Abstract
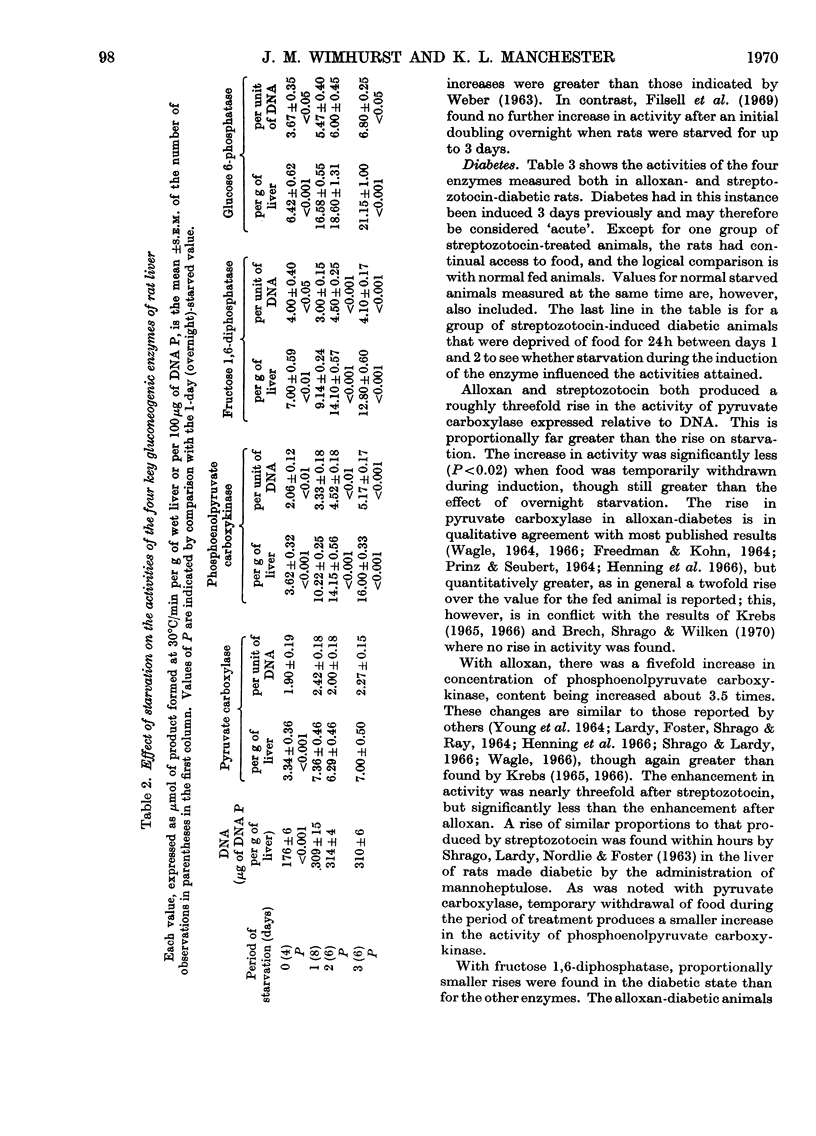
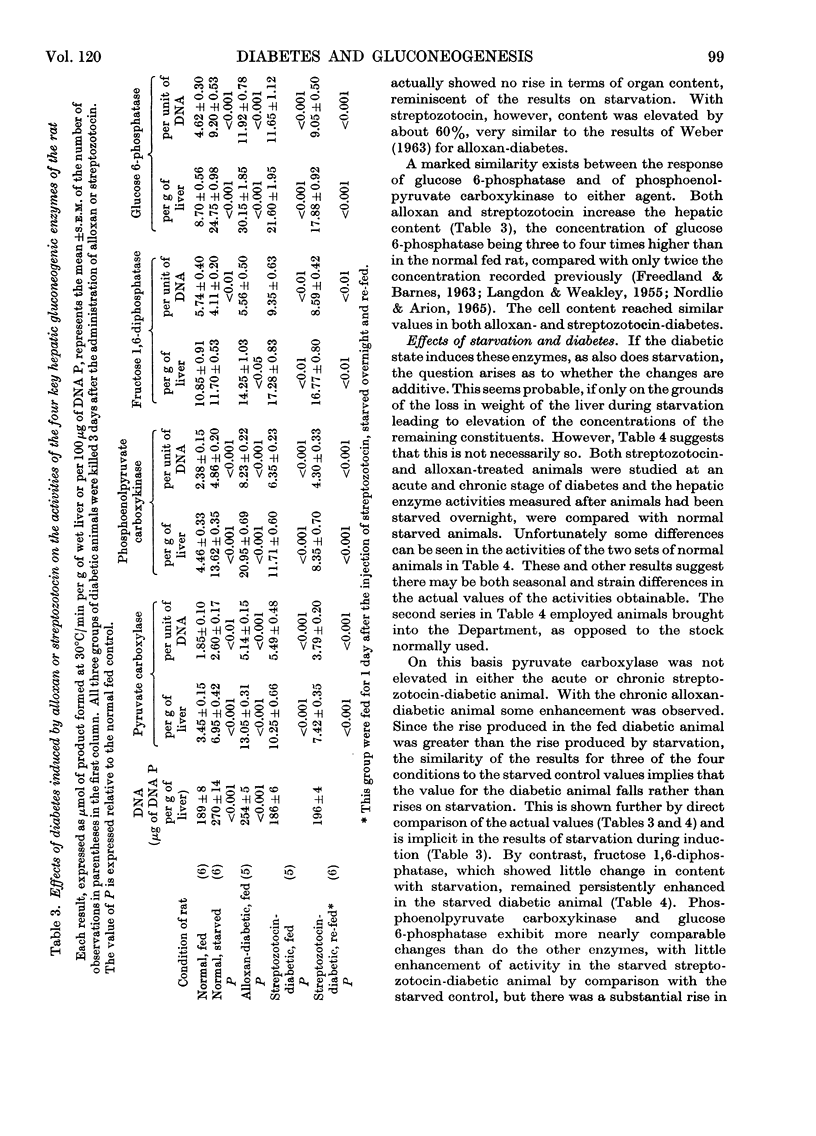
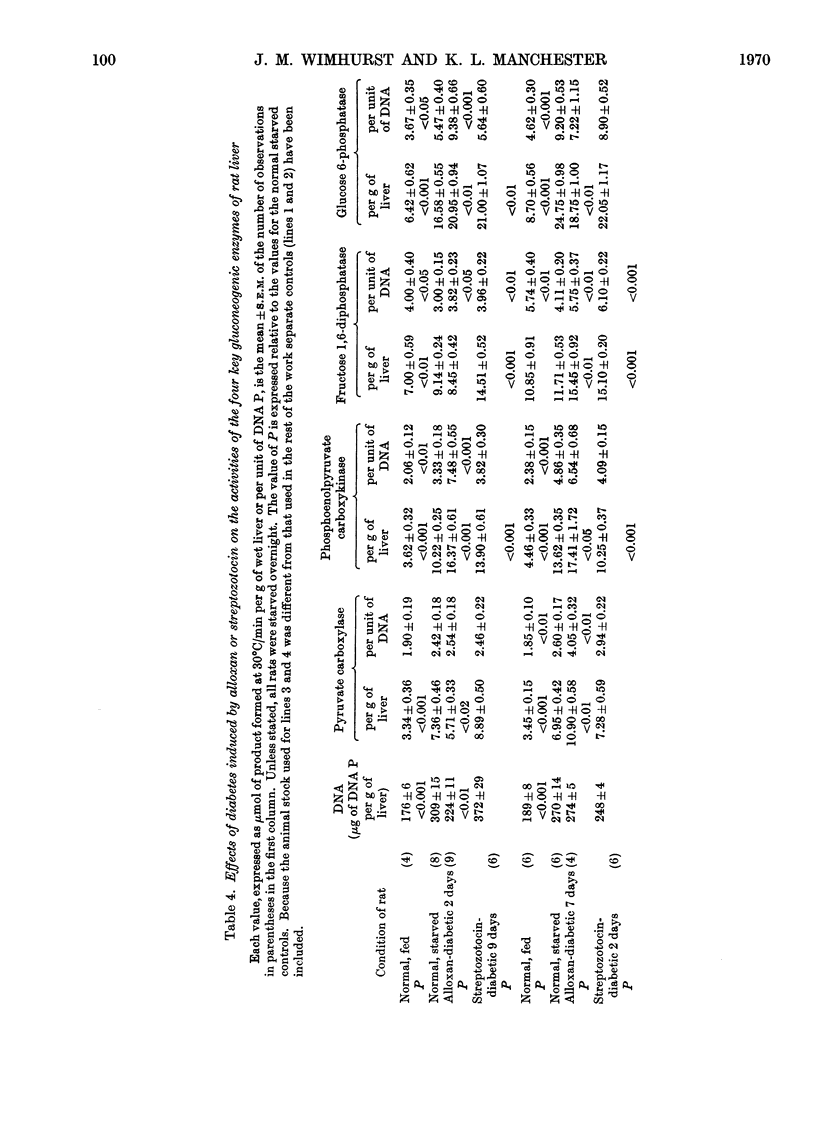
1. Measurements of the activities in rat liver of the four key enzymes involved in gluconeogenesis, i.e. pyruvate carboxylase (EC 6.4.1.1), phosphoenolpyruvate carboxykinase (EC 4.1.1.32), fructose 1,6-diphosphatase (EC 3.1.3.11) and glucose 6-phosphatase (EC 3.1.3.9), have been carried out, all four enzymes being measured in the same liver sample. Changes in activities resulting from starvation and diabetes have been studied. Changes in concentration (activity/unit wet weight of tissue) were compared with changes in the hepatic cellular content (activity/unit of DNA). 2. Each enzyme was found to increase in concentration during starvation for up to 3 days, but only glucose 6-phosphatase and phosphoenolpyruvate carboxykinase showed a significant rise in content. Fructose 1,6-diphosphatase appeared to decrease in content somewhat during the early stages of starvation. 3. There was a marked increase in the concentration of all four enzymes in non-starved rats made diabetic with alloxan or streptozotocin, for the most part similar responses being found for the two diabetogenic agents. On starvation, however, the enzyme contents in the diabetic animals tended to fall, often with streptozotocin-treated animals to values no greater than for the normal overnight-starved rat. Deprivation of food during the period after induction of diabetes with streptozotocin lessened the rise in enzyme activity. 4. The results are compared with other published values and factors such as substrate and activator concentrations likely to influence activity in vivo are considered. 5. Lack of correlation of change in fructose 1,6-diphosphatase with the other enzymes questions whether it should be included in any postulation of control of gluconeogenic enzymes by a single gene unit.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arion W. J., Nordlie R. C. Biological regulation of inorganic pyrophosphate-glucose phosphotransferase and glucose 6-phosphatase. Activation by triamcinolone, in vivo, in the presence of actinomycin D. J Biol Chem. 1967 May 10;242(9):2207–2210. [PubMed] [Google Scholar]
