gold-silver nanoparticles modified electrochemical sensor array for simultaneous determination of chromium(III) and chromium(VI) in wastewater samples. Chemosphere. 2021;281 doi: 10.1016/j.chemosphere.2021.130880. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Yang X., Pan P., Liu J., Yang Z., Wei J., Xu W., Bao Q., Zhang H., Liao Z. All-printed flexible electrochemical sensor based on polyaniline electronic ink for copper (II), Lead (II) and mercury (II) ion determination. Journal of Electronic Materials. 2020;49(11):6695–6705. doi: 10.1007/s11664-020-08418-x. [DOI] [Google Scholar]
- Zhou Y., Huang X., Hu X., Tong W., Leng Y., Xiong Y. Recent advances in colorimetry/fluorimetry-based dual-modal sensing technologies. Biosensors & Bioelectronics. 2021;190 doi: 10.1016/j.bios.2021.113386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.