- Ashmore J., Hastings A. B., Nesbett F. B. THE EFFECT OF DIABETES AND FASTING ON LIVER GLUCOSE-6-PHOSPHATASE. Proc Natl Acad Sci U S A. 1954 Aug;40(8):673–678. doi: 10.1073/pnas.40.8.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brech W., Shrago E., Wilken D. Studies on pyruvate carboxylase in rat and human liver. Biochim Biophys Acta. 1970 Feb 24;201(2):145–154. doi: 10.1016/0304-4165(70)90288-6. [DOI] [PubMed] [Google Scholar]
- Böttger I., Wieland O., Brdiczka D., Pette D. Intracellular localization of pyruvate carboxylase and phosphoenolpyruvate carboxykinase in rat liver. Eur J Biochem. 1969 Mar;8(1):113–119. doi: 10.1111/j.1432-1033.1969.tb00503.x. [DOI] [PubMed] [Google Scholar]
- Eisenstein A. B., Strack I. Effects of glucagon on carbohydrate synthesis and enzyme activity in rat liver. Endocrinology. 1968 Dec;83(6):1337–1348. doi: 10.1210/endo-83-6-1337. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Park C. R. The role of cyclic AMP in the control of liver metabolism. Adv Enzyme Regul. 1968;6:391–407. doi: 10.1016/0065-2571(68)90024-1. [DOI] [PubMed] [Google Scholar]
- FLECK A., MUNRO H. N. The precision of ultraviolet absorption measurements in the Schmidt-Thannhauser procedure for nucleic acid estimation. Biochim Biophys Acta. 1962 May 14;55:571–583. doi: 10.1016/0006-3002(62)90836-3. [DOI] [PubMed] [Google Scholar]
- FREEDLAND R. A., BARNES J. K. The effect of adrenalectomy on the adaptation of glucose 6-phosphate-metabolizing enzymes in the liver. J Biol Chem. 1963 Jun;238:1915–1918. [PubMed] [Google Scholar]
- FREEDLAND R. A., HARPER A. E. Metabolic adaptations in higher animals. I. Dietary effects on liver glucose-6-phosphatase. J Biol Chem. 1957 Oct;228(2):743–751. [PubMed] [Google Scholar]
- Felicioli R. A., Gabrielli F., Rossi C. A. The synthesis of phosphoenolpyruvate in the gluconeogenesis. Enzyme levels of chick embryo livers. Eur J Biochem. 1967 Dec;3(1):19–24. doi: 10.1111/j.1432-1033.1967.tb19494.x. [DOI] [PubMed] [Google Scholar]
- Filsell O. H., Jarrett I. G., Taylor P. H., Keech D. B. Effects of fasting, diabetes and glucocorticoids on gluconeogenic enzymes in the sheep. Biochim Biophys Acta. 1969 Jun 17;184(1):54–63. doi: 10.1016/0304-4165(69)90098-1. [DOI] [PubMed] [Google Scholar]
- Foster D. O., Lardy H. A., Ray P. D., Johnston J. B. Alteration of rat liver phosphoenolpyruvate carboxykinase activity by L-tryptophan in vivo and metals in vitro. Biochemistry. 1967 Jul;6(7):2120–2128. doi: 10.1021/bi00859a033. [DOI] [PubMed] [Google Scholar]
- HORNBROOK K. R., BURCH H. B., LOWRY O. H. CHANGES IN SUBSTRATE LEVELS IN LIVER DURING GLYCOGEN SYNTHESIS INDUCED BY LACTATE AND HYDROCORTISONE. Biochem Biophys Res Commun. 1965 Jan 18;18:206–211. doi: 10.1016/0006-291x(65)90741-2. [DOI] [PubMed] [Google Scholar]
- Henning H. V., Stumpf B., Ohly B., Seubert W. On the mechanism of gluconeogenesis and its regulation. 3. The glucogenic capacity and the activities of pyruvate carboxylase and PEP-carboxylase of rat kidney and rat liver after cortisol treatment and starvation. Biochem Z. 1966 Apr 27;344(3):274–288. [PubMed] [Google Scholar]
- Junod A., Lambert A. E., Orci L., Pictet R., Gonet A. E., Renold A. E. Studies of the diabetogenic action of streptozotocin. Proc Soc Exp Biol Med. 1967 Oct;126(1):201–205. doi: 10.3181/00379727-126-32401. [DOI] [PubMed] [Google Scholar]
- Krebs H. A. The regulation of the release of ketone bodies by the liver. Adv Enzyme Regul. 1966;4:339–354. doi: 10.1016/0065-2571(66)90027-6. [DOI] [PubMed] [Google Scholar]
- LANGDON R. G., WEAKLEY D. R. The influence of hormonal factors and of diet upon hepatic glucose-6-phosphatase activity. J Biol Chem. 1955 May;214(1):167–174. [PubMed] [Google Scholar]
- Manchester K. L., Harris E. J. Effect of denervation on the synthesis of ribonucleic acid and deoxyribonucleic acid in rat diaphragm muscle. Biochem J. 1968 Jun;108(2):177–183. doi: 10.1042/bj1080177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansford K. R., Opie L. Comparison of metabolic abnormalities in diabetes mellitus induced by streptozotocin or by alloxan. Lancet. 1968 Mar 30;1(7544):670–671. doi: 10.1016/s0140-6736(68)92103-x. [DOI] [PubMed] [Google Scholar]
- NORDLIE R. C., ARION W. J. LIVER MICROSOMAL GLUCOSE 6-PHOSPHATASE, INORGANIC PYROPHOSPHATASE, AND PYROPHOSPHATE-GLUCOSE PHOSPHOTRANSFERASE. 3. ASSOCIATED NUCLEOSIDE TRIPHOSPHATE- AND NUCLEOSIDE DIPHOSPHATE-GLUCOSE PHOSPHOTRANSFERASE ACTIVITIES. J Biol Chem. 1965 May;240:2155–2164. [PubMed] [Google Scholar]
- Prinz W., Seubert W. Effect of insulin on pyruvate carboxylase in alloxan diabetic animals. Biochem Biophys Res Commun. 1964 Aug 11;16(6):582–585. doi: 10.1016/0006-291x(64)90196-2. [DOI] [PubMed] [Google Scholar]
- Reshef L., Hanson R. W., Ballard F. J. Glyceride-glycerol synthesis from pyruvate. Adaptive changes in phosphoenolpyruvate carboxykinase and pyruvate carboxylase in adipose tissue and liver. J Biol Chem. 1969 Apr 25;244(8):1994–2001. [PubMed] [Google Scholar]
- Ross B. D., Hems R., Krebs H. A. The rate of gluconeogenesis from various precursors in the perfused rat liver. Biochem J. 1967 Mar;102(3):942–951. doi: 10.1042/bj1020942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHRAGO E., LARDY H. A., NORDLIE R. C., FOSTER D. O. METABOLIC AND HORMONAL CONTROL OF PHOSPHOENOLPYRUVATE CARBOXYKINASE AND MALIC ENZYME IN RAT LIVER. J Biol Chem. 1963 Oct;238:3188–3192. [PubMed] [Google Scholar]
- Shrago E., Lardy H. A. Paths of carbon in gluconeogenesis and lipogenesis. II. Conversion of precursors to phosphoenolpyruvate in liver cytosol. J Biol Chem. 1966 Feb 10;241(3):663–668. [PubMed] [Google Scholar]
- Start C., Newsholme E. A. The effects of starvation and alloxan-diabetes on the contents of citrate and other metabolic intermediates in rat liver. Biochem J. 1968 Apr;107(3):411–415. doi: 10.1042/bj1070411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMSON R. Y., HEAGY F. C., HUTCHISON W. C., DAVIDSON J. N. The deoxyribonucleic acid content of the rat cell nucleus and its use in expressing the results of tissue analysis, with particular reference to the composition of liver tissue. Biochem J. 1953 Feb;53(3):460–474. doi: 10.1042/bj0530460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNDERWOOD A. H., NEWSHOLME E. A. SOME PROPERTIES OF FRUCTOSE 1,6-DIPHOSPHATASE OF RAT LIVER AND THEIR RELATION TO THE CONTROL OF GLUCONEOGENESIS. Biochem J. 1965 Jun;95:767–774. doi: 10.1042/bj0950767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER G., CANTERO A. Effect of hypophysectomy on liver enzymes involved in glycogenolysis and in glucogenesis. Am J Physiol. 1959 Sep;197:699–701. doi: 10.1152/ajplegacy.1959.197.3.699. [DOI] [PubMed] [Google Scholar]
- Wagle S. R. Studies on mechanism of glucose synthesis in diabetic and normal rat liver. Diabetes. 1966 Jan;15(1):19–23. doi: 10.2337/diab.15.1.19. [DOI] [PubMed] [Google Scholar]
- Wagle S. R. Studies on pyruvate carboxylase activity in alloxan diabetic and normal animals. Biochem Biophys Res Commun. 1964;14:533–536. doi: 10.1016/0006-291x(64)90264-5. [DOI] [PubMed] [Google Scholar]
- Wimhurst J. M., Manchester K. L. Some aspects of the kinetics of rat liver pyruvate carboxylase. Biochem J. 1970 Nov;120(1):79–93. doi: 10.1042/bj1200079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimhurst J. M., Manchester K. L. Suppression of pyruvate carboxylase by glucose in the perfused rat liver. FEBS Lett. 1970 May 25;8(2):91–94. doi: 10.1016/0014-5793(70)80232-0. [DOI] [PubMed] [Google Scholar]
- YOUNG J. W., SHRAGO E., LARDY H. A. METABOLIC CONTROL OF ENZYMES INVOLVED IN LIPOGENESIS AND GLUCONEOGENESIS. Biochemistry. 1964 Nov;3:1687–1692. doi: 10.1021/bi00899a015. [DOI] [PubMed] [Google Scholar]


